The Antimicrobial Properties of Cannabis and Cannabis-Derived Compounds and Relevance to CB2-Targeted Neurodegenerative Therapeutics
Abstract
:1. Introduction
2. The Antimicrobial Properties of Cannabis and Cannabis-Derived Compounds
2.1. Infectious Diseases in Cannabis Users
2.2. Antibacterial Properties of Cannabis and Cannabis-Derived Compounds
2.3. Anti-Fungal Activities of Cannabis, Cannabis-Derived Compounds
2.4. Anti-Viral Activities of Cannabis, Cannabis-Derived Compounds
2.5. Antimicrobials as Therapeutics for Neurological Disorders
3. Translational Considerations for the Development of Cannabis-Informed Antimicrobials
3.1. In Vivo and Ex Vivo Verification of Antimicrobial Activities
3.2. Optimization of the Anti-Microbial Potential of Cannabis-Derived Compounds
3.3. Anti-Microbial Mechanisms
4. Translational Considerations for the Development of Non-Antimicrobial CB2-Engaging CIMPs
5. Emergence of Microbial Resistance to Cannabis and Cannabis-Derived Compounds
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Morcuende, A.; Garcia-Gutierrez, M.S.; Tambaro, S.; Nieto, E.; Manzanares, J.; Femenia, T. Immunomodulatory Role of CB2 Receptors in Emotional and Cognitive Disorders. Front. Psychiatry 2022, 13, 866052. [Google Scholar] [CrossRef] [PubMed]
- Bahji, A.; Breward, N.; Duff, W.; Absher, N.; Patten, S.B.; Alcorn, J.; Mousseau, D.D. Cannabinoids in the management of behavioral, psychological, and motor symptoms of neurocognitive disorders: A mixed studies systematic review. J. Cannabis Res. 2022, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Kibret, B.G.; Horiuchi, Y.; Onaivi, E.S. Potential Role of Cannabinoid Type 2 Receptors in Neuropsychiatric and Neurodegenerative Disorders. Front. Psychiatry 2022, 13, 828895. [Google Scholar] [CrossRef] [PubMed]
- Kibret, B.G.; Ishiguro, H.; Horiuchi, Y.; Onaivi, E.S. New Insights and Potential Therapeutic Targeting of CB2 Cannabinoid Receptors in CNS Disorders. Int. J. Mol. Sci. 2022, 23, 975. [Google Scholar] [CrossRef]
- Collaborators, A.R. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar]
- Krejci, Z. Antibacterial action of Canabis indica. Lek. List. 1952, 7, 500–503. [Google Scholar]
- Rabinovich, A.S.; BIu, A.; Zelepukha, S.I. Isolation and investigation of antibacterial properties of preparations from wild hemp (Cannabis ruderalis) growing in the Ukraine. Mikrobiolohichnyi Zhurnal 1959, 21, 40–48. [Google Scholar]
- Karas, J.A.; Wong, L.J.M.; Paulin, O.K.A.; Mazeh, A.C.; Hussein, M.H.; Li, J.; Velkov, T. The Antimicrobial Activity of Cannabinoids. Antibiotics 2020, 9, 406. [Google Scholar] [CrossRef]
- Ferenczy, L.; Gracza, L.; Jakobey, I. An antibacterial preparatum from hemp (Cannabis sativa L.). Die Nat. 1958, 45, 188. [Google Scholar] [CrossRef]
- Schultz, O.E.; Haffner, G. A sedative active principle from the German common hemp (Cannabis sativa). Arch. Pharm. Ber. Dtsch. Pharm. Ges. 1958, 291, 391–403. [Google Scholar]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.W.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 1–18. [Google Scholar] [CrossRef]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; NacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the Hidden Antibiotic Potential of Cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef]
- Hernandez-Cervantes, R.; Mendez-Diaz, M.; Prospero-Garcia, O.; Morales-Montor, J. Immunoregulatory Role of Cannabinoids during Infectious Disease. Neuroimmunomodulation 2017, 24, 183–199. [Google Scholar] [CrossRef]
- Klahn, P. Cannabinoids-Promising Antimicrobial Drugs orIntoxicants with Benefits? Antibiotics 2020, 9, 297. [Google Scholar] [CrossRef]
- Mahmud, M.S.; Hossain, M.S.; Ahmed, A.; Islam, M.Z.; Sarker, M.E.; Islam, M.R. Antimicrobial and Antiviral (SARS-CoV-2) Potential of Cannabinoids and Cannabis sativa: A Comprehensive Review. Molecules 2021, 26, 7216. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Anti-Microbial Activity of Phytocannabinoids and Endocannabinoids in the Light of Their Physiological and Pathophysiological Roles. Biomedicines 2022, 10, 631. [Google Scholar] [CrossRef]
- Sihag, J.; Di Marzo, V. (Wh)olistic (E)ndocannabinoidome-Microbiome-Axis Modulation through (N)utrition (WHEN) to Curb Obesity and Related Disorders. Lipids Health Dis. 2022, 21, 9. [Google Scholar] [CrossRef]
- Bagaitkar, J.; Demuth, D.R.; Scott, D.A. Tobacco use increases susceptibility to bacterial infection. Tob. Induc. Dis. 2008, 4, 1–10. [Google Scholar] [CrossRef]
- Newman, T.M.; Krishnan, L.P.; Lee, J.; Adami, G.R. Microbiomic differences at cancer-prone oral mucosa sites with marijuana usage. Sci. Rep. 2019, 9, 12697. [Google Scholar] [CrossRef]
- Wright, M.L.; Dunlop, A.L.; Dunn, A.B.; Mitchell, R.M.; Wissel, E.F.; Corwin, E.J. Factors Associated with Vaginal Lactobacillus Predominance Among African American Women Early in Pregnancy. J. Womens Health 2021, 31, 682–689. [Google Scholar] [CrossRef]
- Luo, Z.; Fitting, S.; Robinson, C.; Benitez, A.; Li, M.; Wu, Y.; Fu, X.; Amato, D.; Ning, W.; Funderburg, N.; et al. Chronic cannabis smoking-enriched oral pathobiont drives behavioral changes, macrophage infiltration, and increases beta-amyloid protein production in the brain. EBioMedicine 2021, 74, 103701. [Google Scholar] [CrossRef]
