A Nine-Strain Bacterial Consortium Improves Portal Hypertension and Insulin Signaling and Delays NAFLD Progression In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rat NASH Model of Portal Hypertension (PHT)
2.2. STAM™ Mouse Model of NASH
2.3. Blood Biochemistry
2.4. Histological Analyses
2.5. Liver Hemodynamics
2.6. Western Blot
2.7. Real-Time PCR
2.8. Biological Parameter Analyses
3. Results
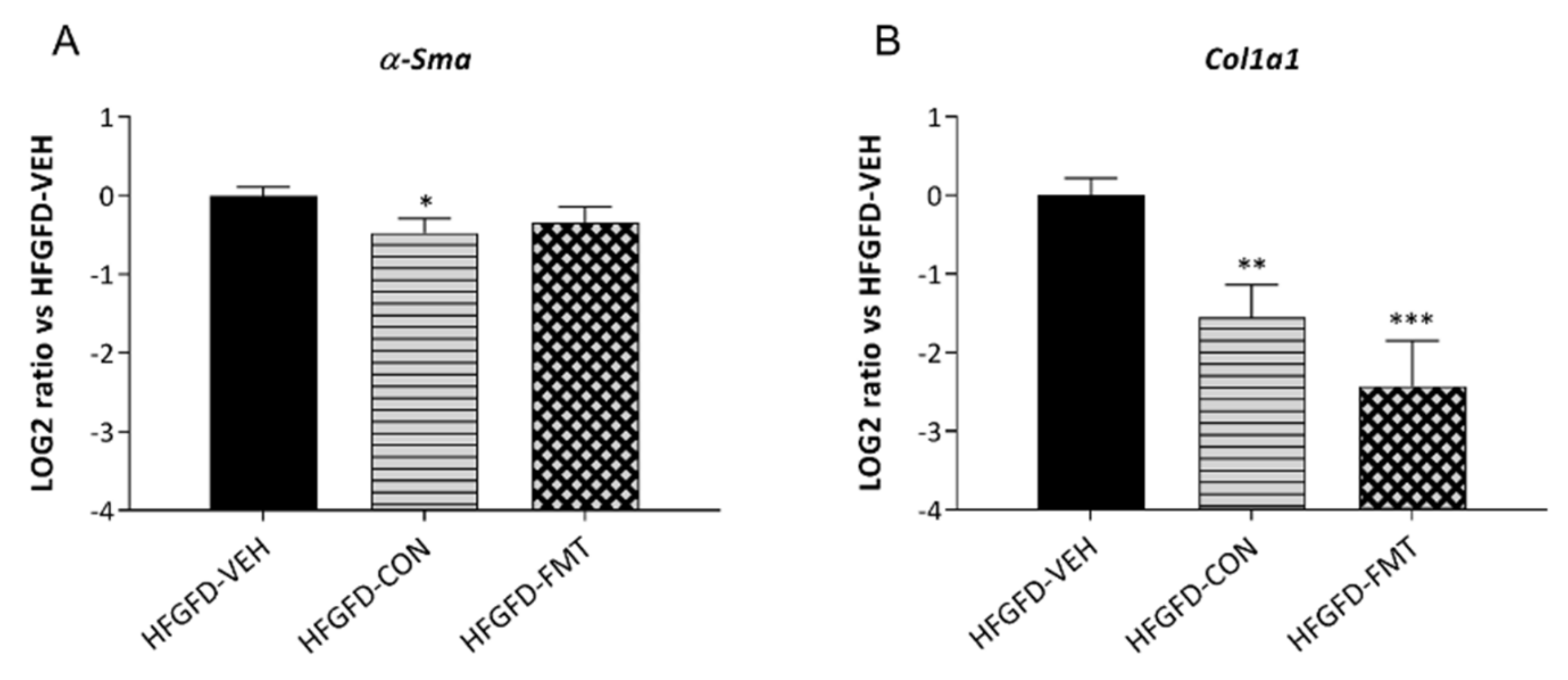
