Nephroprotective Effects of Semaglutide as Mono- and Combination Treatment with Lisinopril in a Mouse Model of Hypertension-Accelerated Diabetic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Uninephrectomy and Adeno-Associated Virus Delivery of Renin
2.3. Drug Treatment
2.4. Blood Pressure
2.5. Blood and Urine Analyses
2.6. Histomorphometry
2.7. Glomerulosclerosis Scoring Using Deep-Learning Computational Analysis
2.8. Filtration Slit Density Analysis
2.9. RNA Sequencing
2.10. Statistics
3. Results
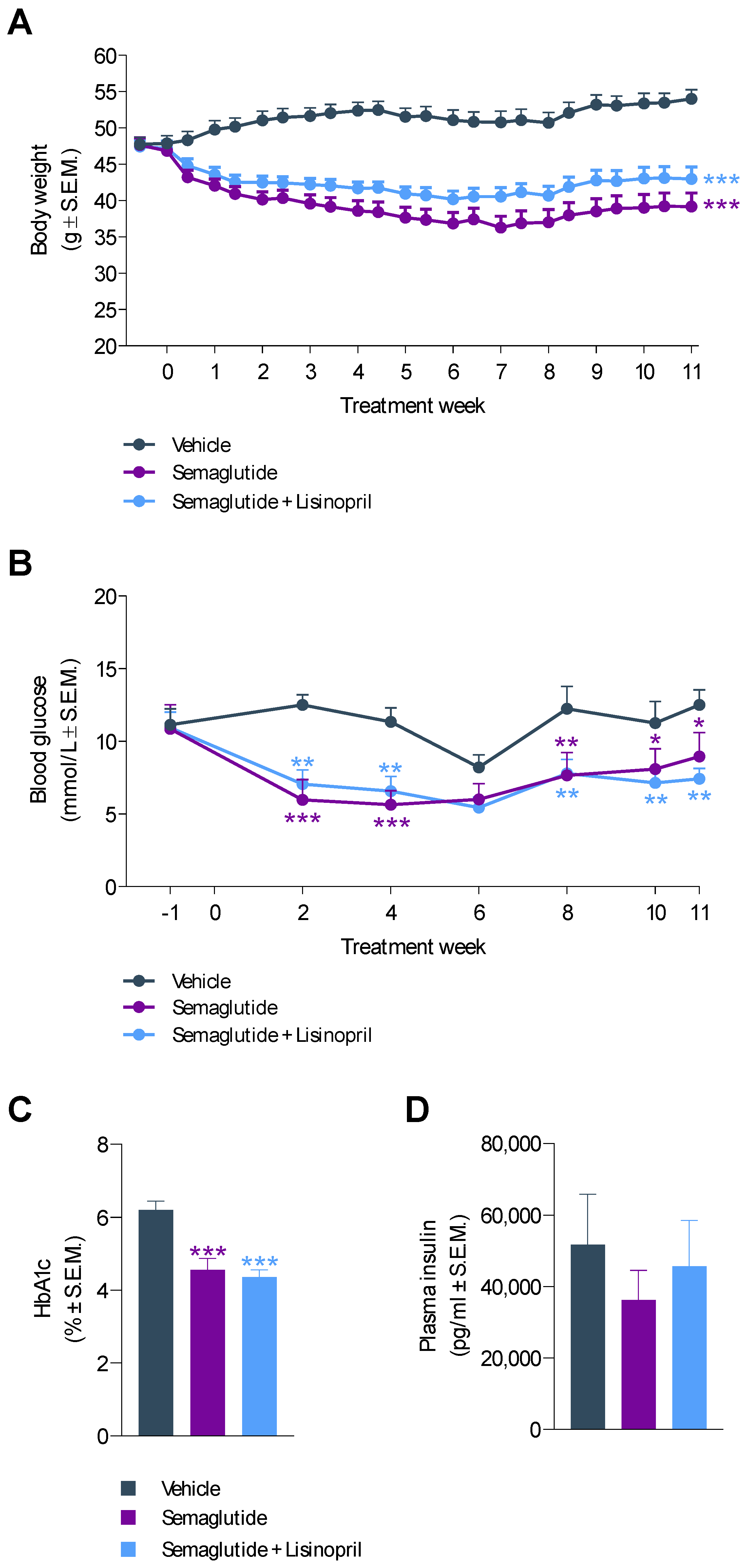
3.1. Semaglutide Improves Obesity and Hyperglycemia in db/db UNx-ReninAAV Mice
3.2. Semaglutide Improves Hypertension in db/db UNx-ReninAAV Mice
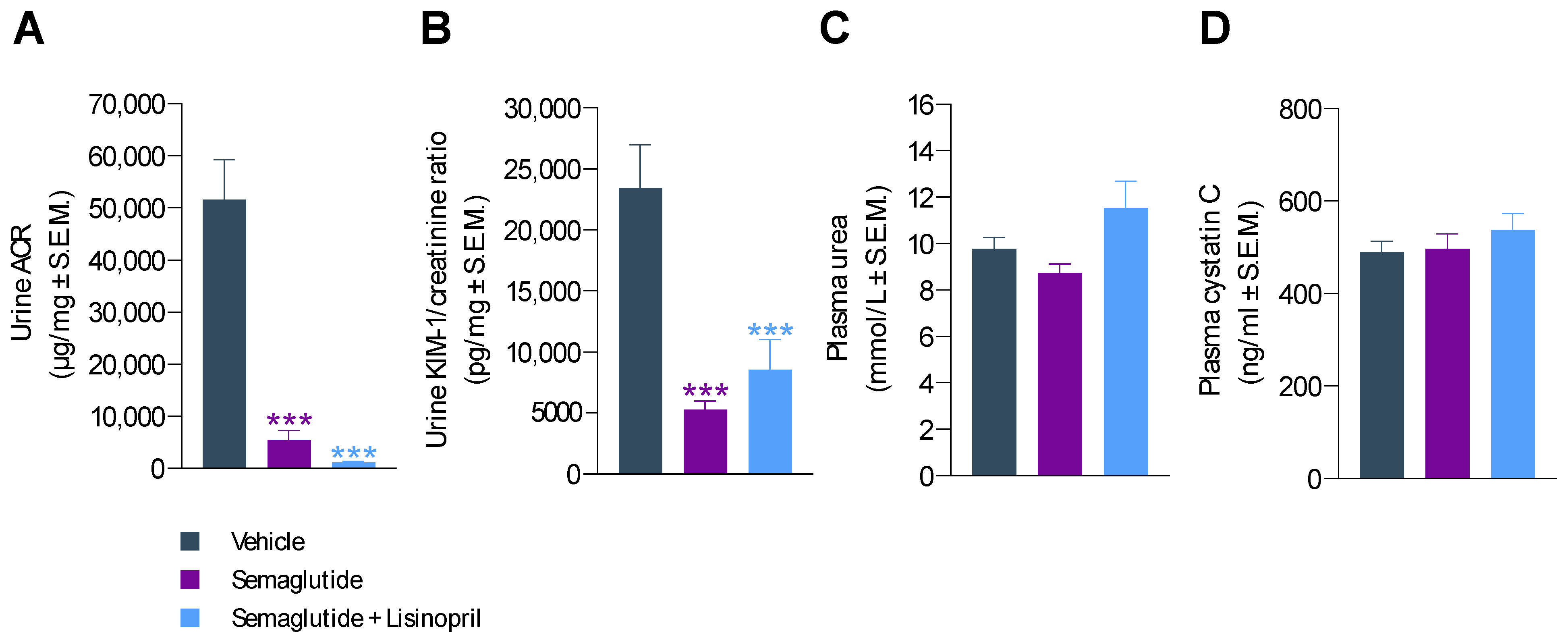
3.3. Semaglutide Improves Urinary Biomarkers in db/db UNx-ReninAAV Mice
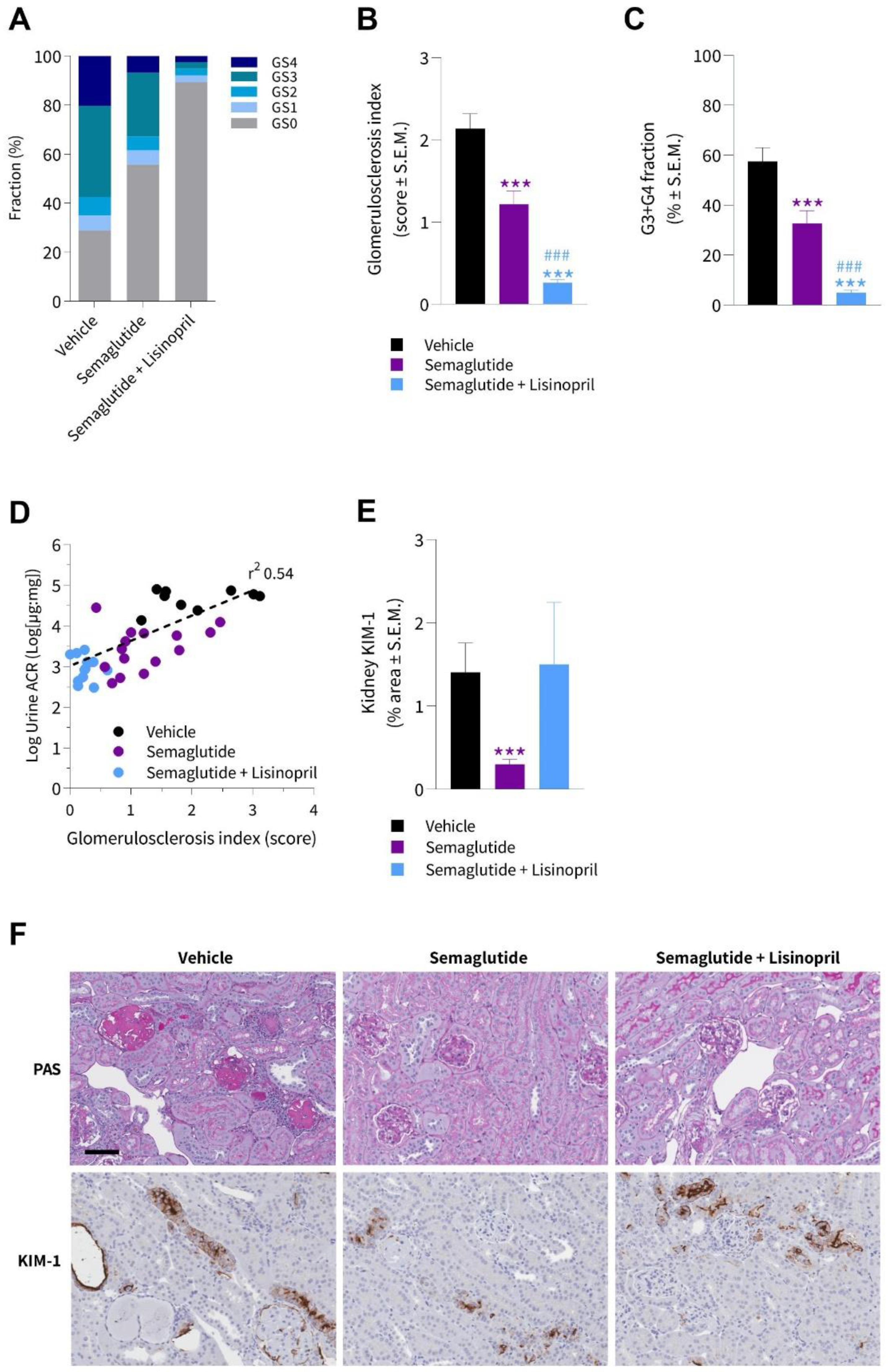
3.4. Semaglutide Improves Kidney Histopathology in db/db UNx-ReninAAV Mice
3.5. Semaglutide Improves Podocyte Health in db/db UNx-ReninAAV Mice
3.6. Semaglutide Promotes Discrete Renal Gene Expression Changes in db/db UNx-ReninAAV Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S.; Rossing, P.; Groop, P.-H.; Cooper, M.E. Diabetic Kidney Disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef] [PubMed]
- Persson, F.; Borg, R.; Rossing, P. A Narrative Review of New Treatment Options for Chronic Kidney Disease in Type 2 Diabetes. Ann. Transl. Med. 2021, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.-H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [PubMed]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors With Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes: A Meta-Analysis. JAMA Cardiol. 2021, 6, 148–158. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Pratley, R.E.; Aroda, V.R.; Lingvay, I.; Lüdemann, J.; Andreassen, C.; Navarria, A.; Viljoen, A. Semaglutide versus Dulaglutide Once Weekly in Patients with Type 2 Diabetes (SUSTAIN 7): A Randomised, Open-Label, Phase 3b Trial. Lancet Diabetes Endocrinol. 2018, 6, 275–286. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, Mortality, and Kidney Outcomes with GLP-1 Receptor Agonists in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cardiovascular Outcome Trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef]
