Metabolic Syndrome and Overactive Bladder Syndrome May Share Common Pathophysiologies
Abstract
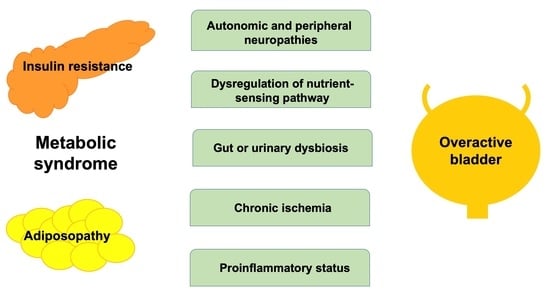
1. Introduction
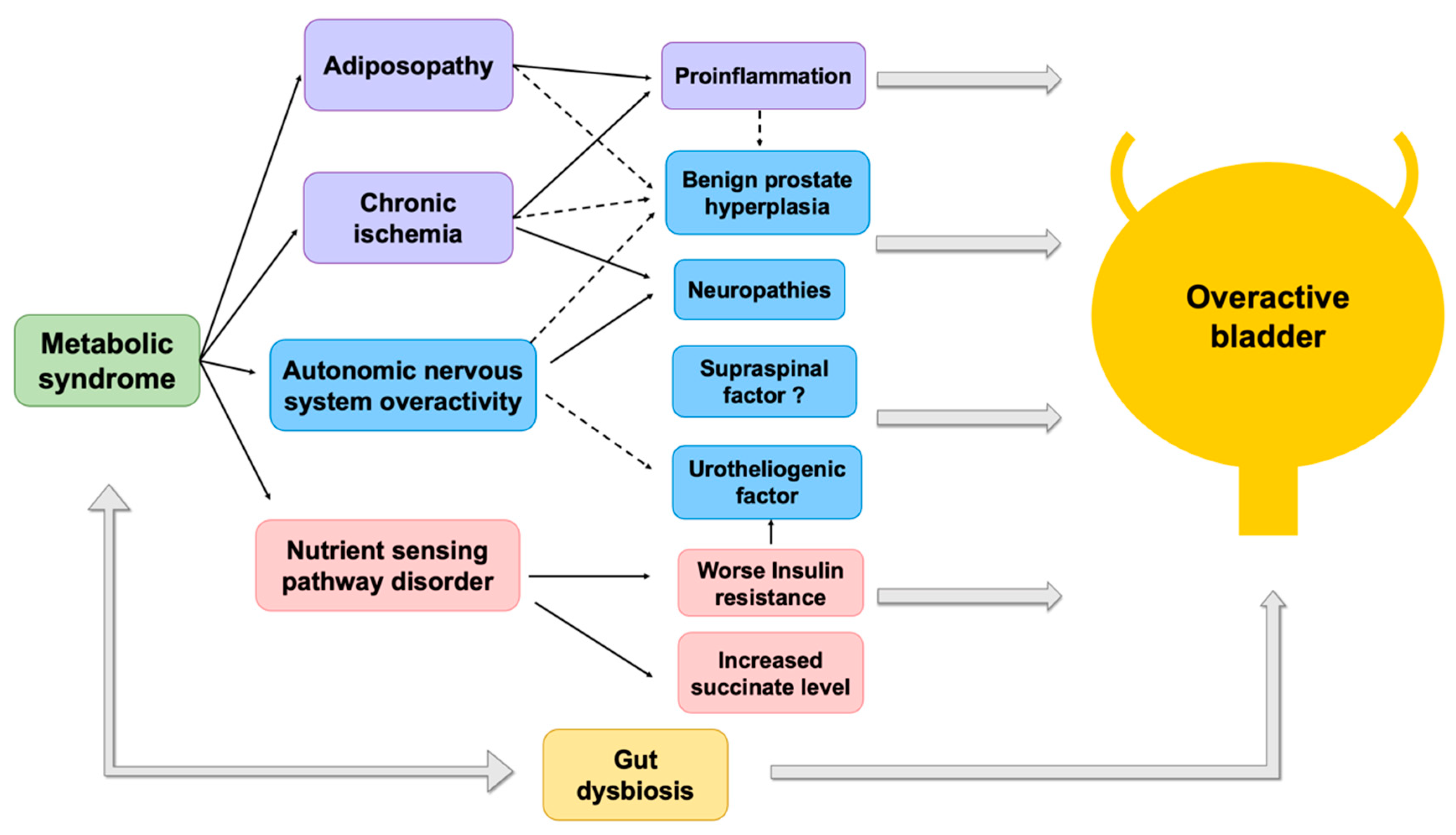
2. Pathophysiology of MetS
3. Pathophysiologies of OAB Associated with MetS
4. Impaired Vesical Afferents and Efferents Activity
5. Chronic Bladder Ischemia
5.1. Pelvis Ischemia
5.2. MetS Associated OAB Secondary to BPH
6. Chronic Low Grade Proinflammatory State
6.1. CRP
6.2. Neurotrophins
6.3. Prostaglandins
7. Dysregulation of Nutrient-Sensing Pathways
7.1. Insulin Resistance at the Bladder Mucosa
7.2. Excessive Succinate Intake
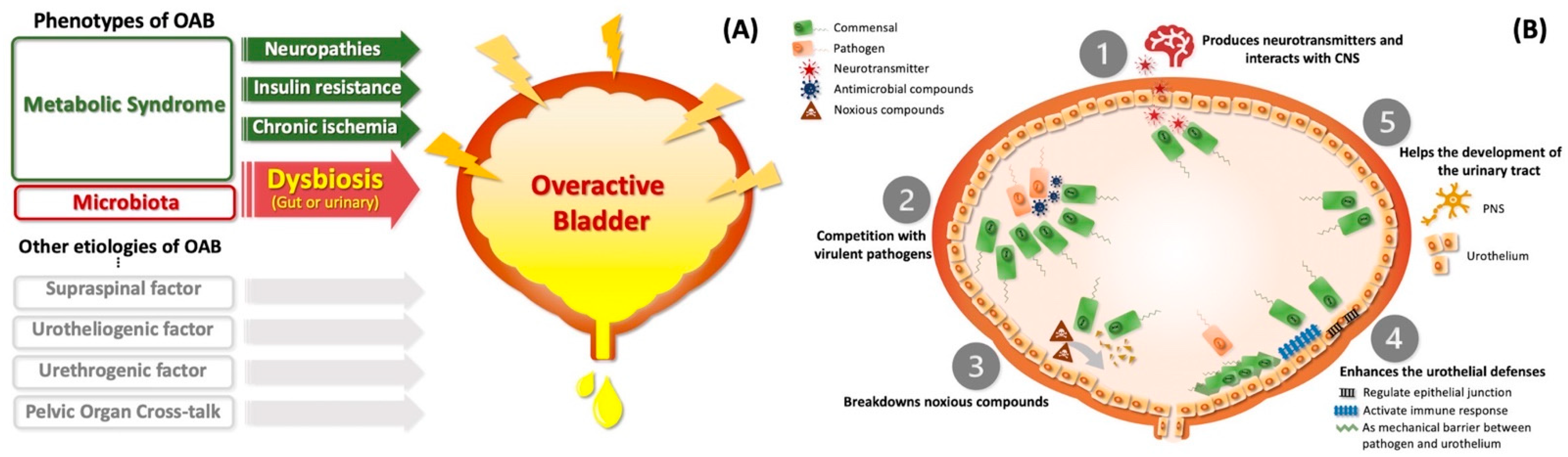
8. The Role of Dysbiosis between MetS and OAB Syndrome in Perspective
8.1. Urinary Microbiome Might Contribute to OAB Syndrome
8.2. MetS Alters the Microbiome and Might Shape a Subtype of OAB
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Falkner, B.; Cossrow, N.D. Prevalence of metabolic syndrome and obesity-associated hypertension in the racial ethnic minorities of the United States. Curr. Hypertens. Rep. 2014, 16, 449. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: A systematic review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, W.S.; Fowke, J.; Dmochowski, R. The Burden of Overactive Bladder on US Public Health. Curr. Bladder Dysfunct. Rep. 2016, 11, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Liu, S.P.; Lee, K.S.; Liao, L.; Wang, J.; Yoo, T.K.; Chu, R.; Sumarsono, B. Prevalence of overactive bladder in China, Taiwan and South Korea: Results from a cross-sectional, population-based study. Low. Urin. Tract Symptoms 2019, 11, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.J. Overactive bladder. In Campbell-Walsh Urology, 11th ed.; Wein, A.J., Kavoussi, L.R., Partin, A.W., Peters, C.A., Eds.; Elsevier: Philadelphia, PA, USA, 2016; Volume III, Chapter 76; pp. 1796–1806. [Google Scholar]
- Peyronnet, B.; Mironska, E.; Chapple, C.; Cardozo, L.; Oelke, M.; Dmochowski, R.; Amarenco, G.; Gamé, X.; Kirby, R.; Van Der Aa, F.; et al. A compressive review of overactive bladder pathophysiology: On the way to tailored treatment. Eur. Urol. 2019, 75, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P. Editorial comment. Neurourol. Urodyn. 