Abstract
Carbon nanomaterials have attracted great interest for their unique physico-chemical properties for various applications, including medicine and, in particular, drug delivery, to solve the most challenging unmet clinical needs. Graphitization is a process that has become very popular for their production or modification. However, traditional conditions are energy-demanding; thus, recent efforts have been devoted to the development of greener routes that require lower temperatures or that use waste or byproducts as a carbon source in order to be more sustainable. In this concise review, we analyze the progress made in the last five years in this area, as well as in their development as drug delivery agents, focusing on active targeting, and conclude with a perspective on the future of the field.
1. Introduction
There are high expectations for the next generation of medicines as we move towards personalized therapy (Figure 1) with targeted delivery for lower side effects [1]. Nanomaterials are well-positioned for a qualitative leap in addressing the most demanding, unsolved issues in medicine, thanks to their innovative properties [2] that enable multimodal imaging [3], combined diagnostics and therapy in theranostics [4], personalized drug delivery at the pathological site [5], and so on. In particular, carbon nanomaterials (CNMs) display unique physico-chemical properties, thanks to their graphitic nature, for unparalleled applications in drug delivery; however, critical steps in terms of thorough biosafety assessments and regulatory requirements must be taken to enable their clinical implementation [6,7]. Graphitization is a popular process not only to produce but also to modify CNMs and enhance their physico-chemical properties, but it is generally an energy-intensive step for which greener alternatives are continuously sought [8]. In this concise review, we will analyze the last 5 years of progress in the development of more sustainable ways to graphitize CNMs and their applications in drug delivery for the most challenging unmet needs in medicine and conclude with a perspective on the future of this exciting area of research.
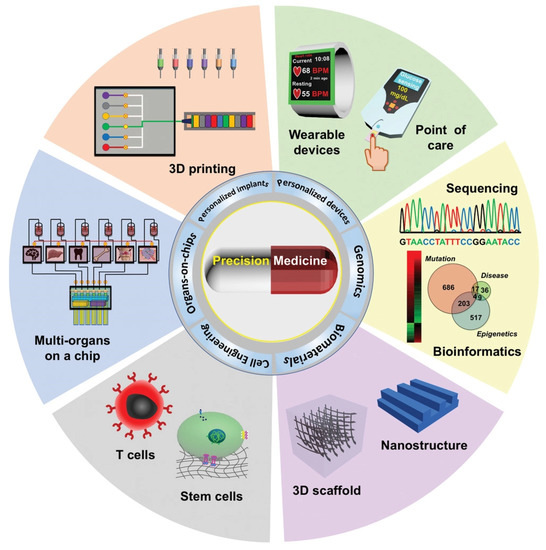
Figure 1.
Various approaches to enable precision medicine. Reproduced from [9] under a Creative Commons licence.
2. Carbon Nanomaterials (CNMs)
CNMs come in different sizes, morphology, curvature, and reactivity. The diverse members of this family (Figure 2) have been discovered through the years since the 1960s [10], with new ones continuously being produced, thanks to top-down or bottom-up approaches [11,12,13]. A common feature is that they are mainly composed of carbon atoms that are typically sp2 hybridized and that are arranged in a honeycomb lattice [14]. However, exceptions exist, such as nanodiamonds that feature a large portion of sp3 hybridization, as the name suggests [15]. A simple way to understand nanocarbon structure is to consider the two-dimensional graphene sheets [16] as a common building block so that, depending on how it is folded, they will yield different CNMs [17]. For example, fullerenes are soccer-ball-shaped [18], and carbon nano-onions (CNOs) are concentric fullerenes [19]. Carbon nanotubes (CNTs) are tubular in shape, and they occur as single-walled [20] or multi-walled [21]. Nanohorns (CNHs) consist of clusters of nanocones [22], while nanodots (CNDs) have been attracting increasing attention, thanks to their ultra-small size (<10 nm) and luminescence [23]. Graphene nanoribbons (GNRs) display yet another morphology, as the name suggests, and they are highly researched for the possibility they offer to fine-tune their electronic properties and, in particular, the bandgap through the modulation of structural features, such as width, orientation, backbone and edge structure, heteroatom doping, and, in general, their quality [24].
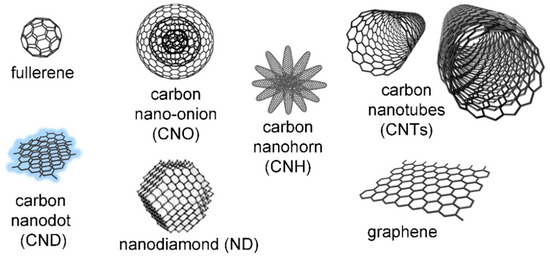
Figure 2.
Carbon nanomaterials (not to scale), reproduced from [25]. The nano-onion is reproduced from [26]; copyright © 2022 with permission from Elsevier.
In spite of the large number of scientific studies on CNMs, often, it is difficult to anticipate which type is the best performer for specific applications, and even more so in the case of biological uses where there is a high degree of biomolecular complexity [27,28,29,30]. Interactions between CNMs and biomolecules, such as proteins [31] and DNA [32], are very relevant in this regard for their key roles in the determination of the dynamic corona on their surface [33], which, in turn, affects their fate in vivo [34], spanning their biodistribution [35] to immune response [36,37] and biodegradation [38,39].
All CNMs share many properties, such as low density, high mechanical resistance, good electronic conductivity, and the ability to undergo various types of chemical functionalization to fine-tune their properties [40], especially for clinical applications [41]. Unsurprisingly, many reviews exist for their successful use in biomedicine [42,43] and, in particular, oncology [44], theranostics [45], and bioimaging [46]. In drug delivery, they have been mainly investigated for cancer therapy and, more recently, also for atherosclerosis [47], microbial infections [48,49], and neurological disorders [50].
Nevertheless, concerns exist over their biosafety profile [51] and immunogenicity [52]; the relevant studies are further complicated by the high heterogeneity of this class of nanomaterials [53]. Various committees are thus tackling the development of unified standards to assist with their correct classification [54] and a general framework to enable their reliable risk assessment for the responsible advancement of nanotechnology [55]. This is not an easy task; however, international standards organizations (e.g., ISO (ISO/TC 229 Nanotechnologies) and IEC (IEC/TC 113 Nanotechnology for electrotechnical products and systems)) have already released various standards for graphene. Countries such as the USA, the UK, Korea, Japan, and especially China play an active role in graphene research, and a number of new standards are being continuously issued, especially in China, to assist the industrialization of graphene-related materials (Table 1) [56].

Table 1.
Graphene-related standards that are publicly available. Reproduced from [56]; copyright © 2022, with permission from Elsevier. Data updated to February 2020.
To speed up research on CNMs, cutting-edge in silico approaches such as machine-learning [57] are key enablers to fully exploit large datasets for which innovative methods for efficient management are also crucial [58]. Another critical step for the wide implementation of CNMs pertains to cost-effective large-scale production [59], ideally in a sustainable manner, in light of the current climate crisis and the urgent need to lower our impact and preserve the environment. The next section will thus focus on the recent efforts to find graphitization routes that are less energy-intensive than traditional methods.
3. The Graphitization Process
Graphitization is the conversion of amorphous carbon into graphitic carbon. This process requires a drastic rearrangement of chemical bonds to form a layered structure made of condensed six-membered sp2 carbon rings. For this reason, graphitization is a very energy-demanding process, and it is typically performed by heating petroleum coke up to 3000 °C in furnaces to produce synthetic graphite at the industrial level. The introduction of metal catalysts allows us to operate at lower temperatures [60], but metal residues are difficult to separate from the product.
Crystallization is also referred to the transformation of non-graphitic carbon into well-organized graphitic CNMs [61] such as fullerenes [62], graphene [63], CNTs [64,65], CNHs [66], CNOs [67], graphitic quantum dots (GQDs) [68], and graphitized carbon black (GCB). There are a lot of ways currently used to prepare this kind of structure, such as arc-discharge, chemical vapor deposition (CVD), laser ablation [69], pyrolysis, and thermal annealing [70], but all these methods involve the use of high amounts of energy and non-sustainable carbon sources. This limits the application of these materials on a large scale in technological and biomedical fields; therefore, the finding of green and economical routes to high-quality CNMs is a crucial challenge today.
4. Greener Alternatives to Traditional Graphitization for CNM Production
In recent years, great efforts have been devoted to finding green and economical ways to prepare CNMs in order to make them more sustainable and widely accessible. The literature in this field is in continuous evolution as many research groups are working hard to provide a solution to the problem. Therefore, in this review, we will focus on the most recent advances organized by the type of carbon source, in particular: waste, natural sources, atmospheric particulate, graphite or coal-derived products, and CO2. Table 2 reports the type of CNM, the green process employed, the carbon source used, and the investigated application for the most recent works discussing the green preparation of CNMs.

Table 2.
Recent examples of green methods to produce graphitic carbon nanostructures using green materials and/or lower energy-demanding processes.
4.1. CNMs from Waste
The valorization of waste is currently a popular topic, especially for organic and plastic waste, which could be potentially used as carbon sources to produce CNMs in a greener and more economical way [142]. Examples were reported for both classes of waste.
As organic waste, waste frying oil has been used as a precursor for the preparation of CNOs with high electrochemical performance through traditional flame pyrolysis [72]. Microwave-assisted pyrolysis of fish scales produced oxidized CNOs with good light emission properties to fabricate a blue LED [73]. The nickel-catalyzed carbonization of rice husk, followed by chemical activation, was used to produce porous CNOs with a high degree of graphitization and excellent electrochemical properties to make supercapacitors [82]. Even barbeque grease has been used as a carbon source to produce CNTs by chemical vapor deposition [98].
As plastic waste, polymers such as polyethylene terephthalate (PET), polypropylene (PP), and polyethylene (PE) from plastic objects have been used to prepare CNMs with various methods. The catalytic thermal decomposition of PET from plastic bottles led to various CNMs, including fullerenes and CNTs, depending on the reaction conditions [143]. Graphene nanosheets suitable for energy applications were obtained by a combination of heating treatments and pyrolysis [138]. Fullerenes [84] and graphene [136,137] were prepared by thermal decomposition for industrial dye removal, and CNTs were prepared by catalyzed pyrolysis for use as lubricants [119]. Finally, numerous recent examples of CNTs prepared from plastic waste employed CVD with a 2-step reactor [101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117]. In brief, the typical reactor consists of two chambers: in the first chamber, plastic sources are pyrolyzed to produce hydrocarbon gases, which then act as precursors for the catalytic decomposition and deposition that happen in the second chamber. In some cases, there is a condensation step after the first chamber to allow further re-cracking of the heaviest hydrocarbon to light gases in order to increase the yield of the process. Table 3 summarizes the main characteristics of the recently reported processes with the best results obtained in terms of CNT quality. A similar process was employed to produce graphene sheets with a high graphitization degree used to make electrodes from various types of plastic waste [139].

Table 3.
Recently reported processes for the CVD formation of CNTs from plastic waste. 1 LDPE = low-density PE. 2 MP = mixed plastics. 3 HDPE = high-density PE.
4.2. CNMs from Sustainable Natural Sources
Natural sources are becoming very popular for the preparation of CNMs, especially for GQDs. For example, the hydrothermal treatment of fruit [123], cotton [124], starch [125], or lemon juice [126] leads to GQDs with excellent and tunable optical properties, used for bioimaging or heavy-metal sensing. To the same end, citric acid [127] and casein [128] can be used through pyrolysis, grape seeds extract through microwave treatment [131], or salicylic acid by means of UV-induced polymerization [130]. The hydrothermal carbonization of citric acid produced CNOs [81], while the pyrolysis of flaxseed oil led to CNOs with good optical properties for photocatalysis and Al(III) detection [71].
Another popular approach is based on using natural carbon precursors for the CVD synthesis of CNTs because it is one of the best methods to control CNT morphology. Plant extracts were used for a metal-free CVD approach towards CNTs of different types and graphitization degrees, depending on the temperature and the plant used [99]. Coconut and olive oil acted as a carbon source for spray pyrolysis (that is a variant of CVD), with the latter producing the best CNTs in terms of the level of graphitization [118].
