Elevated Vascular Sympathetic Neurotransmission and Remodelling Is a Common Feature in a Rat Model of Foetal Programming of Hypertension and SHR
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.1.1. Experimental Model of FPH
2.1.2. Experimental Model of Spontaneous Hypertension
2.1.3. Experimental Protocol
2.2. Chemicals
2.3. Haemodynamic Parameters Measurement
2.4. [3H]-Noradrenaline Release Experiments
2.5. Immunohistochemistry
2.6. Histology
2.7. Statistics
3. Results
3.1. Influence of Foetal Undernutrition on Vascular Morphology
3.2. Influence of Foetal Undernutrition in Sympathetic Postganglionic Nerves Activation
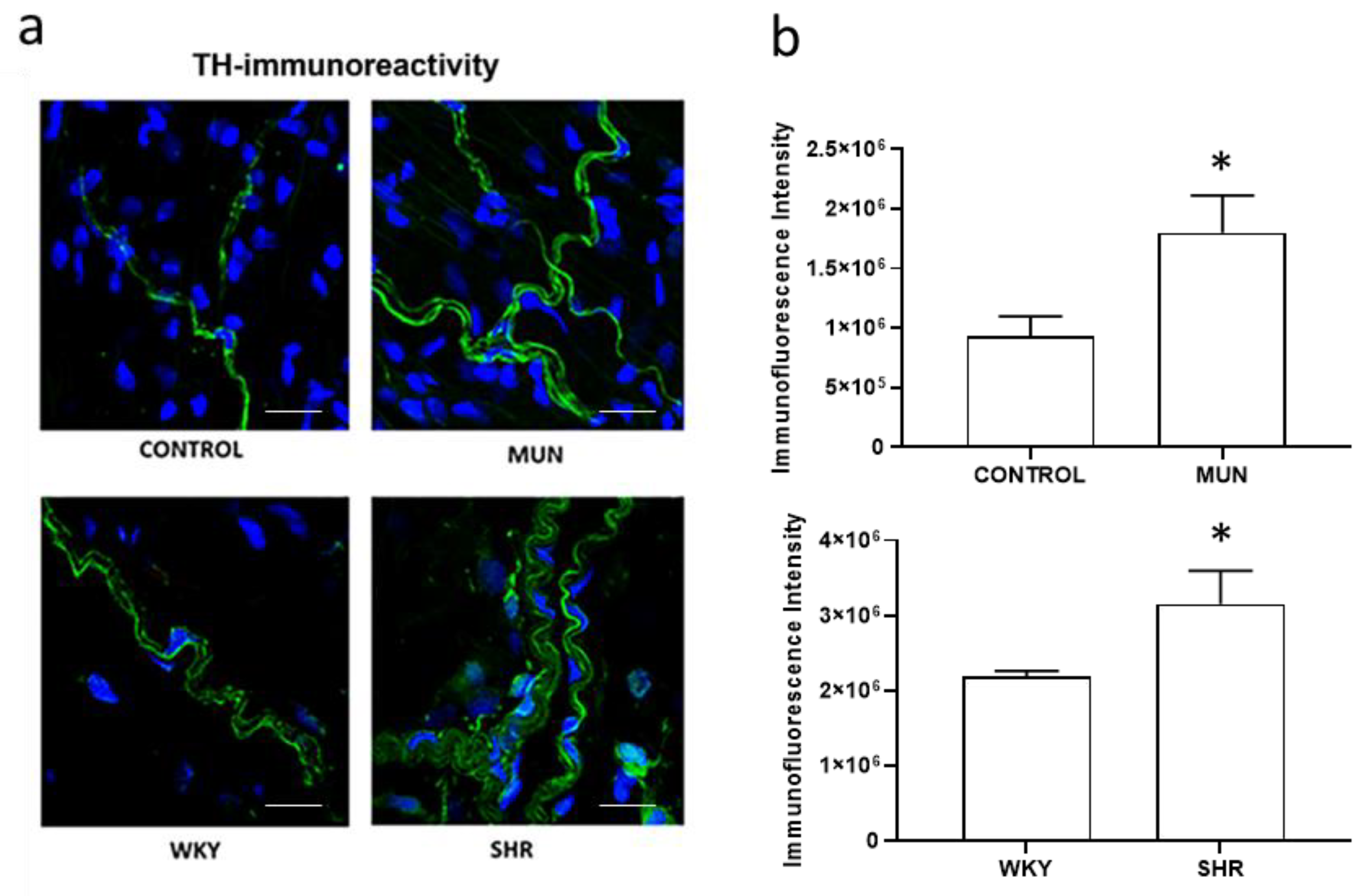
3.3. Influence of Foetal Undernutrition on Perivascular Sympathetic Innervation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Linden, E.L.; Collard, D.; Beune, E.J.A.J.; Nieuwkerk, P.T.; Galenkamp, H.; Haafkens, J.A.; van Charante, E.P.M.; van den Born, B.-J.H.; Agyemang, C. Determinants of suboptimal blood pressure control in a multi-ethnic population: The Healthy Life in an Urban Setting (HELIUS) study. J. Clin. Hypertens. 2021, 23, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Dasgupta, I. The role of the kidney and the sympathetic nervous system in hypertension. Pediatr. Nephrol. 2015, 30, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Zaman, M.A.; Calhoun, D.A. Pathogenesis of hypertension. Ann. Inter. Med. 2003, 139, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Bertoli, S.; Seravalle, G. Sympathetic nervous system: Role in hypertension and in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2012, 21, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Ram, V.S. Evidence for a critical role of the sympathetic nervous system in hypertension. J. Am. Soc. Hypertens. 2016, 10, 457–466. [Google Scholar] [CrossRef]
- Manolis, A.J.; Poulimenos, L.E.; Kallistratos, M.S.; Gavras, I.; Gavras, H. Sympathetic overactivity in hypertension and cardiovascular disease. Curr. Vasc. Pharmacol. 2014, 12, 4–15. [Google Scholar]
- Alexander, B.T.; Dasinger, J.H.; Intapad, S. Fetal programming and cardiovascular pathology. Compr. Physiol. 2015, 5, 997–1025. [Google Scholar]
- Barker, D.J.; Osmond, C. Low birth weight and hypertension. BMJ 1988, 297, 134–135. [Google Scholar] [CrossRef]
- Barker, D.J.; Clark, P.M. Fetal undernutrition and disease in later life. Rev. Reprod. 1997, 2, 105–112. [Google Scholar] [CrossRef]
- Alexander, B.T. Fetal programming of hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1–R10. [Google Scholar] [CrossRef]
- Edwards, L.J.; McMillen, I.C. Maternal undernutrition increases arterial blood pressure in the sheep fetus during late gestation. J. Physiol. 2001, 533, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Tintu, A.N.; Noble, F.A.; Rouwet, E.V. Hypoxia disturbs fetal hemodynamics and growth. Endothelium 2007, 14, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Leitzke, A.; Goyal, D.; Gheorghe, C.P.; Longo, L.D. Antenatal maternal hypoxic stress: Adaptations in fetal lung Renin-Angiotensin system. Reprod. Sci. 2011, 18, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Svitok, P.; Molcan, L.; Stebelova, K.; Vesela, A.; Sedlackova, N.; Ujhazy, E.; Mach, M.; Zeman, M. Prenatal hypoxia in rats increased blood pressure and sympathetic drive of the adult offspring. Hypertens. Res. 2016, 39, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.T. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 2003, 41, 457–462. [Google Scholar] [CrossRef]
