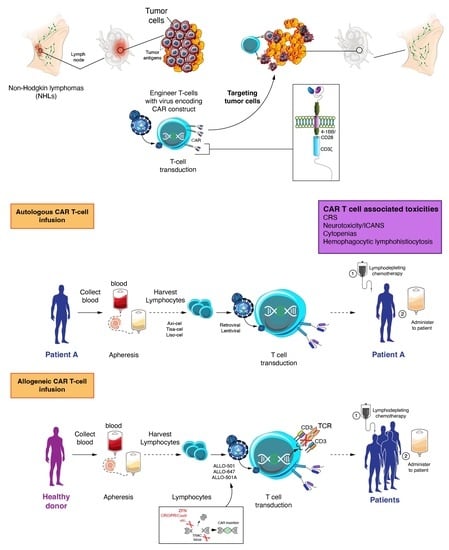
CAR T-Based Therapies in Lymphoma: A Review of Current Practice and Perspectives
Abstract
:1. Introduction
2. Efficacy of Autologous CAR T Cell Therapy in Lymphoma
2.1. Aggressive Lymphoma
2.1.1. Large B-Cell Lymphoma
- Pivotal clinical trials in ≥2 lines
- Real-world evidence and outpatient setting
- Randomized clinical trials in earlier lines of therapy
- Primary and secondary CNS involvement
2.1.2. Mantle Cell Lymphoma (MCL)
2.1.3. T-Cell Lymphoma
2.2. Indolent Lymphoma
2.2.1. Follicular Lymphoma and Marginal Zone Lymphoma
2.2.2. Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma
2.3. Hodgkin Lymphoma
3. CAR T Cell Associated Toxicities
3.1. Cytokine Release Syndrome (CRS)
3.1.1. CRS Definition and Severity
3.1.2. CRS Management
3.2. Neurotoxicity/ICANS
3.2.1. ICANS Grading
3.2.2. ICANS Management
3.3. Other Toxicities Associated with Auto-CAR T Cell CD19 Products
4. Allogeneic CAR T Cell Development and Activity in NHL
5. Conclusions and Future Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surveillance, Epidemiology, and End Results Program. Available online: https://seer.cancer.gov/statfacts/html/nhl.html (accessed on 7 July 2022).
- Globocan. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/34-non-hodgkin-lymphoma-fact-sheet.pdf (accessed on 7 July 2022).
- Savoldo, B.; Ramos, C.A.; Liu, E.; Mims, M.P.; Keating, M.J.; Carrum, G.; Kamble, R.T.; Bollard, C.M.; Gee, A.P.; Mei, Z.; et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor–modified T cells in lymphoma patients. J. Clin. Investig. 2011, 121, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Hematology/Oncology (Cancer) Approvals and Safety Notifications. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications (accessed on 7 July 2022).
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- Chapuy, B. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef]
- Sarkozy, C.; Traverse-Glehen, A.; Coiffier, B. Double-hit and double-protein-expression lymphomas: Aggressive and refractory lymphomas. Lancet Oncol. 2015, 16, e555–e567. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Coiffier, B.; Lepage, E.; Brière, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van Den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef]
- Gisselbrecht, C.; Glass, B.; Mounier, N.; Singh Gill, D.; Linch, D.C.; Trneny, M.; Bosly, A.; Ketterer, N.; Shpilberg, O.; Hagberg, H.; et al. Salvage Regimens With Autologous Transplantation for Relapsed Large B-Cell Lymphoma in the Rituximab Era. J. Clin. Oncol. 2010, 28, 4184–4190. [Google Scholar] [CrossRef]
- Crump, M.; Neelapu, S.S.; Farooq, U.; Van Den Neste, E.; Kuruvilla, J.; Westin, J.; Link, B.K.; Hay, A.; Cerhan, J.R.; Zhu, L.; et al. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 2017, 130, 1800–1808. [Google Scholar] [CrossRef]
- Jacobson, C.; Locke, F.L.; Ghobadi, A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Hill, B.T.; Timmerman, J.M.; Deol, A.; et al. Long-Term (≥4 Year and ≥5 Year) Overall Survival (OS) By 12- and 24-Month Event-Free Survival (EFS): An Updated Analysis of ZUMA-1, the Pivotal Study of Axicabtagene Ciloleucel (Axi-Cel) in Patients (Pts) with Refractory Large B-Cell Lymphoma (LBCL). Blood 2021, 138, 1764. [Google Scholar] [CrossRef]
- Schuster, S.J.; Tam, C.S.; Borchmann, P.; Worel, N.; McGuirk, J.P.; Holte, H.; Waller, E.K.; Jaglowski, S.; Bishop, M.R.; Damon, L.E.; et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021, 22, 1403–1415. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.E.; Purev, E.; Maloney, D.G.; Andreadis, C.; Sehgal, A.R.; et al. Two-Year Follow-up of Transcend NHL 001, a Multicenter Phase 1 Study of Lisocabtagene Maraleucel (liso-cel) in Relapsed or Refractory (R/R) Large B-Cell Lymphomas (LBCL). Blood 2021, 138, 2840. [Google Scholar] [CrossRef]
- Schuster, S.J.; Zhang, J.; Yang, H.; Agarwal, A.; Tang, W.; Martinez-Prieto, M.; Bollu, V.; Kuzan, D.; Maziarz, R.T.; Kersten, M.J. Comparative efficacy of tisagenlecleucel and lisocabtagene maraleucel among adults with relapsed/refractory large B-cell lymphomas: An indirect treatment comparison. Leuk. Lymphoma 2022, 63, 845–854. [Google Scholar] [CrossRef]
- Oluwole, O.O.; Jansen, J.P.; Lin, V.W.; Chan, K.; Keeping, S.; Navale, L.; Locke, F.L. Comparing Efficacy, Safety, and Preinfusion Period of Axicabtagene Ciloleucel versus Tisagenlecleucel in Relapsed/Refractory Large B Cell Lymphoma. Biol. Blood Marrow Transplant. 2020, 26, 1581–1588. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Ma, Q.; Yang, H.; Signorovitch, J.; Wu, E. A Review of Two Regulatory Approved Anti-CD19 CAR T-Cell Therapies in Diffuse Large B-Cell Lymphoma: Why Are Indirect Treatment Comparisons Not Feasible? Adv. Ther. 2020, 37, 3040–3058. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Ma, Q.; Yang, H.; Signorovitch, J.; Wu, E. Letter to the Editor Regarding “Comparing Efficacy, Safety, and Preinfusion Period of Axicabtagene Ciloleucel versus Tisagenlecleucel in Relapsed/Refractory Large B Cell Lymphoma”. Biol. Blood Marrow Transplant. 2020, 26, e333–e334. [Google Scholar] [CrossRef]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Hunter, B.D.; Redd, R.; Rodig, S.J.; Chen, P.-H.; Wright, K.; Lipschitz, M.; Ritz, J.; Kamihara, Y.; Armand, P.; et al. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J. Clin. Oncol. 2020, 38, 3095–3106. [Google Scholar] [CrossRef]