4.3. Atmospheric Particulate as Carbon Source for CNMs
Great efforts are being devoted to the valorization of carbon soot—which is normally considered a pollutant—as a source for functional CNMs, and indeed, it was found to contain CNMs [144]. For this purpose, there are also examples of flying ashes used as carbon sources and/or catalysts for the CVD growth of CNTs as they contain alumina, silica, iron oxides, and other metal compounds that can catalyze CNT growth [100,145].
Recent works reported the isolation of CNOs from diesel soot by a Soxhlet purification of the as-collected particulate, to be used for bioimaging and Cr(VI) sensing [74], strain sensing [75], and dye removal [76]. Graphene nanosheets for the photocatalytic removal of dyes were also obtained by Soxhlet purification of diesel pollutant soot [132,133]. However, this kind of purification involves organic solvents, such as acetone, toluene, or petroleum ether, that are best avoided.
Candle soot is another good and costless source of CNMs, demonstrated to obtain CNOs with good photothermal properties for cancer therapy [79]. Finally, from a candle doped with iron acetylacetonate, magnetic CNOs were obtained and used for the efficient removal of bisphenol A from water [80].
4.4. Greener Routes to Prepare CNMs from Graphite- or Coal-Based Products
Even if graphite- or coal-based products are not green starting materials, it is important to develop low energy-demanding processes that can be useful in combination with recent advances to obtain synthetic graphite in greener ways [146].
Electrochemical treatment of a graphite rod produced GQDs that were marked with technetium-99m for radioimaging [120], while gamma irradiation of graphite produced GQDs that were suitable for photodynamic therapy after functionalization with urea [122]. Electrochemical treatment of wood charcoal yielded GQDs that could be used as a peroxidase mimic [121]. GQDs could also be obtained by mild oxidation of coal tar pitch with hydrogen peroxide [129]. A simple mechanical process such as ball milling produced CNOs starting from graphite powder [83]. Reduced graphene oxide (rGO) was obtained from recycled charcoal by chemical graphitization and oxidation, followed by green exfoliation by sonication and reduction with ascorbic acid [119]. In a similar way, rGO for Ni(II) removal was obtained using Tecoma stans leaf extract to reduce GO [135]. Finally, in a recent work, a catalytic reduction process to obtain a very high-quality rGO was reported [134]. In this case, the use of a Fischer–Tropsch catalyst in the absence of hydrogen allowed it to operate at the very low temperature of 250 °C, with oxygen as the only byproduct.
4.5. CO2 as Carbon Source to Produce CNMs
CO2 is the main greenhouse gas that affects global warming, with the removal and recycling of CO2 produced by human activities being one of the biggest challenges for scientists. For example, in a recent work, a high-quality synthetic graphite was produced by the chemical reduction of CO2 with LiAlH4 [146].
CO2 has been envisaged as a carbon source for the production of CNMs. The first chemical synthesis of CNTs from CO2 was reported by Motiei et al. [147]. They reacted Mg with supercritical carbon dioxide to obtain MgO and carbon, with a small yield of fullerenes and CNTs with good crystallinity. CO2 was also used as the only precursor gas [148,149] or as a co-precursor [150] for the production of CNTs and graphene by CVD. Recently, the synthesis of bilayered graphene with a very high graphitization degree was reported, using CO2 as the carbon source for CVD [140].
An approach that is attracting great attention is the electrolysis of CO2 in molten salt, introduced by The Licht C2CNT (carbon dioxide to CNT) group [151]. Various CNMs were recently obtained with this method, including CNOs, CNTs, graphene, carbon nanofibers, and other more exotic structures termed carbon nanodragons or nanoflowers [77,78,85,86,87,88,89,90,91,92,93,94,95,96,97,141]. Briefly, experiments were carried out in an alumina crucible containing an electrolyte (typically lithium carbonate), which was heated up to melting. Then, electrodes were immersed in the electrolyte, and a constant current was applied across the electrodes. Carbon products accumulated at the cathode, and they could be easily recovered, while oxygen was produced as a byproduct. A transition metal-based nucleation agent could be added to the cathode or in the molten salt as a catalyst or could originate from the erosion of the anode during the process. This technique is almost costless (excluding the electrodes), and it is very versatile as a large variety of CNMs can be obtained under varying reaction parameters, such as electrode composition, nucleation agent, electrolysis time, and current density [78]. For example, the presence of metal nucleation agents (typically iron, nickel, or chromium) usually leads to the formation of CNTs, while in their absence, CNOs [77] or graphene [141] are produced. The use of labeled 13CO2 revealed that carbonate was transformed into CNMs but was continuously renewed by atmospheric CO2, which was the real carbon source [152].
5. Applications of CNMs for Targeted Drug Delivery
CNMs are very interesting as drug delivery agents because of their high versatility. They are typically hydrophobic and well uptaken by cells, and they can also cross biological membranes such as the blood–brain barrier (BBB) [153,154]. In addition, as nanomaterials, passive targeting is possible due to the enhanced permeability retention (EPR) effect [155]. However, this approach has proved to be very effective in experimental studies on animal models but demonstrated limited efficacy in humans [156], with active targeting and personalized medicine holding promise to enable significant progress in the clinic.
CNMs can be functionalized covalently or non-covalently using organic chemistry with a plethora of molecules and biomolecules, and combined approaches are possible. For example, it is possible to append a drug and a targeting agent or multiple drugs for combined therapy or a drug and a fluorescent probe for bioimaging in theranostics. In many cases, CNMs can also act as photothermal and photodynamic agents, besides being drug delivery vehicles, in order to enhance the effectiveness of cancer therapies [157].
Having targeted drug delivery is very important to enhance the effect of drugs and reduce the side effects caused by chemotherapeutics at physiological rather than pathological sites. Differences in microenvironmental conditions in cancer cells, especially cancer stem cells relative to normal cells, are often exploited to this end [158]. In this work, we discuss the last 5 years of progress in using CNMs as drug delivery agents, with a focus on molecular targeting (Table 4).

Table 4.
Recent examples of the use of CNMs in molecular-targeted drug delivery.
5.1. Fullerenes
Fullerene is a quasi-spherical and very hydrophobic carbon nanoparticle made of carbon atoms, with C60 being the most common. Due to its hydrophobic nature, it is a promising drug carrier to cross the BBB. For example, in a recent work, C60 was functionalized with the KLVFF peptide to specifically target the amyloid-β peptide involved in Alzheimer’s disease [159]. In this case, fullerenes were bound to an upconversion nanoparticle and acted as therapeutic agents, thanks to their ability to produce radical oxygen species (ROS) under near-infrared (NIR) light irradiation to prevent amyloid fibrillation and to act as radical scavengers in the dark and reduce the side effects of this approach.
However, the hydrophobic nature of fullerenes can be a problem for biological applications because they tend to aggregate and accumulate in organs such as liver, heart, and adrenal glands, potentially leading to toxicity [192]. For this reason, fullerenes are often functionalized with hydrophilic groups or molecules to increase their dispersibility in water. A theranostic agent based on a water-dispersible fullerene was developed to treat disc diseases, which are notably difficult therapeutic targets [160]. Fullerene was functionalized with hydroxyl and succinyl groups and bound to cyanine-5 for imaging and to the FIFIFK peptide to target the formyl peptide receptor 1 (FPR-1) specifically expressed in activated macrophages/monocytes under inflammatory insult. Fullerene acted as a radical scavenger and successfully inhibited inflammation, leading to pain alleviation in a mice model (Figure 3).
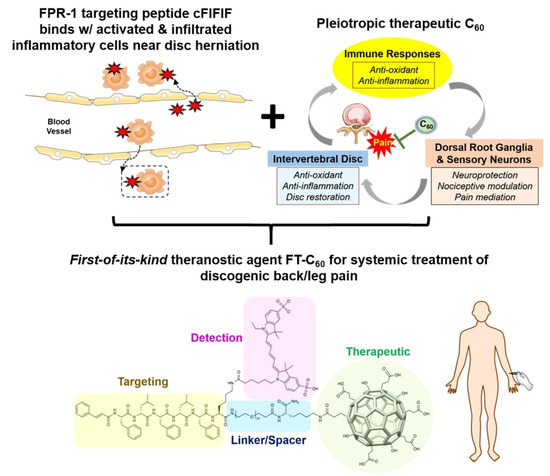
Figure 3.
Schematic illustration of the therapeutic and targeting mechanism of the FIFIFK-functionalized C60 to treat discogenic pain. Reprinted with permission from [160]. Copyright © 2022, American Chemical Society.
Fullerenols are polyhydroxy fullerenes that present fibrinolytic activity that is enhanced using a biomimetic carrier based on platelet and red-blood-cell membranes [161]. This approach has led to prolonged circulation time, better biocompatibility without toxic effects, and an enhanced affinity for fibrin due to the membrane proteins of the carrier. Finally, an innovative dual-responsive nanovehicle for the targeted delivery of the anticancer drug doxorubicin (DOX) has been developed [162]. Briefly, fullerene was non-covalently functionalized with the cationic and magnetic surfactant hexadecyltrimethylammonium trichloromonobromoferrate (CTAF), and this allowed the electrostatic interaction with anionic DNA to obtain a compact histone-like structure. Then, the complex was enveloped in a disulfide-modified hyaluronic acid (HA) shield. DNA served as an electrostatic scaffold to load DOX, while the disulfide-HA acted as both a redox trigger for drug delivery and a targeting vector due to the affinity of HA for the CD44 receptor that is overexpressed in many cancer cells.
5.2. CNOs
CNOs are multi-layered fullerenes, so they share some of their properties. However, CNOs can have different structures, for example, a more spherical or polyhedral shape or different numbers of layers. CNOs show very low toxicity and, differing from fullerenes, good light emission properties, so they have been studied as bioimaging probes [193].
A recent work reported a strategy to overcome drug resistance in cancer based on blocking P-glycoprotein (P-gp), an ATP-binding membrane protein involved in the cellular efflux of many drugs that is overexpressed in multi-drug resistant cancer cells [163]. The result was achieved by co-delivering a P-gp inhibitor (HM30181A) and DOX using silica-CNO nanoparticles coated with fucoidan. While silica rendered CNOs more water-dispersible, selectivity was ensured by the affinity of fucoidan for P-selectin and a NIR-induced drug release (Figure 4).
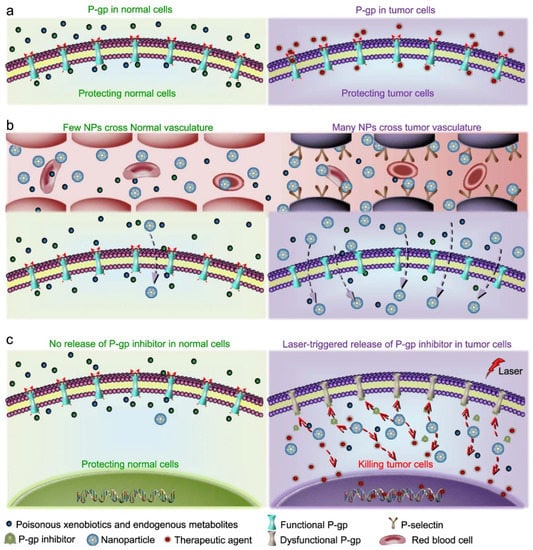
Figure 4.
Schematic illustration of the strategy for targeting multi-drug resistant cancer cells and inhibiting the P-gp pump using CNOs. Reproduced from [163] under a Creative Commons license.
5.3. CNTs
CNTs have excellent conductivity and anisotropic morphology; they are ideally suited for the stimulation of nerve [194,195,196] and cardiac [197,198,199] cells but also have the stigma of being associated with asbestos fibers’ toxicity [200], thus limiting their biological use. This has posed many limits to their clinical implementation, and, in 2019, they were also added to the ChemSec SIN list of nanomaterials of very high concern [201]. However, their toxicity strongly depends on a plethora of factors, including their functionalization, length, and purity, and grouping them all together is unjustified [53].
