The Role of Adiponectin in the Resolution of Male-Obesity-Associated Secondary Hypogonadism after Metabolic Surgery and Its Impact on Cardiovascular Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Measurements
2.3. Statistics
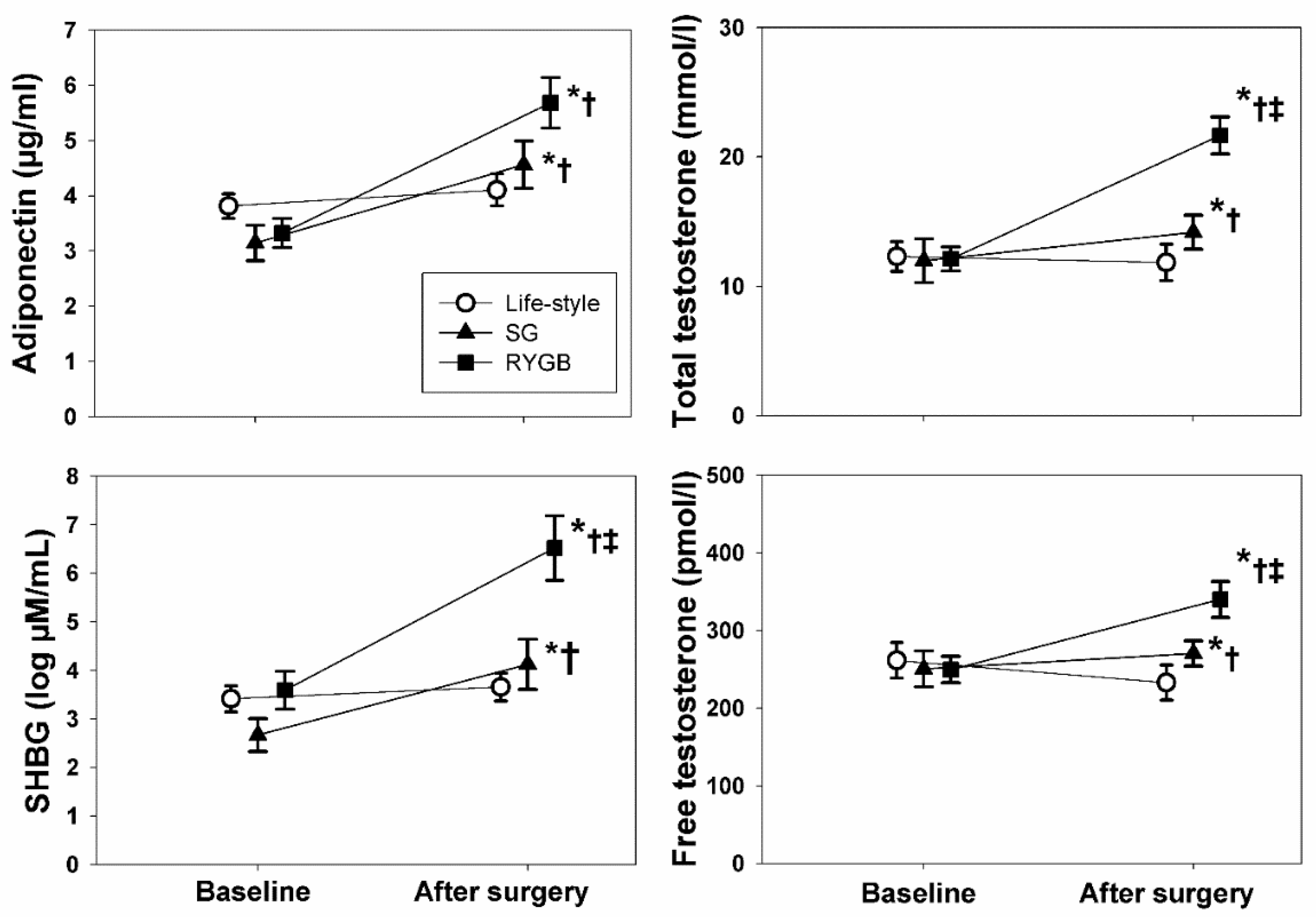
3. Results
3.1. Baseline Characteristics in Patients with and without MOSH
3.2. Changes at Follow-Up Depending on the Presence of MOSH and Its Resolution
3.3. Correlation of Adiponectin Changes at Follow-Up with Other Variables
3.4. Effects of the Type of Therapy
3.5. Ancillary Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderón, B.; Gómez-Martín, J.M.; Vega-Piñero, B.; Martín-Hidalgo, A.; Galindo, J.; Luque-Ramírez, M.; Escobar-Morreale, H.; Botella-Carretero, J.I. Prevalence of male secondary hypogonadism in moderate to severe obesity and its relationship with insulin resistance and excess body weight. Andrology 2016, 4, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Santacruz, E.; Luque-Ramirez, M.; Botella Carretero, J.I. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: A systematic review and meta-analysis. Hum. Reprod. Update 2017, 23, 390–408. [Google Scholar] [CrossRef] [PubMed]
- Rigon, F.A.; Ronsoni, M.F.; Hohl, A.; van de Sande-Lee, S. Effects of Bariatric Surgery in Male Obesity-Associated Hypogonadism. Obes. Surg. 2019, 29, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.J.; Chacko, E.C.; Pappachan, J.M. Male Obesity-related Secondary Hypogonadism–Pathophysiology, Clinical Implications and Management. Eur. Endocrinol. 2019, 15, 83–90. [Google Scholar] [CrossRef]
- Khan, S.M.; Hamnvik, O.P.; Brinkoetter, M.; Mantzoros, C.S. Leptin as a modulator of neuroendocrine function in humans. Yonsei Med. J. 2012, 53, 671–679. [Google Scholar] [CrossRef]
- George, J.T.; Millar, R.P.; Anderson, R.A. Hypothesis: Kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology 2010, 91, 302–307. [Google Scholar] [CrossRef]
- Teerds, K.J.; de Rooij, D.G.; Keijer, J. Functional relationship between obesity and male reproduction: From humans to animal models. Hum. Reprod. Update 2011, 17, 667–683. [Google Scholar] [CrossRef]
- Calderon, B.; Huerta, L.; Galindo, J.; Gonzalez Casbas, J.M.; Escobar-Morreale, H.F.; Martin-Hidalgo, A.; Botella-Carretero, J.I. Lack of Improvement of Sperm Characteristics in Obese Males after Obesity Surgery Despite the Beneficial Changes Observed in Reproductive Hormones. Obes. Surg. 2019, 29, 2045–2050. [Google Scholar] [CrossRef]
- Di Vincenzo, A.; Busetto, L.; Vettor, R.; Rossato, M. Obesity, Male Reproductive Function and Bariatric Surgery. Front. Endocrinol. 2018, 9, 769. [Google Scholar] [CrossRef]