- Dodic, M.; May, C.N.; Wintour, E.M.; Coghlan, J.P. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin. Sci. 1998, 94, 149–155. [Google Scholar] [CrossRef]
- Gwathmey, T.M.; Shaltout, A.H.; Rose, J.C.; Diz, D.I.; Chappell, M.C. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension 2011, 57, 620–626. [Google Scholar] [CrossRef]
- Nguyen, P.; Khurana, S.; Peltsch, H.; Grandbois, J.; Eibl, J.; Crispo, J.; Ansell, D.; Tai, T.C. Prenatal glucocorticoid exposure programs adrenal PNMT expression and adult hypertension. J. Endocrinol. 2015, 227, 117–127. [Google Scholar] [CrossRef]
- Nykjaer, C.; Alwan, N.A.; Greenwood, D.C.; Simpson, N.A.; Hay, A.W.; White, K.L.; Cade, J.E. Maternal alcohol intake prior to and during pregnancy and risk of adverse birth outcomes: Evidence from a British cohort. J. Epidemiol. Community Health 2014, 68, 542–549. [Google Scholar] [CrossRef]
- Nordentoft, M.; Lou, H.C.; Hansen, D.; Nim, J.; Pryds, O.; Rubin, P.; Hemmingsen, R. Intrauterine growth retardation and premature delivery: The influence of maternal smoking and psychosocial factors. Am. J. Public Health 1996, 86, 347–354. [Google Scholar] [CrossRef]
- Banderali, G.; Martelli, A.; Landi, M.; Moretti, F.; Betti, F.; Radaelli, G.; Lassandro, C.; Verduci, E. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: A descriptive review. J. Transl. Med. 2015, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Sprauve, M.E.; Lindsay, M.K.; Drews-Botsch, C.D.; Graves, W. Racial patterns in the effects of tobacco use on fetal growth. Am. J. Obstet. Gynecol. 1999, 181, S22–S27. [Google Scholar] [CrossRef]
- Poston, L.; Igosheva, N.; Mistry, H.D.; Seed, P.T.; Shennan, A.H.; Rana, S.; Karumanchi, S.A.; Chappell, L.C. Role of oxidative stress and antioxidant supplementation in pregnancy disorders. Am. J. Clin. Nutr. 2011, 94, 1980S–1985S. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, P.; Ramiro-Cortijo, D.; Reyes-Hernández, C.G.; López de Pablo, A.L.; González, M.C.; Arribas, S.M. Implication of oxidative stress in fetal programming of cardiovascular disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef]
- Nuyt, A.M. Mechanisms underlying developmental programming of elevated blood pressure and vascular dysfunction: Evidence from human studies and experimental animal models. Clin. Sci. 2008, 114, 1–17. [Google Scholar] [CrossRef]
- Lesage, J.; Blondeau, B.; Grino, M.; Bréant, B.; Dupouy, J.P. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology 2001, 142, 1692–1702. [Google Scholar] [CrossRef]
- Chisari, A.N.; Giovambattista, A.; Perelló, M.; Gaillard, R.C.; Spinedi, E.S. Maternal undernutrition induces neuroendocrine immune dysfunction in male pups at weaning. Neuroimmunomodulation 2001, 9, 41–48. [Google Scholar] [CrossRef]