3.1. The Nine-Strain Bacterial Consortium Improves Portal Hypertension (PHT), Endothelial Dysfunction (ED), and Fibrotic Markers in the Rat NASH Model of PHT
3.1.1. Microbiota-Based Treatments Significantly Improved Body Weight
3.1.2. The Metabolic Profile Improved in the Treatment Groups
3.1.3. No Treatment Group Significantly Reversed the NASH Histological Pattern
3.1.4. Microbiota-Based Treatments Significantly Reduced Portal Pressure and Improved Liver Hemodynamics
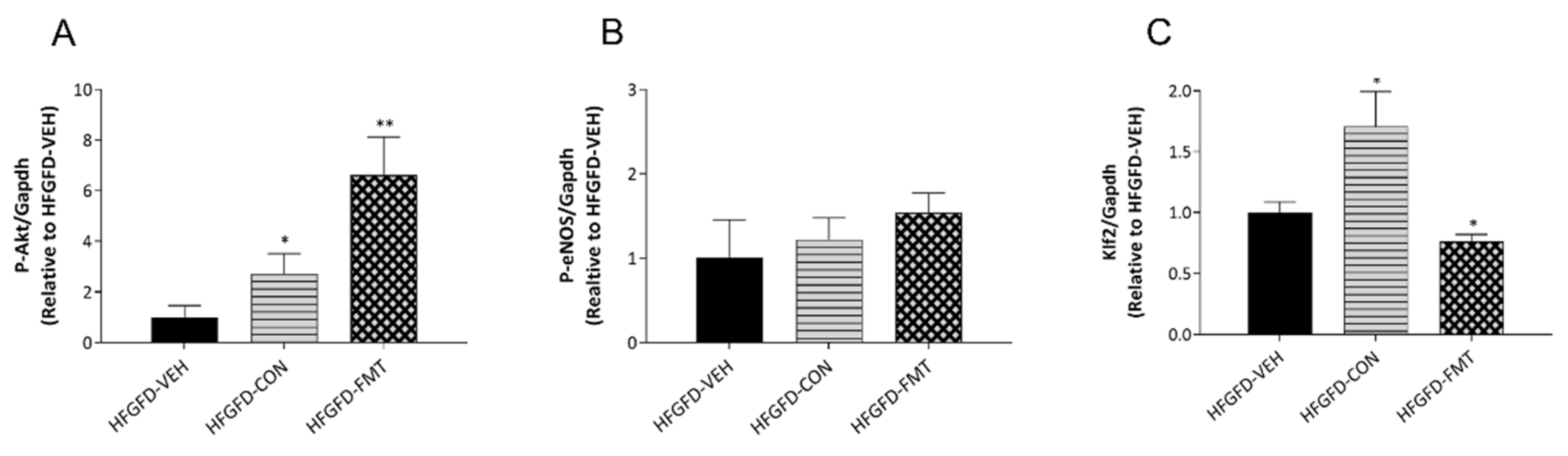
3.1.5. Features of ED Were Improved in Animals after Receiving Microbiota-Based Treatments