- Kivimaki, M.; Kuosma, E.; Ferrie, J.E.; Luukkonen, R.; Nyberg, S.T.; Alfredsson, L.; Batty, G.D.; Brunner, E.J.; Fransson, E.; Goldberg, M.; et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: Pooled analysis of individual-level data for 120,813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017, 2, e277–e285. [Google Scholar] [CrossRef]
- Berrington de Gonzalez, A.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef]
- Boido, A.; Ceriani, V.; Cetta, F.; Lombardi, F.; Pontiroli, A.E. Bariatric surgery and prevention of cardiovascular events and mortality in morbid obesity: Mechanisms of action and choice of surgery. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 437–443. [Google Scholar] [CrossRef]
- Sjostrom, L. Bariatric surgery and reduction in morbidity and mortality: Experiences from the SOS study. Int. J. Obes. 2008, 32 (Suppl. S7), S93–S97. [Google Scholar] [CrossRef]
- Sjostrom, L.; Gummesson, A.; Sjostrom, C.D.; Narbro, K.; Peltonen, M.; Wedel, H.; Bengtsson, C.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): A prospective, controlled intervention trial. Lancet Oncol. 2009, 10, 653–662. [Google Scholar] [CrossRef]
- De Maddalena, C.; Vodo, S.; Petroni, A.; Aloisi, A.M. Impact of testosterone on body fat composition. J. Cell. Physiol. 2012, 227, 3744–3748. [Google Scholar] [CrossRef]
- Cobeta, P.; Osorio, A.; Cuadrado-Ayuso, M.; Garcia-Moreno, F.; Pestana, D.; Galindo, J.; Botella-Carretero, J.I. Sleeve Gastrectomy and Gastric Bypass Decrease the Carotid Intima-Media Thickness in Obese Men: Association with Weight Loss, Cardiovascular Risk Factors, and Circulating Testosterone. Obes. Surg. 2020, 30, 851–859. [Google Scholar] [CrossRef]
- Bots, M.L.; Hoes, A.W.; Koudstaal, P.J.; Hofman, A.; Grobbee, D.E. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation 1997, 96, 1432–1437. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Polak, J.F.; Kavousi, M.; Mathiesen, E.B.; Volzke, H.; Tuomainen, T.P.; Sander, D.; Plichart, M.; Catapano, A.L.; Robertson, C.M.; et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): A meta-analysis of individual participant data. Lancet 2012, 379, 2053–2062. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Ebrahimi-Mamaeghani, M.; Mohammadi, S.; Arefhosseini, S.R.; Fallah, P.; Bazi, Z. Adiponectin as a potential biomarker of vascular disease. Vasc. Health Risk Manag. 2015, 11, 55–70. [Google Scholar]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. BioMed Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef]