1999, 18, 551. [Google Scholar] [CrossRef]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Balkau, B.; Charles, M.A. Comment on the provisional report from the WHO consultation. Diabet. Med. 1999, 16, 442–443. [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Bay, H.E. “Sick fat”, metabolic disease, and atherosclerosis. Am. J. Med. 2009, 122, S26–S37. [Google Scholar]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Meng, E.; Lin, W.Y. Pathophsiology of overactive bladder. LUTS 2012, 4, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, W.S.; Dmochowski, R.; Wein, A.; Bruehl, S. Does central sensitization help explain idiopathic overactive bladder? Nat. Rev. Urol. 2016, 13, 481–491. [Google Scholar] [CrossRef]
- Lohsiriwat, S.; Hirunsai, M.; Chaiyaprasithi, B. Effect of caffeine on bladder function in patients with overactive bladder symptoms. Urol. Ann. 2011, 3, 14–18. [Google Scholar] [CrossRef]
- Huang, A.J.; Grady, D.; Mendes, W.B.; Hernandez, C.; Schembri, M.; Subak, L.L. A randomized controlled trial of device guided, slow-paced respiration in women with overactive bladder syndrome. J. Urol. 2019, 202, 787–794. [Google Scholar] [CrossRef]
- Rohrmann, S.; Smit, E.; Giovannucci, E.; Platz, E.A. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III). Int. J. Obes. 2005, 29, 310–316. [Google Scholar] [CrossRef]
- Yu, H.-J.; Liu, C.-Y.; Lee, K.-L.; Lee, W.-C.; Chen, T.H.-H. Overactive bladder syndrome among community-dwelling adults in Taiwan: Prevalence, correlates, perception, and treatment seeking. Urol. Int. 2006, 77, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Lukacz, E.S.; Liu, I.L.; Nager, C.W.; Luber, K.M. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care 2007, 30, 2536–2541. [Google Scholar] [CrossRef]
- Bunn, F.; Kirby, M.; Pinkney, E.; Cardozo, L.; Chapple, C.; Chester, K.; Cruz, F.; Haab, F.; Kelleher, C.; Milsom, I.; et al. Is there a link between overactive bladder and the metabolic syndrome in women? A systematic review of observational studies. Int. J. Clin. Pract. 2015, 69, 199–217. [Google Scholar] [CrossRef]
- Zacche, M.M.; Giarenis, I.; Thiagamoorthy, G.; Robinson, D.; Cardozo, L. Is there an association between aspects of the metabolic syndrome and overactive bladder? A prospective cohort study in women with lower urinary tract symptoms. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 217, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Markland, A.D.; Shan, L.; Brady, S.S.; Schreiner, P.J.; Sidney, S.; Eeden, S.K.V.D.; Lewis, C.E. Characterizing the spectrum of bladder health and lower urinary tract symptoms (LUTS) among women: Results from the CARDIA study. Urology 2021, 158, 88–94. [Google Scholar] [CrossRef]
