AI Prediction of Neuropathic Pain after Lumbar Disc Herniation—Machine Learning Reveals Influencing Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Study Inclusion Criteria
2.3. Therapy Sequence
2.4. Content and Structure of Learning and Test Group
2.5. Artificial Intelligence-Based Prediction Model
3. Results
3.1. Prediction Accuracy
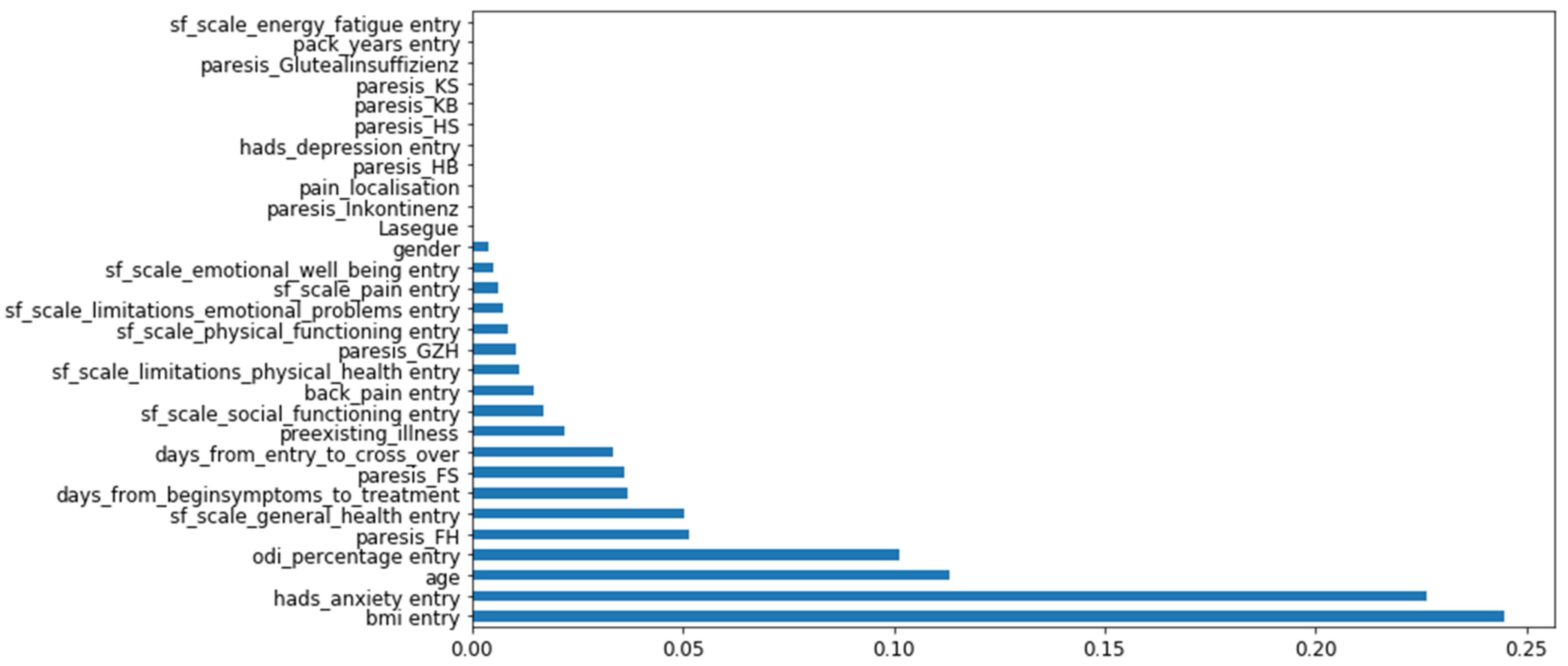
3.2. Feature Importance
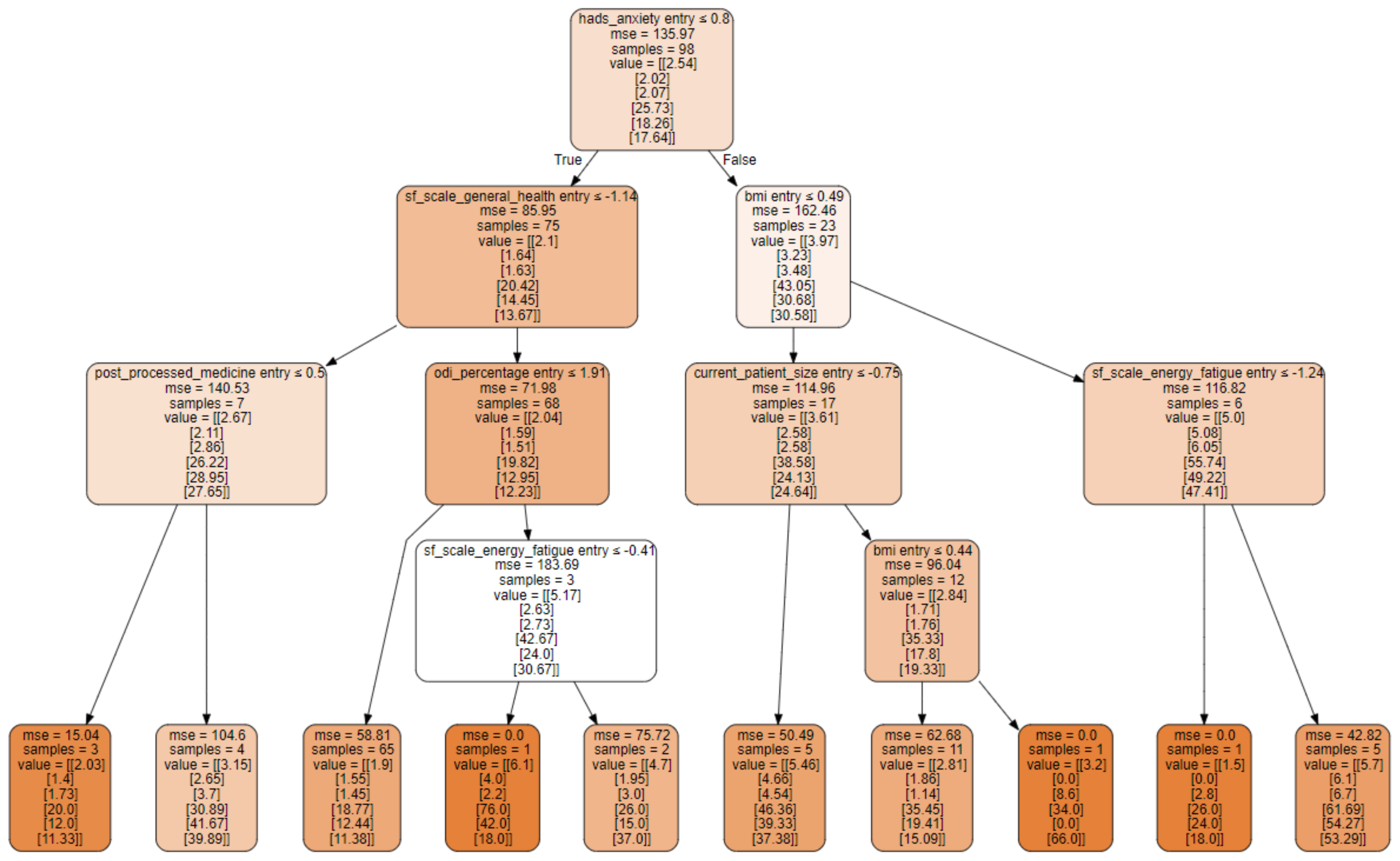
3.3. Decision Tree Structure
4. Discussion
4.1. Interpretation of the Results in Relation to the Study Hypothesis
4.2. Results in the Context of MCID
4.3. Feature Importance Provides Clues to the Pathogenesis of Neuropathic Pain
4.4. Role of Machine Learning as an Assisting Tool
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lurie, J.D.; Tosteson, T.D.; Tosteson, A.N.; Zhao, W.; Morgan, T.S.; Abdu, W.A.; Herkowitz, H.; Weinstein, J.N. Surgical versus nonoperative treatment for lumbar disc herniation: Eight-year results for the spine patient outcomes research trial. Spine 2014, 39, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Tosteson, T.D.; Lurie, J.D.; Tosteson, A.N.; Hanscom, B.; Skinner, J.S.; Abdu, W.A.; Hilibrand, A.S.; Boden, S.D.; Deyo, R.A. Surgical vs nonoperative treatment for lumbar disk herniation: The Spine Patient Outcomes Research Trial (SPORT): A randomized trial. JAMA 2006, 296, 2441–2450. [Google Scholar] [CrossRef] [Green Version]