Recent works have reported the functionalization of CNTs with folic acid (FA) to target cancer cells overexpressing the FA receptor [164,165,166,167]. For example, a dual therapeutic agent was obtained by firstly oxidating CNTs to make them more water-dispersible and coating them with a biocompatible poly(N-vinyl pyrrole) polymer to enhance their photothermal properties. After that, FA was covalently linked to the composite, and the final material was impregnated with DOX to obtain a combined chemo-photothermal treatment with fewer side effects than free DOX [164]. A similar result was obtained with oxidized CNTs covered with bovine serum albumin as a nucleation site for growing gold nanoparticles to enhance their photothermal properties. Then, FA was linked to the gold nanoparticles via an S-functionalized PEG linker to ensure selective targeting, and DOX was adsorbed on the surface of the material [165]. A similar targeting approach was used to selectively deliver docetaxel and coumarin-6 to lung cancer cells using oxidized CNTs coated with FA-functionalized chitosan [167]. Finally, with a more sophisticated approach, an injectable hydrogel based on silk proteins and oxidized CNTs was developed for the NIR-triggered delivery of DOX to cancer cells overexpressing the FA receptor [166]. Oxidized CNTs were covalently linked to FA, and DOX was adsorbed on their surface; then, a silk-CNT hydrogel was formed. These recent examples manifest the increasing interest in the combination of CNTs with hydrogel biomaterials for therapeutic use and controlled delivery kinetics of bioactives [202].
DOX targeting of prostate cancer cells was achieved by simply using oxidized CNTs that were non-covalently functionalized with prostate-homing peptides and the drug [168]. Another cell-penetrating peptide, BR2, which is highly specific for cancer cells, was used for the selective co-delivery of DOX and surviving siRNA (short-interfering RNA) [169]. Oxidized CNTs were covalently linked to the targeting peptide and to betaine-modified polyethylenimine (PEI) to bind siRNA through electrostatic interactions and allow its pH-responsive endosomal escape. Betaine was necessary to reduce the side effects of PEI. The delivery of siRNA with cancer drugs is a novel gene therapeutic strategy to increase the effectiveness of cancer treatments. Another similar example was reported for the delivery of MBD1 siRNA using oxidized CNTs functionalized with a PEG-modified LyP-1 cyclic peptide, which bound specifically to p32 proteins overexpressed in pancreatic cancer cells [170]. CNTs were also covalently functionalized with PEI to bind a plasmid containing MBD1 siRNA and the gene encoding green fluorescent protein (GFP) for theranostics.
Another strategy for molecular targeting is the use of DNA sequences. A recent work reported the delivery of 5-fluoruracil (5-FU) together with an aptamer-siRNA chimera [171]. In this case, the targeting molecule was the AS1411 aptamer, a DNA sequence with anticancer properties for the specific binding of nucleolin, which is overexpressed in some cancer cells. This approach was studied as a treatment for the peritoneal dissemination of gastric cancer, which is known to be very difficult to treat. In another recent work, a more complex DNA-based targeting was achieved by the formation of a hydrogel based on DNA, silica nanoparticles, and CNTs for the more efficient delivery of DOX [172]. In this case, selective targeting was achieved by enriching DNA with CG-GC and aptamer sequences, and DOX was loaded through intercalation in the DNA scaffold. The hydrogel with CNTs was proven to be more cytotoxic for cancer cells compared to the gel without CNTs.
In another work, candesartan was used as both a drug and a targeting molecule as it has a strong affinity and inhibition ability for the angiotensin II type-1 receptor (AT1R) expressed on the surface of neovascular endothelial cells and many tumor cells [173]. Oxidized CNTs were functionalized with PEI as a linker, and candesartan was covalently bound to the linker. PEI also acted as a cationic scaffold to bind vascular endothelial growth factor (VEGF) siRNA, and the co-delivery of candesartan and VEGF siRNA provided a strong inhibition of tumor vascularization.
Finally, a novel and smart nanobot based on CNTs for the highly selective and fine-controlled delivery of DOX has been reported [174]. Briefly, DOX is encapsulated inside oxidized CNTs, and both ends are capped with Fe3O4 nanoparticles via a glutathione linker bound to CNTs with an acid-sensitive amide bond. The same glutathione linker is also used to functionalize CNTs with transferrin or an anti-epithelial cell adhesion molecule antibody (anti-EpCAM mAb) as a targeting vector for cancer cells. Fe3O4 nanoparticles allow the magnetic control of the bot and also self-propulsion due to the oxygen produced by the decomposition of the H2O2 naturally present in the human body, especially in the cancer environment, when catalyzed by the same nanoparticles. The acidic pH of lysosomes cleaves the amide bond linking CNTs and glutathione, freeing the CNTs’ end from the metal nanoparticles and allowing the specific release of DOX in cancer cells (Figure 5).
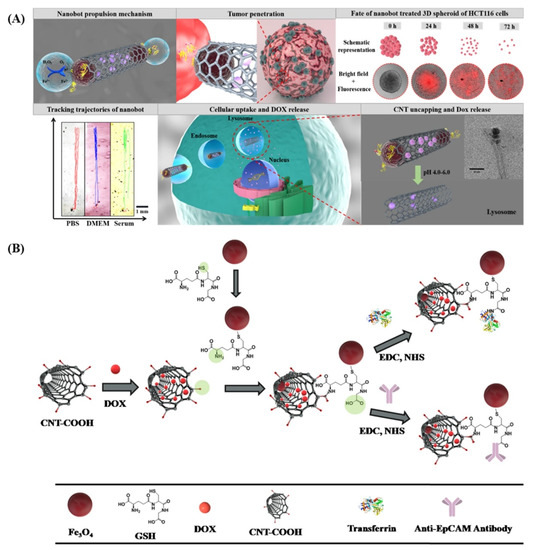
Figure 5.
(A) Schematic representation of the oxygen-induced autonomous propulsion mechanism and deep penetration of nanobots in the tumor, the fate of 3D spheroids treated with nanobots, trajectories of nanobots in physiologically relevant media, followed by an illustration of the DOX-loaded nanobot targeting the transferrin/EpCAM receptor and entry into cancer cells, and, finally, the mechanism of triggered drug release under acidic endo/lysosomal conditions. (B) Schematic illustration of the step-by-step synthesis of CNT-DOX-Fe3O4-Tf/CNT-DOX-Fe3O4-mAb. Reproduced from [174], under a Creative Commons licence.
5.4. GQDs
GQDs are a new class of CNMs that is particularly known for their light emission properties, suitable for bioimaging. This feature, combined with their CNM nature and wide possibilities of functionalization, makes GQDs an ideal theranostic agent for drug delivery and bioimaging.
As an example, nitrogen-doped GQDs synthetized with a hydrothermal method from citric acid and urea were non-covalently loaded with methotrexate (MTX) [175]. This anticancer drug acts as a targeting agent as well due to its affinity for FA receptors, and MTT assays suggest that MTX-loaded GQDs are highly biocompatible and more effective than free MTX.
FA-functionalized GQDs were used for the targeted delivery of IR780 iodide, an effective photothermal agent and bioimaging probe [176]. However, the rigidity of the IR780 iodide molecule rendered it very insoluble in biocompatible solvents, while the loading on GQDs via π-π stacking interactions with the graphitic sp2 structure increased water solubility by over 2400-fold and the so-obtained composite resulted in an efficient theranostic platform for NIR fluorescence imaging and photothermal therapy. The FA-based targeting strategy was also used for the co-delivery of NO and a Pt(IV) prodrug [177]. In this case, the delivery platform was a nitrogen-doped GQD decorated with FA and a dinuclear Ru-Pt complex in which Ru(VI) acted as the NO carrier and Pt(IV) as a prodrug of a Pt(II) drug. NIR irradiation induced the release of NO and the Pt(IV) prodrug that was readily reduced to a Pt(II) active drug in the tumor environment, and the combination with the photothermal properties of GQDs produced a very synergic therapeutic agent.
A smart nano-antibiotic was produced using sulfur-doped GQDs (SGQDs) covalently linked to a CO-releasing molecule (CORM-401) and then electrostatically adsorbed with HA [178]. Secreted bacterial hyaluronidase damaged the HA shield, allowing GQD–CORM to penetrate bacterial cells. Then, under white light irradiation, the GQDs acted as ROS producers, and the subsequent release of CO-induced membrane and metabolic damage led to bacterial death. This novel combined photodynamic/CO gas therapy has been proven to be very safe for somatic cells and non-targeted bacteria and represents a new solution to overcome antibiotic resistance, besides being a diagnostic device for bacterial infections based on GQD fluorescence.
Another utilization of GQDs as theranostic agents was reported for the selective delivery of cisplatin using GQDs linked covalently to a single-chain variable fragment of an antibody that was specifically engineered to bind epidermal growth factor receptor (scFvB10) [179]. The molecular targeting, combined with the pH-dependent release of cisplatin, yielded a very selective nanocarrier that allowed the avoidance of the systematic side effects typical of cisplatin. In another work, a similar therapeutic strategy was reported using a GE11 peptide to target the VEGF receptor and simultaneously deliver cisplatin and DOX for the selective and synergic treatment of nasopharyngeal carcinoma [180].
Finally, a novel biomimetic treatment for atherosclerotic diseases has been developed (Figure 6) [181]. Briefly, monocytes were used as selective carriers to target macrophages in the atherosclerotic plaque, and their membrane was engineered with a C18GVFHQTVSR peptide (C18P). This carefully designed peptide displayed a hydrophobic terminus that could be digested by matrix metalloproteinase-9 (MMP-9), which is overexpressed by macrophages in atherosclerotic plaque, and a hydrophobic C18 chain that was inserted in the cell membrane of the carrier monocyte. The free amino group of the peptide was linked to GQDs via an amide bond, and miRNA223 was linked to GQDs via a disulfide bond. The biomimetic carrier could successfully reach the atherosclerotic plaque as monocytes were continuously recruited there, and, after the selective cleavage of the C18P by MMP-9, GQD-miRNA entered the macrophages. The disulfide bond was then cleaved by γ-interferon-inducible lysosomal thiol reductase in the lysosomes, and miRNA223 was released, with the successful suppression of inflammatory pathways that lead to the formation of atherosclerotic plaque. This kind of smart approach can represent a valid alternative to the currently used statins, which are not completely effective in preventing ischemic strokes due to non-specific distribution.
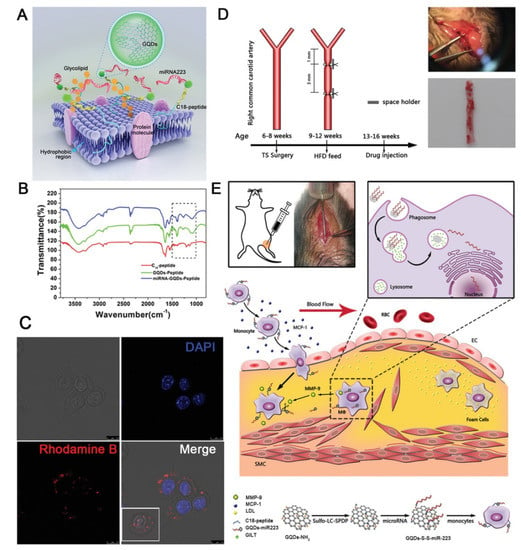
Figure 6.
(A) Structure of the surface-engineered monocyte. (B) FT-IR spectra of C18P, GQDs-C18P, and miRNA223-GQDs-C18P, respectively. (C) Confocal fluorescence images of the surface-engineered monocyte. (D) Animal modeling process: partial ligation of the RCCA in Apoe−/− mice 6–8 weeks of age; continued feeding with a high-fat diet for the 4 weeks after surgery; femoral vein injection after feeding with a high-fat diet for 4 weeks. Top, optical photograph of the surgery; bottom, microscopic image of the oil-red-O-positive area. Scale bar = 100 μm. (E) Delivery mechanism of monocyte-C18P-GQDs-miR223 in vivo. Reproduced with permission from [181]. © 2022 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
5.5. Graphene-Based 2D Materials
Graphene-based 2D materials are a wide class of CNMs that include, for example, graphene, graphene oxide (GO), and reduced graphene oxide (rGO). They have attracted scientists’ attention in biomedicine because of their electronic properties, especially in the field of biosensing [203]; they are also very interesting for drug delivery due to their biocompatibility and wide possibilities for covalent or non-covalent functionalization.