- Butner, K.L.; Nickols-Richardson, S.M.; Clark, S.F.; Ramp, W.K.; Herbert, W.G. A review of weight loss following Roux-en-Y gastric bypass vs restrictive bariatric surgery: Impact on adiponectin and insulin. Obes. Surg. 2010, 20, 559–568. [Google Scholar] [CrossRef]
- Kotidis, E.V.; Koliakos, G.G.; Baltzopoulos, V.G.; Ioannidis, K.N.; Yovos, J.G.; Papavramidis, S.T. Serum ghrelin, leptin and adiponectin levels before and after weight loss: Comparison of three methods of treatment—A prospective study. Obes. Surg. 2006, 16, 1425–1432. [Google Scholar] [CrossRef]
- Malin, S.K.; Bena, J.; Abood, B.; Pothier, C.E.; Bhatt, D.L.; Nissen, S.; Brethauer, S.A.; Schauer, P.R.; Kirwan, J.P.; Kashyap, S.R. Attenuated improvements in adiponectin and fat loss characterize type 2 diabetes non-remission status after bariatric surgery. Diabetes Obes. Metab. 2014, 16, 1230–1238. [Google Scholar] [CrossRef]
- Herder, C.; Peltonen, M.; Svensson, P.A.; Carstensen, M.; Jacobson, P.; Roden, M.; Sjostrom, L.; Carlsson, L. Adiponectin and bariatric surgery: Associations with diabetes and cardiovascular disease in the Swedish Obese Subjects Study. Diabetes Care 2014, 37, 1401–1409. [Google Scholar] [CrossRef]
- Gardener, H.; Sjoberg, C.; Crisby, M.; Goldberg, R.; Mendez, A.; Wright, C.B.; Elkind, M.S.; Sacco, R.L.; Rundek, T. Adiponectin and carotid intima-media thickness in the northern Manhattan study. Stroke 2012, 43, 1123–1125. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Cha, Y.; Chen, Z.P.; Ding, H.Y. Adiponectin levels in non-obese first-degree relatives of type 2 diabetes patients and non-diabetic subjects: A 5-year follow-up study. J. Int. Med. Res. 2010, 38, 792–802. [Google Scholar] [CrossRef]
- Gasbarrino, K.; Gorgui, J.; Nauche, B.; Cote, R.; Daskalopoulou, S.S. Circulating adiponectin and carotid intima-media thickness: A systematic review and meta-analysis. Metabolism 2016, 65, 968–986. [Google Scholar] [CrossRef]
- Martin, L.J. Implications of adiponectin in linking metabolism to testicular function. Endocrine 2014, 46, 16–28. [Google Scholar] [CrossRef]
- Laughlin, G.A.; Barrett-Connor, E.; May, S. Sex-specific association of the androgen to oestrogen ratio with adipocytokine levels in older adults: The Rancho Bernardo Study. Clin. Endocrinol. 2006, 65, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Clarke, S.; Stanworth, R.; Channer, K.S.; Jones, T.H. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur. J. Endocrinol. 2007, 156, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Gomez-Martin, J.M.; Aracil, E.; Galindo, J.; Escobar-Morreale, H.F.; Balsa, J.A.; Botella-Carretero, J.I. Improvement in cardiovascular risk in women after bariatric surgery as measured by carotid intima-media thickness: Comparison of sleeve gastrectomy versus gastric bypass. Surg. Obes. Relat. Dis. 2017, 13, 848–854. [Google Scholar] [CrossRef]