- Vehaskari, V.M.; Stewart, T.; Lafont, D.; Soyez, C.; Seth, D.; Manning, J. Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am. J. Physiol. Ren. Physiol. 2004, 287, F262–F267. [Google Scholar] [CrossRef]
- Manning, J.; Vehaskari, V.M. Postnatal modulation of prenatally programmed hypertension by dietary Na and ACE inhibition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R80–R84. [Google Scholar] [CrossRef]
- Mesquita, F.F.; Gontijo, J.A.; Boer, P.A. Expression of renin-angiotensin system signalling compounds in maternal protein-restricted rats: Effect on renal sodium excretion and blood pressure. Nephrol. Dial. Transplant. 2010, 25, 380–388. [Google Scholar] [CrossRef]
- Bogdarina, I.; Welham, S.; King, P.J.; Burns, S.P.; Clark, A.J. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007, 100, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shi, A.; Zhu, D.; Bo, L.; Zhong, Y.; Wang, J.; Xu, Z.; Mao, C. High sucrose intake during gestation increases angiotensin II type 1 receptor-mediated vascular contractility associated with epigenetic alterations in aged offspring rats. Peptides 2016, 86, 133–144. [Google Scholar] [CrossRef]
- Goyal, R.; Leitzke, A.; Gheorghe, C.P.; Longo, L.D. Brain renin-angiotensin system: Fetal epigenetic programming by maternal protein restriction during pregnancy. Reprod. Sci. 2010, 17, 227–238. [Google Scholar] [CrossRef]
- De Mello, W.C. Intracellular angiotensin II as a regulator of muscle tone in vascular resistance vessels. Pathophysiological implications. Peptides 2016, 78, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.A.; De Brito Alves, J.L.; Nogueira, V.O.; Wanderley, A.G.; Costa-Silva, J.H. Maternal low-protein diet induces changes in the cardiovascular autonomic modulation in male rat offspring. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Siddique, K.; Baum, M.; Smith, S.A. Prenatal programming of hypertension induces sympathetic overactivity in response to physical stress. Hypertension 2013, 61, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Vehaskari, V.M.; Aviles, D.H.; Manning, J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001, 59, 238–245. [Google Scholar] [CrossRef]
- Silvagni, A.; Barros, V.G.; Mura, C.; Antonelli, M.C.; Carboni, E. Prenatal restraint stress differentially modifies basal and stimulated dopamine and noradrenaline release in the nucleus accumbens shell: An ‘in vivo’ microdialysis study in adolescent and young adult rats. Eur. J. Neurosci. 2008, 28, 744–758. [Google Scholar] [CrossRef]
- Vieira-Rocha, M.S.; Sousa, J.B.; Rodriguez-Rodriguez, P.; Morato, M.; Arribas, S.M.; Diniz, C. Insights into sympathetic nervous system and GPCR interplay in fetal programming of hypertension: A bridge for new pharmacological strategies. Drug Discov. Today 2020, 25, 739–747. [Google Scholar] [CrossRef]
- Okamoto, K.; Aoki, K. Development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 1963, 27, 282–293. [Google Scholar] [CrossRef]