A redox-responsive DOX carrier based on GO nanosheets was recently reported [182]. Briefly, GO nanosheets were functionalized with cystamine-modified HA through the free amino group of cystamine, and DOX was adsorbed on the material. The nanocarrier entered cancer cells via the CD44 receptor due to the presence of HA, and it was trapped in lysosomes, but a NIR trigger disrupted them thanks to the photothermal effect of GO nanosheets. Finally, the high concentration of glutathione in cancer cells’ cytoplasm cleaved the disulfide bond, and DOX was released, thanks to a NIR trigger, resulting in fine spatiotemporally controlled selectivity and an enhanced effect (Figure 7). In another recent work, DOX was successfully delivered to cancer cells with high selectivity using a composite material based on rGO functionalized with polydopamine to enhance the photothermal effect of rGO, then coated with mesoporous silica to increase DOX loading, and, finally, covered with HA to increase biocompatibility and ensure the selectivity of NIR-induced drug delivery to CD44 overexpressing cancer cells [183].
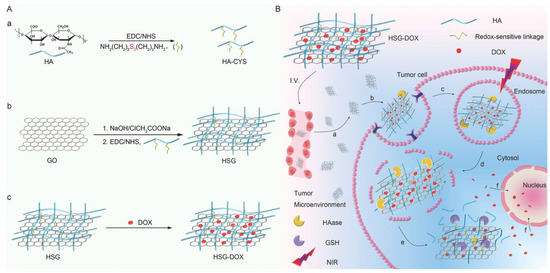
Figure 7.
(A) Synthesis of bioreducible HA-GO-DOX (HSG-DOX) nanosheets. (a,b) HA is bound to graphene oxide (GO) using cystamine as a redox-sensitive linker or adipic dihydrazide as a redox-insensitive linker. (c) DOX loading on the so-modified graphene. (B) NIR-controlled endo/lysosomal escape and rapid release of DOX in cytoplasm induced by glutathione: (a) accumulation of HSG-DOX within the tumor site through passive and active targeting; (b,c) receptor-mediated cellular internalization; (d) hyaluronidase-mediated HA degradation in endosomes and NIR-mediated endo/lysosomal escape; (e) GSH triggered HA detachment and rapid DOX release in cytoplasm; (f) accumulation of DOX in nucleus for DNA damage-mediated apoptosis and cytotoxicity. Reproduced with permission from [182]. © 2022 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
GO was also used for the molecular-targeted delivery of camptothecine by functionalization with an FA-bound PEG that guaranteed the desired selectivity for cancer cells [184]. However, the drug was only adsorbed on the material’s surface, and the release without any other trigger was too slow, especially in the acidic pH typical of cancer cells. Another recent work solved this problem with a redox-sensitive delivery system for DOX based on GO that was non-covalently functionalized with PEG-FA as the targeting molecule and with PEG-PLGA linked to DOX via a disulfide bond [185]. PEG polymers improved GO biocompatibility and stability in water, while the glutathione-sensitive disulfide bond increased the release rate and selectivity of DOX from GO. Cytotoxicity was higher for cancer cells and lower for normal cells compared to the free drug and non-targeted carriers.
A targeted carrier for the delivery of DOX was obtained with the functionalization of rGO with a cyclic RGDfC peptide via the thiol-maleimide click reaction [186]. The carrier alone showed an IC50 similar to free DOX in HeLa cells due to the too-slow release of the drug from rGO, but the use of a NIR trigger increased the release rate and improved the IC50, thanks to the photothermal properties of rGO, which also helped in killing cancer cells. A similar combined approach was developed to deliver berberine derivatives to cancer cells using AS1411-bound GO nanosheets as the targeted carrier [187].
A recent work reported another combined DOX/NIR approach with a smarter theranostic agent [188]. The carrier was based on GO functionalized with Fe3O4 and Cetuximab (CET), an anti-epidermal growth factor receptor, for dual magnetic and molecular targeting. In particular, magnetic nanoparticles were synthetized by chemical co-precipitation directly on GO, and carboxylic groups of the CNM were functionalized with avidin via an amidation reaction. Then the free biotin-binding sites on avidin were exploited to strongly bind biotin–PEG–CET for targeting and biotin–PEG–QD for bioimaging.
Another dual-targeted approach was developed to treat glioma with DOX, combining a magnetic targeting Fe3O4 and molecular brain targeting using transferrin, whose receptor is overexpressed on glioma cells and on the vascular endothelial cells of the BBB [189]. In this case, transferrin was bound to the magnetic nanoparticles, and this targeting strategy proved to be a promising solution to overcome the great obstacle represented by the BBB. Similar transferrin-based brain targeting was used to deliver puerarin to treat Parkinson’s disease (PD) [190]. This nanocarrier was tested in vivo on mice, and it gave excellent results in limiting PD-induced neuronal damage and also acted as a good antioxidant.
Finally, a novel dual gene therapy was studied for the treatment of pancreatic cancer, which is one of the most lethal types of cancer [191]. GO functionalized with FA as the targeting molecule was used as a carrier to deliver HDAC 1 siRNA and G12C mutant K-Ras siRNA together to pancreatic cancer cells, with a NIR-triggered release. The silencing of the two genes led to a strong inhibition in tumor cells’ proliferation without side effects on normal cells as selective targeting was guaranteed by the presence of FA and G12C mutant K-Ras siRNA, which is a mutation that is typical of pancreatic cancer cells.
6. Conclusions
CNMs have attracted wide interest in the scientific community for their engineering and application as drug delivery agents, thanks to their high surface area and drug-loading capacity, as well as ease of functionalization for active targeting of the pathological site and unique physico-chemical properties that enable bioimaging and photodynamic therapy. Despite their promising performance in vitro and in animal models, there are still many challenges to overcome for their clinical implementation, such as long-term toxicity assessments, cost-effective and sustainable production on a large scale, and the development of unified standards for their commercialization and correct classification. Nevertheless, the inclusion of targeting agents on their surface for the selective delivery of bioactive compounds to the pathological site has proven to be a very promising strategy thus far, as it has enabled the lowering of the dose of chemotherapeutics, with consequently reduced side effects. Clearly, further opportunities for development lie in personalized medicine, enabled by the biomarker profiling of patients’ samples. Furthermore, the ability of CNMs to cross the BBB is another key advantage in treating neurological disorders and neurodegenerative diseases; besides cancer, there has been recent interest in the use of CNMs as innovative therapeutic agents. To this end, their engineering to enable biodegradation or excretion appears to be a research area offering ample opportunities for development to enable the full unlocking of CNM potential to provide new solutions to unmet clinical needs.
Author Contributions
Writing—original draft preparation, D.M.; writing—review and editing, S.M.; visualization, D.M. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Trieste (FRA2022). This article was inspired by COST Action EsSENce CA19118, supported by COST (European Cooperation in Science and Technology; www.cost.eu, accessed on 21 May 2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Manuela Bisiacchi for her kind technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mateo, J.; Steuten, L.; Aftimos, P.; André, F.; Davies, M.; Garralda, E.; Geissler, J.; Husereau, D.; Martinez-Lopez, I.; Normanno, N.; et al. Delivering precision oncology to patients with cancer. Nat. Med. 2022, 28, 658–665. [Google Scholar] [CrossRef]
- Marchesan, S.; Prato, M. Nanomaterials for (nano)medicine. ACS Med. Chem. Lett. 2013, 4, 147–149. [Google Scholar] [CrossRef] [Green Version]
- Rosenkrans, Z.T.; Ferreira, C.A.; Ni, D.; Cai, W. Internally responsive nanomaterials for activatable multimodal imaging of cancer. Adv. Healthc. Mater. 2021, 10, e2000690. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, G.; Palem, V.V.; Sundaram, T.; Sundaram, V.; Kishore, S.C.; Bellucci, S. Nanomaterials: Synthesis and applications in theranostics. Nanomaterials 2021, 11, 3228. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Marchesan, S.; Melchionna, M.; Prato, M. Carbon nanostructures for nanomedicine: Opportunities and challenges. Full. Nanotub. Carbon Nanostruct. 2014, 22, 190–195. [Google Scholar] [CrossRef]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomedicine 2018, 14, 2433–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazaka, K.; Jacob, M.V.; Ostrikov, K. Sustainable life cycles of natural-precursor-derived nanocarbons. Chem. Rev. 2016, 116, 163–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Lee, J.; Zhang, S.; Benyshek, C.; Dokmeci, M.R.; Khademhosseini, A. Engineering precision medicine. Adv. Sci. 2019, 6, 1801039. [Google Scholar] [CrossRef] [Green Version]
- Speranza, G. Carbon nanomaterials: Synthesis, functionalization and sensing applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef]
- Jolly, A.; Miao, D.; Daigle, M.; Morin, J.-F. Emerging bottom-up strategies for the synthesis of graphene nanoribbons and related structures. Angew. Chem. Int. Ed. 2020, 59, 4624–4633. [Google Scholar] [CrossRef]
- Bruno, F.; Sciortino, A.; Buscarino, G.; Soriano, M.L.; Ríos, Á.; Cannas, M.; Gelardi, F.; Messina, F.; Agnello, S. A comparative study of top-down and bottom-up carbon nanodots and their interaction with mercury ions. Nanomaterials 2021, 11, 1265. [Google Scholar] [CrossRef] [PubMed]
- Ventrella, A.; Camisasca, A.; Fontana, A.; Giordani, S. Synthesis of green fluorescent carbon dots from carbon nano-onions and graphene oxide. RSC Adv. 2020, 10, 36404–36412. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Basso, L.; Cazzanelli, M.; Orlandi, M.; Miotello, A. Nanodiamonds: Synthesis and application in sensing, catalysis, and the possible connection with some processes occurring in space. Appl. Sci. 2020, 10, 4094. [Google Scholar] [CrossRef]
- Bottari, G.; Herranz, M.Á.; Wibmer, L.; Volland, M.; Rodríguez-Pérez, L.; Guldi, D.M.; Hirsch, A.; Martín, N.; D’Souza, F.; Torres, T. Chemical functionalization and characterization of graphene-based materials. Chem. Sov. Rev. 2017, 46, 4464–4500. [Google Scholar] [CrossRef] [Green Version]
- Calvaresi, M.; Quintana, M.; Rudolf, P.; Zerbetto, F.; Prato, M. Rolling up a graphene sheet. ChemPhysChem 2013, 14, 3447–3453. [Google Scholar] [CrossRef] [PubMed]
- Mchedlov-Petrossyan, N.O. Fullerenes in liquid media: An unsettling intrusion into the solution chemistry. Chem. Rev. 2013, 113, 5149–5193. [Google Scholar] [CrossRef] [PubMed]
- Giordani, S.; Camisasca, A.; Maffeis, V. Carbon nano-onions: A valuable class of carbon nanomaterials in biomedicine. Curr. Med. Chem. 2019, 26, 6915–6929. [Google Scholar] [CrossRef]