- Simeon, D.T.; Bain, B.C.; Wyatt, G.E.; LeFranc, E.; Ricketts, H.; Chambers, C.C.; Tucker, M.B. Characteristics of Jamaicans who smoke marijuana before sex and their risk status for sexually transmitted diseases. West Indian Med. J. 1996, 45, 9–13. [Google Scholar]
- Ly, T.D.A.; Hoang, V.T.; Louni, M.; Dao, T.H.; Badiaga, S.; Tissot-Dupont, H.; Brouqui, P.; Colson, P.; Gautret, P. Epidemiological serosurvey and molecular characterization of sexually transmitted infections among 1890 sheltered homeless people in Marseille: Cross-sectional one day-surveys (2000–2015). J. Infect. 2021, 82, 60–66. [Google Scholar] [CrossRef]
- Benedict, K.; Thompson, G.R.; Jackson, B.R. Cannabis Use and Fungal Infections in a Commercially Insured Population, United States, 2016. Emerg. Infect. Dis. 2020, 26, 1308–1310. [Google Scholar] [CrossRef]
- Johnson, A.B.; Wang, G.S.; Wilson, K.; Cline, D.M.; Craven, T.E.; Slaven, S.; Raghavan, V.; Mistry, R.D. Association between secondhand marijuana smoke and respiratory infections in children. Pediatr. Res. 2021, 91, 1769–1774. [Google Scholar] [CrossRef]
- Elhendawy, M.A.; Wanas, A.S.; Radwan, M.M.; Azzaz, N.A.; Toson, E.S.; ElSohly, M.A. Chemical and Biological Studies of Cannabis sativa Roots. Med. Cannabis Cannabinoids 2019, 1, 104–111. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Garnaes, J.; Tunjic, S.; Mokkapati, V.R.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Lavorgna, M.; Nugnes, R.; Orlo, E.; Isidori, M. Comparative assessment of antimicrobial, antiradical and cytotoxic activities of cannabidiol and its propyl analogue cannabidivarin. Sci. Rep. 2021, 11, 22494. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef] [PubMed]
- Abichabki, N.; Zacharias, L.V.; Moreira, N.C.; Bellissino-Rodrigues, F.; Moreira, F.L.; Benzi, J.R.L.; Ogasawara, T.M.C.; Ferreira, J.C.; Ribeiro, C.M.; Pavan, F.R.; et al. Potential cannabidiol (CBD) repurposing as antibacterial and promising therapy of CBD plus polymyxin B (PB) against PB-resistant gram-negative bacilli. Sci. Rep. 2022, 12, 6454. [Google Scholar] [CrossRef]
- Gu, Z.; Singh, S.; Niyogi, R.G.; Lamont, G.J.; Wang, H.; Lamont, R.J.; Scott, D.A. Marijuana-Derived Cannabinoids Trigger a CB2/PI3K Axis of Suppression of the Innate Response to Oral Pathogens. Front. Immunol. 2019, 10, 2288. [Google Scholar] [CrossRef]
- Di Onofrio, V.; Gesuele, R.; Maione, A.; Liguori, G.; Liguori, R.; Guida, M.; Nigro, R.; Galiero, E. Prevention of Pseudomonas aeruginosa Biofilm Formation on Soft Contact Lenses by Allium sativum Fermented Extract (BGE) and Cannabinol Oil Extract (CBD). Antibiotics 2019, 8, 258. [Google Scholar] [CrossRef]
- Palmieri, S.; Maggio, F.; Pellegrini, M.; Ricci, A.; Serio, A.; Paparella, A.; Lo Sterzo, C. Effect of the Distillation Time on the Chemical Composition, Antioxidant Potential and Antimicrobial Activity of Essential Oils from Different Cannabis sativa L. Cultivars. Molecules 2021, 26, 4770. [Google Scholar] [CrossRef]
- Muscara, C.; Smeriglio, A.; Trombetta, D.; Mandalari, G.; La Camera, E.; Grassi, G.; Circosta, C. Phytochemical characterization and biological properties of two standardized extracts from a non-psychotropic Cannabis sativa L. cannabidiol (CBD)-chemotype. Phytother Res. 2021, 35, 5269–5281. [Google Scholar] [CrossRef]
- Muscara, C.; Smeriglio, A.; Trombetta, D. Antioxidant and antimicrobial activity of two standardized extracts from a new Chinese accession of non-psychotropic Cannabis sativa L. Phytother Res. 2021, 35, 1099–1112. [Google Scholar] [CrossRef]
- Martinenghi, L.D.; Jonsson, R.; Lund, T.; Jenssen, H. Isolation, Purification, and Antimicrobial Characterization of Cannabidiolic Acid and Cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900. [Google Scholar] [CrossRef]
- Pellegrini, M.; Palmieri, S.; Ricci, A.; Serio, A.; Paparella, A.; Lo Sterzo, C. In vitro antioxidant and antimicrobial activity of Cannabis sativa L. cv ‘Futura 75’ essential oil. Nat. Prod. Res. 2020, 35, 6020–6024. [Google Scholar] [CrossRef]
- Galal Osman, A.; Elokely, K.M.; Yadav, V.K.; Carvalho, P.; Radwan, M.; Slade, D.; Gul, W.; Khan, S.; Dale, O.R.; Husni, A.S.; et al. Bioactive products from singlet oxygen photooxygenation of cannabinoids. Eur. J. Med. Chem. 2018, 143, 983–996. [Google Scholar] [CrossRef]
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical Characterization and Evaluation of the Antibacterial Activity of Essential Oils from Fibre-Type Cannabis sativa L. (Hemp). Molecules 2019, 24, 2302. [Google Scholar] [CrossRef]
- Wassmann, C.S.; Hojrup, P.; Klitgaard, J.K. Cannabidiol is an effective helper compound in combination with bacitracin to kill Gram-positive bacteria. Sci. Rep. 2020, 10, 4112. [Google Scholar] [CrossRef]
- Marini, E.; Magi, G.; Ferretti, G.; Bacchetti, T.; Giuliani, A.; Pugnaloni, A.; Rippo, M.R.; Facinelli, B. Attenuation of Listeria monocytogenes Virulence by Cannabis sativa L. Essential Oil. Front. Cell. Infect. Microbiol. 2018, 8, 293. [Google Scholar] [CrossRef]
- Jin, S.; Lee, M.Y. The ameliorative effect of hemp seed hexane extracts on the Propionibacterium acnes-induced inflammation and lipogenesis in sebocytes. PLoS ONE 2018, 13, e0202933. [Google Scholar]