- Trippodo, N.C.; Frohlich, E.D. Similarities of genetic (spontaneous) hypertension. Man and rat. Circ. Res. 1981, 48, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Sasamura, H.; Hayashi, K.; Ishiguro, K.; Nakaya, H.; Saruta, T.; Itoh, H. Prevention and regression of hypertension: Role of renal microvascular protection. Hypertens. Res. 2009, 32, 658–664. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sousa, J.B.; Vieira-Rocha, M.S.; Sá, C.; Ferreirinha, F.; Correia-de-Sá, P.; Fresco, P.; Diniz, C. Lack of endogenous adenosine tonus on sympathetic neurotransmission in spontaneously hypertensive rat mesenteric artery. PLoS ONE 2014, 9, e105540. [Google Scholar] [CrossRef] [PubMed]
- Arribas, S.M.; Briones, A.M.; Bellingham, C.; González, M.C.; Salaices, M.; Liu, K.; Wang, Y.; Hinek, A. Heightened aberrant deposition of hard-wearing elastin in conduit arteries of prehypertensive SHR is associated with increased stiffness and inward remodeling. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2299–H2307. [Google Scholar] [CrossRef]
- González, J.M.; Briones, A.M.; Somoza, B.; Daly, C.J.; Vila, E.; Starcher, B.; McGrath, J.C.; González, M.C.; Arribas, S.M. Postnatal alterations in elastic fiber organization precede resistance artery narrowing in SHR. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H804–H812. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Rodríguez, P.; de Pablo, A.L.; Condezo-Hoyos, L.; Martín-Cabrejas, M.A.; Aguilera, Y.; Ruiz-Hurtado, G.; Gutierrez-Arzapalo, P.Y.; Ramiro-Cortijo, D.; Fernández-Alfonso, M.S.; González, M.C.; et al. Fetal undernutrition is associated with perinatal sex-dependent alterations in oxidative status. J. Nutr. Biochem. 2015, 26, 1650–1659. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Howells, D.W.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Muñoz-Valverde, D.; Rodríguez, P.R.; Gutierrez-Arzapalo, P.Y.; De Pablo, A.L.L.; González, M.C.; López-Giménez, R.; Somoza, B.; Arribas, S.M. Effect of Fetal Undernutrition and Postnatal Overfeeding on Rat Adipose Tissue and Organ Growth at Early Stages of Postnatal Development. Physiol. Res. 2015, 64, 547–559. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, P.; De Pablo, L.L.; García-Prieto, C.F.; Somoza, B.; Quintana-Villamandos, B.; De Diego, J.J.G.; Gutierrez-Arzapalo, P.Y.; Ramiro-Cortijo, D.; Gonzalez-Garcia, M.C.; Arribas, S.M. Long term effects of fetal undernutrition on rat heart. Role of hypertension and oxidative stress. PLoS ONE 2017, 12, e0171544. [Google Scholar] [CrossRef]
- Sousa, J.B.; Vieira-Rocha, M.S.; Arribas, S.M.; González, M.C.; Fresco, P.; Diniz, C. Endothelial and Neuronal Nitric Oxide Activate Distinct Pathways on Sympathetic Neurotransmission in Rat Tail and Mesenteric Arteries. PLoS ONE 2015, 10, e0129224. [Google Scholar] [CrossRef]
- Diniz, C.; Fresco, P.; Leal, S.; Gonçalves, J. Adenosine receptors involved in modulation of noradrenaline release in isolated rat tail artery. Eur. J. Pharmacol. 