- Yang, F.; Wang, M.; Zhang, D.; Yang, J.; Zheng, M.; Li, Y. Chirality pure carbon nanotubes: Growth, sorting, and characterization. Chem. Rev. 2020, 120, 2693–2758. [Google Scholar] [CrossRef] [PubMed]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [Green Version]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, properties, functionalization, and applications of carbon nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Luo, H.; Yu, G. Preparation, bandgap engineering, and performance control of graphene nanoribbons. Chem. Mater. 2022, 34, 3588–3615. [Google Scholar] [CrossRef]
- Adorinni, S.; Cringoli, M.C.; Perathoner, S.; Fornasiero, P.; Marchesan, S. Green approaches to carbon nanostructure-based biomaterials. Appl. Sci. 2021, 11, 2490. [Google Scholar] [CrossRef]
- Ugarte, D. Onion-like graphitic particles. Carbon 1995, 33, 989–993. [Google Scholar] [CrossRef]
- Vicentini, N.; Gatti, T.; Salerno, M.; Gomez, Y.S.H.; Bellon, M.; Gallio, S.; Marega, C.; Filippini, F.; Menna, E. Effect of different functionalized carbon nanostructures as fillers on the physical properties of biocompatible poly(L-lactic acid) composites. Mater. Chem. Phys. 2018, 214, 265–276. [Google Scholar] [CrossRef]
- Piovesana, S.; Iglesias, D.; Melle-Franco, M.; Kralj, S.; Cavaliere, C.; Melchionna, M.; Laganà, A.; Capriotti, A.L.; Marchesan, S. Carbon nanostructure morphology templates nanocomposites for phosphoproteomics. Nano Res. 2020, 13, 380–388. [Google Scholar] [CrossRef]
- Tonellato, M.; Piccione, M.; Gasparotto, M.; Bellet, P.; Tibaudo, L.; Vicentini, N.; Bergantino, E.; Menna, E.; Vitiello, L.; Di Liddo, R. Commitment of autologous human multipotent stem cells on biomimetic poly-l-lactic acid-based scaffolds is strongly influenced by structure and concentration of carbon nanomaterial. Nanomaterials 2020, 10, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias, D.; Melle-Franco, M.; Kurbasic, M.; Melchionna, M.; Abrami, M.; Grassi, M.; Prato, M.; Marchesan, S. Oxidized nanocarbons-tripeptide supramolecular hydrogels: Shape matters! ACS Nano 2018, 12, 5530–5538. [Google Scholar] [CrossRef]
- Marchesan, S.; Prato, M. Under the lens: Carbon nanotube and protein interaction at the nanoscale. Chem. Commun. 2015, 51, 4347–4359. [Google Scholar] [CrossRef]
- Sun, H.; Ren, J.; Qu, X. Carbon nanomaterials and DNA: From molecular recognition to applications. Acc. Chem. Res. 2016, 49, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Pinals, R.L.; Yang, D.; Lui, A.; Cao, W.; Landry, M.P. Corona exchange dynamics on carbon nanotubes by multiplexed fluorescence monitoring. J. Am. Chem. Soc. 2019, 142, 1254–1264. [Google Scholar] [CrossRef]
- Palmieri, V.; Perini, G.; De Spirito, M.; Papi, M. Graphene oxide touches blood: In vivo interactions of bio-coronated 2D materials. Nanoscale Horiz. 2019, 4, 273–290. [Google Scholar] [CrossRef]
- Cai, K.; Wang, A.Z.; Yin, L.; Cheng, J. Bio-nano interface: The impact of biological environment on nanomaterials and their delivery properties. J. Control. Release 2017, 263, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Kostarelos, K.; Bianco, A.; Prato, M. The winding road for carbon nanotubes in nanomedicine. Mater. Today 2015, 18, 12–19. [Google Scholar] [CrossRef]
- Gazzi, A.; Fusco, L.; Orecchioni, M.; Ferrari, S.; Franzoni, G.; Yan, J.S.; Rieckher, M.; Peng, G.; Lucherelli, M.A.; Vacchi, I.A.; et al. Graphene, other carbon nanomaterials and the immune system: Toward nanoimmunity-by-design. J. Phys. Mater. 2020, 3, 034009. [Google Scholar] [CrossRef]
- Chen, M.; Qin, X.; Zeng, G. Biodegradation of carbon nanotubes, graphene, and their derivatives. Trends Biotechnol. 2017, 35, 836–846. [Google Scholar] [CrossRef]
- Rozhin, P.; Abdel Monem Gamal, J.; Giordani, S.; Marchesan, S. Carbon nanomaterials (CNMs) and enzymes: From nanozymes to cnm-enzyme conjugates and biodegradation. Materials 2022, 15, 1037. [Google Scholar] [CrossRef]
- Marchesan, S.; Melchionna, M.; Prato, M. Wire up on carbon nanostructures! How to play a winning game. ACS Nano 2015, 9, 9441–9450. [Google Scholar] [CrossRef] [Green Version]
- Loh, K.P.; Ho, D.; Chiu, G.N.C.; Leong, D.T.; Pastorin, G.; Chow, E.K.H. Clinical applications of carbon nanomaterials in diagnostics and therapy. Adv. Mater. 2018, 30, 1802368. [Google Scholar] [CrossRef] [PubMed]
- Plonska-Brzezinska, M.E. Carbon nanomaterials: Perspective of their applications in biomedicine. Curr. Med. Chem. 2019, 26, 6832–6833. [Google Scholar] [CrossRef] [PubMed]
- Teradal, N.L.; Jelinek, R. Carbon nanomaterials in biological studies and biomedicine. Adv. Healthc. Mater. 2017, 6, 1700574. [Google Scholar] [CrossRef] [PubMed]
- Mehra, N.K.; Jain, A.K.; Nahar, M. Carbon nanomaterials in oncology: An expanding horizon. Drug Discov. Today 2018, 23, 1016–1025. [Google Scholar] [CrossRef]
- Augustine, S.; Singh, J.; Srivastava, M.; Sharma, M.; Das, A.; Malhotra, B.D. Recent advances in carbon based nanosystems for cancer theranostics. Biomater. Sci. 2017, 5, 901–952. [Google Scholar] [CrossRef]
- Bartelmess, J.; Quinn, S.; Giordani, S. Carbon nanomaterials: Multi-functional agents for biomedical fluorescence and raman imaging. Chem. Soc. Rev. 2015, 44, 4672–4698. [Google Scholar] [CrossRef]
- Rezaee, M.; Behnam, B.; Banach, M.; Sahebkar, A. The yin and yang of carbon nanomaterials in atherosclerosis. Biotechnol. Adv. 2018, 36, 2232–2247. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B. Antibacterial carbon-based nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef]
- Unal, M.A.; Bayrakdar, F.; Nazir, H.; Besbinar, O.; Gurcan, C.; Lozano, N.; Arellano, L.M.; Yalcin, S.; Panatli, O.; Celik, D.; et al. Graphene oxide nanosheets interact and interfere with SARS-CoV-2 surface proteins and cell receptors to inhibit infectivity. Small 2021, 17, 2101483. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mohammadinejad, R.; Kailasa, S.K.; Ahmadi, Z.; Afshar, E.G.; Pardakhty, A. Carbon dots as versatile nanoarchitectures for the treatment of neurological disorders and their theranostic applications: A review. Adv. Coll. Interface Sci. 2020, 278, 102123. [Google Scholar] [CrossRef]
- Gupta, N.; Rai, D.B.; Jangid, A.K.; Kulhari, H. A review of theranostics applications and toxicities of carbon nanomaterials. Curr. Drug Metab. 2019, 20, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular toxicity and immunological effects of carbon-based nanomaterials. Particle Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Fadeel, B.; Kostarelos, K. Grouping all carbon nanotubes into a single substance category is scientifically unjustified. Nat. Nanotechnol. 2020, 15, 164. [Google Scholar] [CrossRef] [Green Version]
- Graphene Standards. Available online: https://www.thegraphenecouncil.org/page/GrapheneStandards (accessed on 21 May 2022).
- Xiarchos, I.; Morozinis, A.K.; Kavouras, P.; Charitidis, C.A. Nanocharacterization, materials modeling, and research integrity as enablers of sound risk assessment: Designing responsible nanotechnology. Small 2020, 16, 2001590. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qu, B.; Andreeva, D.V.; Ye, C.; Novoselov, K.S. Graphene standardization: The lesson from the East. Mater. Today 2021, 47, 9–15. [Google Scholar] [CrossRef]
- Kotzabasaki, M.; Sotiropoulos, I.; Charitidis, C.; Sarimveis, H. Machine learning methods for multi-walled carbon nanotubes (mwcnt) genotoxicity prediction. Nanoscale Adv. 2021, 3, 3167–3176. [Google Scholar] [CrossRef]
- Romanos, N.; Kalogerini, M.; Koumoulos, E.P.; Morozinis, A.; Sebastiani, M.; Charitidis, C. Innovative data management in advanced characterization: Implications for materials design. Mater. Today Commun. 2019, 20, 100541. [Google Scholar] [CrossRef]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon nanotubes and related nanomaterials: Critical advances and challenges for synthesis toward mainstream commercial applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zhang, H.; Li, K.; Zhang, J.; Sun, M.; Su, B. Catalytic graphitization of coke carbon by iron: Understanding the evolution of carbon structure, morphology and lattice fringes. Fuel 2020, 279, 118531. [Google Scholar] [CrossRef]
- Flygare, M.; Svensson, K. Quantifying crystallinity in carbon nanotubes and its influence on mechanical behaviour. Mater. Today Commun. 2019, 18, 39–45. [Google Scholar] [CrossRef]
- Mochida, I.; Egashira, M.; Korai, Y.; Yokogawa, K. Structural changes of fullerene by heat-treatment up to graphitization temperature. Carbon 1997, 35, 1707–1712. [Google Scholar] [CrossRef]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety assessment of graphene-based materials: Focus on human health and the environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Qian, D.; Dickey, E.C. Purification and structural annealing of multiwalled carbon nanotubes at graphitization temperatures. Carbon 2001, 39, 1681–1687. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, G. Single-walled carbon nanohorns and their applications. Nanoscale 2010, 2, 2538–2549. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, J.P.; Fragoso, A. Preparation and characterization of carbon nano-onions by nanodiamond annealing and functionalization by radio-frequency Ar/O2 plasma. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 327–334. [Google Scholar] [CrossRef]
- Li, L.; Wu, G.; Yang, G.; Peng, J.; Zhao, J.; Zhu, J.-J. Focusing on luminescent graphene quantum dots: Current status and future perspectives. Nanoscale 2013, 5, 4015–4039. [Google Scholar] [CrossRef] [Green Version]
- Yogesh, G.K.; Shukla, S.; Sastikumar, D.; Koinkar, P. Progress in pulsed laser ablation in liquid (plal) technique for the synthesis of carbon nanomaterials: A review. Appl. Phys. A 2021, 127, 810. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotube: An overview of synthesis, properties, functionalization, characterization, and the application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Tripathi, K.M.; Tran, T.S.; Kim, Y.J.; Kim, T. Green fluorescent onion-like carbon nanoparticles from flaxseed oil for visible light induced photocatalytic applications and label-free detection of Al(III) ions. ACS Sustain. Chem. Eng. 2017, 5, 3982–3992. [Google Scholar] [CrossRef]
- Jung, S.; Myung, Y.; Das, G.S.; Bhatnagar, A.; Park, J.-W.; Tripathi, K.M.; Kim, T. Carbon nano-onions from waste oil for application in energy storage devices. New J. Chem. 2020, 44, 7369–7375. [Google Scholar] [CrossRef]
- Xin, Y.; Odachi, K.; Shirai, T. Fabrication of ultra-bright carbon nano-onions via a one-step microwave pyrolysis of fish scale waste in seconds. Green Chem. 2022, 24, 3969–3976. [Google Scholar] [CrossRef]
- Gunture; Dalal, C.; Kaushik, J.; Garg, A.K.; Sonkar, S.K. Pollutant-soot-based nontoxic water-soluble onion-like nanocarbons for cell imaging and selective sensing of toxic cr(VI). ACS Appl. Bio Mater. 2020, 3, 3906–3913. [Google Scholar] [CrossRef]