- He, Q.; Wang, Z.; Liu, G.; Daneshgari, F.; MacLennan, G.T.; Gupta, S. Metabolic syndrome, inflammation and lower urinary tract symptoms: Possible translational links. Prostate Cancer Prostatic Dis. 2016, 19, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Chueh, K.-S.; Chuang, S.-M.; Long, C.-Y.; Lu, J.-H.; Juan, Y.-S. Bladder Hyperactivity Induced by Oxidative Stress and Bladder Ischemia: A Review of Treatment Strategies with Antioxidants. Int. J. Mol. Sci. 2021, 22, 6014. [Google Scholar] [CrossRef] [PubMed]
- Leiria, L.O.; Sollon, C.; Báu, F.R.; Mónica, F.Z.; D’Ancona, C.L.; De Nucci, G.; Grant, A.D.; Anhê, G.F.; Antunes, E. Insulin relaxes bladder via PI3K/AKT/eNOS pathway activation in mucosa: Unfolded protein response-dependent insulin resistance as a cause of obesity-associated overactive bladder. J. Physiol. 2013, 591, 2259–2273. [Google Scholar] [CrossRef]
- Flores, M.V.; Mossa, A.H.; Cammisotto, P.; Campeau, L. Succinate decreases bladder function in a rat model associated with metabolic syndrome. Neurourol. Urodyn. 2018, 37, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, S.; Russell, S.; McVary, K.T. Metabolic syndrome and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Curr. Urol. Rep. 2006, 7, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.; McCance, D.R.; Atkinson, A.; Bassett, J.; Ennis, C.N.; Sheridan, B.; Bell, P.M. Effect of the alpha-adrenergic blocker, doxazosin, on endothelial function and insulin action. Metabolism 2003, 52, 1147–1152. [Google Scholar] [CrossRef]
- Yang, S.S.; Wang, C.C.; Cheng, C.H.; Chen, Y.T. α1-adrenergic blockers in young men with primary bladder neck obstruction. J. Urol. 2002, 168, 571–574. [Google Scholar] [CrossRef]
- Tong, Y.C.; Cheng, J.T. Alterations of M2,3-muscarinic receptor protein and mRNA expression in the bladder of the fructose fed obese rat. J. Urol. 2007, 178, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.D.; Chien, C.T.; Yu, H.J. Alterations in peripheral purinergic and muscarinic signaling of rat bladder after long-term fructose-induced metabolic syndrome. Eur. J. Nutr. 2013, 52, 347–359. [Google Scholar] [CrossRef]
- Lee, W.C.; Chiang, P.H.; Tain, Y.L.; Wu, C.C.; Chuang, Y.C. Sensory dysfunction of bladder mucosa and bladder oversensitivity in a rat model of metabolic syndrome. PLoS ONE 2012, 7, e45578. [Google Scholar] [CrossRef]
- Thurmond, P.; Yang, J.-H.; Azadzoi, K.M. LUTS in pelvic ischemia: A new concept in voiding dysfunction. Am. J. Physiol.-Ren. Physiol. 2016, 310, F738–F743. [Google Scholar] [CrossRef]