- Gildea, L.; Ayariga, J.A.; Ajayi, O.S.; Xu, J.; Villafane, R.; Samuel-Foo, M. Cannabis sativa CBD Extract Shows Promising Antibacterial Activity against Salmonella typhimurium and S. newington. Molecules 2022, 27, 2669. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-Bacterial Properties of Cannabigerol toward Streptococcus mutans. Front. Microbiol. 2021, 12, 656471. [Google Scholar] [CrossRef]
- Feldman, M.; Sionov, R.V.; Mechoulam, R.; Steinberg, D. Anti-Biofilm Activity of Cannabidiol against Candida albicans. Microorganisms 2021, 9, 441. [Google Scholar] [CrossRef]
- Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Carradori, S.; Di Simone, S.; Ciferri, M.C.; Zengin, G.; Ak, G.; et al. Water Extract from Inflorescences of Industrial Hemp Futura 75 Variety as a Source of Anti-Inflammatory, Anti-Proliferative and Antimycotic Agents: Results from In Silico, In Vitro and Ex Vivo Studies. Antioxidants 2020, 9, 437. [Google Scholar] [CrossRef]
- Raj, V.; Park, J.G.; Cho, K.H.; Choi, P.; Kim, T.; Ham, J.; Lee, J. Assessment of antiviral potencies of cannabinoids against SARS-CoV-2 using computational and in vitro approaches. Int. J. Biol. Macromol. 2021, 168, 474–485. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Muchiri, R.N.; Bates, T.A.; Weinstein, J.B.; Leier, H.C.; Farley, S.; Tafesse, F.G. Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants. J. Nat. Prod. 2022, 85, 176–184. [Google Scholar] [CrossRef]
- Nguyen, L.C.; Yang, D.; Nicolaescu, V.; Best, T.J.; Ohtsuki, T.; Chen, S.N.; Friesen, J.B.; Drayman, N.; Mohamed, A.; Dann, C.; et al. Cannabidiol Inhibits SARS-CoV-2 Replication and Promotes the Host Innate Immune Response. bioRxiv 2021. [Google Scholar] [CrossRef]
- Nguyen, L.C.; Yang, D.; Nicolaescu, V.; Best, T.J.; Gula, H.; Saxena, D.; Gabbard, J.D.; Chen, S.N.; Ohtsuki, T.; Friesen, J.B.; et al. Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses. Sci. Adv. 2022, 8, eabi6110. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Ge, X.; Xua, H.; Ma, K.; Zhang, W.; Zan, Y.; Efferth, T.; Xue, Z.; Hua, X. Phytochemicals with activity against methicillin-resistant Staphylococcus aureus. Phytomedicine 2022, 100, 154073. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Sikora, V.; Semerdjieva, I.B.; Kacaniova, M.; Astatkie, T.; Dincheva, I. Grinding and Fractionation during Distillation Alter Hemp Essential Oil Profile and Its Antimicrobial Activity. Molecules 2020, 25, 3943. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, F.; Schinzari, M.; Lucchi, A.; Mandrioli, M.; Toschi, T.G.; De Cesare, A.; Manfreda, G. Preliminary data on the antimicrobial effect of Cannabis sativa L. variety Futura 75 against food-borne pathogens in vitro as well as against naturally occurring microbial populations on minced meat during storage. Ital. J. Food Saf. 2020, 9, 8581. [Google Scholar] [CrossRef] [PubMed]
- Galletta, M.; Reekie, T.A.; Nagalingam, G.; Bottomly, A.L.; Harry, E.J.; Kassiou, M.; Triccas, J.A. Rapid Antibacterial Activity of Cannabichromenic Acid against Methicillin-Resistant Staphylococcus aureus. Antibiotics 2020, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Napiroon, T.; Tanruean, K.; Poolprasert, P.; Bacher, M.; Balslev, H.; Poopath, M.; Santimaleeworagun, W. Cannabinoids from inflorescences fractions of Trema orientalis (L.) Blume (Cannabaceae) against human pathogenic bacteria. PeerJ 2021, 9, e11446. [Google Scholar] [CrossRef] [PubMed]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-Biofilm Activity of Cannabigerol against Streptococcus mutans. Microorganisms 2021, 9, 2031. [Google Scholar] [CrossRef]
- Aqawi, M.; Steinberg, D.; Feuerstein, O.; Friedman, M.; Gingichashvili, S. Cannabigerol Effect on Streptococcus mutans Biofilms-A Computational Approach to Confocal Image Analysis. Front. Microbiol. 2022, 13, 880993. [Google Scholar] [CrossRef]
- Aqawi, M.; Gallily, R.; Sionov, R.V.; Zaks, B.; Friedman, M.; Steinberg, D. Cannabigerol Prevents Quorum Sensing and Biofilm Formation of Vibrio harveyi. Front. Microbiol. 2020, 11, 858. [Google Scholar] [CrossRef]
- Silvestri, C.; Pagano, E.; Lacroix, S.; Venneri, T.; Cristiano, C.; Caligno, A.; Parisi, O.A.; Izzo, A.A.; Di Marzo, V.; Borrelli, F. Fish Oil, Cannabidiol and the Gut Microbiota: An Investigation in a Murine Model of Colitis. Front. Pharmacol. 2020, 11, 585096. [Google Scholar] [CrossRef]
- Skinner, C.M.; Nookaew, I.; Ewing, L.E.; Wongsurawat, T.; Jenjaroenpun, P.; Quick, C.M.; Yee, E.U.; Piccolo, B.D.; ElSohly, M.; Walker, L.A.; et al. Potential Probiotic or Trigger of Gut Inflammation-The Janus-Faced Nature of Cannabidiol-Rich Cannabis Extract. J. Diet. Suppl. 2020, 17, 543–560. [Google Scholar] [CrossRef]
- Wasim, K.; Haq, I.; Ashraf, M. Antimicrobial studies of the leaf of Cannabis sativa L. Pak. J. Pharm. Sci. 1995, 8, 29–38. [Google Scholar]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Gorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef]
- Tazi, N.; Pigeon, X.; Mbuyi-Boisvert, J.M.; Giret, S.; Béland, F.; Rouabhia, M. Effect of Cannabis Smoke Condensate on C. albicans Growth and Biofilm Formation. Microorganisms 2021, 9, 2348. [Google Scholar] [CrossRef]