- Hui, E.; Xu, A.; Chow, W.S.; Lee, P.C.; Fong, C.H.; Cheung, S.C.; Tse, H.F.; Chau, M.T.; Cheung, B.M.; Lam, K.S. Hypoadiponectinemia as an independent predictor for the progression of carotid atherosclerosis: A 5-year prospective study. Metab. Syndr. Relat. Disord. 2014, 12, 517–522. [Google Scholar] [CrossRef]
- Sirbu, A.; Stanca, I.; Copaescu, C.; Martin, S.; Albu, A.; Barbu, C.; Fica, S. Association of serum adiponectin and insulin-like growth factor I levels with parameters of cardiac remodeling in severely obese patients. J. Endocrinol. Investig. 2013, 36, 686–692. [Google Scholar]
- Ballantyne, G.H.; Gumbs, A.; Modlin, I.M. Changes in insulin resistance following bariatric surgery and the adipoinsular axis: Role of the adipocytokines, leptin, adiponectin and resistin. Obes. Surg. 2005, 15, 692–699. [Google Scholar] [CrossRef]
- Hindle, A.K.; Edwards, C.; McCaffrey, T.; Fu, S.W.; Brody, F. Reactivation of adiponectin expression in obese patients after bariatric surgery. Surg. Endosc. 2010, 24, 1367–1373. [Google Scholar] [CrossRef]
- de Luis, D.A.; Calvo, S.G.; Pacheco, D.; Ovalle, H.F.; Aller, R. Adiponectin gene variant RS rs266729: Relation to lipid profile changes and circulating adiponectin after bariatric surgery. Surg. Obes. Relat. Dis. 2018, 14, 1402–1408. [Google Scholar] [CrossRef]
- Illan-Gomez, F.; Gonzalvez-Ortega, M.; Orea-Soler, I.; Alcaraz-Tafalla, M.S.; Aragon-Alonso, A.; Pascual-Diaz, M.; Perez-Paredes, M.; Lozano-Almela, M.L. Obesity and inflammation: Change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes. Surg. 2012, 22, 950–955. [Google Scholar] [CrossRef]
- Thakkar, H.; Vincent, V.; Sukhla, S.; Sra, M.; Kanga, U.; Aggarwal, S.; Singh, A. Improvements in cholesterol efflux capacity of HDL and adiponectin contribute to mitigation in cardiovascular disease risk after bariatric surgery in a cohort with morbid obesity. Diabetol. Metab. Syndr. 2021, 13, 46. [Google Scholar] [CrossRef]
- Askarpour, M.; Alizadeh, S.; Hadi, A.; Symonds, M.E.; Miraghajani, M.; Sheikhi, A.; Ghaedi, E. Effect of Bariatric Surgery on the Circulating Level of Adiponectin, Chemerin, Plasminogen Activator Inhibitor-1, Leptin, Resistin, and Visfatin: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2020, 52, 207–215. [Google Scholar] [CrossRef]