- Gacci, M.; Corona, G.; Sebastianelli, A.; Serni, S.; De Nunzio, C.; Maggi, M.; Vignozzi, L.; Novara, G.; McVary, K.T.; Kaplan, S.A.; et al. Male lower urinary tract symptoms and cardiovascular events: A systemic review and meta-analysis. Eur. Urol. 2016, 70, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.-H.; Yip, J.S.Y.; Cheng, N.M.Y.; Kwan, C.-H.; Li, K.-M.; Teoh, J.Y.C.; Chiu, P.K.F.; Wong, J.H.-M.; Chan, E.S.Y.; Chan, C.-K.; et al. The cardiovascular risk factors in men with lower urinary tract symptoms. World J. Urol. 2019, 37, 727–733. [Google Scholar] [CrossRef]
- Azadzoi, K.M.; Tarcan, T.; Siroky, M.B.; Krane, R.J. Atherosclerosis-induced chronic ischemia causes bladder fibrosis and non-compliance in the rabbit. J. Urol. 1999, 161, 1626–1635. [Google Scholar] [CrossRef]
- Azadzoi, K.M.; Tarcan, T.; Kozlowski, R.; Krane, R.J.; Siroky, M.B. Overactivity and structural changes in the chronically ischemic bladder. J. Urol. 1999, 162, 1768–1778. [Google Scholar] [CrossRef]
- Azadzoi, K.M.; Yalla, S.V.; Siroky, M.B. Oxidative stress and neurodegeneration in the ischemic overactive bladder. J. Urol. 2007, 178, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.-C.; Chung, S.-D.; Chien, C.-T.; Yu, H.-J. Sulforaphane improves ischemia-induced detrusor overactivity by downregulating the enhancement of associated endoplasmic reticulum stress, autophagy, and apoptosis in rat bladder. Sci. Rep. 2016, 6, 36110. [Google Scholar] [CrossRef]
- Koritsiadis, G.; Tyritzis, S.I.; Koutalellis, G.; Lazaris, A.C.; Stravodimos, K. The effect of alpha-blocker treatment on bladder hypoxia inducible factor-1 alpha regulation during lower urinary tract obstruction. Int. Braz. J. Urol. 2010, 36, 86–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ngai, H.-Y.; Yuen, K.-K.S.; Ng, C.-M.; Cheng, C.-H.; Chu, S.-K.P. Metabolic syndrome and benign prostatic hyperplasia: An update. Asian J. Urol. 2017, 4, 164–173. [Google Scholar] [CrossRef]
- Nandeesha, H.; Koner, B.C.; Dorairajan, L.N.; Sen, S.K. Hyperinsulinemiaand dyslipidaemia in non-diabetic benign prostatic hyperplasia. Clin. Chim. Acta 2006, 370, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.K.; Carter, H.B.; Partin, A.W.; Windham, B.G.; Metter, E.J.; Ferrucci, L.; Landis, P.; Platz, E.A. Metabolic factors associated with benign prostatic hyperplasia. J. Clin. Endocrinol. Metab. 2006, 91, 2562–2568. [Google Scholar] [CrossRef]
- Bhide, A.A.; Cartwright, R.; Khullar, V.; Digesu, G.A. Biomarkers in overactive bladder. Int. Urogynecol. J. 2013, 24, 1065–1072. [Google Scholar] [CrossRef]
- Kupelian, V.; McVary, K.T.; Barry, M.J.; Link, C.L.; Rosen, R.C.; Aiyer, L.P.; Mollon, P.; McKinlay, J.B. Associated of C-reactive protein and lower urinary tract symptoms in men and women: Results from Boston area community health survey. Urology 2009, 73, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.-M.; Lin, H.-H.; Kuo, H.-C. The role of C-reactive protein in women with lower urinary tract symptoms. Int. Urogynecol. J. 2012, 23, 935–940. [Google Scholar] [CrossRef]