- McDew-White, M.; Lee, E.; Alvarez, X.; Sestak, K.; Ling, B.J.; Byrareddy, S.N.; Okeoma, C.M.; Mohan, M. Cannabinoid control of gingival immune activation in chronically SIV-infected rhesus macaques involves modulation of the indoleamine-2,3-dioxygenase-1 pathway and salivary microbiome. EBioMedicine 2022, 75, 103769. [Google Scholar] [CrossRef]
- DeMarino, C.; Cowen, M.; Khatkar, P.; Cotto, B.; Branscome, H.; Kim, Y.; Sharif, S.A.; Agbottah, E.T.; Zhou, W.; Costiniuk, C.T.; et al. Cannabinoids Reduce Extracellular Vesicle Release from HIV-1 Infected Myeloid Cells and Inhibit Viral Transcription. Cells 2022, 11, 723. [Google Scholar] [CrossRef]
- Pluskota-Karwatka, D.; Hoffmann, M.; Barciszewski, J. Reducing SARS-CoV-2 pathological protein activity with small molecules. J. Pharm. Anal. 2021, 11, 383–397. [Google Scholar] [CrossRef]
- Chatow, L.; Nudel, A.; Nesher, I.; Hayo Hemo, D.; Rozenberg, P.; Voropaev, H.; Winkler, I.; Levy, R.; Kerem, Z.; Yaniv, Z.; et al. In Vitro Evaluation of the Activity of Terpenes and Cannabidiol against Human Coronavirus E229. Life 2021, 11, 290. [Google Scholar] [CrossRef]
- Esposito, G.; Pesce, M.; Seguella, L.; Sanseverino, W.; Lu, J.; Corpetti, C.; Sarnelli, G. The potential of cannabidiol in the COVID-19 pandemic. Br. J. Pharmacol. 2020, 177, 4967–4970. [Google Scholar] [CrossRef]
- Wang, B.; Kovalchuk, A.; Li, D.; Rodriguez-Juarez, R.; Ilnytskyy, Y.; Kovalchuk, I.; Kovalchuk, O. In search of preventive strategies: Novel high-CBD Cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Aging 2020, 12, 22425–22444. [Google Scholar]
- Costiniuk, C.T.; Jenabian, M.A. Acute inflammation and pathogenesis of SARS-CoV-2 infection: Cannabidiol as a potential anti-inflammatory treatment? Cytokine Growth Factor Rev. 2020, 53, 63–65. [Google Scholar] [CrossRef]
- Khalsa, J.H.; Bunt, G.; Maggirwar, S.B.; Kottilil, S. COVID-19 and Cannabidiol (CBD). J. Addict. Med. 2020, 15, 355–356. [Google Scholar] [CrossRef]
- Janecki, M.; Graczyk, M.; Lewandowska, A.A.; Pawlak, L. Anti-Inflammatory and Antiviral Effects of Cannabinoids in Inhibiting and Preventing SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 4170. [Google Scholar] [CrossRef]
- Vallee, A. Cannabidiol and SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 870787. [Google Scholar] [CrossRef]
- Sansores-Espana, L.D.; Melgar-Rodriguez, S.; Olivares-Sagredo, K.; Cafferata, E.A.; Martínez-Aguilar, V.M.; Vernal, R.; Paula-Lima, A.C.; Díaz-Zúñiga, J. Oral-Gut-Brain Axis in Experimental Models of Periodontitis: Associating Gut Dysbiosis with Neurodegenerative Diseases. Front. Aging 2021, 2, 781582. [Google Scholar] [CrossRef]
- Narengaowa Kong, W.; Lan, F.; Awan, U.F.; Qing, H.; Ni, J. The Oral-Gut-Brain AXIS: The Influence of Microbes in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 633735. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, X.; Ye, Y.; Cafferata, E.A.; Martínez-Aguilar, V.M.; Vernal, R.; Paula-Lima, A.C.; Díaz-Zúñiga, J. Gut Microbiota: A Novel Therapeutic Target for Parkinson’s Disease. Front. Immunol. 2022, 13, 937555. [Google Scholar] [CrossRef]
- Shannon, K.M. Infections and Changes in Commensal Bacteria and the Pathogenesis of Parkinson’s Disease. J. Parkinsons Dis. 2022. [Google Scholar] [CrossRef]
- Eicher, T.P.; Mohajeri, M.H. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients 2022, 14, 2661. [Google Scholar] [CrossRef]
- Thu Thuy Nguyen, V.; Endres, K. Targeting gut microbiota to alleviate neuroinflammation in Alzheimer’s disease. Adv. Drug. Deliv. Rev. 2022, 188, 114418. [Google Scholar] [CrossRef]
- de Rijke, T.J.; Doting, M.H.E.; van Hemert, S.; De Deyn, P.P.; van Munster, B.C.; Harmsen, H.J.M.; Sommer, I.E.C. A Systematic Review on the Effects of Different Types of Probiotics in Animal Alzheimer’s Disease Studies. Front. Psychiatry 2022, 13, 879491. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.; Kanagasingam, S.; Welbury, R.; Singhrao, S.K. Periodontitis as a Risk Factor for Alzheimer’s Disease: The Experimental Journey So Far, with Hope of Therapy. Adv. Exp. Med. Biol. 2022, 1373, 241–260. [Google Scholar] [PubMed]
- Nara, P.L.; Sindelar, D.; Penn, M.S.; Potempa, J.; Griffin, W.S.T. Porphyromonas gingivalis Outer Membrane Vesicles as the Major Driver of and Explanation for Neuropathogenesis, the Cholinergic Hypothesis, Iron Dyshomeostasis, and Salivary Lactoferrin in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, 1417–1450. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Negro Alvarez, S.E.; Hernandez, E.B. The potential use of tetracyclines in neurodegenerative diseases and the role of nano-based drug delivery systems. Eur. J. Pharm. Sci. 2022, 175, 106237. [Google Scholar] [CrossRef]
- Bello-Medina, P.C.; Corona-Cervantes, K.; Zavala Torres, N.G.; González, A.; Pérez-Morales, M.; González-Franco, D.A.; Gómez, A.; García-Mena, J.; Díaz-Cintra, S.; Pacheco-López, G. Chronic-Antibiotics Induced Gut Microbiota Dysbiosis Rescues Memory Impairment and Reduces beta-Amyloid Aggregation in a Preclinical Alzheimer’s Disease Model. Int. J. Mol. Sci. 2022, 23, 8209. [Google Scholar] [CrossRef]