- Pasquini, M.C.; Locke, F.L.; Herrera, A.F.; Siddiqi, T.; Ghobadi, A.; Komanduri, K.V.; Hu, Z.-H.; Dong, H.; Hematti, P.; Nikiforow, S.; et al. Post-Marketing Use Outcomes of an Anti-CD19 Chimeric Antigen Receptor (CAR) T Cell Therapy, Axicabtagene Ciloleucel (Axi-Cel), for the Treatment of Large B Cell Lymphoma (LBCL) in the United States (US). Blood 2019, 134, 764. [Google Scholar] [CrossRef]
- Pasquini, M.C.; Hu, Z.-H.; Curran, K.; Laetsch, T.; Locke, F.; Rouce, R.; Pulsipher, M.A.; Phillips, C.L.; Keating, A.; Frigault, M.J.; et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020, 4, 5414–5424. [Google Scholar] [CrossRef]
- Riedell, P.A.; Walling, C.; Nastoupil, L.J.; Pennisi, M.; Maziarz, R.T.; McGuirk, J.P.; Oluwole, O.O.; Bachanova, V.; Hwang, W.-T.; Schuster, S.J.; et al. A Multicenter Retrospective Analysis of Outcomes and Toxicities with Commercial Axicabtagene Ciloleucel and Tisagenlecleucel for Relapsed/Refractory Aggressive B-Cell Lymphomas. Biol. Blood Marrow Transplant. 2020, 26, S41–S42. [Google Scholar] [CrossRef]
- Svoboda, J.; Ballard, H.J.; Chong, E.A.; LaRose, M.I.; Bair, S.M.; Namoglu, E.C.; Hughes, M.E.; Nasta, S.D.; Landsburg, D.J.; Barta, S.K.; et al. Use of Bendamustine for Lymphodepletion before Tisagenlecleucel (anti-CD19 CAR T cells) for Aggressive B-Cell Lymphomas. Blood 2019, 134 (Suppl. 1), 1606. [Google Scholar] [CrossRef]
- Le Gouill, S.; Bachy, E.; di Blasi, R.; Cartron, G.; Beauvais, D.; le Bras, F.; Gros, F.-X.; Choquet, S.; Bories, P.; Rubio, M.-T.; et al. First Results of DLBCL Patients Treated with CAR-T-Cells and Enrolled in DESCAR-T Registry, a French Real-Life Database for CAR-T-Cells in Hematologic Malignancies. In Proceedings of the EHA, Virtual, 9–17 June 2021. [Google Scholar]
- Jaglowski, S.; Hu, Z.-H.; Zhang, Y.; Kamdar, M.; Ghosh, M.; Lulla, P.; Sasine, J.; Perales, M.-A.; Hematti, P.; Nikiforow, S.; et al. Tisagenlecleucel Chimeric Antigen Receptor (CAR) T-Cell Therapy for Adults with Diffuse Large B-Cell Lymphoma (DLBCL): Real World Experience from the Center for International Blood & Marrow Transplant Research (CIBMTR) Cellular Therapy (CT) Registry. Blood 2019, 134, 766. [Google Scholar] [CrossRef]
- Sesques, P.; Ferrant, E.; Safar, V.; Wallet, F.; Tordo, J.; Dhomps, A.; Karlin, L.; Brisou, G.; Vercasson, M.; Hospital-Gustem, C.; et al. Commercial anti-CD19 CAR T cell therapy for patients with relapsed/refractory aggressive B cell lymphoma in a European center. Am. J. Hematol. 2020, 95, 1324–1333. [Google Scholar] [CrossRef]
- Kuhnl, A.; Roddie, C.; Martinez-Cibrian, N.; Menne, T.F.; Linton, K.; Lugthart, S.; Chaganti, S.; Sanderson, R.; Marzolini, M.A.V.; Norman, J.; et al. Real-World Data of High-Grade Lymphoma Patients Treated with CD19 CAR-T in England. Blood 2019, 134, 767. [Google Scholar] [CrossRef]
- Stolz, S.; Roncador, M.; Rösler, W.; Zenz, T.; Manz, M.G.; Müller, A.M.; Widmer, C.C. Introducing innovative cellular therapies into the clinic: A 2-year retrospective experience of a chimeric antigen receptor T-cell programme at a single centre in Switzerland. Swiss Med. Wkly. 2022, 152, w30186. [Google Scholar] [CrossRef]
- Casadei, B.; Argnani, L.; Guadagnuolo, S.; Pellegrini, C.; Stefoni, V.; Broccoli, A.; Nanni, L.; Morigi, A.; Lolli, G.; Guarino, M.; et al. Real World Evidence of CAR T-Cell Therapies for the Treatment of Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma: A Monocentric Experience. Cancers 2021, 13, 4789. [Google Scholar] [CrossRef]
- Jain, M.D.; Jacobs, M.T.; Nastoupil, L.J.; Spiegel, J.Y.; Feng, G.; Lin, Y.; Lunning, M.A.; Dahiya, S.; Lekakis, L.J.; Reagan, P.M.; et al. Characteristics and Outcomes of Patients Receiving Bridging Therapy While Awaiting Manufacture of Standard of Care Axicabtagene Ciloleucel CD19 Chimeric Antigen Receptor (CAR) T-Cell Therapy for Relapsed/Refractory Large B-Cell Lymphoma: Results from the US Lymphoma CAR-T Consortium. Blood 2019, 134, 245. [Google Scholar] [CrossRef]
- Pinnix, C.C.; Gunther, J.R.; Dabaja, B.S.; Strati, P.; Fang, P.; Hawkins, M.C.; Adkins, S.; Westin, J.; Ahmed, S.; Fayad, L.; et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Adv. 2020, 4, 2871–2883. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Santomasso, B.D.; Nastoupil, L.J.; Adkins, S.; Lacchetti, C.; Schneider, B.J.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Chimeric Antigen Receptor T-Cell Therapy: ASCO Guideline. J. Clin. Oncol. 2021, 39, 3978–3992. [Google Scholar] [CrossRef]