- Peul, W.C.; van Houwelingen, H.C.; van den Hout, W.B.; Brand, R.; Eekhof, J.A.; Tans, J.T.; Thomeer, R.T.; Koes, B.W. Surgery versus prolonged conservative treatment for sciatica. N. Engl. J. Med. 2007, 356, 2245–2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, C.S.; Rasoulinejad, P.; Taylor, D.; Sequeira, K.; Miller, T.; Watson, J.; Rosedale, R.; Bailey, S.I.; Gurr, K.R.; Siddiqi, F.; et al. Surgery versus Conservative Care for Persistent Sciatica Lasting 4 to 12 Months. N. Engl. J. Med. 2020, 382, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, A.; Webb, K.M.; Cowperthwaite, M.C. One-year outcomes of early-crossover patients in a cohort receiving nonoperative care for lumbar disc herniation. J. Neurosurg. Spine 2017, 27, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, D.; Zhao, W.; Lurie, J.D. What Are Long-term Predictors of Outcomes for Lumbar Disc Herniation? A Randomized and Observational Study. Clin. Orthop. Relat. Res. 2015, 473, 1920–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazanec, D.; Okereke, L. Interpreting the Spine Patient Outcomes Research Trial. Medical vs surgical treatment of lumbar disk herniation: Implications for future trials. Cleve Clin. J. Med. 2007, 74, 577–583. [Google Scholar] [CrossRef]