- Webberley, T.S.; Masetti, G.; Bevan, R.J.; Kerry-Smith, J.; Jack, A.A.; Michael, D.R.; Thomas, S.; Glymenaki, M.; Li, J.; McDonald, J.A.K. The Impact of Probiotic Supplementation on Cognitive, Pathological and Metabolic Markers in a Transgenic Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2022, 16, 843105. [Google Scholar] [CrossRef]
- Scott, D.A.; Dukka, H.; Saxena, D. Potential Mechanisms Underlying Marijuana-Associated Periodontal Tissue Destruction. J. Dent. Res. 2022, 101, 133–142. [Google Scholar] [CrossRef]
- Nemergut, M.; Batkova, T.; Vigasova, D.; Bartos, M.; Hlozankova, M.; Schenkmayerova, A.; Liskova, B.; Sheardova, K.; Vyhnalek, M.; Hort, J.; et al. Increased occurrence of Treponema spp. and double-species infections in patients with Alzheimer’s disease. Sci. Total Env. 2022, 844, 157114. [Google Scholar] [CrossRef]
- Dardiotis, E.; Tsouris, Z.; Mentis, A.A.; Siokas, V.; Michalopoulou, A.; Sokratous, M.; Dastamani, M.; Bogdanos, D.P.; Deretzi, G.; Kountouras, J.H. pylori and Parkinson’s disease: Meta-analyses including clinical severity. Clin. Neurol. Neurosurg. 2018, 175, 16–24. [Google Scholar] [CrossRef]
- Liu, N.Y.; Sun, J.H.; Jiang, X.F.; Li, H. Helicobacter pylori infection and risk for developing dementia: An evidence-based meta-analysis of case-control and cohort studies. Aging 2021, 13, 22571–22587. [Google Scholar] [CrossRef]
- Vasudevan, K.; Stahl, V. Cannabinoids infused mouthwash products are as effective as chlorhexidine on inhibition of total-culturable bacterial content in dental plaque samples. J. Cannabis Res. 2020, 2, 20. [Google Scholar] [CrossRef]
- Vasudevan, K.; Stahl, V. CBD-supplemented Polishing Powder Enhances Tooth Polishing by Inhibiting Dental Plaque Bacteria. J Int. Soc. Prev. Community Dent. 2020, 10, 766–770. [Google Scholar]
- Chen, Y.; Yang, B.; Xu, J.; Zheng, T.; Fan, H.; Yang, G.Z. Photo-activated DNA binding and antimicrobial activities of alkaloids from Glycosmis pentaphylla. Yao Xue Xue Bao 2012, 47, 1646–1652. [Google Scholar]
- Newman, M.A.; von Roepenack-Lahaye, E.; Parr, A.; Daniels, M.J.; Dow, J.M. Induction of hydroxycinnamoyl-tyramine conjugates in pepper by Xanthomonas campestris, a plant defense response activated by hrp gene-dependent and hrp gene-independent mechanisms. Mol. Plant Microbe. Interact 2001, 14, 785–792. [Google Scholar] [CrossRef]
- Zacares, L.; Lopez-Gresa, M.P.; Fayos, J.; Primo, J.; Belles, J.M.; Conejero, V. Induction of p-coumaroyldopamine and feruloyldopamine, two novel metabolites, in tomato by the bacterial pathogen Pseudomonas syringae. Mol. Plant Microbe. Interact 2007, 20, 1439–1448. [Google Scholar] [CrossRef]
- Newman, M.A.; von Roepenack-Lahaye, E.; Parr, A.; Daniels, M.J.; Dow, J.M. Prior exposure to lipopolysaccharide potentiates expression of plant defenses in response to bacteria. Plant J. 2002, 29, 487–495. [Google Scholar] [CrossRef]
- Hussein, M.; Allobawi, R.; Levou, I.; Blaskovich, M.A.T.; Rao, G.G.; Li, J.; Velkov, T. Mechanisms Underlying Synergistic Killing of Polymyxin B in Combination with Cannabidiol against Acinetobacter baumannii: A Metabolomic Study. Pharmaceutics 2022, 14, 786. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Pérez, C.J.; Desimone, M.F. Collagen Hydrogels Loaded with Silver Nanoparticles and Cannabis sativa Oil. Antibiotics 2021, 10, 1420. [Google Scholar] [CrossRef]
- Kosgodage, U.S.; Matewele, P.; Awamaria, B.; Kraev, I.; Warde, P.; Mastroianni, G.; Nunn, A.V.; Guy, G.W.; Bell, J.D.; Inal, J.M.; et al. Cannabidiol Is a Novel Modulator of Bacterial Membrane Vesicles. Front. Cell Infect Microbiol. 2019, 9, 324. [Google Scholar] [CrossRef]
- Aghamahdi, F.; Shafiee, A.; Rostami, S.; Mokhames, Z.; Safavi, M.; Yaslianifard, S.; Siami, Z.; Kabir, K.; Azizi, G.; Bakhtiyari, M.; et al. Comparative study of CNR1 and CNR2 cannabinoid receptors expression levels in COVID-19 patients with and without diabetes mellitus: Recommendations for future research targets. Diabetes Metab. Syndr. 2022, 16, 102499. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Matsabisa, M.G.; Muhammad, A.; Abarshi, M.M.; Amaku, J.F.; Katsayal, S.B.; Nde, A.L. Targeting of Protein’s Messenger RNA for Viral Replication, Assembly and Release in SARS-CoV-2 Using Whole Genomic Data from South Africa: Therapeutic Potentials of Cannabis sativa L. Front. Pharmacol. 2021, 2314. [Google Scholar] [CrossRef]
- Altyar, A.E.; Youssef, F.S.; Kurdi, M.M.; Bifari, R.J.; Ashour, M.L. The Role of Cannabis sativa L. as a Source of Cannabinoids against Coronavirus 2 (SARS-CoV-2): An In Silico Study to Evaluate Their Activities and ADMET Properties. Molecules 2022, 27, 2797. [Google Scholar] [CrossRef]
- Fernandes, M.F.; Chan, J.Z.; Hung, C.C.J.; Tomczewski, M.V.; Duncan, R.E. Effect of cannabidiol on apoptosis and cellular interferon and interferon-stimulated gene responses to the SARS-CoV-2 genes ORF8, ORF10 and M protein. Life Sci. 2022, 301, 120624. [Google Scholar] [CrossRef]
- Klann, E.M.; Dissanayake, U.; Gurrala, A.; Farrer, M.; Shukla, A.W.; Ramirez-Zamora, A.; Mai, V.; Vedam-Mai, V. The Gut-Brain Axis and Its Relation to Parkinson’s Disease: A Review. Front. Aging Neurosci. 2021, 13, 782082. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Barichello De Quevedo, C.E.; Petronilho, F. Microbiota-gut-brain axis in the Alzheimer’s disease pathology-an overview. Neurosci. Res. 2022, 181, 17–21. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ibrahim, A.K.; Radwan, M.M.; Slade, D.; Chandra, S.; Khan, I.A.; ElSohly, M.A. Microbial Biotransformation of Cannabidiol (CBD) from Cannabis sativa. Planta Med. 2021, 88, 389–397. [Google Scholar] [CrossRef]