- Chung, S.D.; Liu, H.T.; Lin, H.; Kuo, H.C. Elevation of serum c-reactive protein in patients with OAB and IC/BPS implies chronic inflammation in the urianry bladder. Neurol. Urodyn. 2011, 30, 417–420. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Ebrahimi, M. Serum high-sensitivity C-reactive protein as a biomarker in patients with metabolic syndrome: Evidence-base study with 7284 subjects. Eur. J. Clin. Nutr. 2016, 70, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.W.; Li, R.; Geetha, T.; Tao, Y.X.; Babu, J.R. Nerve growth factor in metabolic complications and Alzheimer’s disease: Physioloy and therapeutic potential. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165858. [Google Scholar] [CrossRef]
- Atanassova, P.; Hrischev, P.; Orbetzova, M.; Nikolov, P.; Nikolova, J.; Georgieva, E. Expression of leptin, NGF and adiponectin in metabolic syndrome. Folia Biol. 2014, 62, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, H.; Cengiz, H. Association between metabolic syndrome and serum nerve growth factor levels in women with overactive bladder. Gynecol. Obstet. Investig. 2018, 83, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Pawelzik, S.C.; Avignon, A.; Idborg, H.; Boegner, C.; Stanke-Labesque, F.; Jakobsson, P.J.; Sultan, A.; Bäck, M. Urinary prostaglandin D2 and E2 metabolites associated with abdominal obesity, glucose metabolism, and triglycerides in obese subjects. Prostaglandins Other Lipid Mediat. 2019, 145, 106361. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Tamura, Y.; Minami, M.; Higuchi, S.; Fujikawa, R.; Ikedo, T.; Nagata, M.; Arai, H.; Murayama, T.; Yokode, M. The prostaglandin E2 receptor EP4 regulates obesity-related inflammation and insulin sensitivity. PLoS ONE 2015, 10, e0136304. [Google Scholar] [CrossRef]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Uzun, H.; Yilmaz, A.; Kemik, A.; Zorba, O.U.; Kalkan, M. Association of insulin resistance with overactive bladder in female patients. Int. Neurourol. J. 2012, 16, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-C.; Wu, K.L.H.; Tain, Y.-L.; Leu, S.; Cheng, Y.-T.; Chan, J.Y.H. Impaired Insulin signaling at the bladder mucosa facilitates metabolic syndrome-associated bladder overactivity in rats with maternal and post-weaning fructose exposure. J. Formos. Med. Assoc. 2022, in press. [Google Scholar]