- Beck, J.; Westin, O.; Brisby, H.; Baranto, A. Association of extended duration of sciatic leg pain with worse outcome after lumbar disc herniation surgery: A register study in 6216 patients. J. Neurosurg. Spine 2021, 34, 759–767. [Google Scholar] [CrossRef]
- Wirries, A.; Geiger, F.; Hammad, A.; Oberkircher, L.; Blumcke, I.; Jabari, S. Artificial intelligence facilitates decision-making in the treatment of lumbar disc herniations. Eur. Spine J. 2020, 30, 2176–2184. [Google Scholar] [CrossRef]
- McHorney, C.A.; Ware, J.E., Jr.; Lu, J.R.; Sherbourne, C.D. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care 1994, 32, 40–66. [Google Scholar] [CrossRef]
- Osthus, H.; Cziske, R.; Jacobi, E. Cross-cultural adaptation of a German version of the Oswestry Disability Index and evaluation of its measurement properties. Spine 2006, 31, E448–E453. [Google Scholar] [CrossRef]
- Hinz, A.; Brähler, E. Normative values for the hospital anxiety and depression scale (HADS) in the general German population. J. Psychosom. Res. 2011, 71, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Koehrsen, W. Feature-Selector. Available online: https://github.com/WillKoehrsen/feature-selector (accessed on 4 May 2022).