- Wassmann, C.S.; Rolsted, A.P.; Lyngsie, M.C.; Torres-Puig, S.; Kronborg, T.; Vestergaard, M.; Ingmer, H.; Pontoppidan, S.P.; Klitgaard, J.K. The menaquinone pathway is important for susceptibility of Staphylococcus aureus to the antibiotic adjuvant, cannabidiol. Microbiol. Res. 2022, 257, 126974. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, N.; Roy, A. Role of cannabinoids in various diseases: A review. Curr. Pharm. Biotechnol. 2022, 23, 1346–1358. [Google Scholar] [CrossRef]
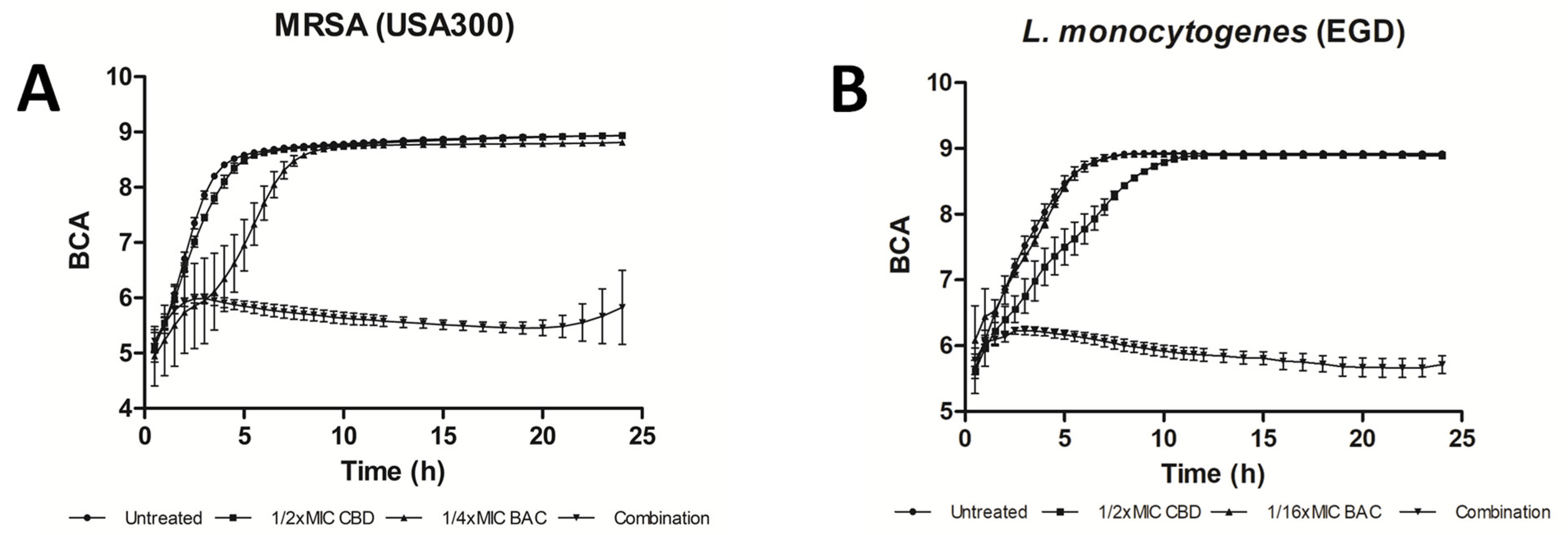
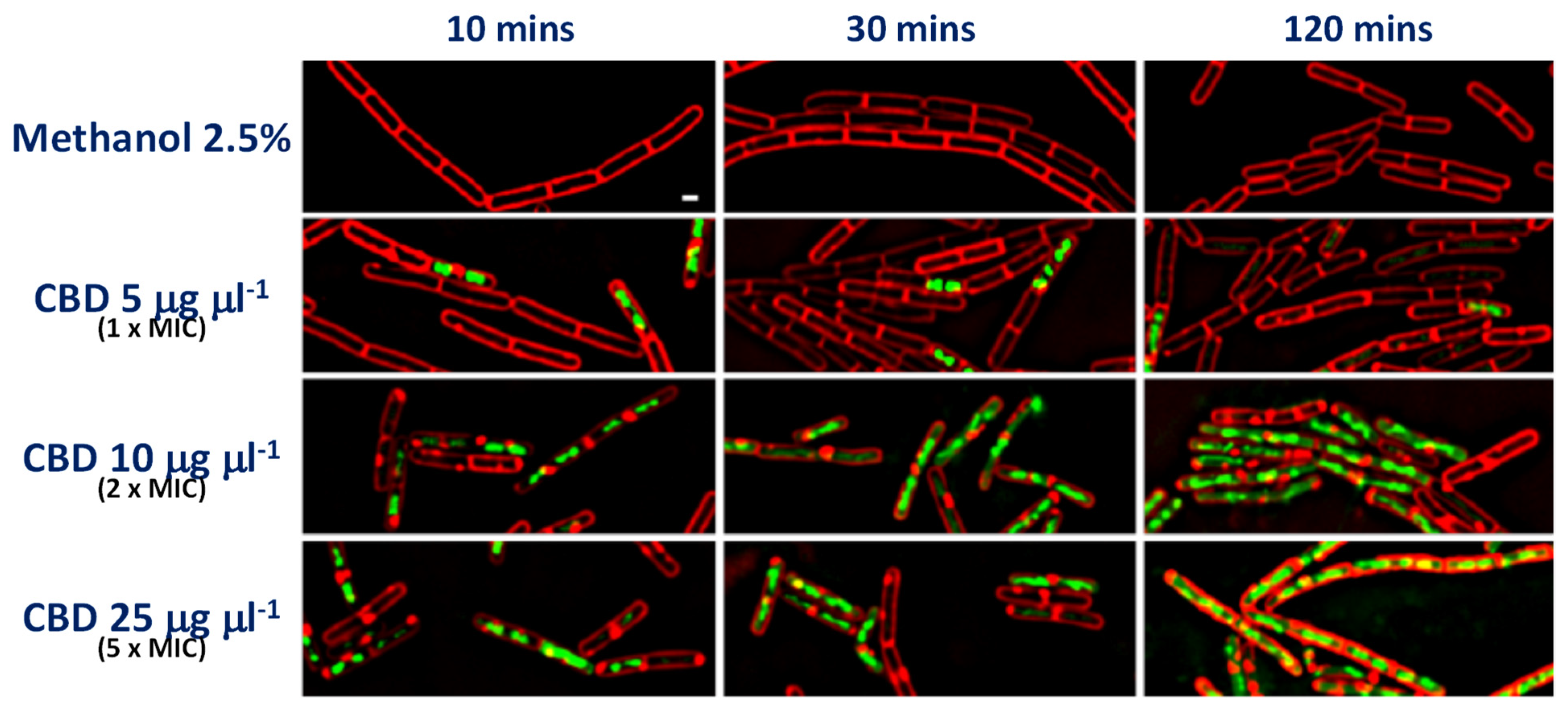
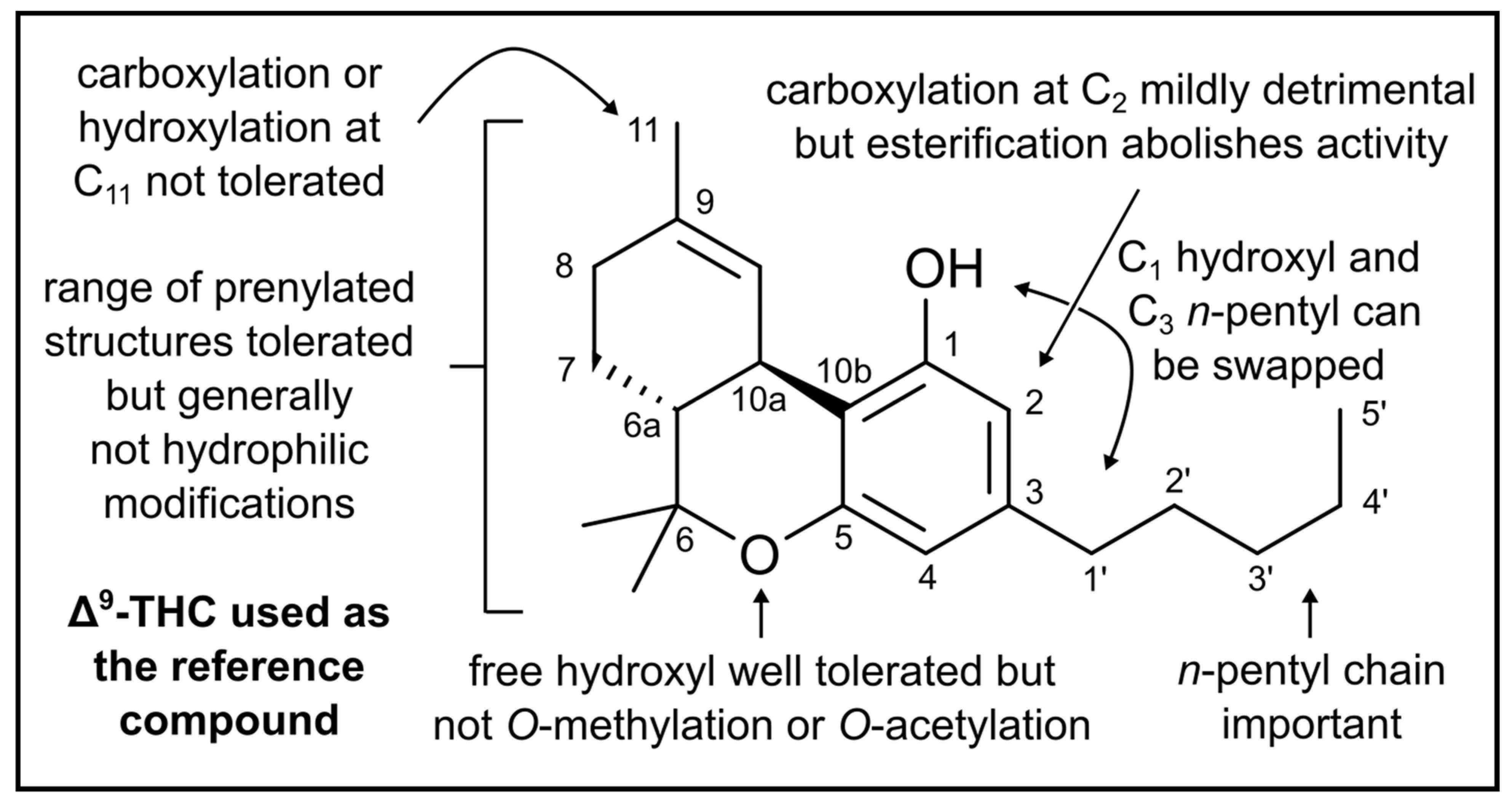
| Compound | Source | Target | Strain(s) | Efficacy | Reference |
|---|---|---|---|---|---|
| p-coumaroyltyramine | Roots of C. sativa, high CBD variety | Escherichia coli | ATCC 35218 | 0.8 μg/mL * | Elhendawy et al., 2019 [26] |
| Water extract | C. sativa, Futura 75 | Escherichia coli | ATCC 10536 | 7.1 mg/mL ** | Ferrante et al., 2019 [27] |
| Hemp stem Ag-nanoparticles | C. sativa, USO-31 | Escherichia coli | UTI 89 | 12.5 µg/mL ** 25 µg/mL *** | Singh et al., 2018 [28] |
| CBD | Commercial | Escherichia coli | ATCC 13762 | 29 µM * | Russo et al., 2021 [29] |
| Cannabidivarin (CBDV) | Commercial | Escherichia coli | ATCC 13762 | 35 µM * | Russo et al., 2021 [29] |
| Essential oils | C. sativa Futura 75 | Helicobacter pylori | 14 strains, variant Ab sensitivity patterns | 8–64 µg/mL ** 8–64 µg/mL *** | Zengin et al., 2018 [30] |
| Cannabidiol (CBD) | Commercial | Legionella pneumophila | MMX 7515 | 1 µg/mL ** | Blaskovich et al., 2021 [11] |
| CBD | Commercial | Moraxella catarrhalis | MMX 3782 | 1 µg/mL ** | Blaskovich et al., 2021 [11] |
| CBD | Commercial | Moraxella catarrhalis | ATCC 25238 | 164 µg/mL ** | Abichabki et al., 2022 [31] |
| CBD | Commercial | Neisseria gonorrhoeae | ATCC 49226 | 1 µg/mL ** | Blaskovich et al., 2021 [11] |
| CBD | Commercial | Neisseria meningitidis | ATCC 13090 | 0.25 µg/mL ** | Blaskovich et al., 2021 [11] |
| CBD | Commercial | Neisseria meningitidis | ATCC 13077 | 128 µg/mL ** | Abichabki et al., 2022 [31] |
| CBD | Commercial | Porphyromonas gingivalis | ATCC 33277 | 5 µg/mL * | Gu et al., 2019 [32] |
| Water extract | C. sativa, Futura 75 | Pseudomonas aeruginosa | ATCC 15442 | 7.1 mg/mL ** | Ferrante et, 2019 al [27] |
| Hemp stem Ag-nanoparticles | C. sativa, USO-31 | Pseudomonas aeruginosa | PAO1 | 6.25 µg/mL ** 12.5 µg/mL *** | Singh et al., 2018 [28] |
| Cannabinol oil extract | Commercial | Pseudomonas aeruginosa | ATCC 9027 | 2% ** | Di Onofrio et al., 2019 [33] |
| Essential oils | C. sativa (Futura 75, Carmagnola Lemon, Gran Sasso Kush, Carmagnola, Kompolti) | Pseudomonas fluorescens | P34 | 0.3–2.5 µL/mL ** | Palmieri et al., 2021 [34] |
| Compound | Source | Strain(s) | Antibiotic Sensitivity | Efficacy | Reference |
|---|---|---|---|---|---|
| Hexane extracts | C. sativa, Fibrante | 19 clinical strains | All MRSA | 4.9–78.1 μg/mL *** | Muscara et al., 2021 [35] |
| Hexane extracts | C. sativa, Fibrante | ATCC 6538 | Methicillin-sensitive | 4.9 μg/mL ** 4.9–19.5 *** | Muscara et al., 2021 [35] |
| Hexane extracts | C. sativa, C-309 | 19 clinical strains | All MRSA | 39.1–78.1 μg/mL *** | Muscara et al., 2021 [36] |
| Hexane extracts | C. sativa, C-309 | ATCC 6538 | Methicillin- sensitive | 39.1 μg/mL ** 39.1–78.1μg/mL *** | Muscara et al., 2021 [36] |
| CBD | Purified from Cannabis sativa, fiber types | USA300 | MRSA | 1 μg/mL ** | Martinenghi et al., 2020 [37] |
| CBD | Purified from Cannabis sativa, fiber types | ATCC 25923 | Methicillin- sensitive | 1 μg/mL ** | Martinenghi et al., 2020 [37] |
| Cannabidiolic acid (CBDA) | Purified from Cannabis sativa, fiber types | USA300 | MRSA | 4 μg/mL ** | Martinenghi et al., 2020 [37] |
| CBDA | Purified from Cannabis sativa, fiber types | ATCC 25923 | Methicillin- sensitive | 2 μg/mL ** | Martinenghi et al., 2020 [37] |
| Essential oil | C. sativa, Futura 75 | STA 32, St 47, St 39 | 1.25–5 μL/mL ** 1.25–5 μL/mL *** | Pellegrini et al., 2020 [38] | |
| Essential oils | C. sativa (Futura 75, Carmagnola Lemon, Gran Sasso Kush, Carmagnola, Kompolti) | STA 32, St 47 | 0.156–20 µL/mL ** | Palmieri et al., 2021 [34] | |
| Water extract | C. sativa L., Futura 75 | ATCC 6538s | Disinfectant testing strain | 3.6 mg/mL ** | Ferrante et al. 2019 [27] |
| Essential oils | C. sativa L., Futura 75 | ATCC 29213, 101TV, 104, 105 | Variant Ab sensitivity patterns | 8 mg/mL ** 16 mg/mL *** 16–24 mg/mL **** | Zengin et al., 2018 [30] |
| Oxygenated derivatives of Δ9-THC and its isomer Δ8-THC | - | Not presented | Not noted | 2.5–5 μg/mL * | Galal Osman et al., 2018 [39] |
| Oxygenated derivatives of Δ9-THC and its isomer Δ8-THC | - | Not presented | MRSA | 2.5–10 μg/mL ** | Galal Osman et al., 2018 [39] |
| CBD | Commercial | ATCC 25923, ATCC 43300, NRS-1, VRS1 | MMSA, MRSA, MRSA (vancomycin intermediate) and VRSA, respectively | 1–4 µg/mL ** | Blaskovich et al., 2021 [11] |