- Lau, J.; Bloch, P.; Schä, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; Mcguire, J.; Steensgaard, B.; et al. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J. Med. Chem. 2015, 58, 7370–7380. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Harlan, S.M.; Heinz-Taheny, K.M.; Sullivan, J.M.; Wei, T.; Baker, H.E.; Jaqua, D.L.; Qi, Z.; Cramer, M.S.; Shiyanova, T.L.; Breyer, M.D.; et al. Progressive Renal Disease Established by Renin-Coding Adeno-Associated Virus-Driven Hypertension in Diverse Diabetic Models. J. Am. Soc. Nephrol. 2018, 29, 477–491. [Google Scholar] [CrossRef]
- Østergaard, M.v.; Secher, T.; Christensen, M.; Salinas, C.G.; Roostalu, U.; Skytte, J.L.; Rune, I.; Hansen, H.H.; Jelsing, J.; Vrang, N.; et al. Therapeutic Effects of Lisinopril and Empagliflozin in a Mouse Model of Hypertension-Accelerated Diabetic Kidney Disease. Am. J. Physiol. Ren. Physiol. 2021, 321, F149–F161. [Google Scholar] [CrossRef]
- Sembach, F.E.; Agidius, H.M.; Fink, L.N.; Secher, T.; Aarup, A.; Jelsing, J.; Vrang, N.; Feldt-Rasmussen, B.; Rigbolt, K.T.G.; Nielsen, J.C.; et al. Integrative Transcriptomic Profiling of a Mouse Model of Hypertension-Accelerated Diabetic Kidney Disease. Dis. Models Mech. 2021, 14, dmm049086. [Google Scholar] [CrossRef]
- Ninomiya, H.; Inomata, T.; Ogihara, K. Obstructive Uropathy and Hydronephrosis in Male KK-Ay Mice: A Report of Cases. J. Vet. Med. Sci. 1999, 61, 53–57. [Google Scholar] [CrossRef][Green Version]
- Springer, D.A.; Allen, M.; Hoffman, V.; Brinster, L.; Starost, M.F.; Bryant, M.; Eckhaus, M. Investigation and Identification of Etiologies Involved in the Development of Acquired Hydronephrosis in Aged Laboratory Mice with the Use of High-Frequency Ultrasound Imaging. Pathobiol. Aging Age-Relat. Dis. 2014, 4, 24932. [Google Scholar] [CrossRef][Green Version]
- Sembach, F.E.; Fink, L.N.; Johansen, T.; Boland, B.B.; Secher, T.; Thrane, S.T.; Nielsen, J.C.; Fosgerau, K.; Vrang, N.; Jelsing, J.; et al. Impact of Sex on Diabetic Nephropathy and the Renal Transcriptome in UNx Db/Db C57BLKS Mice. Physiol. Rep. 2019, 7, e14333. [Google Scholar] [CrossRef]
- Harlan, S.M.; Ostroski, R.A.; Coskun, T.; Yantis, L.D.; Breyer, M.D.; Heuer, J.G. Viral Transduction of Renin Rapidly Establishes Persistent Hypertension in Diverse Murine Strains. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R467–R474. [Google Scholar] [CrossRef]