- Roumaud, P.; Martin, L.J. Roles of leptin, adiponectin and resistin in the transcriptional regulation of steroidogenic genes contributing to decreased Leydig cells function in obesity. Horm. Mol. Biol. Clin. Investig. 2015, 24, 25–45. [Google Scholar] [CrossRef]
- Elsaied, M.A.; Masallat, D.; Abdel-Hamid, I.A. Correlation of Adiponectin with Testosterone in Patients with and without Type 2 Diabetes and Erectile Dysfunction. Am. J. Mens. Health 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Rasul, S.; Ilhan, A.; Reiter, M.H.; Baumgartner-Parzer, S.; Kautzky-Willer, A. Relations of adiponectin to levels of metabolic parameters and sexual hormones in elderly type 2 diabetic patients. Gend. Med. 2011, 8, 93–102. [Google Scholar] [CrossRef]
- Frederiksen, L.; Hojlund, K.; Hougaard, D.M.; Mosbech, T.H.; Larsen, R.; Flyvbjerg, A.; Frystyk, J.; Brixen, K.; Andersen, M. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur. J. Endocrinol. 2012, 166, 469–476. [Google Scholar] [CrossRef]
- Lanfranco, F.; Zitzmann, M.; Simoni, M.; Nieschlag, E. Serum adiponectin levels in hypogonadal males: Influence of testosterone replacement therapy. Clin. Endocrinol. 2004, 60, 500–507. [Google Scholar] [CrossRef]
- Genchi, V.A.; Rossi, E.; Lauriola, C.; D’Oria, R.; Palma, G.; Borrelli, A.; Caccioppoli, C.; Giorgino, F.; Cignarelli, A. Adipose Tissue Dysfunction and Obesity-Related Male Hypogonadism. Int. J. Mol. Sci. 2022, 23, 8194. [Google Scholar] [CrossRef]
- Gautier, A.; Bonnet, F.; Dubois, S.; Massart, C.; Grosheny, C.; Bachelot, A.; Aubé, C.; Balkau, B.; Ducluzeau, P.H. Associations between Visceral Adipose Tissue, Inflammation and Sex Steroid Concentrations in Men. Clin. Endocrinol. 2013, 78, 373–378. [Google Scholar] [CrossRef]
- Hales, D.B. Testicular Macrophage Modulation of Leydig Cell Steroidogenesis. J. Reprod. Immunol. 2002, 57, 6–18. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, G.; Li, J.; Zhang, J.; Li, F.; Li, K.; Gao, B.; Zeng, L.; Shan, W.; Lin, P.; et al. Hyperleptinemia Directly Affects Testicular Maturation at Different Sexual Stages in Mice, and Suppressor of Cytokine Signaling 3 Is Involved in This Process. Reprod. Biol. Endocrinol. 2014, 12, 15. [Google Scholar] [CrossRef]
- Otani, M.; Kogo, M.; Furukawa, S.; Wakisaka, S.; Maeda, T. The Adiponectin Paralog C1q/TNF-Related Protein 3 (CTRP3) Stimulates Testosterone Production through the CAMP/PKA Signaling Pathway. Cytokine 2012, 58, 238–244. [Google Scholar] [CrossRef]
- Molina-Vega, M.; Asenjo-Plaza, M.; García-Ruiz, M.C.; Varea-Marineto, E.; Casal-Nievas, N.; Alvarez-Millan, J.J.; Cabezas-Sanchez, P.; Cardona-Díaz, F.; Queipo-Ortuño, M.I.; Castellano-Castillo, D.; et al. Cross-Sectional, Primary Care–Based Study of the Prevalence of Hypoandrogenemia in Nondiabetic Young Men with Obesity. Obesity 2019, 27, 1584–1590. [Google Scholar] [CrossRef]