2004, 504, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Diniz, C.; Goncalves, J. Facilitation of noradrenaline release by activation of adenosine A(2A) receptors triggers both phospholipase C and adenylate cyclase pathways in rat tail artery. Cardiovasc. Res. 2004, 63, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Pereira, C.; Sousa, J.B.; Vieira-Rocha, M.S.; Fresco, P.; Gonçalves, J.; Diniz, C. Differential inhibition of noradrenaline release mediated by inhibitory A(1)-adenosine receptors in the mesenteric vein and artery from normotensive and hypertensive rats. Neurochem. Int. 2013, 62, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Pereira, C.; Arribas, S.M.; Fresco, P.; González, M.C.; Gonçalves, J.; Diniz, C. Impaired inhibitory function of presynaptic A1-adenosine receptors in SHR mesenteric arteries. J. Pharmacol. Sci. 2013, 122, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Arribas, S.M.; Hillier, C.; González, C.; McGrory, S.; Dominiczak, A.; McGrath, J. Cellular Aspects of Vascular Remodeling in Hypertension Revealed by Confocal Microscopy. Hypertension 1997, 30, 1455–1464. [Google Scholar] [CrossRef]
- Sousa, J.B.; Fresco, P.; Diniz, C. Imaging receptors with Laser Scanning Confocal Microscopy: Qualitative and quantitative analysis. In Microscopy: Advances in Scientific Research and Education; Méndez-Villas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2014; pp. 201–208. ISBN 978-84-942134-3-4. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibish, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- De Fontgalland, D.; Wattchow, D.A.; Costa, M.; Brookes, S.J. Immunohistochemical characterization of the innervation of human colonic mesenteric and submucosal blood vessels. Neurogastroenterol. Motil. 2008, 20, 1212–1226. [Google Scholar] [CrossRef]
- Rook, W.; Johnson, C.D.; Coney, A.M.; Marshall, J.M. Prenatal hypoxia leads to increased muscle sympathetic nerve activity, sympathetic hyperinnervation, premature blunting of neuropeptide Y signaling, and hypertension in adult life. Hypertension 2014, 64, 1321–1327. [Google Scholar] [CrossRef]
- Luff, S.E.; Young, S.B.; McLachlan, E.M. Hyperinnervation of mesenteric arteries in spontaneously hypertensive rats by sympathetic but not primary afferent axons. J. Vasc. Res. 2005, 42, 348–358. [Google Scholar] [CrossRef]
- Blakely, R.D.; Bauman, A.L. Biogenic amine transporters: Regulation in flux. Curr. Opin. Neurobiol. 2000, 10, 328–336. [Google Scholar] [CrossRef]
- Zhou, J. Norepinephrine transporter inhibitors and their therapeutic potential. Drugs Future 2004, 29, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Rocha, M.S.; Rodríguez-Rodríguez, P.; Sousa, J.B.; González, M.C.; Arribas, S.M.; López de Pablo, A.L.; Diniz, C. Vascular angiotensin AT1 receptor neuromodulation in fetal programming of hypertension. Vascul. Pharmacol. 2019, 117, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Whall, C.W., Jr.; Myers, M.M.; Halpern, W. Norepinephrine sensitivity, tension development and neuronal uptake in resistance arteries from spontaneously hypertensive and normotensive rats. Blood Vessels 1980, 17, 1–15. [Google Scholar] [PubMed]
- Zsoter, T.T.; Wolchinsky, C. Norepinephrine uptake in arteries of spontaneously hypertensive rats. Clin. Investig. Med. 1983, 6, 191–195. [Google Scholar]
- IJzerman, R.G.; Stehouwer, C.D.; de Geus, E.J.; van Weissenbruch, M.M.; Delemarre-van de Waal, H.A.; Boomsma, D.I. Low birth weight is associated with increased sympathetic activity: Dependence on genetic factors. Circulation 2003, 108, 566–571. [Google Scholar] [CrossRef]