- Caron, K.M.I.; James, L.R.; Kim, H.S.; Morham, S.G.; Sequeira Lopez, M.L.S.; Gomez, R.A.; Reudelhuber, T.L.; Smithies, O. A Genetically Clamped Renin Transgene for the Induction of Hypertension. Proc. Natl. Acad. Sci. USA 2002, 99, 8248–8252. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Rupprecht, L.E.; Fortin, S.M.; de Jonghe, B.C.; Hayes, M.R. The Role of Nausea in Food Intake and Body Weight Suppression by Peripheral GLP-1 Receptor Agonists, Exendin-4 and Liraglutide. Neuropharmacology 2012, 62, 1916–1927. [Google Scholar] [CrossRef]
- Østergaard, M.V.; Sembach, F.E.; Skytte, J.L.; Roostalu, U.; Secher, T.; Overgaard, A.; Fink, L.N.; Vrang, N.; Jelsing, J.; Hecksher-Sørensen, J. Automated Image Analyses of Glomerular Hypertrophy in a Mouse Model of Diabetic Nephropathy. Kidney360 2020, 1, 469–479. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015, Munich, Germany, 5–9 October 2015; Lecture Notes in Computer Science; Navab, N., Hornegger, J., Wells, W., Frangi, A., Eds.; Springer: Cham, Switzerland, 2015; Volume 9351, pp. 234–241. [Google Scholar]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the Inception Architecture for Computer Vision. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Maric, C.; Sandberg, K.; Hinojosa-Laborde, C. Glomerulosclerosis and Tubulointerstitial Fibrosis Are Attenuated with 17β-Estradiol in the Aging Dahl Salt Sensitive Rat. J. Am. Soc. Nephrol. 2004, 15, 1546–1556. [Google Scholar] [CrossRef]
- Siegerist, F.; Ribback, S.; Dombrowski, F.; Amann, K.; Zimmermann, U.; Endlich, K.; Endlich, N. Structured Illumination Microscopy and Automatized Image Processing as a Rapid Diagnostic Tool for Podocyte Effacement. Sci. Rep. 2017, 7, 11473. [Google Scholar] [CrossRef]
- Hansen, H.H.; Ægidius, H.M.; Oró, D.; Evers, S.S.; Heebøll, S.; Eriksen, P.L.; Thomsen, K.L.; Bengtsson, A.; Veidal, S.S.; Feigh, M.; et al. Human Translatability of the GAN Diet-Induced Obese Mouse Model of Non-Alcoholic Steatohepatitis. BMC Gastroenterol. 2020, 20, 210. [Google Scholar] [CrossRef]