- Lee, W.-C.; Leu, S.; Wu, K.L.H.; Tain, Y.-L.; Chuang, Y.-C.; Chan, J.Y.H. Tadalafil ameliorates bladder overactivity by restoring insulin-activated detrusor relaxation via the bladder mucosal IRS/PI3K/AKT/eNOS pathway in fructose-fed rats. Sci. Rep. 2021, 11, 8202. [Google Scholar] [CrossRef] [PubMed]
- Sadagopan, N.; Li, W.; Roberds, S.; Major, T.; Preston, G.M.; Yu, Y.; Tones, M.A. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am. J. Hypertens. 2007, 20, 1209–1215. [Google Scholar]
- Fernández-Veledo, S.; Vendrell, J. Gut microbiota-derived succinate: Friend or foe in human metabolic diseases? Rev. Endocr. Metab. Disord. 2019, 20, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Prieto, F.J.; Martinez-Tellez, B.; Ortiz-Alvarez, L.; Di, X.; Jurado-Fasoli, L.; Xu, H.; Ceperuelo-Mallafré, V.; Núñez-Roa, C.; Kohler, I.; Segura-Carretero, A.; et al. Elevated plasma succinate levels are linked to higher cardiovascular disease risk factors in young adults. Cardiovasc. Diabetol. 2021, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.H.; Flores, M.V.; Cammisotto, P.G.; Campeau, L. Succinate, increased in metabolic syndrome, activates GPR91 receptor signaling in urothelial cells. Cell. Signal. 2017, 37, 31–39. [Google Scholar] [CrossRef]
- Mossa, A.H.; Flores, M.V.; Nguyen, H.; Cammisotto, P.G.; Campeau, L. Beta-3 Adrenoceptor Signaling Pathways in Urothelial and Smooth Muscle Cells in the Presence of Succinate. J. Pharmacol. Exp. Ther. 2018, 367, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Urinology Think Tank Writing Group. Urine: Waste product or biologically active tissue? Neurourol. Urodyn. 2018, 37, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Govender, Y.; Gabriel, I.; Minassian, V.; Fichorova, R. The Current Evidence on the Association Between the Urinary Microbiome and Urinary Incontinence in Women. Front. Cell. Infect. Microbiol. 2019, 9, 133. [Google Scholar] [CrossRef]
- Whiteside, S.A.; Razvi, H.; Dave, S.; Reid, G.; Burton, J.P. The microbiome of the urinary tract—A role beyond infection. Nat. Rev. Urol. 2015, 12, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Kondo, M.; Takezawa, K.; Kiuchi, H.; Sekii, Y.; Inagaki, Y.; Soda, T.; Fukuhara, S.; Fujita, K.; Uemura, M.; et al. Bladder urothelium converts bacterial lipopolysaccharide information into neural signaling via an ATP-mediated pathway to enhance the micturition reflex for rapid defense. Sci. Rep. 2020, 10, 21167. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.M.; Hilt, E.E. The female urinary microbiome: A comparison of women with and without urgency urinary incontinence. MBio 2014, 5, e01283-14. [Google Scholar] [CrossRef] [PubMed]