- English, K.M.; Mandour, O.; Steeds, R.P.; Diver, M.J.; Jones, T.H.; Channer, K.S. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur. Heart J. 2000, 21, 890–894. [Google Scholar] [CrossRef]
- Lorigo, M.; Mariana, M.; Oliveira, N.; Lemos, M.C.; Cairrao, E. Vascular Pathways of Testosterone: Clinical Implications. J. Cardiovasc. Trans. Res. 2020, 13, 55–72. [Google Scholar] [CrossRef]
- Mangolim, A.S.; Brito, L.A.R.; Nunes-Nogueira, V.S. Effectiveness of testosterone replacement in men with obesity: A systematic review and meta-analysis. Eur. J. Endocrinol. 2021, 186, 123–135. [Google Scholar] [CrossRef]

| MOSH (n = 30) | No MOSH (n = 30) | |
|---|---|---|
| Age (y) | 47 ± 9 | 49 ± 8 |
| BMI (Kg/m2) | 45.8 ± 6.5 | 42.4 ±5.4 * |
| EBW (kg) | 64.6 ± 20.3 | 54.3 ± 18.0 * |
| cIMT (mm) | 0.64 ± 0.11 | 0.68 ± 0.12 |
| Systolic BP (mmHg) | 136 ± 14 | 137 ± 19 |
| Diastolic BP (mmHg) | 82 ± 11 | 83 ± 11 |
| LDL (mmol/L) | 3.1 ± 0.8 | 3.0 ± 0.9 |
| HDL (mmol/L) | 1.0 ± 0.8 | 1.0 ± 0.4 |
| Glucose (mmol/L) | 6.6 ± 2.3 | 6.2 ± 1.8 |
| Insulin (mU/L) | 24 ± 14 | 23 ± 14 |
| HOMA-IR | 6.8 ± 4.7 | 7.3 ± 7.4 |
| TT (ng/dL) | 253 ± 163 | 440 ± 105 * |
| SHBG (µmol/dL) | 24.5 ± 12.5 | 32.6 ± 13.6 * |
| FT (pmol/L) | 179 ± 46 | 324 ± 68 * |
| APN (µg/mL) | 3.7 ± 1.7 | 3.9 ± 1.5 |
| No MOSH (n = 30) | MOSH Resolved (n = 20) | MOSH Persisted (n = 10) | |
|---|---|---|---|
| EWL (kg) | 44.5 ± 42.0 | 50.6 ± 30.7 | 13.4 ± 27.5 *,† |
| BMI (Kg/m2) | −8.1 ± 8.2 | −9.8 ± 6.6 | −1.9 ± 6.4 *,† |
| Systolic BP (mmHg) | −5.4 ± 25.2 | −11.2 ± 9.6 | −1.6 ± 18.4 |
| Diastolic BP (mmHg) | −1.3 ± 14.1 | −10.9 ± 11.4 *,‡ | −0.7 ± 4.7 |
| LDL (mmol/L) | −0.08 ± 0.88 | −0.14 ± 1.01 | −0.12 ± 0.49 |
| HDL (mmol/L) | −1.05 ± 0.37 | −0.97 ± 0.25 | −0.97 ± 0.28 |
| Glucose (mmol/L) | −0.6 ± 2.1 | −1.0 ± 2.3 | 0.2 ± 0.5 |
| Insulin (mU/L) | −7.5 ± 11.3 | −10.1 ± 10.3 | −8.5 ± 17.6 |
| HOMA-IR | −1.1 ± 7.1 | −3.2 ± 3.8 | 4.9 ± 14.8 |
| Δ APN | Δ TT | Δ SHBG | Δ FT | |
|---|---|---|---|---|
| Δ cIMT | −0.283 * | −0.428 * | −0.347 ** | −0.269 * |
| Δ BMI | −0.266 * | −0.583 ** | −0.691 ** | −0.337 ** |
| Δ Systolic BP | −0.211 | −0.394 ** | −0.205 | −0.419 ** |
| Δ Diastolic BP | −0.059 | −0.291 * | −0.185 | −0.342 ** |
| Δ LDL | −0.230 | −0.099 | −0.060 | −0.035 |
| Δ HDL | 0.046 | 0.442 ** | 0.355 ** | 0.324 * |
| Δ Glucose | −0.135 | −0.421 ** | −0.273 * | −0.379 ** |
| Δ Insulin | −0.158 | −0.516 ** | −0.405 ** | −0.399 ** |
| Δ HOMA-IR | −0.157 | −0.508 ** | −0.378 ** | −0.395 ** |