- Hayden, P.J.; Roddie, C.; Bader, P.; Basak, G.W.; Bonig, H.; Bonini, C.; Chabannon, C.; Ciceri, F.; Corbacioglu, S.; Ellard, R.; et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann. Oncol. 2022, 33, 259–275. [Google Scholar] [CrossRef]
- Godwin, J.E.; Mattar, B.; Maris, M.; Bachier, C.; Stevens, D.; Hoda, D.; Varela, J.C.; Cherry, M.; Fanning, S.; Essell, J.; et al. Outreach: Results from a Phase 2 Study of Lisocabtagene Maraleucel (liso-cel) Administered As Inpatient (Inpt) or Outpatient (Outpt) Treatment in the Nonuniversity Setting in Patients (Pts) with R/R Large B-Cell Lymphoma (LBCL). Blood 2021, 138, 1762. [Google Scholar] [CrossRef]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.; Perales, M.-A.; Kersten, M.J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.P.; Pagel, J.M.; et al. Primary Analysis of ZUMA-7: A Phase 3 Randomized Trial of Axicabtagene Ciloleucel (Axi-Cel) Versus Standard-of-Care Therapy in Patients with Relapsed/Refractory Large B-Cell Lymphoma. Blood 2021, 138, 2. [Google Scholar] [CrossRef]
- Bishop, M.R.; Dickinson, M.; Purtill, D.; Barba, P.; Santoro, A.; Hamad, N.; Kato, K.; Sureda, A.; Greil, R.; Thieblemont, C.; et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 629–639. [Google Scholar] [CrossRef]
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): Results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022, 399, 2294–2308. [Google Scholar] [CrossRef]
- Crump, M.; Kuruvilla, J.; Couban, S.; MacDonald, D.A.; Kukreti, V.; Kouroukis, C.T.; Rubinger, M.; Buckstein, R.; Imrie, K.R.; Federico, M.; et al. Randomized Comparison of Gemcitabine, Dexamethasone, and Cisplatin Versus Dexamethasone, Cytarabine, and Cisplatin Chemotherapy Before Autologous Stem-Cell Transplantation for Relapsed and Refractory Aggressive Lymphomas: NCIC-CTG LY.12. J. Clin. Oncol. 2014, 32, 3490–3496. [Google Scholar] [CrossRef]
- Van Imhoff, G.W.; McMillan, A.; Matasar, M.J.; Radford, J.; Ardeshna, K.M.; Kuliczkowski, K.; Kim, W.; Hong, X.; Goerloev, J.S.; Davies, A.; et al. Ofatumumab Versus Rituximab Salvage Chemoimmunotherapy in Relapsed or Refractory Diffuse Large B-Cell Lymphoma: The ORCHARRD Study. J. Clin. Oncol. 2017, 35, 544–551. [Google Scholar] [CrossRef]
- Elsawy, M.; Chavez, J.C.; Avivi, I.; Larouche, J.-F.; Wannesson, L.; Cwynarski, K.; Osman, K.; Davison, K.; Rudzki, J.D.; Dahiya, S.; et al. Patient-Reported Outcomes in a Phase 3, Randomized, Open-Label Study Evaluating the Efficacy of Axicabtagene Ciloleucel (Axi-Cel) Versus Standard of Care Therapy in Patients with Relapsed/Refractory Large B-Cell Lymphoma (ZUMA-7). Blood 2021, 138, 430. [Google Scholar] [CrossRef]
- Abramson, J.S.; Solomon, S.R.; Arnason, J.E.; Johnston, P.B.; Glass, B.; Crotta, A.; Montheard, S.; Previtali, A.; Liu, F.F.; Braverman, J.; et al. Improved Quality of Life (QOL) with Lisocabtagene Maraleucel (liso-cel), a CD19-Directed Chimeric Antigen Receptor (CAR) T Cell Therapy, Compared with Standard of Care (SOC) As Second-Line (2L) Treatment in Patients (Pts) with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL): Results from the Phase 3 Transform Study. Blood 2021, 138, 3845. [Google Scholar] [CrossRef]
- Karschnia, P.; Rejeski, K.; Winkelmann, M.; Schöberl, F.; Bücklein, V.L.; Blumenberg, V.; Schmidt, C.; Blobner, J.; von Bergwelt-Baildon, M.; Tonn, J.-C.; et al. Toxicities and Response Rates of Secondary CNS Lymphoma After Adoptive Immunotherapy With CD19-Directed Chimeric Antigen Receptor T Cells. Neurology 2022, 98, 884. [Google Scholar] [CrossRef]
- Ghafouri, S.; Timmerman, J.; Larson, S.; Mead, M.D. Axicabtagene Ciloleucel CAR T-cell therapy for relapsed/refractory secondary CNS non-Hodgkin lymphoma: Comparable outcomes and toxicities, but shorter remissions may warrant alternative consolidative strategies? Bone Marrow Transplant. 2021, 56, 974–977. [Google Scholar] [CrossRef]
- Bennani, N.N.; Maurer, M.J.; Nastoupil, L.J.; Jain, M.D.; Chavez, J.C.; Cashen, A.F.; Dahiya, S.; Lekakis, L.J.; Reagan, P.M.; Oluwole, O.O.; et al. Experience with Axicabtagene Ciloleucel (Axi-cel) in Patients with Secondary CNS Involvement: Results from the US Lymphoma CAR T Consortium. Blood 2019, 134, 763. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Xun, R.; Liu, M.; Sun, Z.; Huang, J. Efficacy and Safety of Axicabtagene Ciloleucel and Tisagenlecleucel Administration in Lymphoma Patients With Secondary CNS Involvement: A Systematic Review. Front. Immunol. 2021, 12, 693200. [Google Scholar] [CrossRef]
- Frigault, M.J.; Dietrich, J.; Gallagher, K.; Roschewski, M.; Jordan, J.T.; Forst, D.; Plotkin, S.R.; Cook, D.; Casey, K.S.; Lindell, K.A.; et al. Safety and efficacy of tisagenlecleucel in primary CNS lymphoma: A phase 1/2 clinical trial. Blood 2022, 139, 2306–2315. [Google Scholar] [CrossRef]
- Siddiqi, T.; Wang, X.; Blanchard, M.S.; Wagner, J.R.; Popplewell, L.L.; Budde, L.E.; Stiller, T.L.; Clark, M.C.; Lim, L.; Vyas, V.; et al. CD19-directed CAR T-cell therapy for treatment of primary CNS lymphoma. Blood Adv. 2021, 5, 4059–4063. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. Three-Year Follow-Up of KTE-X19 in Patients With Relapsed/Refractory Mantle Cell Lymphoma, Including High-Risk Subgroups, in the ZUMA-2 Study. J. Clin. Oncol. 2022. [Google Scholar] [CrossRef]