| Cannabigerol (CBG) | Lab synthesized | USA300 | MRSA | 4 µg/mL **** | Farha et al., 2020 [12] |
| Various essential oils | Multiple sources | ATCC 6538, 18As, 386 | Ciprofloxacin sensitive | 2–32 µg/mL ** | Iseppi et al., 2019 [40] |
| Various terpenes | Commercial | ATCC 6538, 18As, 386 | Ciprofloxacin sensitive | 4–32 µg/mL ** | Iseppi et al., 2019 [40] |
| CBD | Commercial | ATCC 6538, 18As, 386 | Ciprofloxacin sensitive | 8–32 µg/mL ** | Iseppi et al., 2019 [40] |
| CBD | Commercial | MRSA USA300 | MRSA | 4 µg/mL ** | Wassman et al., 2020 [41] |
| CBD | Commercial | ATCC 6538 | Ciprofloxacin sensitive | 1.8 µM * | Russo et al., 2021 [29] |
| CBDV | Commercial | ATCC 6538 | Ciprofloxacin sensitive | 30.1 µM * | Russo et al., 2021 [29] |
| CBD | Commercial | ATCC 29213, ATCC 43300, N315, ATCC 700698, ATCC 700699, ATCC BAA-976, ATCC BAA-977 | Variant Ab susceptibility patterns | 4 µg/mL** | Abichabki et al., 2022 [31] |
| Compound | Source | Target | Strain(s) | Efficacy | Reference |
|---|---|---|---|---|---|
| Essential oils | Various sources | Bacillus isolates | n = 12, including B. cereus | 0.5–32 µg/mL ** | Iseppi et al., 2019 [40] |
| Various terpenes | Commercial | Bacillus isolates | n = 12, including B. cereus | 1–32 µg/mL ** | Iseppi et al., 2019 [40] |
| CBD | Commercial | Bacillus isolates | n = 12, including B. cereus | 2–16 µg/mL ** | Iseppi et al., 2019 [40] |
| Essential oils | C. sativa (Futura 75, Carmagnola Lemon, Gran Sasso Kush, Carmagnola, Kompolti) | Brochothrix thermosphacta | B1 | 0.31–20 µg/mL ** | Palmieri et al., 2021 [34] |
| CBD | Commercial | Cutibacterium acnes | ATCC 6919 | 1–2 µg/mL ** | Blaskovich et al., 2021 [11] |
| CBD | Commercial | Clostridioides difficile | M7404 | 2–4 µg/mL ** | Blaskovich et al., 2021 [11] |
| CBD | Commercial | Enterococcus casseliflavus | ATCC 12361 | 4 µg/mL ** | Abichabki et al., 2022 [31] |
| CBD | Commercial | Enterococcus faecium | ATCC 35667, ATCC 700221, ATCC 19434, MMX 485 | 0.5–1 µg/mL ** | Blaskovich et al., 2021 [11] |
| Essential oils | Various sources | Enterococcus faecium | V5, EQ19 | 1–32 µg/mL ** | Iseppi et al., 2019 [40] |
| Various terpenes | Commercial | Enterococcus faecium | V5, EQ19 | 1–16 µg/mL ** | Iseppi et al., 2019 [40] |
| CBD | Commercial | Enterococcus faecium | V5, EQ19 | 1–4 µg/mL ** | Iseppi et al., 2019 [40] |
| Essential oils | C. sativa (Futura 75, Carmagnola Lemon, Gran Sasso Kush, Carmagnola, Kompolti) | Enterococcus faecium | ATCC 19434 | 1.25->20 µg/mL ** | Palmieri et al., 2021 [34] |
| CBD | Commercial | Enterococcus faecalis | NCTC 7171, ATCC 51559,ATCC 29212, ATCC 51299 | 2–4 µg/mL ** | Abichabki et al., 2022 [31] |
| CBD | Commercial | Enterococcus faecalis | ATCC 29212, clinical isolate, MMX 486 | 1–4 µg/mL ** | Blaskovich et al., 2021 [11] |
| CBD | Commercial | Enterococcus faecalis | 13-327129 | 8 µg/ml | Wassman et al., 2020 [41] |
| Essential oils | Various sources | Enterococcus faecalis | ATCC 29212, V3, V4, v6 | 0.5–32 µg/mL ** | Iseppi et al., 2019 [40] |
| Various terpenes | Commercial | Enterococcus faecalis | ATCC 29212, V3, V4, v6 | 0.5–16 µg/mL ** | Iseppi et al., 2019 [40] |
| CBD | Commercial | Enterococcus faecalis | ATCC 29212, V3, V4, v6 | 1–4 µg/mL ** | Iseppi et al., 2019 [40] |
| CBD | Commercial | Enterococcus gallinarum | ATCC 12359 | 4 µg/mL ** | Abichabki et al., 2022 [31] |
| Essential oils | Various sources | Enterococcus hirae | ATCC 10541 | 2–32 µg/mL ** | Iseppi et al., 2019 [40] |
| Various terpenes | Commercial | Enterococcus hirae | ATCC 10541 | 1–8 µg/mL ** | Iseppi et al., 2019 [40] |
| CBD | Commercial | Enterococcus hirae | ATCC 10541 | 2 µg/mL ** | Iseppi et al., 2019 [40] |
| CBD | Commercial | Filifactor alocis | ATCC 35896 | 1 µg/mL * | Gu et al., 2019 [32] |
| Essential oil-derived α-pinene and myrcene | C. sativa, Futura 75 | Listeria monocytogenes | 11 clinical isolates | ≥1024 µg/mL *** | Marini et al. 2018 [42] |
| CBD | Commercial | Listeria monocytogenes | EGD | 4 µg/ml | Wassman et al., 2020 [41] |
| Essential oils | C. sativa (Futura 75, Carmagnola Lemon, Gran Sasso Kush, Carmagnola, Kompolti) | Listeria monocytogenes | ATCC 7644, ATCC 19114, LM4 | 0.6->20 µL/mL ** | Palmieri et al., 2021 [34] |
| Essential oils | Various sources | Listeria monocytogenes | NCTC 10888, ATCC 13932, ATCC 5008, 70, 139 | 2–32 µg/mL ** | Iseppi et al., 2019 [40] |
| Various terpenes | Commercial | Listeria monocytogenes | NCTC 10888, ATCC 13932, ATCC 5008, 70, 139 | 0.5–4 µg/mL ** | Iseppi et al., 2019 [40] |
| CBD | Commercial | Listeria monocytogenes | NCTC 10888, ATCC 13932, ATCC 5008, 70, 139 | 1–4 µg/mL ** | Iseppi et al., 2019 [40] |
| Essential oil | C. sativa L, Futura 75 | Listeria monocytogenes | ATCC 19114, LM 4, ATCC 7644 | 2.5–5 μL/mL ** 2.5–5 μL/mL *** | Pellegrini et al., 2020 [38] |
| CBD | Commercial | Micrococcus luteus | CCT 2688 | 4 µg/mL ** | Abichabki et al., 2022 [31] |
| Hexane extract | C. sativa (unspecified hemp variety) seeds | Propionibacterium acnes | KCTC strain | 20% extract ** | Jin et al., 2018 [43] |
| CBD | Commercial | Rhodococcus equi | ATCC 6939 | 4 µg/mL ** | Abichabki et al., 2022 [31] |