- Mann, J.F.E.; Ørsted, D.D.; Brown-Frandsen, K.; Marso, S.P.; Poulter, N.R.; Rasmussen, S.; Tornøe, K.; Zinman, B.; Buse, J.B. LEADER Steering Committee and Investigators Liraglutide and Renal Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 839–848. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes (REWIND): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Cox, E.J.; Neumiller, J.J.; Tuttle, K.R. Incretin Drugs in Diabetic Kidney Disease: Biological Mechanisms and Clinical Evidence. Nat. Rev. Nephrol. 2020, 17, 227–244. [Google Scholar] [CrossRef]
- Harlan, S.M.; Heinz-Taheny, K.M.; Overstreet, J.M.; Breyer, M.D.; Harris, R.C.; Heuer, J.G. Pathological and Transcriptome Changes in the ReninAAV Db/Db UNx Model of Advanced Diabetic Kidney Disease Exhibit Features of Human Disease. Toxicol. Pathol. 2018, 46, 991–998. [Google Scholar] [CrossRef]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Rønne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide Lowers Body Weight in Rodents via Distributed Neural Pathways. JCI Insight 2020, 5, e133429. [Google Scholar] [CrossRef]
- Liu, D.; Gu, J.; Shao, W.; Pang, J.; Qian, X.; Jin, T. Comparison of Beneficial Metabolic Effects of Liraglutide and Semaglutide in Male C57BL/6J Mice. Can. J. Diabetes 2022, 46, 216–224. [Google Scholar] [CrossRef]
- Hansen, H.H.; Hansen, G.; Secher, T.; Feigh, M.; Veidal, S.; Fosgerau, K.; Jelsing, J.; Vrang, N. Animal Models of Type 2 Diabetes, Obesity and Nonalcoholic Steatohepatitis–Clinical Translatability and Applicability in Preclinical Drug Development. In Translational Research Methods in Diabetes, Obesity, and Nonalcoholic Fatty Liver Disease; Krentz, A., Weyer, C., Hompesch, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 369–403. ISBN 978-3-030-11748-1. [Google Scholar]
- Dalbøge, L.S.; Almholt, D.L.C.; Neerup, T.S.R.; Vassiliadis, E.; Vrang, N.; Pedersen, L.; Fosgerau, K.; Jelsing, J. Characterisation of Age-Dependent Beta Cell Dynamics in the Male Db/Db Mice. PLoS ONE 2013, 8, e82813. [Google Scholar] [CrossRef]
- Henriksen, E.J.; Jacob, S. Modulation of Metabolic Control by Angiotensin Converting Enzyme (ACE) Inhibition. J. Cell. Physiol. 2003, 196, 171–179. [Google Scholar] [CrossRef]
- Hirata, K.; Kume, S.; Araki, S.i.; Sakaguchi, M.; Chin-Kanasaki, M.; Isshiki, K.; Sugimoto, T.; Nishiyama, A.; Koya, D.; Haneda, M.; et al. Exendin-4 Has an Anti-Hypertensive Effect in Salt-Sensitive Mice Model. Biochem. Biophys. Res. Commun. 2009, 380, 44–49. [Google Scholar] [CrossRef]