- Palomba, M.L.; Gordon, L.I.; Siddiqi, T.; Abramson, J.S.; Kamdar, M.; Lunning, M.A.; Maloney, D.G.; Andreadis, C.; Arnason, J.E.; Ghosh, N.; et al. Safety and Preliminary Efficacy in Patients with Relapsed/Refractory Mantle Cell Lymphoma Receiving Lisocabtagene Maraleucel in Transcend NHL 001. Blood 2020, 136, 10–11. [Google Scholar] [CrossRef]
- Wang, Y.; Jain, P.; Locke, F.L.; Munoz, J.; Maurer, M.J.; Beitinjaneh, A.; Frank, M.J.; Dahiya, S.; McGuirk, J.P.; Jacobs, M.T.; et al. Brexucabtagene Autoleucel for Relapsed/Refractory Mantle Cell Lymphoma: Real World Experience from the US Lymphoma CAR T Consortium. Blood 2021, 138, 744. [Google Scholar] [CrossRef]
- Iacoboni, G.; Rejeski, K.; Villacampa, G.; van Doesum, J.A.; Chiappella, A.; Bonifazi, F.; Lopez-Corral, L.; van Aalderen, M.; Kwon, M.; Martínez-Cibrian, N.; et al. Real-world evidence of brexucabtagene autoleucel for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 2022, 6, 3606–3610. [Google Scholar] [CrossRef]
- Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Rahbarizadeh, F. CAR-T cell therapy in T-cell malignancies: Is success a low-hanging fruit? Stem Cell Res. Ther. 2021, 12, 527. [Google Scholar] [CrossRef]
- Lu, P.; Liu, Y.; Yang, J.; Zhang, X.; Yang, X.; Wang, H.; Wang, L.; Wang, Q.; Jin, D.; Li, Q.; et al. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: First-in-human phase 1 clinical trial. Blood 2022, 140, 321–334. [Google Scholar] [CrossRef]
- Sureda, A.; Zhang, M.; Dreger, P.; Carreras, J.; Fenske, T.; Finel, H.; Schouten, H.; Montoto, S.; Robinson, S.; Smith, S.M.; et al. Allogeneic hematopoietic stem cell transplantation for relapsed follicular lymphoma: A combined analysis on behalf of the Lymphoma Working Party of the EBMT and the Lymphoma Committee of the CIBMTR. Cancer 2018, 124, 1733–1742. [Google Scholar] [CrossRef]
- Freedman, A. Follicular lymphoma: 2018 update on diagnosis and management. Am. J. Hematol. 2018, 93, 296–305. [Google Scholar] [CrossRef]
- Casulo, C.; Byrtek, M.; Dawson, K.L.; Zhou, X.; Farber, C.M.; Flowers, C.R.; Hainsworth, J.D.; Maurer, M.J.; Cerhan, J.R.; Link, B.K.; et al. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J. Clin. Oncol. 2015, 33, 2516–2522. [Google Scholar] [CrossRef]
- Ghione, P.; Ghesquieres, H.; Bobillo, S.; Patel, A.R.; Kanters, S.; Deighton, K.; Dong, H.; Yang, Y.; Ma, L.; Limbrick-Oldfield, E.H.; et al. Outcomes in Later-Lines of Therapy for Relapsed/Refractory Follicular Lymphoma: Results from the International Scholar-5 Study. Hematol. Oncol. 2021, 39. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Wilson, W.H.; Janik, J.E.; Dudley, M.E.; Stetler-Stevenson, M.; Feldman, S.A.; Maric, I.; Raffeld, M.; Nathan, D.-A.N.; Lanier, B.J.; et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010, 116, 4099–4102. [Google Scholar] [CrossRef]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, A.V.; Gauthier, J.; Hay, K.A.; Voutsinas, J.M.; Wu, Q.; Pender, B.S.; Hawkins, R.M.; Vakil, A.; Steinmetz, R.N.; Riddell, S.R.; et al. High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy. Blood 2019, 134, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, C.A.; Chavez, J.C.; Sehgal, A.R.; William, B.M.; Munoz, J.; Salles, G.; Munshi, P.N.; Casulo, C.; Maloney, D.G.; de Vos, S.; et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022, 23, 91–103. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Chavez, J.C.; Sehgal, A.R.; Epperla, N.; Ulrickson, M.L.; Bachy, E.; Munshi, P.N.; Casulo, C.; Maloney, D.G.; de Vos, S.; et al. Long-Term Follow-up Analysis of ZUMA-5: A Phase 2 Study of Axicabtagene Ciloleucel (Axi-Cel) in Patients with Relapsed/Refractory (R/R) Indolent Non-Hodgkin Lymphoma (iNHL). Blood 2021, 138, 93. [Google Scholar] [CrossRef]
- Ghione, P.; Palomba, M.L.; Patel, A.; Bobillo, S.; Deighton, K.; Jacobson, C.A.; Nahas, M.; Hatswell, A.J.; Jung, A.S.; Kanters, S.; et al. Comparative effectiveness of ZUMA-5 (axi-cel) vs. SCHOLAR-5 external control in relapsed/refractory follicular lymphoma. Blood 2022. [Google Scholar] [CrossRef]
- Fowler, N.H.; Dickinson, M.; Dreyling, M.; Martinez-Lopez, J.; Kolstad, A.; Butler, J.; Ghosh, M.; Popplewell, L.; Chavez, J.C.; Bachy, E.; et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: The phase 2 ELARA trial. Nat. Med. 2022, 28, 325–332. [Google Scholar] [CrossRef]
- Brentjens, R.J.; Rivière, I.; Park, J.H.; Davila, M.L.; Wang, X.; Stefanski, J.; Taylor, C.; Yeh, R.; Bartido, S.; Borquez-Ojeda, O.; et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011, 118, 4817–4828. [Google Scholar] [CrossRef]
- Turtle, C.J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Cherian, S.; Chen, X.; Wood, B.; Lozanski, A.; Byrd, J.C.; Heimfeld, S.; et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor–Modified T Cells After Failure of Ibrutinib. J. Clin. Oncol. 2017, 35, 3010–3020. [Google Scholar] [CrossRef]