- Mizuno, M.; Lozano, G.; Siddique, K.; Baum, M.; Smith, S.A. Enalapril attenuates the exaggerated sympathetic response to physical stress in prenatally programmed hypertensive rats. Hypertension 2014, 63, 324–329. [Google Scholar] [CrossRef]
- Marshall, J.M. Interactions between local dilator and sympathetic vasoconstrictor influences in skeletal muscle in acute and chronic hypoxia. Exp. Physiol. 2015, 100, 1400–1411. [Google Scholar] [CrossRef]
- de Brito Alves, J.L.; de Oliveira, J.M.; Ferreira, D.J.; Barros, M.A.; Nogueira, V.O.; Alves, D.S.; Vidal, H.; Leandro, C.G.; Lagranha, C.J.; Pirola, L.; et al. Maternal protein restriction induced-hypertension is associated to oxidative disruption at transcriptional and functional levels in the medulla oblongata. Clin. Exp. Pharmacol. Physiol. 2016, 43, 1177–1184. [Google Scholar] [CrossRef]
- Hiraoka, T.; Kudo, T.; Kishimoto, Y. Catecholamines in experimentally growth-retarded rat fetus. Asia Ocean. J. Obstet. Gynaecol. 1991, 17, 341–348. [Google Scholar] [CrossRef]
- Petry, C.J.; Dorling, M.W.; Wang, C.L.; Pawlak, D.B.; Ozanne, S.E. Catecholamine levels and receptor expression in low protein rat offspring. Diabet. Med. 2000, 17, 848–853. [Google Scholar] [CrossRef]
- Jones, C.T.; Robinson, J.S. Studies on experimental growth retardation in sheep. Plasma catecholamines in fetuses with small placenta. J. Dev. Physiol. 1983, 5, 77–87. [Google Scholar] [PubMed]
- Johansson, S.; Norman, M.; Legnevall, L.; Dalmaz, Y.; Lagercrantz, H.; Vanpée, M. Increased catecholamines and heart rate in children with low birth weight: Perinatal contributions to sympathoadrenal overactivity. J. Intern. Med. 2007, 261, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Butcher, K.S.; Hachinski, V.C.; Wilson, J.X.; Guiraudon, C.; Cechetto, D.F. Cardiac and sympathetic effects of middle cerebral artery occlusion in the spontaneously hypertensive rat. Brain Res. 1993, 621, 79–86. [Google Scholar] [CrossRef]
- Susic, D.; Varagic, J.; Ahn, J.; Matavelli, L.C.; Frohlich, E.D. Beneficial cardiovascular actions of eplerenone in the spontaneously hypertensive rat. J. Cardiovasc. Pharmacol. Ther. 2005, 10, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Darby, J.R.T.; McMillen, I.C.; Morrison, J.L. Maternal undernutrition in late gestation increases IGF2 signalling molecules and collagen deposition in the right ventricle of the fetal sheep heart. J. Physiol. 2018, 596, 2345–2358. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Rocha, M.S.; Rodriguez-Rodriguez, P.; Ferreira-Duarte, M.; Faria, M.; Sousa, J.B.; Morato, M.; Arribas, S.M.; Diniz, C. Fetal Undernutrition Modifies Vascular RAS Balance Enhancing Oxidative Damage and Contributing to Remodeling. Int. J. Mol. Sci. 2022, 23, 1233. [Google Scholar] [CrossRef]
- Renna, N.F.; de Las Heras, N.; Miatello, R.M. Pathophysiology of vascular remodeling in hypertension. Int. J. Hypertens. 2013, 2013, 808353. [Google Scholar] [CrossRef]
- Khorram, O.; Momeni, M.; Ferrini, M.; Desai, M.; Ross, M.G. In utero undernutrition in rats induces increased vascular smooth muscle content in the offspring. Am. J. Obstet. Gynecol. 2007, 196, 486.e1–486.e8. [Google Scholar] [CrossRef]