- Chowdhury, S.N.; Tung, T.T.; Ta, Q.T.H.; Gunture; Castro, M.; Feller, J.F.; Sonkar, S.K.; Tripathi, K.M. Upgrading of diesel engine exhaust waste into onion-like carbon nanoparticles for integrated degradation sensing in nano-biocomposites. New J. Chem. 2021, 45, 3675–3682. [Google Scholar] [CrossRef]
- Gunture; Kaushik, J.; Garg, A.K.; Saini, D.; Khare, P.; Sonkar, S.K. Pollutant diesel soot derived onion-like nanocarbons for the adsorption of organic dyes and environmental assessment of treated wastewater. Ind. Eng. Chem. Res. 2020, 59, 12065–12074. [Google Scholar] [CrossRef]
- Liu, X.; Ren, J.; Licht, G.; Wang, X.; Licht, S. Carbon nano-onions made directly from CO2 by molten electrolysis for greenhouse gas mitigation. Adv. Sustain. Syst. 2019, 3, 1900056. [Google Scholar] [CrossRef]
- Liu, X.; Licht, G.; Wang, X.; Licht, S. Controlled growth of unusual nanocarbon allotropes by molten electrolysis of CO2. Catalysts 2022, 12, 125. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, X.; Jia, H.-R.; Zhu, Y.-X.; Guo, Y.; Gao, G.; Li, Y.-H.; Wu, F.-G. Water-dispersible candle soot–derived carbon nano-onion clusters for imaging-guided photothermal cancer therapy. Small 2019, 15, 1804575. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Q.; Zhong, S.; Chen, J.; Lin, H.; Wu, X.-L. Facile large scale fabrication of magnetic carbon nano-onions for efficient removal of bisphenol a. Mater. Chem. Phys. 2017, 198, 186–192. [Google Scholar] [CrossRef]
- Sang, S.; Yang, S.; Guo, A.; Gao, X.; Wang, Y.; Zhang, C.; Cui, F.; Yang, X. Hydrothermal synthesis of carbon nano-onions from citric acid. Asian J. Chem. 2020, 15, 3428–3431. [Google Scholar] [CrossRef]
- Jin, H.; Wu, S.; Li, T.; Bai, Y.; Wang, X.; Zhang, H.; Xu, H.; Kong, C.; Wang, H. Synthesis of porous carbon nano-onions derived from rice husk for high-performance supercapacitors. Appl. Surf. Sci. 2019, 488, 593–599. [Google Scholar] [CrossRef]
- Patiño-Carachure, C.; Martínez-Vargas, S.; Flores-Chan, J.E.; Rosas, G. Synthesis of carbon nanostructures by graphite deformation during mechanical milling in air. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 869–876. [Google Scholar] [CrossRef]
- Elessawy, N.A.; El-Sayed, E.M.; Ali, S.; Elkady, M.F.; Elnouby, M.; Hamad, H.A. One-pot green synthesis of magnetic fullerene nanocomposite for adsorption characteristics. J. Water Process. Eng. 2020, 34, 101047. [Google Scholar] [CrossRef]
- Douglas, A.; Carter, R.; Li, M.; Pint, C.L. Toward small-diameter carbon nanotubes synthesized from captured carbon dioxide: Critical role of catalyst coarsening. ACS Appl. Mater. Interfaces 2018, 10, 19010–19018. [Google Scholar] [CrossRef]
- Liu, X.; Licht, G.; Licht, S. The green synthesis of exceptional braided, helical carbon nanotubes and nanospiral platelets made directly from CO2. Mater. Today Chem. 2021, 22, 100529. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Lu, S.; Tu, J.; Jiao, S. Electrochemical graphitization conversion of CO2 through soluble navo3 homogeneous catalyst in carbonate molten salt. Electrochim. Acta 2020, 331, 135461. [Google Scholar] [CrossRef]
- Hu, L.; Song, Y.; Ge, J.; Zhu, J.; Han, Z.; Jiao, S. Electrochemical deposition of carbon nanotubes from CO2 in CaCl2–NaCl-based melts. J. Mater. Chem. A 2017, 5, 6219–6225. [Google Scholar] [CrossRef]
- Arcaro, S.; Berutti, F.A.; Alves, A.K.; Bergmann, C.P. Mwcnts produced by electrolysis of molten carbonate: Characteristics of the cathodic products grown on galvanized steel and nickel chrome electrodes. Appl. Surf. Sci. 2019, 466, 367–374. [Google Scholar] [CrossRef]
- Hu, L.; Yang, W.; Yang, Z.; Xu, J. Fabrication of graphite via electrochemical conversion of CO2 in a CaCl2 based molten salt at a relatively low temperature. RSC Adv. 2019, 9, 8585–8593. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, X.; Licht, G.; Licht, S. Calcium metaborate induced thin walled carbon nanotube syntheses from CO2 by molten carbonate electrolysis. Sci. Rep. 2020, 10, 15146. [Google Scholar] [CrossRef]
- Wang, X.; Sharif, F.; Liu, X.; Licht, G.; Lefler, M.; Licht, S. Magnetic carbon nanotubes: Carbide nucleated electrochemical growth of ferromagnetic cnts from CO2. J. CO2 Util. 2020, 40, 101218. [Google Scholar] [CrossRef]
- Wang, X.; Licht, G.; Liu, X.; Licht, S. One pot facile transformation of CO2 to an unusual 3-D nano-scaffold morphology of carbon. Sci. Rep. 2020, 10, 21518. [Google Scholar] [CrossRef]
- Ren, J.; Johnson, M.; Singhal, R.; Licht, S. Transformation of the greenhouse gas CO2 by molten electrolysis into a wide controlled selection of carbon nanotubes. J. CO2 Util. 2017, 18, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yuan, D.; Wu, H.; Li, W.; Gu, D. A novel route to synthesize carbon spheres and carbon nanotubes from carbon dioxide in a molten carbonate electrolyzer. Inorg. Chem. Front. 2018, 5, 208–216. [Google Scholar] [CrossRef]
- Johnson, M.; Ren, J.; Lefler, M.; Licht, G.; Vicini, J.; Liu, X.; Licht, S. Carbon nanotube wools made directly from CO2 by molten electrolysis: Value driven pathways to carbon dioxide greenhouse gas mitigation. Mater. Today Energy 2017, 5, 230–236. [Google Scholar] [CrossRef]
- Wang, X.; Licht, G.; Liu, X.; Licht, S. CO2 utilization by electrolytic splitting to carbon nanotubes in non-lithiated, cost-effective, molten carbonate electrolytes. Adv. Sustain. Syst. 2022, 6, 2100481. [Google Scholar] [CrossRef]
- Chan, K.F.; Ranade, A.K.; Desai, P.; Hazan, M.A.; Azman, N.F.I.; Shaharifin, S.; Kalita, G.; Mamat, M.S.; Tanemura, M.; Yaakob, Y. Upcycling the barbeque grease into carbon nanomaterials. Carbon Trends 2022, 6, 100143. [Google Scholar] [CrossRef]
- Tripathi, N.; Pavelyev, V.; Islam, S.S. Synthesis of carbon nanotubes using green plant extract as catalyst: Unconventional concept and its realization. Appl. Nanosci. 2017, 7, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Salah, N.; Abdel-wahab, M.S.; Alshahrie, A.; Alharbi, N.D.; Khan, Z.H. Carbon nanotubes of oil fly ash as lubricant additives for different base oils and their tribology performance. RSC Adv. 2017, 7, 40295–40302. [Google Scholar] [CrossRef] [Green Version]
- Yao, D.; Wang, C.-H. Pyrolysis and in-line catalytic decomposition of polypropylene to carbon nanomaterials and hydrogen over fe- and ni-based catalysts. Appl. Energy 2020, 265, 114819. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Awadallah, A.E. Production of nanostructure carbon materials via non-oxidative thermal degradation of real polypropylene waste plastic using La2O3 supported Ni and Ni–Cu catalysts. Polym. Degrad. Stab. 2019, 167, 157–169. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Awadallah, A.E. Production of nanostructured carbon materials using Fe–Mo/MgO catalysts via mild catalytic pyrolysis of polyethylene waste. Chem. Eng. J. 2018, 354, 802–816. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Durbach, S.; Coville, N.J. Synthesis of multi-walled carbon nanotubes from plastic waste using a stainless-steel cvd reactor as catalyst. Nanomaterials 2017, 7, 284. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Enein, A.A.; Adel-Rahman, H.; Haggar, A.M.; Awadallah, A.E. Simple method for synthesis of carbon nanotubes over Ni-Mo/Al2O3 catalyst via pyrolysis of polyethylene waste using a two-stage process. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 211–222. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Awadallah, A.E.; Abdel-Rahman, A.A.H.; Haggar, A.M. Synthesis of multi-walled carbon nanotubes via pyrolysis of plastic waste using a two-stage process. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 443–450. [Google Scholar] [CrossRef]
- Bajad, G.; Vijayakumar, R.P.; Rakhunde, P.; Hete, A.; Bhade, M. Processing of mixed-plastic waste to fuel oil, carbon nanotubes and hydrogen using multi–core reactor. Chem. Eng. Process. 2017, 121, 205–214. [Google Scholar] [CrossRef]
- Jia, J.; Veksha, A.; Lim, T.-T.; Lisak, G. In situ grown metallic nickel from x–Ni (x = La, Mg, Sr) oxides for converting plastics into carbon nanotubes: Influence of metal–support interaction. J. Clean. Prod. 2020, 258, 120633. [Google Scholar] [CrossRef]
- Liu, X.; Sun, H.; Wu, C.; Patel, D.; Huang, J. Thermal chemical conversion of high-density polyethylene for the production of valuable carbon nanotubes using Ni/AAO membrane catalyst. Energy Fuels 2018, 32, 4511–4520. [Google Scholar] [CrossRef]
- Liu, X.; Shen, B.; Wu, Z.; Parlett, C.M.A.; Han, Z.; George, A.; Yuan, P.; Patel, D.; Wu, C. Producing carbon nanotubes from thermochemical conversion of waste plastics using ni/ceramic based catalyst. Chem. Eng. Sci. 2018, 192, 882–891. [Google Scholar] [CrossRef] [Green Version]
- Panahi, A.; Wei, Z.; Song, G.; Levendis, Y.A. Influence of stainless-steel catalyst substrate type and pretreatment on growing carbon nanotubes from waste postconsumer plastics. Ind. Eng. Chem. Res. 2019, 58, 3009–3023. [Google Scholar] [CrossRef]
- Yao, D.; Wu, C.; Yang, H.; Zhang, Y.; Nahil, M.A.; Chen, Y.; Williams, P.T.; Chen, H. Co-production of hydrogen and carbon nanotubes from catalytic pyrolysis of waste plastics on ni-fe bimetallic catalyst. Energy Convers. Manag. 2017, 148, 692–700. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Hu, Q.; Chen, Y.; Chen, H.; Williams, P.T. Carbon nanotubes from post-consumer waste plastics: Investigations into catalyst metal and support material characteristics. Appl. Catal. B 2021, 280, 119413. [Google Scholar] [CrossRef]
- Zhao, N.; Wu, Q.; Zhang, X.; Yang, T.; Li, D.; Zhang, X.; Ma, C.; Liu, R.; Xin, L.; He, M. Chemical vapor deposition growth of single-walled carbon nanotubes from plastic polymers. Carbon 2022, 187, 29–34. [Google Scholar] [CrossRef]
- Haggar, A.M.; Awadallah, A.E.; Aboul-Enein, A.A.; Sayed, G.H. Non-oxidative conversion of real low density polyethylene waste into hydrogen and carbon nanomaterials over mgo supported bimetallic co-mo catalysts with different total co-mo contents. Chem. Eng. Sci. 2022, 247, 117092. [Google Scholar] [CrossRef]
- Yao, D.; Li, H.; Mohan, B.C.; Prabhakar, A.K.; Dai, Y.; Wang, C.-H. Conversion of waste plastic packings to carbon nanomaterials: Investigation into catalyst material, waste type, and product applications. ACS Sustain. Chem. Eng. 2022, 10, 1125–1136. [Google Scholar] [CrossRef]
- Moo, J.G.S.; Veksha, A.; Oh, W.-D.; Giannis, A.; Udayanga, W.D.C.; Lin, S.-X.; Ge, L.; Lisak, G. Plastic derived carbon nanotubes for electrocatalytic oxygen reduction reaction: Effects of plastic feedstock and synthesis temperature. Electrochem. Commun. 2019, 101, 11–18. [Google Scholar] [CrossRef]
- Hamid, Z.A.; Azim, A.A.; Mouez, F.A.; Rehim, S.S.A. Challenges on synthesis of carbon nanotubes from environmentally friendly green oil using pyrolysis technique. J. Anal. Appl. Pyrolysis 2017, 126, 218–229. [Google Scholar] [CrossRef]