- Gauthier, J.; Hirayama, A.V.; Purushe, J.; Hay, K.A.; Lymp, J.; Li, D.H.; Yeung, C.C.S.; Sheih, A.; Pender, B.S.; Hawkins, R.M.; et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood 2020, 135, 1650–1660. [Google Scholar] [CrossRef]
- Porter, D.L.; Hwang, W.-T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalez, V.; et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015, 7, 303ra139. [Google Scholar] [CrossRef]
- Gill, S.I.; Vides, V.; Frey, N.V.; Metzger, S.; O’Brien, M.; Hexner, E.; Mato, A.R.; Lacey, S.F.; Melenhorst, J.J.; Pequignot, E.; et al. Prospective Clinical Trial of Anti-CD19 CAR T Cells in Combination with Ibrutinib for the Treatment of Chronic Lymphocytic Leukemia Shows a High Response Rate. Blood 2018, 132, 298. [Google Scholar] [CrossRef]
- Siddiqi, T.; Soumerai, J.D.; Dorritie, K.A.; Stephens, D.M.; Riedell, P.A.; Arnason, J.; Kipps, T.J.; Gillenwater, H.H.; Gong, L.; Yang, L.; et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood 2022, 139, 1794–1806. [Google Scholar] [CrossRef]
- Wierda, W.G.; Dorritie, K.A.; Munoz, J.; Stephens, D.M.; Solomon, S.R.; Gillenwater, H.H.; Gong, L.; Yang, L.; Ogasawara, K.; Thorpe, J.; et al. Transcend CLL 004: Phase 1 Cohort of Lisocabtagene Maraleucel (liso-cel) in Combination with Ibrutinib for Patients with Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL). Blood 2020, 136, 39–40. [Google Scholar] [CrossRef]
- Flinn, I.; Marris, M.; Wierda, W.G.; Coutre, S.; Pagel, J.M.; Byrd, J.C.; Goyal, L.; Goodman, K.; Zheng, Y.; Milletti, F.; et al. ZUMA-8: A phase 1/2 multicenter study evaluating KTE-X19 in patients (pts) with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). J. Clin. Oncol. 2019, 37, TPS7566. [Google Scholar] [CrossRef]
- Kittai, A.S.; Bond, D.A.; William, B.; Saad, A.; Penza, S.; Efebera, Y.; Larkin, K.; Wall, S.A.; Choe, H.K.; Bhatnagar, B.; et al. Clinical activity of axicabtagene ciloleucel in adult patients with Richter syndrome. Blood Adv. 2020, 4, 4648–4652. [Google Scholar] [CrossRef]
- Benjamini, O.; Shimoni, A.; Besser, M.; Shem-Tov, N.; Danylesko, I.; Yerushalmi, R.; Merkel, D.G.; Tadmor, T.; Lavie, D.; Fineman, R.; et al. Safety and Efficacy of CD19-CAR T Cells in Richter’s Transformation after Targeted Therapy for Chronic Lymphocytic Leukemia. Blood 2020, 136 (Suppl. 1), 40. [Google Scholar] [CrossRef]
- Ortiz-Maldonado, V.; Frigola, G.; Español-Rego, M.; Balagué, O.; Martínez-Cibrián, N.; Magnano, L.; Giné, E.; Pascal, M.; Correa, J.G.; Martínez-Roca, A.; et al. Results of ARI-0001 CART19 Cells in Patients With Chronic Lymphocytic Leukemia and Richter’s Transformation. Front. Oncol. 2022, 12, 828471. [Google Scholar] [CrossRef]
- Wang, C.M.; Wu, Z.Q.; Wang, Y.; Guo, Y.L.; Dai, H.R.; Wang, X.H.; Li, X.; Zhang, Y.J.; Zhang, W.Y.; Chen, M.X.; et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clin. Cancer Res. 2017, 23, 1156–1166. [Google Scholar] [CrossRef]
- Ramos, C.A.; Grover, N.S.; Beaven, A.W.; Lulla, P.D.; Wu, M.-F.; Ivanova, A.; Wang, T.; Shea, T.C.; Rooney, C.M.; Dittus, C.; et al. Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J. Clin. Oncol. 2020, 38, 3794–3804. [Google Scholar] [CrossRef]
- Sang, W.; Wang, X.; Geng, H.; Li, T.; Li, D.; Zhang, B.; Zhou, Y.; Song, X.; Sun, C.; Yan, D.; et al. Anti-PD-1 Therapy Enhances the Efficacy of CD30-Directed Chimeric Antigen Receptor T Cell Therapy in Patients With Relapsed/Refractory CD30+ Lymphoma. Front. Immunol. 2022, 13, 858021. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 2016, 127, 3321–3330. [Google Scholar] [CrossRef]
- Hay, K.A.; Hanafi, L.-A.; Li, D.; Gust, J.; Liles, W.C.; Wurfel, M.M.; López, J.A.; Chen, J.; Chung, D.; Harju-Baker, S.; et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor–modified T-cell therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef]
- Teachey, D.T.; Lacey, S.F.; Shaw, P.A.; Melenhorst, J.J.; Maude, S.L.; Frey, N.; Pequignot, E.; Gonzalez, V.E.; Chen, F.; Finklestein, J.; et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016, 6, 664–679. [Google Scholar] [CrossRef]
- Giavridis, T.; van der Stegen, S.J.C.; Eyquem, J.; Hamieh, M.; Piersigilli, A.; Sadelain, M. CAR T cell–induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 2018, 24, 731–738. [Google Scholar] [CrossRef]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef]
- Singh, N.; Hofmann, T.J.; Gershenson, Z.; Levine, B.L.; Grupp, S.A.; Teachey, D.T.; Barrett, D.M. Monocyte lineage–derived IL-6 does not affect chimeric antigen receptor T-cell function. Cytotherapy 2017, 19, 867–880. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Karschnia, P.; Jordan, J.T.; Forst, D.A.; Arrillaga-Romany, I.C.; Batchelor, T.T.; Baehring, J.M.; Clement, N.F.; Gonzalez Castro, L.N.; Herlopian, A.; Maus, M.V.; et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood 2019, 133, 2212–2221. [Google Scholar] [CrossRef]