- Suzuki, H.; Aono, S.; Inoue, S.; Imajo, Y.; Nishida, N.; Funaba, M.; Harada, H.; Mori, A.; Matsumoto, M.; Higuchi, F. Clinically significant changes in pain along the Pain Intensity Numerical Rating Scale in patients with chronic low back pain. PLoS ONE 2020, 15, e0229228. [Google Scholar] [CrossRef] [Green Version]
- Bahreini, M.; Safaie, A.; Mirfazaelian, H.; Jalili, M. How much change in pain score does really matter to patients? Am. J. Emerg. Med. 2020, 38, 1641–1646. [Google Scholar] [CrossRef]
- Olsen, M.F.; Bjerre, E.; Hansen, M.D.; Tendal, B.; Hilden, J.; Hróbjartsson, A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: Systematic review of empirical studies. J. Clin. Epidemiol. 2018, 101, 87–106. [Google Scholar] [CrossRef] [Green Version]
- Mannion, A.; Junge, A.; Grob, D.; Dvorak, J.; Fairbank, J. Development of a German version of the Oswestry Disability Index. Part 2: Sensitivity to change after spinal surgery. Eur. Spine J. 2006, 15, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hooff, M.L.; Mannion, A.F.; Staub, L.P.; Ostelo, R.W.; Fairbank, J.C. Determination of the Oswestry Disability Index score equivalent to a “satisfactory symptom state” in patients undergoing surgery for degenerative disorders of the lumbar spine—a Spine Tango registry-based study. Spine J. 2016, 16, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Mi, J.; Peng, Y.; Han, H.; Liu, Z. Causal Associations of Obesity With the Intervertebral Degeneration, Low Back Pain, and Sciatica: A Two-Sample Mendelian Randomization Study. Front. Endocrinol. 2021, 12, 740200. [Google Scholar] [CrossRef]
- Hozumi, J.; Sumitani, M.; Matsubayashi, Y.; Abe, H.; Oshima, Y.; Chikuda, H.; Takeshita, K.; Yamada, Y. Relationship between Neuropathic Pain and Obesity. Pain Res. Manag. 2016, 2016, 2487924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, N.; Wilson, P.J.; Reddy, R. Use of machine learning to model surgical decision-making in lumbar spine surgery. Eur. Spine J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Saravi, B.; Hassel, F.; Ülkümen, S.; Zink, A.; Shavlokhova, V.; Couillard-Despres, S.; Boeker, M.; Obid, P.; Lang, G.M. Artificial Intelligence-Driven Prediction Modeling and Decision Making in Spine Surgery Using Hybrid Machine Learning Models. J. Pers. Med. 2022, 12, 509. [Google Scholar] [CrossRef]
- Wirries, A.; Geiger, F.; Hammad, A.; Redder, A.; Oberkircher, L.; Ruchholtz, S.; Bluemcke, I.; Jabari, S. Combined Artificial Intelligence Approaches Analyzing 1000 Conservative Patients with Back Pain-A Methodological Pathway to Predicting Treatment Efficacy and Diagnostic Groups. Diagnostics 2021, 11, 1934. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, D.S.; Hwang, S.W.; Easa, J.E.; Resnick, D.K.; Baisden, J.L.; Bess, S.; Cho, C.H.; DePalma, M.J.; Dougherty, P., II; Fernand, R. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J. 2014, 14, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Peul, W.C.; van den Hout, W.B.; Brand, R.; Thomeer, R.T.; Koes, B.W.; Leiden-The Hague Spine Intervention Prognostic Study Group. Prolonged conservative care versus early surgery in patients with sciatica caused by lumbar disc herniation: Two year results of a randomised controlled trial. BMJ 2008, 336, 1355–1358. [Google Scholar] [CrossRef] [Green Version]