- Sarno, M.; Senatore, A.; Scarpa, D.; Cirillo, C. “Green” synthesis of nanocarbons for reduced friction and wear. Lubricants 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- De Menezes, F.D.; dos Reis, S.R.R.; Pinto, S.R.; Portilho, F.L.; do Vale Chaves e Mello, F.; Helal-Neto, E.; da Silva de Barros, A.O.; Alencar, L.M.R.; de Menezes, A.S.; dos Santos, C.C.; et al. Graphene quantum dots unraveling: Green synthesis, characterization, radiolabeling with 99mTc, in vivo behavior and mutagenicity. Mater. Sci. Eng. C 2019, 102, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Nirala, N.R.; Khandelwal, G.; Kumar, B.; Vinita; Prakash, R.; Kumar, V. One step electro-oxidative preparation of graphene quantum dots from wood charcoal as a peroxidase mimetic. Talanta 2017, 173, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, S.P.; Syrgiannis, Z.; Budimir, M.D.; Milivojević, D.D.; Jovanovic, D.J.; Pavlović, V.B.; Papan, J.M.; Bartenwerfer, M.; Mojsin, M.M.; Stevanović, M.J.; et al. Graphene quantum dots as singlet oxygen producer or radical quencher—The matter of functionalization with urea/thiourea. Mater. Sci. Eng. C 2020, 109, 110539. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, D.; Gu, B.; Gao, B.; Wang, T.; Guo, Q.; Wang, G. Biomass-derived nitrogen doped graphene quantum dots with color-tunable emission for sensing, fluorescence ink and multicolor cell imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 227, 117671. [Google Scholar] [CrossRef]
- Chen, W.; Shen, J.; Lv, G.; Li, D.; Hu, Y.; Zhou, C.; Liu, X.; Dai, Z. Green synthesis of graphene quantum dots from cotton cellulose. ChemistrySelect 2019, 4, 2898–2902. [Google Scholar] [CrossRef]
- Chen, W.; Li, D.; Tian, L.; Xiang, W.; Wang, T.; Hu, W.; Hu, Y.; Chen, S.; Chen, J.; Dai, Z. Synthesis of graphene quantum dots from natural polymer starch for cell imaging. Green Chem. 2018, 20, 4438–4442. [Google Scholar] [CrossRef]
- Hoan, B.T.; Tam, P.D.; Pham, V.-H. Green synthesis of highly luminescent carbon quantum dots from lemon juice. J. Nanotechnol. 2019, 2019, 2852816. [Google Scholar] [CrossRef] [Green Version]
- Qu, C.; Zhang, D.; Yang, R.; Hu, J.; Qu, L. Nitrogen and sulfur co-doped graphene quantum dots for the highly sensitive and selective detection of mercury ion in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, Y.; Yang, H.; Zhao, K.; Li, J.; Deng, A. Fluorescent nitrogen and sulfur co-doped carbon dots from casein and their applications for sensitive detection of Hg2+ and biothiols and cellular imaging. Anal. Chim. Acta 2017, 964, 150–160. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.; He, H.; Huang, G.; Xing, B.; Jia, J.; Zhang, C. Green preparation of high yield fluorescent graphene quantum dots from coal-tar-pitch by mild oxidation. Nanomaterials 2018, 8, 844. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Tang, Y.; Wang, G.; Mao, J.; Liu, Z.; Sun, T.; Wang, M.; Chen, D.; Yang, Y.; Li, J.; et al. Green, rapid, and universal preparation approach of graphene quantum dots under ultraviolet irradiation. ACS Appl. Mater. Interfaces 2017, 9, 14470–14477. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene quantum dots for cell proliferation, nucleus imaging, and photoluminescent sensing applications. Sci. Rep. 2017, 7, 15858. [Google Scholar] [CrossRef]
- Singh, A.; Khare, P.; Verma, S.; Bhati, A.; Sonker, A.K.; Tripathi, K.M.; Sonkar, S.K. Pollutant soot for pollutant dye degradation: Soluble graphene nanosheets for visible light induced photodegradation of methylene blue. ACS Sustain. Chem. Eng. 2017, 5, 8860–8869. [Google Scholar] [CrossRef]
- Khare, P.; Singh, A.; Verma, S.; Bhati, A.; Sonker, A.K.; Tripathi, K.M.; Sonkar, S.K. Sunlight-induced selective photocatalytic degradation of methylene blue in bacterial culture by pollutant soot derived nontoxic graphene nanosheets. ACS Sustain. Chem. Eng. 2018, 6, 579–589. [Google Scholar] [CrossRef]
- Yoon, J.-C.; Dai, X.; Kang, K.-N.; Hwang, J.; Kwak, M.-J.; Ding, F.; Jang, J.-H. Graphitization with suppressed carbon loss for high-quality reduced graphene oxide. ACS Nano 2021, 15, 11655–11666. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Hosny, M.; El-Maghrabi, N.; Fawzy, M. Facile synthesis of reduced graphene oxide by tecoma stans extracts for efficient removal of ni (II) from water: Batch experiments and response surface methodology. Sustain. Environ. Res. 2022, 32, 22. [Google Scholar] [CrossRef]
- El Essawy, N.A.; Ali, S.M.; Farag, H.A.; Konsowa, A.H.; Elnouby, M.; Hamad, H.A. Green synthesis of graphene from recycled pet bottle wastes for use in the adsorption of dyes in aqueous solution. Ecotoxicol. Environ. Saf. 2017, 145, 57–68. [Google Scholar] [CrossRef]
- Ezzat, M.N.; Ali, Z.T.A. Green approach for fabrication of graphene from polyethylene terephthalate (pet) bottle waste as reactive material for dyes removal from aqueous solution: Batch and continuous study. Sustain. Mater. Technol. 2022, 32, e00404. [Google Scholar] [CrossRef]
- Pandey, S.; Karakoti, M.; Dhali, S.; Karki, N.; SanthiBhushan, B.; Tewari, C.; Rana, S.; Srivastava, A.; Melkani, A.B.; Sahoo, N.G. Bulk synthesis of graphene nanosheets from plastic waste: An invincible method of solid waste management for better tomorrow. Waste Manag. 2019, 88, 48–55. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.; Chen, N.; Ji, B.; Qu, L. Trash to treasure: Converting plastic waste into a useful graphene foil. Nanoscale 2017, 9, 9089–9094. [Google Scholar] [CrossRef]
- Gong, P.; Tang, C.; Wang, B.; Xiao, T.; Zhu, H.; Li, Q.; Sun, Z. Precise CO2 reduction for bilayer graphene. ACS Cent. Sci. 2022, 8, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Licht, G.; Licht, S. Transformation of the greenhouse gas carbon dioxide to graphene. J. CO2 Util. 2020, 36, 288–294. [Google Scholar] [CrossRef]
- Ahamed, A.; Veksha, A.; Yin, K.; Weerachanchai, P.; Giannis, A.; Lisak, G. Environmental impact assessment of converting flexible packaging plastic waste to pyrolysis oil and multi-walled carbon nanotubes. J. Hazard. Mater. 2020, 390, 121449. [Google Scholar] [CrossRef] [PubMed]
- El Essawy, N.A.; Konsowa, A.H.; Elnouby, M.; Farag, H.A. A novel one-step synthesis for carbon-based nanomaterials from polyethylene terephthalate (PET) bottles waste. J. Air. Waste. Manag. Assoc. 2017, 67, 358–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulay, M.R.; Chauhan, A.; Patel, S.; Balakrishnan, V.; Halder, A.; Vaish, R. Candle soot: Journey from a pollutant to a functional material. Carbon 2019, 144, 684–712. [Google Scholar] [CrossRef]
- Li, F.; Zhou, C.; Yang, P.; Wang, B.; Hu, J.; Wei, J.; Yu, Q. Direct synthesis of carbon nanotubes on fly ash particles to produce carbon nanotubes/fly ash composites. Front. Struct. Civ. Eng. 2019, 13, 1405–1414. [Google Scholar] [CrossRef]
- Liang, C.; Chen, Y.; Wu, M.; Wang, K.; Zhang, W.; Gan, Y.; Huang, H.; Chen, J.; Xia, Y.; Zhang, J.; et al. Green synthesis of graphite from CO2 without graphitization process of amorphous carbon. Nat. Commun. 2021, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Motiei, M.; Rosenfeld Hacohen, Y.; Calderon-Moreno, J.; Gedanken, A. Preparing carbon nanotubes and nested fullerenes from supercritical CO2 by a chemical reaction. J. Am. Chem. Soc. 2001, 123, 8624–8625. [Google Scholar] [CrossRef] [PubMed]
- Allaedini, G.; Tasirin, S.M.; Aminayi, P. Synthesis of CNTs via chemical vapor deposition of carbon dioxide as a carbon source in the presence of nimgo. J. Alloys Compd. 2015, 647, 809–814. [Google Scholar] [CrossRef]
- Strudwick, A.J.; Weber, N.E.; Schwab, M.G.; Kettner, M.; Weitz, R.T.; Wünsch, J.R.; Müllen, K.; Sachdev, H. Chemical vapor deposition of high quality graphene films from carbon dioxide atmospheres. ACS Nano 2015, 9, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Sugime, H.; Noda, S. CO2-assisted growth of millimeter-tall single-wall carbon nanotube arrays and its advantage against h2o for large-scale and uniform synthesis. Carbon 2018, 136, 143–149. [Google Scholar] [CrossRef]
- Ren, J.; Li, F.-F.; Lau, J.; González-Urbina, L.; Licht, S. One-pot synthesis of carbon nanofibers from CO2. Nano Lett. 2015, 15, 6142–6148. [Google Scholar] [CrossRef]
- Ren, J.; Licht, S. Tracking airborne CO2 mitigation and low cost transformation into valuable carbon nanotubes. Sci. Rep. 2016, 6, 27760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perini, G.; Palmieri, V.; Ciasca, G.; De Spirito, M.; Papi, M. Unravelling the potential of graphene quantum dots in biomedicine and neuroscience. Int. J. Mol. Sci. 2020, 21, 3712. [Google Scholar] [CrossRef]
- Tupone, M.G.; Panella, G.; d’Angelo, M.; Castelli, V.; Caioni, G.; Catanesi, M.; Benedetti, E.; Cimini, A. An update on graphene-based nanomaterials for neural growth and central nervous system regeneration. Int. J. Mol. Sci. 2021, 22, 13047. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the epr effect and strategies to improve the therapeutic effects of nanomedicines by using epr effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Bort, G.; Lux, F.; Dufort, S.; Crémillieux, Y.; Verry, C.; Tillement, O. EPR-mediated tumor targeting using ultrasmall-hybrid nanoparticles: From animal to human with theranostic aguix nanoparticles. Theranostics 2020, 10, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.R.; Aramesh, N.; Bilal, M.; Xiao, J.; Kim, H.W.; Yan, B. Carbon nanomaterials as emerging nanotherapeutic platforms to tackle the rising tide of cancer—A review. Bioorg. Med. Chem. 2021, 51, 116493. [Google Scholar] [CrossRef] [PubMed]
- Clara, J.A.; Monge, C.; Yang, Y.; Takebe, N. Targeting signalling pathways and the immune microenvironment of cancer stem cells—A clinical update. Nat. Rev. Clin. Oncol. 2020, 17, 204–232. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Gao, N.; Wang, X.; Ren, J.; Qu, X. Near-infrared switchable fullerene-based synergy therapy for alzheimer’s disease. Small 2018, 14, 1801852. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, R.; Zhang, Y.; Li, T.; Dai, J.; Nannapuneni, N.; Chastanet, T.R.; Chen, M.; Shen, F.H.; Jin, L.; et al. A new formyl peptide receptor-1 antagonist conjugated fullerene nanoparticle for targeted treatment of degenerative disc diseases. ACS Appl. Mater. Interfaces 2019, 11, 38405–38416. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, Y.; Liang, H.; Xia, S.; Liang, W.; Kong, J.; Liang, Y.; Chen, X.; Mao, M.; Chen, Z.; et al. Intrinsic biotaxi solution based on blood cell membrane cloaking enables fullerenol thrombolysis in vivo. ACS Appl. Mater. Interfaces 2020, 12, 14958–14970. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Hao, J.; Dong, S. Magnetic fullerene-DNA/hyaluronic acid nanovehicles with magnetism/reduction dual-responsive triggered release. Biomacromolecules 2017, 18, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, Y.; Yin, Y.; Zhang, J.; Su, W.; White, A.M.; Bin, J.; Xu, J.; Zhang, Y.; Stewart, S.; et al. Carbon nano-onion-mediated dual targeting of p-selectin and p-glycoprotein to overcome cancer drug resistance. Nat. Commun. 2021, 12, 312. [Google Scholar] [CrossRef]