- Khorram, O.; Khorram, N.; Momeni, M.; Han, G.; Halem, J.; Desai, M.; Ross, M.G. Maternal undernutrition inhibits angiogenesis in the offspring: A potential mechanism of programmed hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R745–R753. [Google Scholar] [CrossRef][Green Version]
- Khorram, O.; Ghazi, R.; Chuang, T.D.; Han, G.; Naghi, J.; Ni, Y. Pearce, W.J. Excess maternal glucocorticoids in response to in utero undernutrition inhibit offspring angiogenesis. Reprod. Sci. 2014, 21, 601–611. [Google Scholar] [CrossRef]
- Gutierrez-Arzapalo, P.Y.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; López de Pablo, Á.L.; López-Giménez, M.R.; Condezo-Hoyos, L.; Greenwald, S.E.; González, M.D.C.; Arribas, S.M. Role of fetal nutrient restriction and postnatal catch-up growth on structural and mechanical alterations of rat aorta. J. Physiol. 2018, 596, 5791–5806. [Google Scholar] [CrossRef] [PubMed]
- Somoza, B.; Abderrahim, F.; González, J.M.; Conde, M.V.; Arribas, S.M.; Starcher, B.; Regadera, J.; Fernández-Alfonso, M.S.; Díaz-Gil, J.J.; González, M.C. Short-term treatment of spontaneously hypertensive rats with liver growth factor reduces carotid artery fibrosis, improves vascular function, and lowers blood pressure. Cardiovasc. Res. 2006, 69, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.M.; Pang, S.C. The correlation between the development of sympathetic innervation and the development of medial hypertrophy in jejunal arteries in normotensive and spontaneously hypertensive rats. J. Auton. Nerv. Syst. 1983, 8, 25–32. [Google Scholar] [CrossRef]
- Cai, X.N.; Wang, C.Y.; Cai, Y.; Peng, F. Effects of renal denervation on blood-pressure response to hemorrhagic shock in spontaneously hypertensive rats. Chin. J. Traumatol. 2018, 21, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Pladys, P.; Lahaie, I.; Cambonie, G.; Thibault, G.; Lê, N.L.; Abran, D.; Nuyt, A.M. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr. Res. 2004, 55, 1042–1099. [Google Scholar] [CrossRef] [PubMed]
- Spann, M.N.; Smerling, J.; Gustafsson, H.; Foss, S.; Altemus, M.; Monk, C. Deficient maternal zinc intake-but not folate-is associated with lower fetal heart rate variability. Early Hum. Dev. 2015, 91, 169–172. [Google Scholar] [CrossRef]
- Sucharita, S.; Dwarkanath, P.; Thomas, T.; Srinivasan, K.; Kurpad, A.V.; Vaz, M. Low maternal vitamin B12 status during pregnancy is associated with reduced heart rate variability indices in young children. Matern. Child Nutr. 2014, 10, 226–233. [Google Scholar] [CrossRef]
- Kitagawa, H.; Yamazaki, T.; Akiyama, T.; Mori, H.; Sunagawa, K. Effects of ketamine on exocytotic and non-exocytotic noradrenaline release. Neurochem. Int. 2003, 42, 261–267. [Google Scholar] [CrossRef]
- Sinclair, M.D. A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice. Can. Vet. J. 2003, 44, 885–897. [Google Scholar]
- Briones, A.M.; González, J.M.; Somoza, B.; Giraldo, J.; Daly, C.J.; Vila, E.; González, M.C.; McGrath, J.C.; Arribas, S.M. Role of elastin in spontaneously hypertensive rat small mesenteric artery remodelling. J. Physiol. 2003, 552, 185–195. [Google Scholar] [CrossRef]