- Obstfeld, A.E.; Frey, N.V.; Mansfield, K.; Lacey, S.F.; June, C.H.; Porter, D.L.; Melenhorst, J.J.; Wasik, M.A. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: Clinicopathological insights. Blood 2017, 130, 2569–2572. [Google Scholar] [CrossRef]
- Schubert, M.-L.; Schmitt, M.; Wang, L.; Ramos, C.A.; Jordan, K.; Müller-Tidow, C.; Dreger, P. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann. Oncol. 2021, 32, 34–48. [Google Scholar] [CrossRef]
- Alvi, R.M.; Frigault, M.J.; Fradley, M.G.; Jain, M.D.; Mahmood, S.S.; Awadalla, M.; Lee, D.H.; Zlotoff, D.A.; Zhang, L.; Drobni, Z.D.; et al. Cardiovascular Events Among Adults Treated With Chimeric Antigen Receptor T-Cells (CAR-T). J. Am. Coll. Cardiol. 2019, 74, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Seethapathy, H.; Strohbehn, I.A.; Frigault, M.J.; O’Donnell, E.K.; Jacobson, C.A.; Motwani, S.S.; Parikh, S.M.; Curhan, G.C.; Reynolds, K.L.; et al. Acute Kidney Injury and Electrolyte Abnormalities After Chimeric Antigen Receptor T-Cell (CAR-T) Therapy for Diffuse Large B-Cell Lymphoma. Am. J. Kidney Dis. 2020, 76, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Santomasso, B.D.; Park, J.H.; Salloum, D.; Riviere, I.; Flynn, J.; Mead, E.; Halton, E.; Wang, X.; Senechal, B.; Purdon, T.; et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov. 2018, 8, 958–971. [Google Scholar] [CrossRef]
- Gust, J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial Activation and Blood–Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef]
- Nair, R.; Drillet, G.; Lhomme, F.; Le Bras, A.; Michel, L.; Rossi, J.; Sherman, M.; Xue, A.; Kerber, A.; Jittapiromsak, N.; et al. Acute leucoencephalomyelopathy and quadriparesis after CAR T-cell therapy. Haematologica 2020, 106, 1504–1506. [Google Scholar] [CrossRef]
- Parker, K.R.; Migliorini, D.; Perkey, E.; Yost, K.E.; Bhaduri, A.; Bagga, P.; Haris, M.; Wilson, N.E.; Liu, F.; Gabunia, K.; et al. Single-Cell Analyses Identify Brain Mural Cells Expressing CD19 as Potential Off-Tumor Targets for CAR-T Immunotherapies. Cell 2020, 183, 126–142.e17. [Google Scholar] [CrossRef]
- Jain, M.D.; Bachmeier, C.A.; Phuoc, V.H.; Chavez, J.C. Axicabtagene ciloleucel (KTE-C19), an anti-CD19 CAR T therapy for the treatment of relapsed/refractory aggressive B-cell non-Hodgkin’s lymphoma. Ther. Clin. Risk Manag. 2018, 14, 1007–1017. [Google Scholar] [CrossRef]
- Jain, T.; Bar, M.; Kansagra, A.J.; Chong, E.A.; Hashmi, S.K.; Neelapu, S.S.; Byrne, M.; Jacoby, E.; Lazaryan, A.; Jacobson, C.A.; et al. Use of Chimeric Antigen Receptor T Cell Therapy in Clinical Practice for Relapsed/Refractory Aggressive B Cell Non-Hodgkin Lymphoma: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biol. Blood Marrow Transplant. 2019, 25, 2305–2321. [Google Scholar] [CrossRef]
- Cordeiro, A.; Bezerra, E.D.; Hirayama, A.V.; Hill, J.A.; Wu, Q.V.; Voutsinas, J.; Sorror, M.L.; Turtle, C.J.; Maloney, D.G.; Bar, M. Late Events after Treatment with CD19-Targeted Chimeric Antigen Receptor Modified T Cells. Biol. Blood Marrow Transplant. 2020, 26, 26–33. [Google Scholar] [CrossRef]
- Hill, J.A.; Giralt, S.; Torgerson, T.R.; Lazarus, H.M. CAR-T—And a side order of IgG, to go?—Immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev. 2019, 38, 100596. [Google Scholar] [CrossRef]
- Hill, J.A.; Seo, S.K. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood 2020, 136, 925–935. [Google Scholar] [CrossRef]
- Sharma, N.; Reagan, P.M.; Liesveld, J.L. Cytopenia after CAR-T Cell Therapy—A Brief Review of a Complex Problem. Cancers 2022, 14, 1501. [Google Scholar] [CrossRef]
- Chiappella, A.; Guidetti, A.; Dodero, A.; Bramanti, S.; Zinzani, P.L.; Santoro, A.; Casadei, B.; Di Rocco, A.; Carrabba, M.G.; Chiusolo, P.; et al. Real-Life CAR-T Cell Treatment in Large B-Cell Lymphomas Indicates That Axi-Cel and Tisa-Cel Have Similar Outcomes, but Long-Term Cytopenia Is an Emerging Problem. Blood 2021, 138, 3867. [Google Scholar] [CrossRef]
- Spiegel, J.Y.; Dahiya, S.; Jain, M.D.; Tamaresis, J.S.; Nastoupil, L.; Jacobs, M.T.; Ghobadi, A.; Lin, Y.; Lunning, M.; Lekakis, L.J.; et al. Outcomes of Patients with Large B-cell Lymphoma Progressing after Axicabtagene Ciloleucel. Blood 2020, 137, 1832–1835. [Google Scholar] [CrossRef]
- Chong, E.A.; Ruella, M.; Schuster, S.J. Five-Year Outcomes for Refractory B-Cell Lymphomas with CAR T-Cell Therapy. N. Engl. J. Med. 2021, 384, 673–674. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Rossi, J.M.; Jacobson, C.A.; Locke, F.L.; Miklos, D.B.; Reagan, P.M.; Rodig, S.J.; Lekakis, L.J.; Flinn, I.W.; Zheng, L.; et al. CD19-Loss with Preservation of Other B Cell Lineage Features in Patients with Large B Cell Lymphoma Who Relapsed Post-Axi-Cel. Blood 2019, 134, 203. [Google Scholar] [CrossRef]
- Khurana, A.; Lin, Y. Allogeneic Chimeric Antigen Receptor Therapy in Lymphoma. Curr. Treat. Options Oncol. 2022, 23, 171–187. [Google Scholar] [CrossRef]