- Yu, M.; Moreno, C.; Hoagland, K.M.; Dahly, A.; Ditter, K.; Mistry, M.; Roman, R.J. Antihypertensive Effect of Glucagon-like Peptide 1 in Dahl Salt-Sensitive Rats. J. Hypertens. 2003, 21, 1125–1135. [Google Scholar] [CrossRef]
- Skov, J.; Pedersen, M.; Holst, J.J.; Madsen, B.; Goetze, J.P.; Rittig, S.; Jonassen, T.; Frøkiær, J.; Dejgaard, A.; Christiansen, J.S. Short-Term Effects of Liraglutide on Kidney Function and Vasoactive Hormones in Type 2 Diabetes: A Randomized Clinical Trial. Diabetes Obes. Metab. 2016, 18, 581–589. [Google Scholar] [CrossRef]
- Skov, J.; Dejgaard, A.; Frøkiær, J.; Holst, J.J.; Jonassen, T.; Rittig, S.; Christiansen, J.S. Glucagon-like Peptide-1 (GLP-1): Effect on Kidney Hemodynamics and Renin-Angiotensin-Aldosterone System in Healthy Men. J. Clin. Endocrinol. Metab. 2013, 98, E664–E671. [Google Scholar] [CrossRef]
- Asmar, A.; Cramon, P.K.; Simonsen, L.; Asmar, M.; Sorensen, C.M.; Madsbad, S.; Moro, C.; Hartmann, B.; Jensen, B.L.; Holst, J.J.; et al. Extracellular Fluid Volume Expansion Uncovers a Natriuretic Action of GLP-1: A Functional GLP-1-Renal Axis in Man. J. Clin. Endocrinol. Metab. 2019, 104, 2509–2519. [Google Scholar] [CrossRef]
- Asmar, A.; Simonsen, L.; Asmar, M.; Madsbad, S.; Holst, J.J.; Frandsen, E.; Moro, C.; Sorensen, C.M.; Jonassen, T.; Bülow, J. Glucagon-like Peptide-1 Does Not Have Acute Effects on Central or Renal Hemodynamics in Patients with Type 2 Diabetes without Nephropathy. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E744–E753. [Google Scholar] [CrossRef]
- Asmar, A.; Simonsen, L.; Asmar, M.; Madsbad, S.; Holst, J.J.; Frandsen, E.; Moro, C.; Jonassen, T.; Bülow, J. Renal Extraction and Acute Effects of Glucagon-like Peptide-1 on Central and Renal Hemodynamics in Healthy Men. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E641–E649. [Google Scholar] [CrossRef]
- Bjørnholm, K.D.; Ougaard, M.E.; Skovsted, G.F.; Knudsen, L.B.; Pyke, C. Activation of the Renal GLP-1R Leads to Expression of Ren1 in the Renal Vascular Tree. Endocrinol. Diabetes Metab. 2021, 4, e00234. [Google Scholar] [CrossRef]
- Ariel Gomez, R.; Sequeira-Lopez, M.L.S. Renin Cells in Homeostasis, Regeneration and Immune Defence Mechanisms. Nat. Rev. Nephrol. 2018, 14, 231–245. [Google Scholar] [CrossRef]