- Ames, C.P.; Smith, J.S.; Pellisé, F.; Kelly, M.; Alanay, A.; Acaroglu, E.; Pérez-Grueso, F.J.S.; Kleinstück, F.; Obeid, I.; Vila-Casademunt, A. Artificial intelligence based hierarchical clustering of patient types and intervention categories in adult spinal deformity surgery: Towards a new classification scheme that predicts quality and value. Spine 2019, 44, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Durand, W.M.; Lafage, R.; Hamilton, D.K.; Passias, P.G.; Kim, H.J.; Protopsaltis, T.; Lafage, V.; Smith, J.S.; Shaffrey, C.; Gupta, M.; et al. Artificial intelligence clustering of adult spinal deformity sagittal plane morphology predicts surgical characteristics, alignment, and outcomes. Eur. Spine J. 2021, 30, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Harada, G.K.; Siyaji, Z.K.; Mallow, G.M.; Hornung, A.L.; Hassan, F.; Basques, B.A.; Mohammed, H.A.; Sayari, A.J.; Samartzis, D.; An, H.S. Artificial intelligence predicts disk re-herniation following lumbar microdiscectomy: Development of the "RAD" risk profile. Eur. Spine J. 2021, 30, 2167–2175. [Google Scholar] [CrossRef]
- Ogink, P.T.; Groot, O.Q.; Karhade, A.V.; Bongers, M.E.R.; Oner, F.C.; Verlaan, J.J.; Schwab, J.H. Wide range of applications for machine-learning prediction models in orthopedic surgical outcome: A systematic review. Acta Orthop. 2021, 92, 526–531. [Google Scholar] [CrossRef]
- Pedersen, C.F.; Andersen, M.O.; Carreon, L.Y.; Eiskjaer, S. Applied Machine Learning for Spine Surgeons: Predicting Outcome for Patients Undergoing Treatment for Lumbar Disc Herniation Using PRO Data. Glob. Spine J. 2022, 12, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, D.G.; O’Mara, J.W., Jr.; Boden, S.D.; Lauerman, W.C.; Jacobson, A.; Platenberg, C.; Schellinger, D.; Wiesel, S.W. The value of magnetic resonance imaging of the lumbar spine to predict low-back pain in asymptomatic subjects: A seven-year follow-up study. J. Bone Jt. Surg. Am. 2001, 83, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.C.; Brant-Zawadzki, M.N.; Obuchowski, N.; Modic, M.T.; Malkasian, D.; Ross, J.S. Magnetic resonance imaging of the lumbar spine in people without back pain. N. Engl. J. Med. 1994, 331, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| n = 123 | ||||
|---|---|---|---|---|
| Mean | Std | Min | Max | |
| Age | 53.2 | 13.2 | 27 | 83 |
| Days from onset of symptoms to 1st treatment | 29.3 | 27.4 | 1.0 | 84.0 |
| BMI | 27.6 | 6.1 | 17.9 | 54.7 |
| Patient standing height | 173.5 | 9.8 | 152.0 | 196.0 |
| Pack years (smoking) | 7.3 | 12.7 | 0.0 | 47.0 |
| Lasègue | 42.7 | 15.8 | 5.0 | 60.0 |
| SF 36 physical functioning | 24.0 | 21.8 | 0.0 | 95.0 |
| SF 36 limit. physical health | 15.4 | 29.6 | 0.0 | 100.0 |
| SF 36 limit. emotional problems | 50.4 | 46.4 | 0.0 | 100.0 |
| SF 36 energy fatigue | 39.1 | 18.1 | 5.0 | 90.0 |
| SF 36 emotional well being | 60.6 | 19.3 | 20.0 | 100.0 |
| SF 36 social functioning | 54.4 | 26.7 | 0.0 | 100.0 |
| SF 36 pain | 21.9 | 23.7 | 0.0 | 100.0 |
| SF 36 general health | 57.8 | 19.1 | 10.0 | 100.0 |
| ODI percentage | 57.0 | 19.5 | 11.1 | 100.0 |
| HADS anxiety | 6.6 | 3.9 | 0.0 | 15.0 |
| HADS depression | 6.5 | 3.5 | 0.0 | 15.0 |
| Back pain (VAS) | 5.7 | 3.0 | 0.0 | 10.0 |
| Leg pain (VAS) | 5.5 | 3.4 | 1.1 | 10.0 |
| Gender | Male | Female | |