- Gutiérrez-Arzapalo, P.Y.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Gil-Ortega, M.; Somoza, B.; de Pablo, Á.L.L.; González, M.D.C.; Arribas, S.M. Fetal Undernutrition Induces Resistance Artery Remodeling and Stiffness in Male and Female Rats Independent of Hypertension. Biomedicines 2020, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Elmarakby, A.A.; Sullivan, J.C. Sex differences in hypertension: Lessons from spontaneously hypertensive rats (SHR). Clin. Sci. 2021, 135, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Quarti-Trevano, F. The ‘neuroadrenergic hypothesis’ in hypertension: Current evidence. Exp. Physiol. 2010, 95, 581–586. [Google Scholar] [CrossRef] [PubMed]
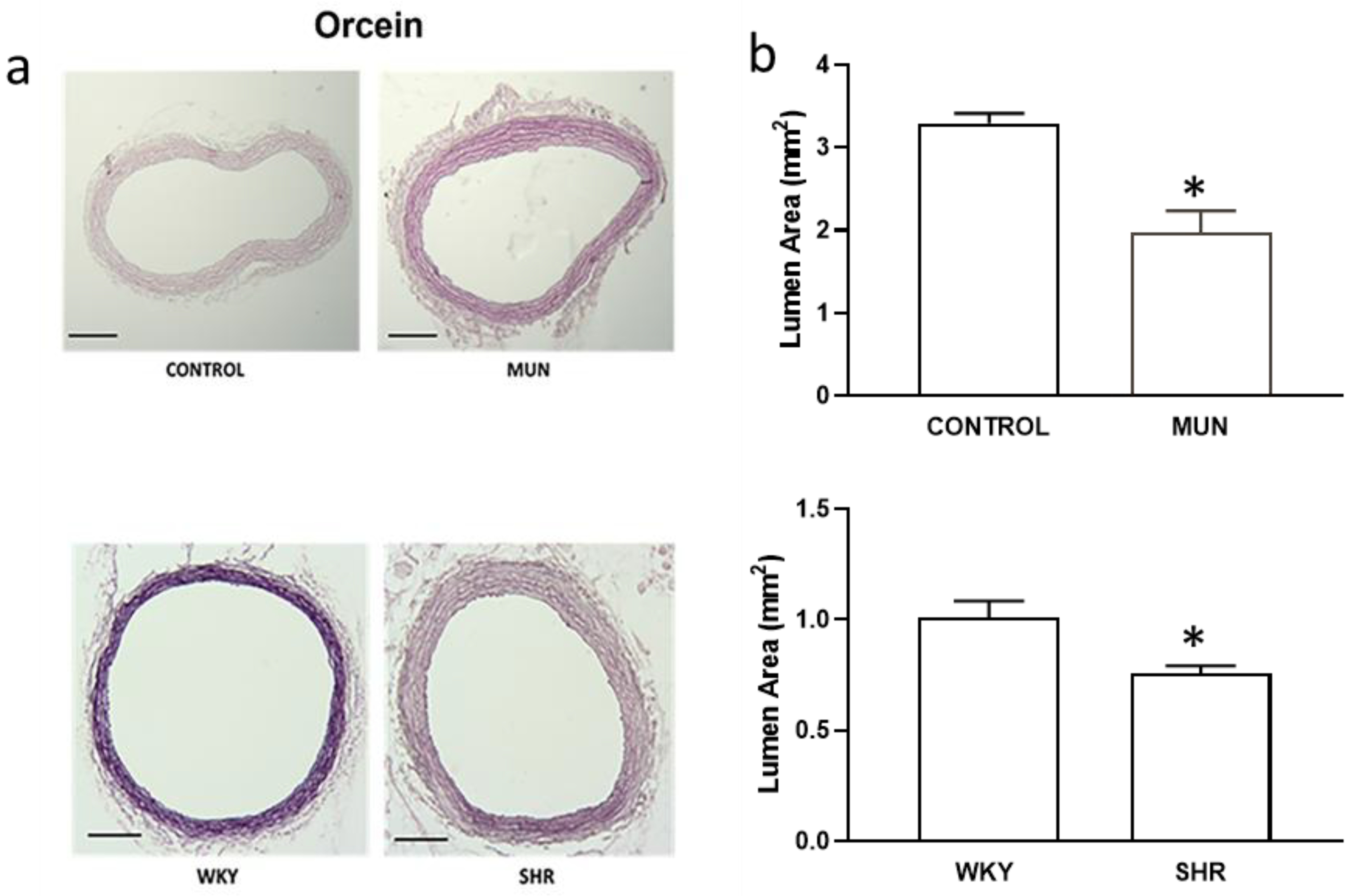
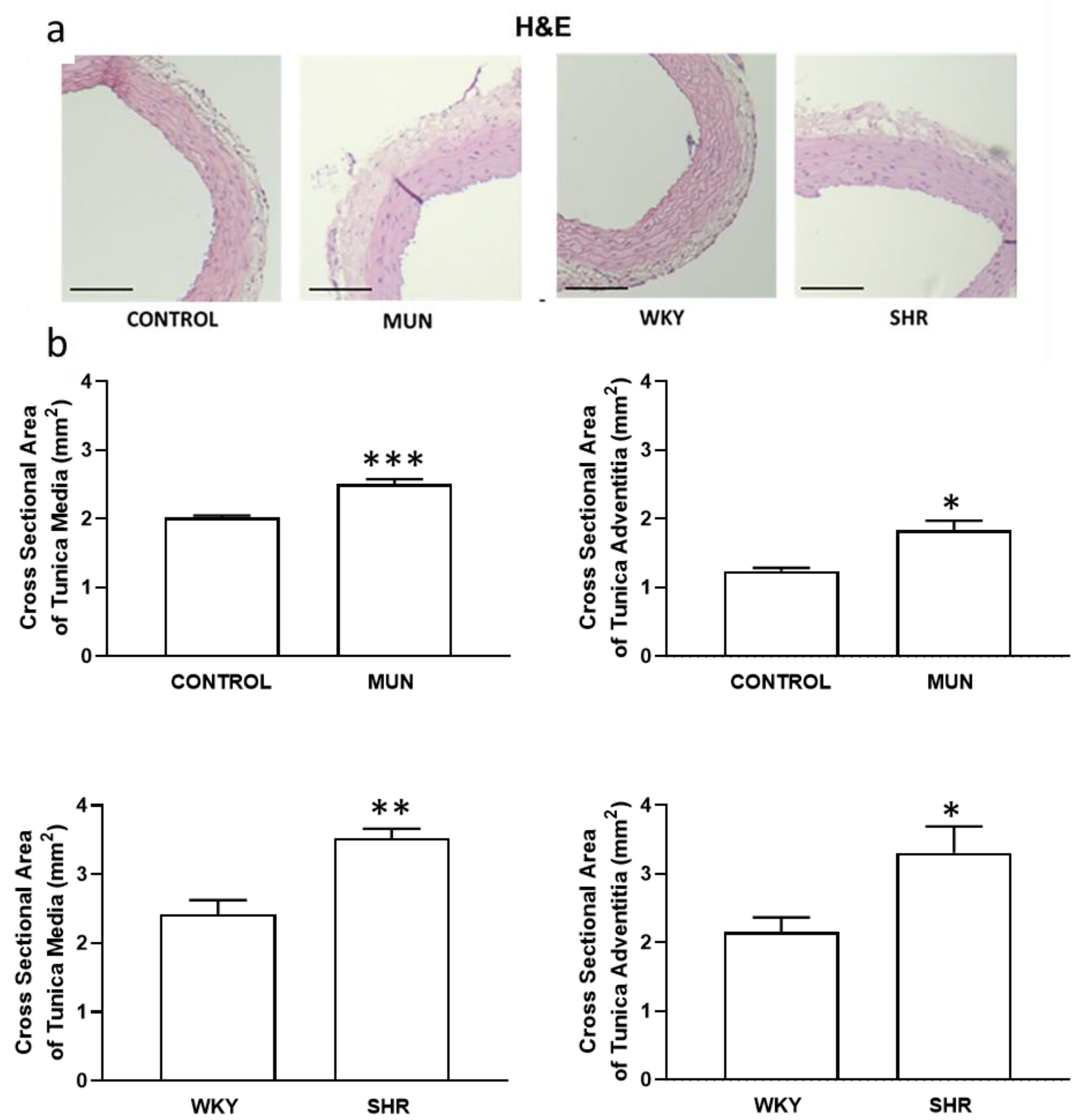
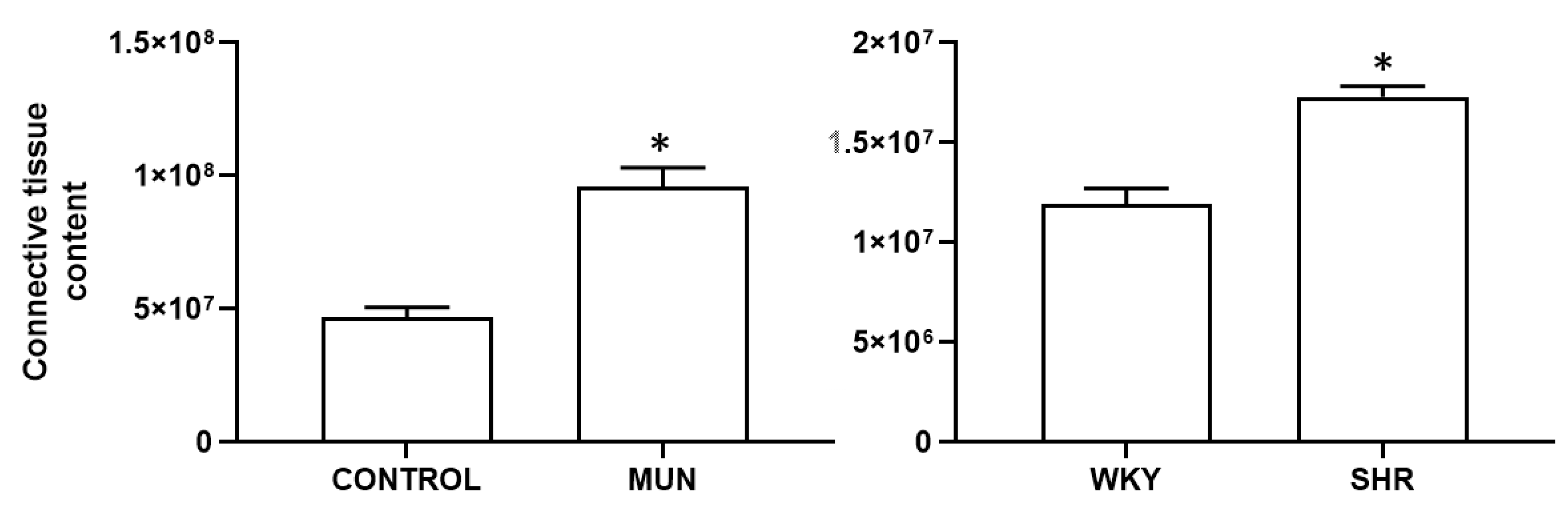
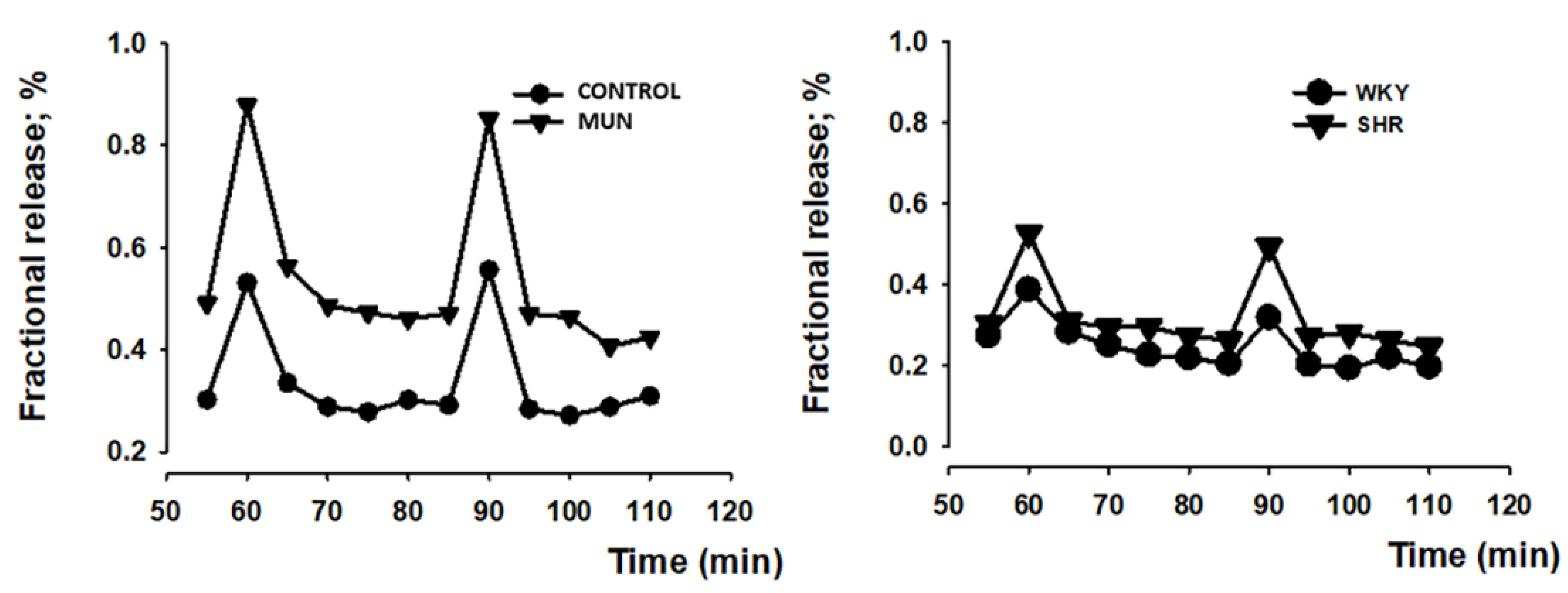
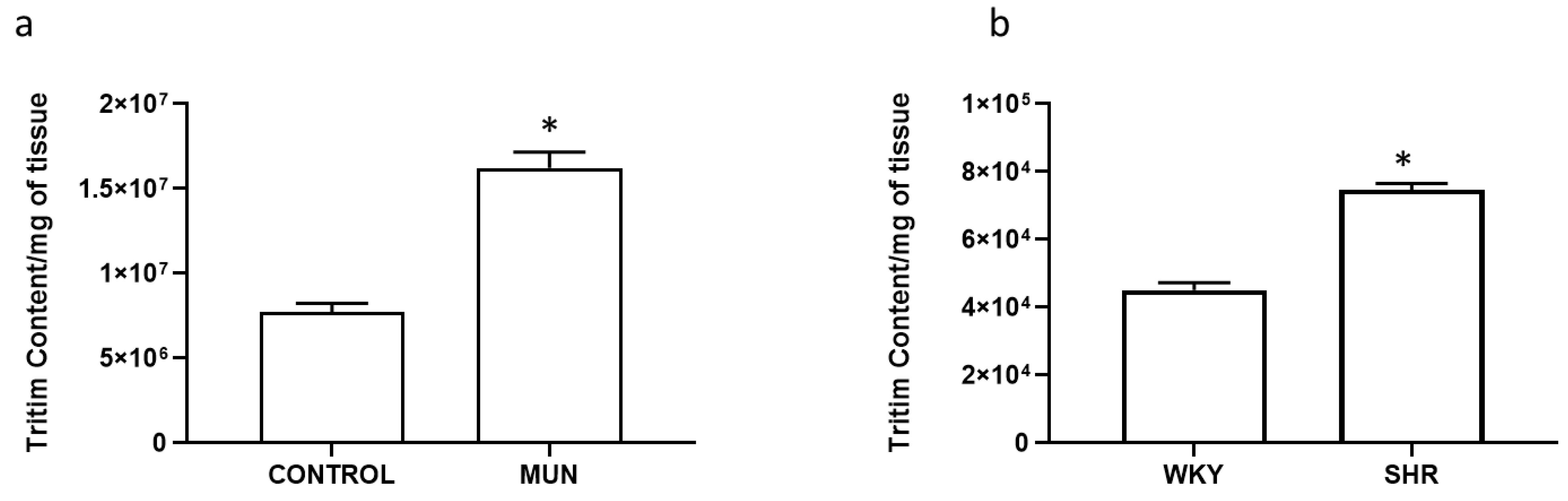

| Mesenteric Artery | DBP (mmHg) | SBP (mmHg) | HR (Beat/min) | n |
|---|---|---|---|---|
| CONTROL | 68.7 ± 4.2 † | 125.6 ± 5.1 | 258 ± 8 | 6 |
| MUN | 90.2 ± 4.5 *# | 151.1 ± 4.1 *# | 253 ± 9 # | 6 |
| WKY | 98.5 ± 5.5 | 133.1 ± 4.3 | 250 ± 10 | 6 |
| SHR | 140.3 ± 5.9 * | 186.2 ± 8.3 * | 318 ± 8 * | 6 |
| Mesenteric Artery | Ratio Media/Lumen | Ratio Adventitia/Lumen | n |
|---|---|---|---|
| CONTROL | 0.626 ± 0.016 | 0.419 ± 0.057 | 6 |
| MUN | 0.989 ± 0.314 * | 0.829 ± 0.303 * | 6 |
| WKY | 0.307 ± 0.092 | 0.266 ± 0.112 | 6 |
| SHR | 0.519 ± 0.165 * | 0.398 ± 0.187 * | 6 |
| Mesenteric Artery | Basal Outflow (b1) (Fractional Rate Outflow; min−1) | Evoked Overflow (S1) (% of Tissue Tritium Content) | n |
|---|---|---|---|
| CONTROL | 0.097 ± 0.008 | 0.220 ± 0.040 | 6 |
| MUN | 0.093 ± 0.006 | 0.347 ± 0.030 * | 6 |
| WKY | 0.088 ± 0.006 | 0.265 ± 0.021 | 6 |
| SHR | 0.073 ± 0.006 | 0.381 ± 0.032 * | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira-Rocha, M.S.; Sousa, J.B.; Rodríguez-Rodríguez, P.; Arribas, S.M.; Diniz, C. Elevated Vascular Sympathetic Neurotransmission and Remodelling Is a Common Feature in a Rat Model of Foetal Programming of Hypertension and SHR. Biomedicines 2022, 10, 1902. https://doi.org/10.3390/biomedicines10081902
Vieira-Rocha MS, Sousa JB, Rodríguez-Rodríguez P, Arribas SM, Diniz C. Elevated Vascular Sympathetic Neurotransmission and Remodelling Is a Common Feature in a Rat Model of Foetal Programming of Hypertension and SHR. Biomedicines. 2022; 10(8):1902. https://doi.org/10.3390/biomedicines10081902
Chicago/Turabian StyleVieira-Rocha, Maria Sofia, Joana Beatriz Sousa, Pilar Rodríguez-Rodríguez, Silvia Madaglena Arribas, and Carmen Diniz. 2022. "Elevated Vascular Sympathetic Neurotransmission and Remodelling Is a Common Feature in a Rat Model of Foetal Programming of Hypertension and SHR" Biomedicines 10, no. 8: 1902. https://doi.org/10.3390/biomedicines10081902
APA StyleVieira-Rocha, M. S., Sousa, J. B., Rodríguez-Rodríguez, P., Arribas, S. M., & Diniz, C. (2022). Elevated Vascular Sympathetic Neurotransmission and Remodelling Is a Common Feature in a Rat Model of Foetal Programming of Hypertension and SHR. Biomedicines, 10(8), 1902. https://doi.org/10.3390/biomedicines10081902