- Gutzwiller, J.P.; Tschopp, S.; Bock, A.; Zehnder, C.E.; Huber, A.R.; Kreyenbuehl, M.; Gutmann, H.; Drewe, J.; Henzen, C.; Goeke, B.; et al. Glucagon-like Peptide 1 Induces Natriuresis in Healthy Subjects and in Insulin-Resistant Obese Men. J. Clin. Endocrinol. Metab. 2004, 89, 3055–3061. [Google Scholar] [CrossRef]
- Hviid, A.V.R.; Sørensen, C.M. Glucagon-like Peptide-1 Receptors in the Kidney: Impact on Renal Autoregulation. Am. J. Physiol. Ren. Physiol. 2020, 318, F443–F454. [Google Scholar] [CrossRef]
- Jensen, E.P.; Poulsen, S.S.; Kissow, H.; Holstein-Rathlou, N.H.; Deacon, C.F.; Jensen, B.L.; Holst, J.J.; Sorensen, C.M. Activation of GLP-1 Receptors on Vascular Smooth Muscle Cells Reduces the Autoregulatory Response in Afferent Arterioles and Increases Renal Blood Flow. Am. J. Physiol. Ren. Physiol. 2015, 308, F867–F877. [Google Scholar] [CrossRef]
- Ricciardi, C.A.; Gnudi, L. Kidney Disease in Diabetes: From Mechanisms to Clinical Presentation and Treatment Strategies. Metabolism 2021, 124, 154890. [Google Scholar] [CrossRef]
- Pyke, C.; Heller, R.S.; Kirk, R.K.; Ørskov, C.; Reedtz-Runge, S.; Kaastrup, P.; Hvelplund, A.; Bardram, L.; Calatayud, D.; Knudsen, L.B. GLP-1 Receptor Localization in Monkey and Human Tissue: Novel Distribution Revealed with Extensively Validated Monoclonal Antibody. Endocrinology 2014, 155, 1280–1290. [Google Scholar] [CrossRef]
- Jensen, E.P.; Møller, S.; Hviid, A.V.; Veedfald, S.; Holst, J.J.; Pedersen, J.; Ørskov, C.; Sorensen, C.M. GLP-1-Induced Renal Vasodilation in Rodents Depends Exclusively on the Known GLP-1 Receptor and Is Lost in Prehypertensive Rats. Am. J. Physiol. Ren. Physiol. 2020, 318, F1409–F1417. [Google Scholar] [CrossRef]
- Barutta, F.; Bellini, S.; Gruden, G. Mechanisms of Podocyte Injury and Implications for Diabetic Nephropathy. Clin. Sci. 2022, 136, 493–520. [Google Scholar] [CrossRef]
- Tesch, F.; Siegerist, F.; Hay, E.; Artelt, N.; Daniel, C.; Amann, K.; Zimmermann, U.; Kavvadas, P.; Grisk, O.; Chadjichristos, C.; et al. Super-Resolved Local Recruitment of CLDN5 to Filtration Slits Implicates a Direct Relationship with Podocyte Foot Process Effacement. J. Cell Mol. Med. 2021, 25, 7631–7641. [Google Scholar] [CrossRef]
- D’Amico, G.; Bazzi, C. Pathophysiology of Proteinuria. Kidney Int. 2003, 63, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Daehn, I.S.; Duffield, J.S. The Glomerular Filtration Barrier: A Structural Target for Novel Kidney Therapies. Nat. Rev. Drug Discov. 2021, 20, 770–788. [Google Scholar] [CrossRef] [PubMed]