| CBD | C. sativa extraction | Salmonella newington | UC1698 | 0.125 µg/mL ** | Gildea et al., 2022 [44] |
| CBD | C. sativa extraction | Salmonella typhimurium | MS1868 | 0.125 µg/mL ** | Gildea et al., 2022 [44] |
| CBD | Commercial | Staphylococcus agalactiae | ATCC 13813 | 4 µg/mL ** | Abichabki et al., 2022 [31] |
| CBD | Commercial | Staphylococcus epidermidis | 933010 3F-16 b4 | 4 µg/ml | Wassman et al., 2020 [41] |
| CBDA | Purified from Cannabis sativa, fiber types | Staphylococcus epidermidis | CA#71, ATCC 51625 | 4 μg/mL ** | Martinenghi et al., 2020 [37] |
| CBD | Purified from Cannabis sativa, fiber types | Staphylococcus epidermidis | CA#71, ATCC 51625 | 2 μg/mL ** | Martinenghi et al., 2020 [37] |
| CBD | Commercial | Staphylococcus epidermidis | ATCC 12228, NRS-60 | 1–8 µg/mL ** | Blaskovich et al., 2021 [11] |
| Essential oils | Various sources | Staphylococcus epidermidis | 18Bs | 1–16 µg/mL ** | Iseppi et al., 2019 [40] |
| Various terpenes | Commercial | Staphylococcus epidermidis | 18Bs | 8–32 µg/mL ** | Iseppi et al., 2019 [40] |
| CBD | Commercial | Staphylococcus epidermidis | 18Bs | 16 µg/mL ** | Iseppi et al., 2019 [40] |
| CBD | Commercial | Staphylococcus epidermidis | ATCC 14990 | 4 µg/mL ** | Abichabki et al., 2022 [31] |
| CBD | Commercial | Staphylococcus lugdunensis | ATCC 43809 | 4 µg/mL ** | Abichabki et al., 2022 [31] |
| CBG | Commercial | Staphylococcus mutans | ATCC 700610 | 2.5 µg/mL ** | Aqawi et al., 2021 [45] |
| CBD | Commercial | Streptococcus pneumoniae | ATCC 33400, ATCC 700677 | 1–4 µg/mL ** | Blaskovich et al., 2021 [11] |
| CBD | Commercial | Streptococcus pneumoniae | ATCC 49619 | 64 µg/mL ** | Abichabki et al., 2022 [31] |
| CBD | Commercial | Staphylococcus pyogenes | ATCC 12344 | 32 µg/mL ** | Abichabki et al., 2022 [31] |
| CBG | Commercial | Streptococcus sanguis | 10556 | 1 µg/mL * | Aqawi et al., 2021 [45] |
| CBD | Commercial | Staphylococcus saprophyticus | ATCC 53050 | 4 µg/mL ** | Abichabki et al., 2022 [31] |
| CBG | Commercial | Streptococcus sobrinus | ATCC 27351 | 5 µg/mL ** | Aqawi et al., 2021 [45] |
| CBG | Commercial | Streptococcus salivarius | ATCC 25975 | 5 µg/mL ** | Aqawi et al., 2021 [45] |
| Compound | Source | Target | Strain | Efficacy | Reference |
|---|---|---|---|---|---|
| Ergost-5-en-3-ol | C. sativa (root) | Cryptococcus neoformans | ATCC 90113 | 13.7 μg/mL * | Elhendawy et al., 2019 [26] |
| Oxygenated derivatives of Δ9-THC and its isomer Δ8-THC | - | Cryptococcus neoformans | Not noted | 2.5–20 μg/mL * | Galal Osman et al., 2018 [39] |
| CBD | Commercial | Candida albicans | SC5314 | 100 μg/mL ****** | Feldman et al., 2019 [46] |
| Water extract | C. sativa, Futura 75 | Candida albicans | YEPGA 6183 | 1.4 mg/mL ** | Ferrante et al., 2019 [27] |
| Water extract | C. sativa, Futura 75 | Trichophyton interdigitale | CCC 202–2000 | 1000 μg/mL ** | Orlando et al., 2020 [47] |
| Water extract | C. sativa, Futura 75 | Trichophyton rubrum | CCC 134–2000 | 500 μg/mL ** | Orlando et al., 2020 [47] |
| Compound | Source | Target | Variant | Microbe | Efficacy | Reference |
|---|---|---|---|---|---|---|
| Δ9-tetrahydrocannabinol (THC) | Lab synthesized | SARS-CoV-2 | βCoV/KOR/KCDC03/2020 | ssRNA virus | 10.25 μM * | Raj et al., 2021 [48] |
| CBD | Lab synthesized | SARS-CoV-2 | βCoV/KOR/KCDC03/2020 | ssRNA virus | 7.91 μM * | Raj et al., 2021 [48] |
| Cannabigerolic acid (CBGA) | Commercial | SARS-CoV-2 | WA1; B.1.1.7; B.1.351 | ssRNA virus | 26–37 μg/mL * | Van Breemen et al., 2022 [49] |
| CBDA | Commercial | SARS-CoV-2 | WA1; B.1.1.7; B.1.351 | ssRNA virus | 11–24 μg/mL * | Van Breemen et al., 2022 [49] |
| CBD | Commercial | SARS-CoV-2 | Not apparent | ssRNA virus | 1.27 μM ***** | Nguyen et al., 2021 [50] |
| 7-OH-CBD | Commercial | SARS-CoV-2 | Not apparent | ssRNA virus | 1.27 μM ***** | Nguyen et al., 2021 [50] |
| CBD | Commercial | SARS-CoV-2 | WA1/2020 | ssRNA virus | 1.2 μM ***** | Nguyen et al., 2022 [51] |
| 7-OH-CBD | Commercial | SARS-CoV-2 | WA1/2020 | ssRNA virus | 2.6 μM ***** | Nguyen et al., 2022 [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, H.; Sloan, L.; Saxena, D.; Scott, D.A. The Antimicrobial Properties of Cannabis and Cannabis-Derived Compounds and Relevance to CB2-Targeted Neurodegenerative Therapeutics. Biomedicines 2022, 10, 1959. https://doi.org/10.3390/biomedicines10081959
Hong H, Sloan L, Saxena D, Scott DA. The Antimicrobial Properties of Cannabis and Cannabis-Derived Compounds and Relevance to CB2-Targeted Neurodegenerative Therapeutics. Biomedicines. 2022; 10(8):1959. https://doi.org/10.3390/biomedicines10081959
Chicago/Turabian StyleHong, HeeJue, Lucy Sloan, Deepak Saxena, and David A. Scott. 2022. "The Antimicrobial Properties of Cannabis and Cannabis-Derived Compounds and Relevance to CB2-Targeted Neurodegenerative Therapeutics" Biomedicines 10, no. 8: 1959. https://doi.org/10.3390/biomedicines10081959
APA StyleHong, H., Sloan, L., Saxena, D., & Scott, D. A. (2022). The Antimicrobial Properties of Cannabis and Cannabis-Derived Compounds and Relevance to CB2-Targeted Neurodegenerative Therapeutics. Biomedicines, 10(8), 1959. https://doi.org/10.3390/biomedicines10081959