|---|---|---|---|
| n = 123 | 69 | 54 | |
| Treatment option | Operation | Conservative | Cross-over |
| n = 123 | 52 | 37 | 34 |
| Neurological deficit on admission | Motor weakness | Sensory deficit | |
| n = 123 | 43 out of 123 | 77 out of 123 |
| Categorical Variables | Continuous Variables on Admission | Target Variables |
|---|---|---|
| motor weakness | Age | ODI after 6 weeks |
| Treatment option | Days from onset of symptoms to 1st treatment | ODI after 6 months |
| SF 36 limitations physical health | ODI after 1 year | |
| SF 36 limitations emotional problems | Leg pain after 6 weeks | |
| SF 36 pain | Leg pain after 6 months | |
| Patient standing height | Leg pain after 1 year | |
| SF 36 emotional well being | ||
| SF 36 social functioning | ||
| SF 36 physical functioning | ||
| SF 36 general health | ||
| Back pain | ||
| Leg pain | ||
| BMI | ||
| HADS anxiety | ||
| ODI | ||
| Days from admission to cross over | ||
| Pre-existing conditions and medication types |
| Machine Learning Algorithm | MAE | SD |
|---|---|---|
| Linear Regression | 9.19 | 0.37 |
| Elastic Net | 9.57 | 0.89 |
| Nearest Neighbour | 8.13 | 0.37 |
| Random Forest | 7.51 | 0.02 |
| Neuronal Net | 8.11 | 0.54 |
| Fold | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAE | 7.33 | 8.10 | 6.23 | 6.34 | 7.71 | 7.61 | 8.68 | 5.75 | 6.76 | 5.70 | 7.02 | 0.97 |
| Fold | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAS leg pain 6 weeks | 2.16 | 1.68 | 1.67 | 1.21 | 1.55 | 1.75 | 2.10 | 2.23 | 2.01 | 1.56 | 1.79 | 0.31 |
| VAS leg pain 6 months | 2.25 | 2.13 | 1.82 | 1.66 | 1.11 | 2.04 | 1.87 | 1.43 | 2.45 | 1.37 | 1.81 | 0.40 |
| VAS leg pain 1 year | 2.58 | 1.91 | 2.07 | 2.25 | 1.58 | 1.85 | 2.25 | 1.52 | 2.06 | 1.60 | 1.97 | 0.33 |
| ODI value 6 weeks | 9.12 | 9.59 | 9.46 | 9.31 | 11.74 | 10.39 | 11.68 | 7.22 | 5.87 | 10.71 | 9.51 | 1.75 |
| ODI value 6 months | 11.96 | 10.22 | 8.84 | 10.35 | 9.20 | 9.26 | 9.72 | 13.79 | 8.83 | 7.99 | 10.02 | 1.62 |
| ODI value 1 year | 9.55 | 8.40 | 7.78 | 11.61 | 8.97 | 8.57 | 7.16 | 9.77 | 7.48 | 7.52 | 8.68 | 1.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wirries, A.; Geiger, F.; Hammad, A.; Bäumlein, M.; Schmeller, J.N.; Blümcke, I.; Jabari, S. AI Prediction of Neuropathic Pain after Lumbar Disc Herniation—Machine Learning Reveals Influencing Factors. Biomedicines 2022, 10, 1319. https://doi.org/10.3390/biomedicines10061319
Wirries A, Geiger F, Hammad A, Bäumlein M, Schmeller JN, Blümcke I, Jabari S. AI Prediction of Neuropathic Pain after Lumbar Disc Herniation—Machine Learning Reveals Influencing Factors. Biomedicines. 2022; 10(6):1319. https://doi.org/10.3390/biomedicines10061319
Chicago/Turabian StyleWirries, André, Florian Geiger, Ahmed Hammad, Martin Bäumlein, Julia Nadine Schmeller, Ingmar Blümcke, and Samir Jabari. 2022. "AI Prediction of Neuropathic Pain after Lumbar Disc Herniation—Machine Learning Reveals Influencing Factors" Biomedicines 10, no. 6: 1319. https://doi.org/10.3390/biomedicines10061319
APA StyleWirries, A., Geiger, F., Hammad, A., Bäumlein, M., Schmeller, J. N., Blümcke, I., & Jabari, S. (2022). AI Prediction of Neuropathic Pain after Lumbar Disc Herniation—Machine Learning Reveals Influencing Factors. Biomedicines, 10(6), 1319. https://doi.org/10.3390/biomedicines10061319