- Nardos, R.; Leung, E.T.; Dahl, E.M.; Davin, S.; Asquith, M.; Gregory, W.T.; Karstens, L. Network-Based Differences in the Vaginal and Bladder Microbial Communities Between Women With and Without Urgency Urinary Incontinence. Front. Cell. Infect. Microbiol. 2022, 12, 759156. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; Van Raalte, D.H.; Herrema, H. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef] [PubMed]
- Leue, C.; Kruimel, J.; Vrijens, D.; Masclee, A.; van Os, J.; Van Koeveringe, G. Functional urological disorders: A sensitized defence response in the bladder-gut-brain axis. Nat. Rev. Urol. 2017, 14, 153–163. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Greenwood-Van Meerveld, B.; Mohammadi, E.; Tyler, K.; Van Gordon, S.; Parker, A.; Towner, R.; Hurst, R. Mechanisms of Visceral Organ Crosstalk: Importance of Alterations in Permeability in Rodent Models. J. Urol. 2015, 194, 804–811. [Google Scholar] [CrossRef]
- Lee, W.-C.; Tain, Y.-L.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Maternal Fructose Exposure Programs Metabolic Syndrome-Associated Bladder Overactivity in Young Adult Offspring. Sci. Rep. 2016, 6, 34669. [Google Scholar] [CrossRef]
- Mitsui, T.; Kira, S.; Ihara, T.; Sawada, N.; Nakagomi, H.; Miyamoto, T.; Shimura, H.; Yokomichi, H.; Takeda, M. Metabolomics approach to male lower urinary tract symptoms: Identification of possible biomarkers and potential targets for new treatments. J. Urol. 2017, 199, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-J.; Hsu, C.-C.; Lee, W.-C.; Chiang, P.-H.; Chuang, Y.-C. Medical diseases affecting lower urinary tract function. Urol. Sci. 2013, 24, 41–45. [Google Scholar] [CrossRef]
- Massaro, A.; Galiano, A.; Scarafile, D.; Vacca, A.; Frassanito, A.; Melaccio, A.; Solimando, A.; Ria, R.; Calamita, G.; Bonomo, M.; et al. Telemedicine DSS-AI multi level platform for monoclonal gammopathy assistance. In Proceedings of the 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Bari, Italy, 1 June–1 July 2020. [Google Scholar]
- De Falco, I.; De Pietro, G.; Sannino, G. A two-step approach for classification in Alzheimer’s disease. Sensors 2022, 22, 3966. [Google Scholar] [CrossRef] [PubMed]
- Laila, U.E.; Mahboob, K.; Khan, A.W.; Khan, F.; Taekeun, W. An ensemble approach to predict early-stage diabetes risk using machine learning: An empirical study. Sensors 2022, 22, 5247. [Google Scholar] [CrossRef] [PubMed]
| Key Concept | Criteria | Obesity | Blood Pressure | Dyslipidemia | Hyperglycemia | Others | |