- Ronco, P. Proteinuria: Is It All in the Foot? J. Clin. Investig. 2007, 117, 2079–2082. [Google Scholar] [CrossRef]
- Butt, L.; Unnersjö-Jess, D.; Höhne, M.; Edwards, A.; Binz-Lotter, J.; Reilly, D.; Hahnfeldt, R.; Ziegler, V.; Fremter, K.; Rinschen, M.M.; et al. A Molecular Mechanism Explaining Albuminuria in Kidney Disease. Nat. Metab. 2020, 2, 461–474. [Google Scholar] [CrossRef] [PubMed]
- White, K.E.; Bilous, R.W.; Cordonnier, D.J.; Pinel, N.; Barro, C.; Halimi, S.; Chopinet, P.; Darcy, P.; Pointet, P.; Hurault de Ligny, B.; et al. Structural Alterations to the Podocyte Are Related to Proteinuria in Type 2 Diabetic Patients. Nephrol. Dial. Transpl. 2004, 19, 1437–1440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, J.X.; Huang, Q. Glucagon-like Peptide-1 Protects Mouse Podocytes against High Glucose-induced Apoptosis, and Suppresses Reactive Oxygen Species Production and Proinflammatory Cytokine Secretion, through Sirtuin 1 Activation in Vitro. Mol. Med. Rep. 2018, 18, 1789–1797. [Google Scholar] [CrossRef]
- Kodera, R.; Shikata, K.; Kataoka, H.U.; Takatsuka, T.; Miyamoto, S.; Sasaki, M.; Kajitani, N.; Nishishita, S.; Sarai, K.; Hirota, D.; et al. Glucagon-like Peptide-1 Receptor Agonist Ameliorates Renal Injury through Its Anti-Inflammatory Action without Lowering Blood Glucose Level in a Rat Model of Type 1 Diabetes. Diabetologia 2011, 54, 965–978. [Google Scholar] [CrossRef]
- Bisgaard, L.S.; Bosteen, M.H.; Fink, L.N.; Sørensen, C.M.; Rosendahl, A.; Mogensen, C.K.; Rasmussen, S.E.; Rolin, B.; Nielsen, L.B.; Pedersen, T.X. Liraglutide Reduces Both Atherosclerosis and Kidney Inflammation in Moderately Uremic LDLr-/- Mice. PLoS ONE 2016, 11, e0168396. [Google Scholar] [CrossRef]
- Ye, Y.; Zhong, X.; Li, N.; Pan, T. Protective Effects of Liraglutide on Glomerular Podocytes in Obese Mice by Inhibiting the Inflammatory Factor TNF-α-Mediated NF-ΚB and MAPK Pathway. Obes. Res. Clin. Pract. 2019, 13, 385–390. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Xu, F.; Sabbisetti, V.; Grgic, I.; Naini, S.M.; Wang, N.; Chen, G.; Xiao, S.; Patel, D.; Henderson, J.M.; et al. Chronic Epithelial Kidney Injury Molecule-1 Expression Causes Murine Kidney Fibrosis. J. Clin. Investig. 2013, 123, 4023–4035. [Google Scholar] [CrossRef]