- Jeyakumar, N.; Smith, M. Custom CARs: Leveraging the Adaptability of Allogeneic CAR Therapies to Address Current Challenges in Relapsed/Refractory DLBCL. Front. Immunol. 2022, 13, 887866. [Google Scholar] [CrossRef]
- Brudno, J.N.; Somerville, R.P.T.; Shi, V.; Rose, J.J.; Halverson, D.C.; Fowler, D.H.; Gea-Banacloche, J.C.; Pavletic, S.Z.; Hickstein, D.D.; Lu, T.L.; et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation without Causing Graft-Versus-Host Disease. J. Clin. Oncol. 2016, 34, 1112–1121. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Munoz, J.; Locke, F.L.; Miklos, D.B.; Brown, R.; McDevitt, J.T.; Mardiros, A.; Demirhan, E.; Konto, C.; Tees, M.T. First-in-human data of ALLO-501 and ALLO-647 in relapsed/refractory large B-cell or follicular lymphoma (R/R LBCL/FL): ALPHA study. J. Clin. Oncol. 2020, 38, 8002. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Nath, R.; Munoz, J.; Tees, M.; Miklos, D.B.; Frank, M.J.; Malik, S.A.; Stevens, D.; Shin, C.R.; Balakumaran, A.; et al. ALPHA Study: ALLO-501 Produced Deep and Durable Responses in Patients with Relapsed/Refractory Non-Hodgkin’s Lymphoma Comparable to Autologous CAR T. Blood 2021, 138, 3878. [Google Scholar] [CrossRef]
- Lekakis, L.J.; Locke, F.L.; Tees, M.; Neelapu, S.S.; Malik, S.A.; Hamadani, M.; Frank, M.J.; Popplewell, L.L.; Abramson, J.S.; de Vos, S.; et al. ALPHA2 Study: ALLO-501A Allogeneic CAR T in LBCL, Updated Results Continue to Show Encouraging Safety and Efficacy with Consolidation Dosing. Blood 2021, 138, 649. [Google Scholar] [CrossRef]
- Locke, F.L.; Malik, S.; Tees, M.T.; Neelapu, S.S.; Popplewell, L.; Abramson, J.S.; McDevitt, J.T.; Shin, C.R.; Demirhan, E.; Konto, C.; et al. First-in-human data of ALLO-501A, an allogeneic chimeric antigen receptor (CAR) T-cell therapy and ALLO-647 in relapsed/refractory large B-cell lymphoma (R/R LBCL): ALPHA2 study. J. Clin. Oncol. 2021, 39, 2529. [Google Scholar] [CrossRef]
- Shah, B.D.; Jacobson, C.A.; Solomon, S.; Jain, N.; Vainorius, M.; Heery, C.R.; He, F.C.; Reshef, R.; Herrera, A.F.; Akard, L.P.; et al. Preliminary safety and efficacy of PBCAR0191, an allogeneic, off-the-shelf CD19-targeting CAR-T product, in relapsed/refractory (r/r) CD19+ NHL. J. Clin. Oncol. 2021, 39, 7516. [Google Scholar] [CrossRef]
- CRISPRTX Website. Available online: https://crisprtx.gcs-web.com/static-files/E5304031-1ceb-4db3-8451-08b1adcd3ee8 (accessed on 7 July 2022).
| CAR T Product | Year of Approval | Clinical Trial | Study Design | Patient Population | Engineering and Manufacturing Characteristics | Dose | Median Time from Leukapheresis to Product Release (Days) | Lympho-Depleting Regimen |
|---|---|---|---|---|---|---|---|---|
| Axi-cel (KTE-019, Yescarta) | 2017 | ZUMA-1 (NCT02348216) | Phase 2 single-arm, open-label, multicenter, international | LBCL ≥ 2 lines | CD28, retrovirus Fresh leukapheresis | 2 × 106 cells/kg (max. 2 × 108 cells/kg) | 17 | Flu 30 mg/m2 + Cy 500 mg/m2 daily × 3d Flu 30 mg/m2 + Cy 500 mg/m2 daily × 3d |
| 2021 | ZUMA-5 (NCT03105336) | Phase 2 single-arm, open-label, multicenter, international | FL ≥ 3 lines | 17 | ||||
| 2022 1 | ZUMA-7 (NCT03391466) | Phase 3 randomized, multicenter, international | LBCL ≥ 1 lines | 13 | ||||
| Brexu-cel (KTE-X19, Tecartus) | 2020 | ZUMA-2 (NCT02601313) | Phase 2 single-arm, open-label, multicenter, international | MCL ≥ 3 lines | CD28, retrovirus Fresh leukapheresis | 16 | ||
| Tisa-cel (CTL019, Kymriah) | 2018 | JULIET (NCT02445248) | Phase 2 single-arm, open-label, multicenter, international | LBCL ≥ 2 lines | 4-1BB, lentivirus Frozen leukapheresis | 0.6–6 × 108 cells | 54 | Flu 25 mg/m2 + Cy 250 mg/m2 daily × 3d or Be 90 mg/m2 daily × 2d |
| Liso-cel (JCAR017, Breyanzi) | 2021 | TRANSCEND (NCT02631044) | Phase 1 single-arm, open-label, multicenter, international | LBCL ≥ 2 lines | 4-1BB, retrovirus Fresh leukapheresis | 50–110 × 106 cells (Separate infusions of CD4+/CD8+ CAR-T cells at 1:1 dose ratio) | 24 | Flu 30 mg/m2 + Cy 300 mg/m2 daily × 3d |
| 2022 1 | TRANSFORM (NCT03575351) | Phase 3 randomized, multicenter, international | LBCL ≥ 1 lines | 26 |
| Variable | ZUMA-1 NCT02348216 | JULIET NCT02445248 | TRANSCEND NCT02631044 | ZUMA-2 NCT02601313 | ZUMA-5 1 NCT03105336 |
|---|---|---|---|---|---|
| Auto-CAR product | Axi-cel | Tisa-cel | Liso-cel | Brexu-cel | Axi-cel |
| Histologic type (%) | DLBCL (76), PMBL (8), tFL (16) | DLBCL (80), HGBL (15), tFL (18), Other (2) | DLBCL (51), HGBL (13), FL grade 3b (1), PMBL (6), tFL (22), tiNHL (7) | MCL | iNHL, including FL (84) and MZL (16) |
| Enrolled patients–no/Infused patients–no (%) | 111/101 (91) | 165/115 (69) | 344/269 (85) 2 | 74/58 (92) | 127/124 (98) |
| Median age, yr (range) | 58 (23–76) | 56 (27–76) | 63 (18–86) | 65 (38–19) | 60 (34–79) |
| Bridging therapy (%patients) | Corticosteroids (NA) | Chemotherapy (93) | Chemotherapy (59) | Any (35) | Any (4) |
| Median prior lines of therapy (range) | 3 (2–4) | 3 (1–6) | 3 (1–8) | 3 (1–5) 3 | 3 (2–4) 4 |
| Best overall response rate (%) | 74 | 53 | 73 | 91 | 94 |
| Complete response rate (%) | 54 | 39 | 53 | 68 | 79 |