- Wang, D.; Ren, Y.; Shao, Y.; Yu, D.; Meng, L. Facile preparation of doxorubicin-loaded and folic acid-conjugated carbon nanotubes@poly(N-vinyl pyrrole) for targeted synergistic chemo–photothermal cancer treatment. Bioconjug. Chem. 2017, 28, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Meng, L.; Fei, Z.; Hou, C.; Long, J.; Zeng, L.; Dyson, P.J.; Huang, P. Multi-layered tumor-targeting photothermal-doxorubicin releasing nanotubes eradicate tumors in vivo with negligible systemic toxicity. Nanoscale 2018, 10, 8536–8546. [Google Scholar] [CrossRef] [PubMed]
- Gangrade, A.; Mandal, B.B. Injectable carbon nanotube impregnated silk based multifunctional hydrogel for localized targeted and on-demand anticancer drug delivery. ACS Biomater. Sci. Eng. 2019, 5, 2365–2381. [Google Scholar] [CrossRef]
- Singh, R.P.; Sharma, G.; Sonali; Singh, S.; Bharti, S.; Pandey, B.L.; Koch, B.; Muthu, M.S. Chitosan-folate decorated carbon nanotubes for site specific lung cancer delivery. Mater. Sci. Eng. C 2017, 77, 446–458. [Google Scholar] [CrossRef]
- Milosavljevic, V.; Krejcova, L.; Guran, R.; Buchtelova, H.; Wawrzak, D.; Richtera, L.; Heger, Z.; Kopel, P.; Adam, V. Exceptional release kinetics and cytotoxic selectivity of oxidised mwcnts double-functionalised with doxorubicin and prostate-homing peptide. Colloids Surf. B 2017, 156, 123–132. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, H.-Y.; Chen, L.-Q.; Du, H.-H.; Cui, J.-H.; Zhang, L.W.; Lee, B.-J.; Cao, Q.-R. Enhanced lysosomal escape of ph-responsive polyethylenimine–betaine functionalized carbon nanotube for the codelivery of survivin small interfering rna and doxorubicin. ACS Appl. Mater. Interfaces 2019, 11, 9763–9776. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.-J.; Xie, Z.-B.; Gao, Y.; Zhang, Y.-F.; Yao, L.; Fu, D.-L. Lyp-1-fMWNTs enhanced targeted delivery of MBD1 siRNA to pancreatic cancer cells. J. Cell. Mol. Med. 2020, 24, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, S.; Wei, X.; Yang, Z.; Liu, D.; Pu, X.; He, S.; Zhang, Y. Construction of aptamer-sirna chimera/PEI/5-FU/carbon nanotube/collagen membranes for the treatment of peritoneal dissemination of drug-resistant gastric cancer. Adv. Healthc. Mater. 2020, 9, 2001153. [Google Scholar] [CrossRef]
- Hu, Y.; Niemeyer, C.M. Designer DNA–silica/carbon nanotube nanocomposites for traceable and targeted drug delivery. J. Mater. Chem. B 2020, 8, 2250–2255. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Su, Y.; Wang, C.; Zhang, F.; Chen, K.; Wang, Y.; Li, M.; Wang, W. Synergistic suppression of tumor angiogenesis by the co-delivering of vascular endothelial growth factor targeted sirna and candesartan mediated by functionalized carbon nanovectors. ACS Appl. Mater. Interfaces 2017, 9, 23353–23369. [Google Scholar] [CrossRef] [PubMed]
- Andhari, S.S.; Wavhale, R.D.; Dhobale, K.D.; Tawade, B.V.; Chate, G.P.; Patil, Y.N.; Khandare, J.J.; Banerjee, S.S. Self-propelling targeted magneto-nanobots for deep tumor penetration and pH-responsive intracellular drug delivery. Sci. Rep. 2020, 10, 4703. [Google Scholar] [CrossRef] [Green Version]
- Khodadadei, F.; Safarian, S.; Ghanbari, N. Methotrexate-loaded nitrogen-doped graphene quantum dots nanocarriers as an efficient anticancer drug delivery system. Mater. Sci. Eng. C 2017, 79, 280–285. [Google Scholar] [CrossRef]
- Li, S.; Zhou, S.; Li, Y.; Li, X.; Zhu, J.; Fan, L.; Yang, S. Exceptionally high payload of the IR780 iodide on folic acid-functionalized graphene quantum dots for targeted photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 22332–22341. [Google Scholar] [CrossRef]
- Shi, S.-W.; Li, Y.-H.; Zhang, Q.-L.; Yang, S.-P.; Liu, J.-G. Targeted and NIR light-controlled delivery of nitric oxide combined with a platinum(IV) prodrug for enhanced anticancer therapy. J. Mater. Chem. B 2019, 7, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, R.S.; He, M.; Xu, Z.; Xu, L.Q.; Kang, Y.; Xue, P. Multifunctional sGQDs-corm@HA nanosheets for bacterial eradication through cascade-activated “nanoknife” effect and photodynamic/CO gas therapy. Biomaterials 2021, 277, 121084. [Google Scholar] [CrossRef]
- Nasrollahi, F.; Koh, Y.R.; Chen, P.; Varshosaz, J.; Khodadadi, A.A.; Lim, S. Targeting graphene quantum dots to epidermal growth factor receptor for delivery of cisplatin and cellular imaging. Mater. Sci. Eng. C 2019, 94, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Long, Z.; Qiu, Q.; Liu, F.; Xu, Y.; Zhang, T.; Guo, R.; Zhong, W.; Huang, S.; Chen, S. Graphene quantum dots-based targeted nanoprobes detecting drug delivery, imaging, and enhanced chemotherapy of nasopharyngeal carcinoma. Bioeng. Transl. Med. 2021, 7, e10270. [Google Scholar] [CrossRef]
- Liu, F.; Ding, N.; Huo, D.; Yang, G.; Wei, K.; Guan, G.; Li, Y.; Yang, J.; Wang, T.; Wang, Y.; et al. Surface-engineered monocyte inhibits atherosclerotic plaque destabilization via graphene quantum dot-mediated microrna delivery. Adv. Healthc. Mater. 2019, 8, 1900386. [Google Scholar] [CrossRef]
- Yin, T.; Liu, J.; Zhao, Z.; Zhao, Y.; Dong, L.; Yang, M.; Zhou, J.; Huo, M. Redox sensitive hyaluronic acid-decorated graphene oxide for photothermally controlled tumor-cytoplasm-selective rapid drug delivery. Adv. Funct. Mater. 2017, 27, 1604620. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, R.; Lu, J.; Zhao, C.; Deng, X.; Wu, Y. Mesoporous silica coated polydopamine functionalized reduced graphene oxide for synergistic targeted chemo-photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, M.; Visani de Luna, L.A.; Fonseca, L.C.; Giorgio, S.; Alves, O.L. Folic-acid-functionalized graphene oxide nanocarrier: Synthetic approaches, characterization, drug delivery study, and antitumor screening. ACS Appl. Nano Mater. 2018, 1, 922–932. [Google Scholar] [CrossRef]
- Huang, C.; Wu, J.; Jiang, W.; Liu, R.; Li, Z.; Luan, Y. Amphiphilic prodrug-decorated graphene oxide as a multi-functional drug delivery system for efficient cancer therapy. Mater. Sci. Eng. C 2018, 89, 15–24. [Google Scholar] [CrossRef]
- Oz, Y.; Barras, A.; Sanyal, R.; Boukherroub, R.; Szunerits, S.; Sanyal, A. Functionalization of reduced graphene oxide via thiol–maleimide “click” chemistry: Facile fabrication of targeted drug delivery vehicles. ACS Appl. Mater. Interfaces 2017, 9, 34194–34203. [Google Scholar] [CrossRef]
- Du, P.; Yan, J.; Long, S.; Xiong, H.; Wen, N.; Cai, S.; Wang, Y.; Peng, D.; Liu, Z.; Liu, Y. Tumor microenvironment and nir laser dual-responsive release of berberine 9-o-pyrazole alkyl derivative loaded in graphene oxide nanosheets for chemo-photothermal synergetic cancer therapy. J. Mater. Chem. B 2020, 8, 4046–4055. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Lin, P.-Y.; Huang, P.-H.; Kuo, C.-Y.; Shalumon, K.T.; Chen, M.-Y.; Chen, J.-P. Magnetic graphene oxide for dual targeted delivery of doxorubicin and photothermal therapy. Nanomaterials 2018, 8, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.-M.; Xu, H.-L.; Liang, J.-X.; Xiang, H.-H.; Liu, R.; Shen, Y.-X. Lactoferrin modified graphene oxide iron oxide nanocomposite for glioma-targeted drug delivery. Mater. Sci. Eng. C 2017, 77, 904–911. [Google Scholar] [CrossRef]
- Xiong, S.; Luo, J.; Wang, Q.; Li, Z.; Li, J.; Liu, Q.; Gao, L.; Fang, S.; Li, Y.; Pan, H.; et al. Targeted graphene oxide for drug delivery as a therapeutic nanoplatform against Parkinson’s disease. Biomater. Sci. 2021, 9, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Hu, K.; Chen, Y.; Yu, M.; Wang, D.; Wang, Q.; Yong, K.-T.; Lu, F.; Liang, Y.; Li, Z. SiRNA delivery with PEGylated graphene oxide nanosheets for combined photothermal and genetherapy for pancreatic cancer. Theranostics 2017, 7, 1133–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Đurašević, S.; Nikolić, G.; Todorović, A.; Drakulić, D.; Pejić, S.; Martinović, V.; Mitić-Ćulafić, D.; Milić, D.; Kop, T.J.; Jasnić, N.; et al. Effects of fullerene C(60) supplementation on gut microbiota and glucose and lipid homeostasis in rats. Food Chem. Toxicol. 2020, 140, 111302. [Google Scholar] [CrossRef] [PubMed]
- Dalal, C.; Saini, D.; Garg, A.K.; Sonkar, S.K. Fluorescent carbon nano-onion as bioimaging probe. ACS Appl. Bio Mater. 2021, 4, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Ballerini, L.; Prato, M. Nanomaterials for stimulating nerve growth. Science 2017, 356, 1010–1011. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yang, W.; Xie, H.; Wang, J.; Zhang, L.; Wang, Z.; Wang, L. Cnt/sericin conductive nerve guidance conduit promotes functional recovery of transected peripheral nerve injury in a rat model. ACS Appl. Mater. Interfaces 2020, 12, 36860–36872. [Google Scholar] [CrossRef]
- Kunisaki, A.; Kodama, A.; Ishikawa, M.; Ueda, T.; Lima, M.D.; Kondo, T.; Adachi, N. Carbon-nanotube yarns induce axonal regeneration in peripheral nerve defect. Sci. Rep. 2021, 11, 19562. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Bosi, S.; Alshatwi, A.; Prato, M. Carbon nanotubes for organ regeneration: An electrifying performance. Nano Today 2016, 11, 398–401. [Google Scholar] [CrossRef]
- Amin, D.R.; Sink, E.; Narayan, S.P.; Abdel-Hafiz, M.; Mestroni, L.; Peña, B. Nanomaterials for cardiac tissue engineering. Molecules 2020, 25, 5889. [Google Scholar] [CrossRef] [PubMed]
- Barrejón, M.; Marchesan, S.; Alegret, N.; Prato, M. Carbon nanotubes for cardiac tissue regeneration: State of the art and perspectives. Carbon 2021, 184, 641–650. [Google Scholar] [CrossRef]
- Chernova, T.; Murphy, F.A.; Galavotti, S.; Sun, X.M.; Powley, I.R.; Grosso, S.; Schinwald, A.; Zacarias-Cabeza, J.; Dudek, K.M.; Dinsdale, D.; et al. Long-fiber carbon nanotubes replicate asbestos-induced mesothelioma with disruption of the tumor suppressor gene CDKN2A (INK4A/ARF). Curr. Biol. 2017, 27, 3302–3314.e3306. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.F.; Lennquist, A. Carbon nanotubes added to the sin list as a nanomaterial of very high concern. Nat. Nanotechnol. 2020, 15, 3–4. [Google Scholar] [CrossRef]
- Iglesias, D.; Bosi, S.; Melchionna, M.; Da Ros, T.; Marchesan, S. The glitter of carbon nanostructures in hybrid/composite hydrogels for medicinal use. Curr. Top. Med. Chem. 2016, 16, 1976–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezapour, M.R.; Myung, C.W.; Yun, J.; Ghassami, A.; Li, N.; Yu, S.U.; Hajibabaei, A.; Park, Y.; Kim, K.S. Graphene and graphene analogs toward optical, electronic, spintronic, green-chemical, energy-material, sensing, and medical applications. ACS Appl. Mater. Interfaces 2017, 9, 24393–24406. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).