- Ougaard, M.E.; Sembach, F.E.; Jensen, H.E.; Pyke, C.; Knudsen, L.B.; Kvist, P.H. Liraglutide Improves the Kidney Function in a Murine Model of Chronic Kidney Disease. Nephron 2020, 144, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Moschovaki Filippidou, F.; Kirsch, A.H.; Thelen, M.; Kétszeri, M.; Artinger, K.; Aringer, I.; Schabhüttl, C.; Mooslechner, A.A.; Frauscher, B.; Pollheimer, M.; et al. Glucagon-Like Peptide-1 Receptor Agonism Improves Nephrotoxic Serum Nephritis by Inhibiting T-Cell Proliferation. Am. J. Pathol. 2020, 190, 400–411. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalbøge, L.S.; Christensen, M.; Madsen, M.R.; Secher, T.; Endlich, N.; Drenic’, V.; Manresa-Arraut, A.; Hansen, H.H.; Rune, I.; Fink, L.N.; et al. Nephroprotective Effects of Semaglutide as Mono- and Combination Treatment with Lisinopril in a Mouse Model of Hypertension-Accelerated Diabetic Kidney Disease. Biomedicines 2022, 10, 1661. https://doi.org/10.3390/biomedicines10071661
Dalbøge LS, Christensen M, Madsen MR, Secher T, Endlich N, Drenic’ V, Manresa-Arraut A, Hansen HH, Rune I, Fink LN, et al. Nephroprotective Effects of Semaglutide as Mono- and Combination Treatment with Lisinopril in a Mouse Model of Hypertension-Accelerated Diabetic Kidney Disease. Biomedicines. 2022; 10(7):1661. https://doi.org/10.3390/biomedicines10071661
Chicago/Turabian StyleDalbøge, Louise S., Michael Christensen, Martin Rønn Madsen, Thomas Secher, Nicole Endlich, Vedran Drenic’, Alba Manresa-Arraut, Henrik H. Hansen, Ida Rune, Lisbeth N. Fink, and et al. 2022. "Nephroprotective Effects of Semaglutide as Mono- and Combination Treatment with Lisinopril in a Mouse Model of Hypertension-Accelerated Diabetic Kidney Disease" Biomedicines 10, no. 7: 1661. https://doi.org/10.3390/biomedicines10071661
APA StyleDalbøge, L. S., Christensen, M., Madsen, M. R., Secher, T., Endlich, N., Drenic’, V., Manresa-Arraut, A., Hansen, H. H., Rune, I., Fink, L. N., & Østergaard, M. V. (2022). Nephroprotective Effects of Semaglutide as Mono- and Combination Treatment with Lisinopril in a Mouse Model of Hypertension-Accelerated Diabetic Kidney Disease. Biomedicines, 10(7), 1661. https://doi.org/10.3390/biomedicines10071661