| Median follow-up (mo) | 51.1 | 40.3 | 29.3 | 35.6 | 30.9 |
| Median duration of response (mo) | 11.1 | NE | 23.1 | 38.6 | NR |
| Median progression-free survival (mo) | 5.9 | 2.9 | 6.8 | 39.6 | NR |
| Progression-free survival at 24 mo (%) | 40 | 35 | 40.6 | 52.9 | 65.6 (18 mo) |
| Progression-free survival among patients with CR at 24 mo (%) | 70 | 80 | 49.5 | 71.8 | NR |
| Median overall survival (mo) | 25.8 | 11.1 | 27.3 | NR | NR |
| Overall survival at 24 mo (%) | 44 (48 mo) | 45 | 50.5 | ~84 | 88 (18 mo) |
| Adverse Events grade ≥3 (%) | 98 | 89 | 79 | 99 | 85 |
| Serious Adverse Events(%) | 48 | 65 | 45 | 68 | 46 |
| Adverse Events of special interest | |||||
| Cytokine release syndrome (CRS) 5 | |||||
| All (%) | 92 | 58 | 42 | 91 | 78 |
| Grade ≥3 (%) | 11 | 17 | 2 | 15 | 6 |
| Tocilizumab | 43 7 | 24 | 18 8 | 59 | 50 (all iNHL) |
| Corticosteroids (%) | 27 7 | 16 | 2 | 22 | 18 (all iNHL) |
| Vasopressors (%) | 13 | 10 | 3 | 16 | 5 (all iNHL) |
| Neurological events 6 | |||||
| All (%) | 67 | 20 | 30 | 63 | 56 |
| Grade ≥3 (%) | 32 7 | 11 | 10 | 31 | 15 |
| Tocilizumab | 43 7 | 20 | NA | 26 | 36 |
| Corticosteroids (%) | 27 | 12 | NA | 38 | 6 |
| Infections grade ≥ 3 (%) | 28 | 19 | 12 | 32 | 18 (all iNHL) |
| Late cytopenia grade ≥ 3 9 (%) | 38 | 32 | 37 | 26 | 33 |
| Immunoglobulin (%) | 31 | 33 | 21 | 32 | 9 (all iNHL) |
| Variable | ZUMA-7 NCT03391466 | BELINDA NCT03391466 | TRANSFORM NCT03575351 |
|---|---|---|---|
| CAR product | Axi-cel | Tisa-cel | Liso-cel |
| Primary end-point definition (Event-free survival) | SD or PD up to day 150, new lymphoma treatment, death | SD or PD disease at week 12, death | SD or PD at week 9, new lymphoma treatment, death |
| Crossover (%) | Not permitted | Allowed (51) | Allowed (55) |
| Manufacturing success (%) | 100 | 97 | 99 |
| Lymphodepleting regimen | Flu 30 mg/m2 + Cy 500 mg/m2 daily × 3 days | Flu 25 mg/m2 + Cy 250 mg/m2 daily × 3 days 1 | Flu 30 mg/m2 + Cy 300 mg/m2 daily × 3 days |
| Enrolled patients–no (assigned to CAR) | 359 (180) | 322 (162) | 182 (92) |
| CAR-infused patients–no (%) | 170 (94) | 155 (96) | 89 (97) 2 |
| Median time to infusion (days) | 13 | 52 | 36 |
| Bridging therapy (%) | Corticosteroids only (36) | Chemotherapy (83) | Chemotherapy (63) |
| Histologic type (%) | |||
| DLBCL (ABC subtype) | 70 (9) | 62 (32) | 58 (23) |
| HGBL | 17 | 24 | 24 |
| PMBL | - | 7 | 9 |
| FL grade 3b | - | 3 | 1 |
| tiNHL | 11 | 17 | 8 |
| Other | 13 | 3 | 1 |
| Secondary CNS involvement | - | 3 | - |
| Median age, yr | 58 (range 21–80) | 59.5 (range 19–79) | 60 (IQR 54–68) |
| Secondary IPI score ≥ 2 (%) | 46 | 65 | 40 |
| Refractory disease 3,4 (%) | 74 | 66 | 45 |
| Best overall response rate (%) | 83 | 46 | 86 |
| Complete response rate (%) | 65 | 28 | 66 |
| Median progression-free survival (mo) | 14.5 | NA | 14.8 |
| Progression-free survival (%) | ~46 (24 mo) | NA | 52 (12 mo) |
| Median event-free survival (mo) | 8.3 | 3 | 10.1 |
| Event-free survival (%) | 40.5 (24 mo) | NA | 45 (12 mo) |
| Median overall survival (mo) | NR | NR | NR |
| Overall survival at 24 months (%) | ~61 (24 mo) | NA | ~79 (12 mo) |
| Median follow-up (mo) | 25 | 10 | 6.2 |
| Adverse Event grade ≥ 3 (%) | 91 | 84 | 92 |
| Serious Adverse Events (%) | 50 | 36 | 48 |
| Adverse Events of special interest | |||
| Cytokine release syndrome | |||
| All (%) | 92 | 61 | 49 |
| Grade ≥ 3 (%) | 6 | 5 | 1 |
| Tocilizumab | 64 | 52 | 23 5 |
| Corticosteroids (%) | 24 | 17 | - |
| Vasopressors (%) | 6 | NA | - |
| Neurological events 6 | |||
| All (%) | 60 | 10 | 12 |
| Grade ≥ 3 (%) | 21 | 2 | 4 |
| Corticosteroids (%) | 32 | NA | 8 7 |
| Infections grade ≥ 3 | 14 | NA | 15 |
| Cytopenia grade ≥ 3 (>30 days) 8 | 29 | NA | 43 |
| Characteristic | Auto-CAR | Allo-CAR |
|---|---|---|
| Cell source and product |
|
|
| Manufacturing process |
|
|
| Availability |
|
|
| Side effects |
|
|
| Repeat dosing | Possible but may require repeat apheresis, under investigation | Possible and can consider alternative donor, under investigation |
| Persistence | Months to years | Weeks to months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheikh, S.; Migliorini, D.; Lang, N. CAR T-Based Therapies in Lymphoma: A Review of Current Practice and Perspectives. Biomedicines 2022, 10, 1960. https://doi.org/10.3390/biomedicines10081960
Sheikh S, Migliorini D, Lang N. CAR T-Based Therapies in Lymphoma: A Review of Current Practice and Perspectives. Biomedicines. 2022; 10(8):1960. https://doi.org/10.3390/biomedicines10081960
Chicago/Turabian StyleSheikh, Semira, Denis Migliorini, and Noémie Lang. 2022. "CAR T-Based Therapies in Lymphoma: A Review of Current Practice and Perspectives" Biomedicines 10, no. 8: 1960. https://doi.org/10.3390/biomedicines10081960
APA StyleSheikh, S., Migliorini, D., & Lang, N. (2022). CAR T-Based Therapies in Lymphoma: A Review of Current Practice and Perspectives. Biomedicines, 10(8), 1960. https://doi.org/10.3390/biomedicines10081960