|---|---|---|---|---|---|---|---|
| WHO (1998) [10] | Consensus Definition | Insulin resistance or diabetes, plus two of the other criteria below | Waist/hip ratio: >0.90 in men, >0.85 in women; or BMI >30 kg/m2 | ≥140/90 mmHg | TG 150 mg/dL; HDL-cholesterol <35 mg/dL in men, <39 mg/dL in women | Insulin resistance ‡ | Microalbuminuria * |
| EGIR (1999) [11] | Hyperinsulinemia | Hyperinsulinemia, plus two of the other criteria below | Waist circumference: ≥94 cm in men, ≥80 cm in women | ≥140/90 mmHg or Rx | TG ≥ 177 mg/dL or HDL-cholesterol <39 mg/dL | Insulin resistance ‡ | |
| NCEP:ATP III (2001) [12] | Any three or more of the criteria below | Waist circumference: >102 cm in men, >88 cm in women | ≥130/85 mmHg | TG ≥ 150 mg/dL; HDL-cholesterol <40 mg/dL in men, <50 mg/dL in women | Fasting glucose ≥110 mg/dL | ||
| NCEP ATP III (2005 revision) [13] | Central obesity | Any three of the criteria below | Waist circumference: >40 inches in men, >35 inches in women | ≥130/85 mmHg or Rx | TG ≥ 150 mg/dL; HDL-cholesterol <40 mg/dL in men, <50 mg/dL in women | Fasting glucose ≥100 mg/dL | |
| IDF (2005) [14] | Central obesity with ethnicity-specific values §, plus two of the other criteria below | Central obesity with ethnicity-specific values § | ≥130/85 mmHg | TG ≥ 150 mg/dL; HDL-cholesterol <40 mg/dL in men, <50 mg/dL in women | Fasting glucose ≥110 mg/dL | ||
| Consensus Definition [15] | Elevated waist circumference (according to country-specific definitions) | ≥130/85 mmHg | TG ≥ 150 mg/dL; HDL-cholesterol <40 mg/dL in men, <50 mg/dL in women | Fasting glucose ≥110 mg/dL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, L.-N.; Hu, J.-C.; Chen, P.-Y.; Lee, W.-C.; Chuang, Y.-C. Metabolic Syndrome and Overactive Bladder Syndrome May Share Common Pathophysiologies. Biomedicines 2022, 10, 1957. https://doi.org/10.3390/biomedicines10081957
Hsu L-N, Hu J-C, Chen P-Y, Lee W-C, Chuang Y-C. Metabolic Syndrome and Overactive Bladder Syndrome May Share Common Pathophysiologies. Biomedicines. 2022; 10(8):1957. https://doi.org/10.3390/biomedicines10081957
Chicago/Turabian StyleHsu, Lin-Nei, Ju-Chuan Hu, Po-Yen Chen, Wei-Chia Lee, and Yao-Chi Chuang. 2022. "Metabolic Syndrome and Overactive Bladder Syndrome May Share Common Pathophysiologies" Biomedicines 10, no. 8: 1957. https://doi.org/10.3390/biomedicines10081957
APA StyleHsu, L.-N., Hu, J.-C., Chen, P.-Y., Lee, W.-C., & Chuang, Y.-C. (2022). Metabolic Syndrome and Overactive Bladder Syndrome May Share Common Pathophysiologies. Biomedicines, 10(8), 1957. https://doi.org/10.3390/biomedicines10081957