| Lifestyle (n = 20) | SG (n = 20) | RYGB (n = 20) | ||||
|---|---|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | Baseline | 6 Months | |
| BMI (Kg/m2) | 44.0 ± 5.4 | 45.2 ±7.1 | 45.0 ±6.9 | 33.2 ± 4.1 *,† | 43.7 ± 7.2 | 31.6 ± 6.5 *,† |
| EBW (kg) | 59.4 ± 17.4 | 3.7 ± 14.1 | 61.6 ± 21.2 | 57.4 ± 18.2 *,† | 56.7 ± 20.8 | 69.4 ± 26.2 *,† |
| cIMT (mm) | 0.66 ± 0.10 | 0.67 ± 0.11 | 0.65 ± 0.11 | 0.60 ± 0.09 *,† | 0.66 ± 0.13 | 0.60 ± 0.12 *,† |
| Systolic BP (mmHg) | 145 ± 16 | 151 ± 12 | 141 ± 17 | 130 ± 12 *,† | 144 ± 16 | 129 ± 17 *,† |
| Diastolic BP (mmHg) | 87 ± 9 | 89 ± 8 | 85 ± 11 | 84 ± 8† | 87 ± 11 | 76 ± 12 *,† |
| LDL (mmol/L) | 3.0 ± 0.8 | 3.2 ± 0.6 | 3.0 ± 0.9 | 2.9 ± 0.8 | 2.1 ± 0.8 †,‡ | 1.9 ± 0.7 |
| HDL (mmol/L) | 1.1 ± 0.4 | 1.0 ± 0.3 | 1.0 ± 0.2 | 1.2 ± 0.2 *,† | 1.0 ± 0.2 | 1.2 ± 0.4 *,† |
| Glucose (mmol/L) | 6.6 ± 2.3 | 7.0 ± 2.9 | 5.9 ± 1.9 | 5.4 ± 0.9 † | 7.2 ± 3.3 | 5.5 ± 1.7 *,† |
| Insulin (mU/L) | 30 ± 16 | 27 ± 14 | 19 ± 9† | 10 ± 6 *,† | 21 ± 14 † | 7 ± 3 *,† |
| HOMA-IR | 9.5 ± 8.0 | 15.2 ± 28.6 | 5.1 ± 3.1† | 2.4 ± 1.6 | 6.4 ± 5.7 † | 3.2 ± 5.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobeta, P.; Pariente, R.; Osorio, A.; Marchan, M.; Cuadrado-Ayuso, M.; Pestaña, D.; Galindo, J.; Botella-Carretero, J.I. The Role of Adiponectin in the Resolution of Male-Obesity-Associated Secondary Hypogonadism after Metabolic Surgery and Its Impact on Cardiovascular Risk. Biomedicines 2022, 10, 2000. https://doi.org/10.3390/biomedicines10082000
Cobeta P, Pariente R, Osorio A, Marchan M, Cuadrado-Ayuso M, Pestaña D, Galindo J, Botella-Carretero JI. The Role of Adiponectin in the Resolution of Male-Obesity-Associated Secondary Hypogonadism after Metabolic Surgery and Its Impact on Cardiovascular Risk. Biomedicines. 2022; 10(8):2000. https://doi.org/10.3390/biomedicines10082000
Chicago/Turabian StyleCobeta, Pilar, Roberto Pariente, Alvaro Osorio, Marta Marchan, Marta Cuadrado-Ayuso, David Pestaña, Julio Galindo, and José I. Botella-Carretero. 2022. "The Role of Adiponectin in the Resolution of Male-Obesity-Associated Secondary Hypogonadism after Metabolic Surgery and Its Impact on Cardiovascular Risk" Biomedicines 10, no. 8: 2000. https://doi.org/10.3390/biomedicines10082000
APA StyleCobeta, P., Pariente, R., Osorio, A., Marchan, M., Cuadrado-Ayuso, M., Pestaña, D., Galindo, J., & Botella-Carretero, J. I. (2022). The Role of Adiponectin in the Resolution of Male-Obesity-Associated Secondary Hypogonadism after Metabolic Surgery and Its Impact on Cardiovascular Risk. Biomedicines, 10(8), 2000. https://doi.org/10.3390/biomedicines10082000