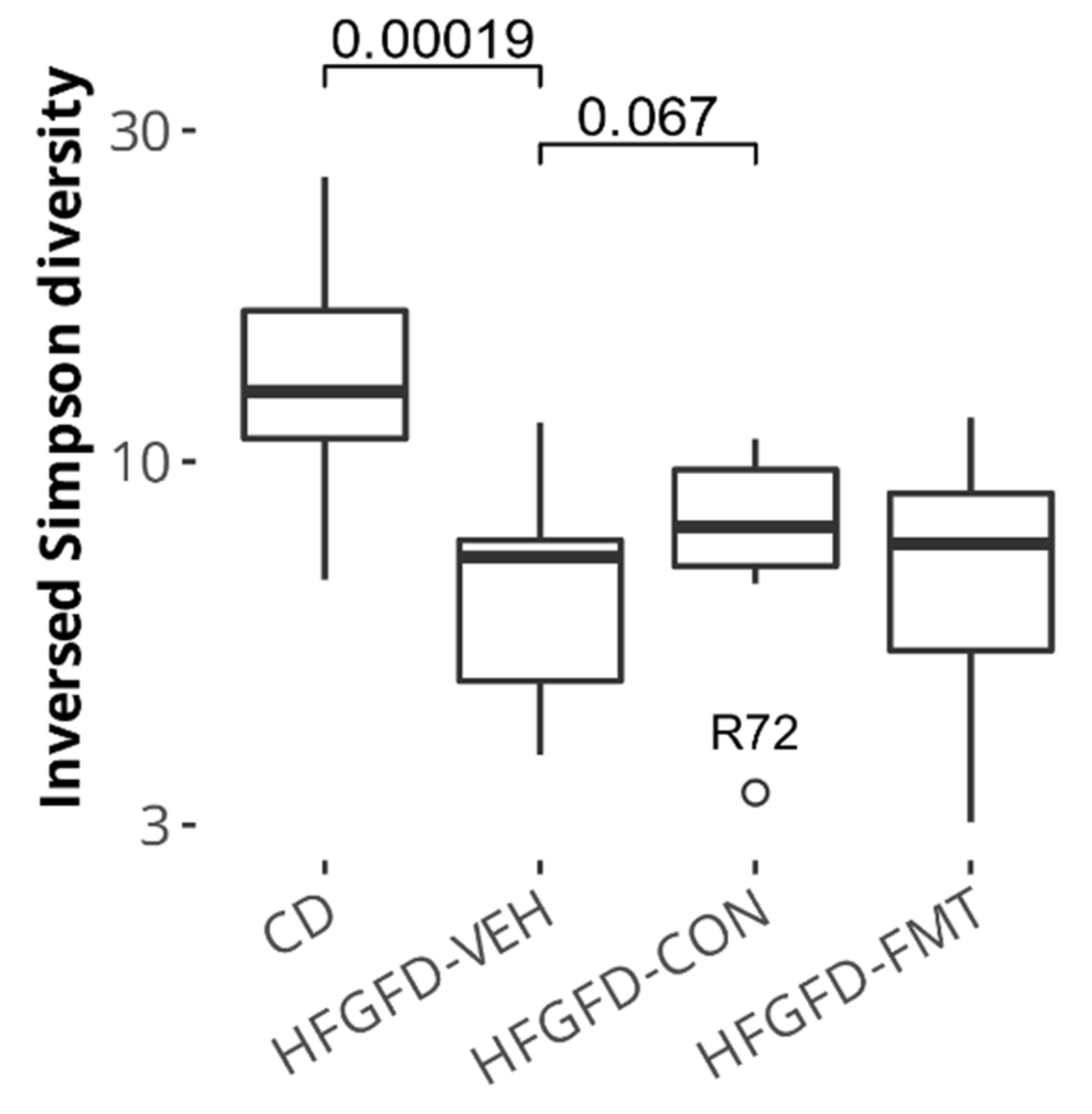
3.1.6. Microbiota-Based Treatments Improved Cecal Species Diversity and Induced Composition Shifts
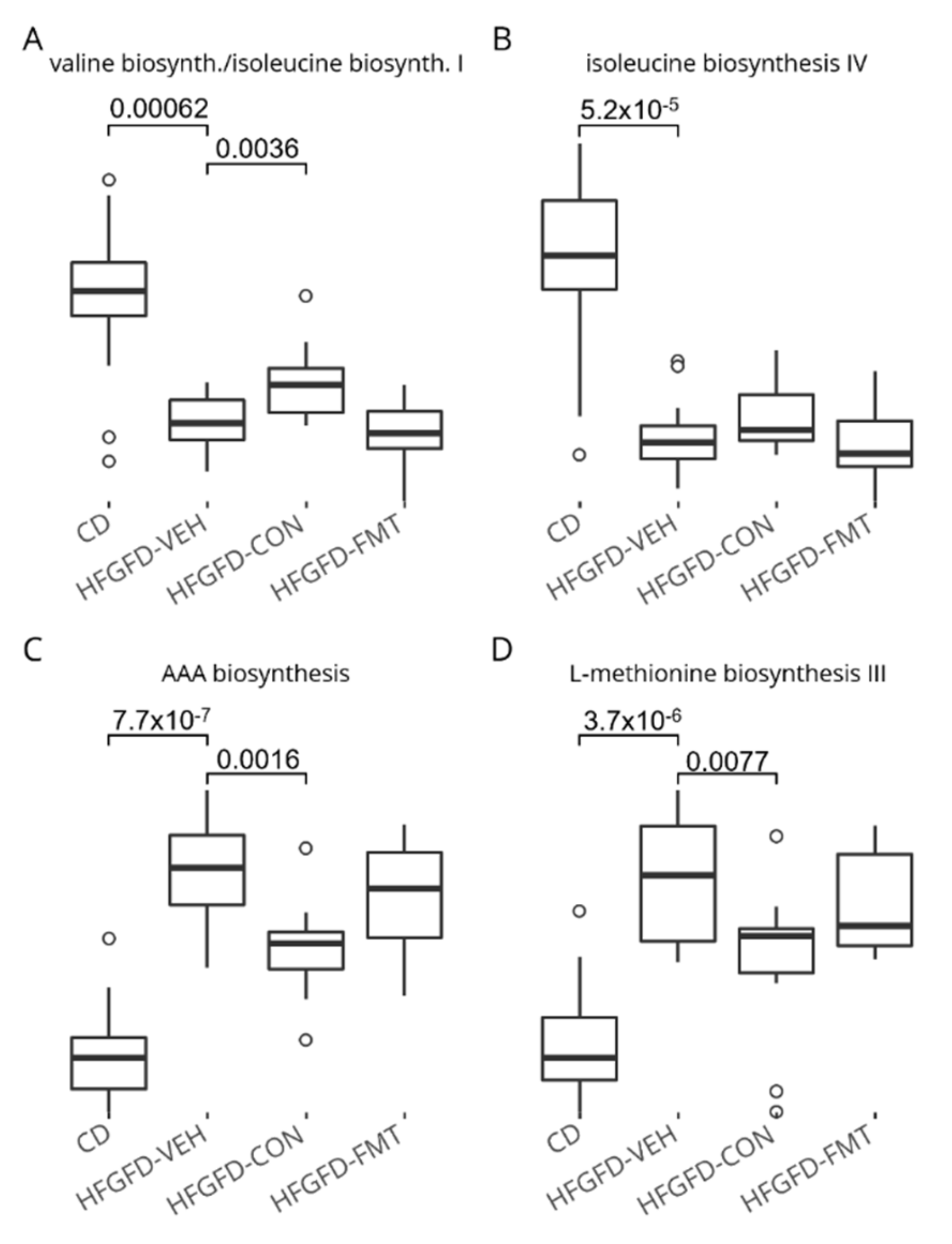
3.1.7. The Nine-Strain Bacterial Consortium Induced Functional Microbiome Shifts
3.1.8. Microbiota-Based Treatments Induced Differential Hepatic Gene Expression
3.2. The Nine-Strain Bacterial Consortium Delays Disease Progression and Improves NAFLD Disease Markers in the STAM™ Mouse Model
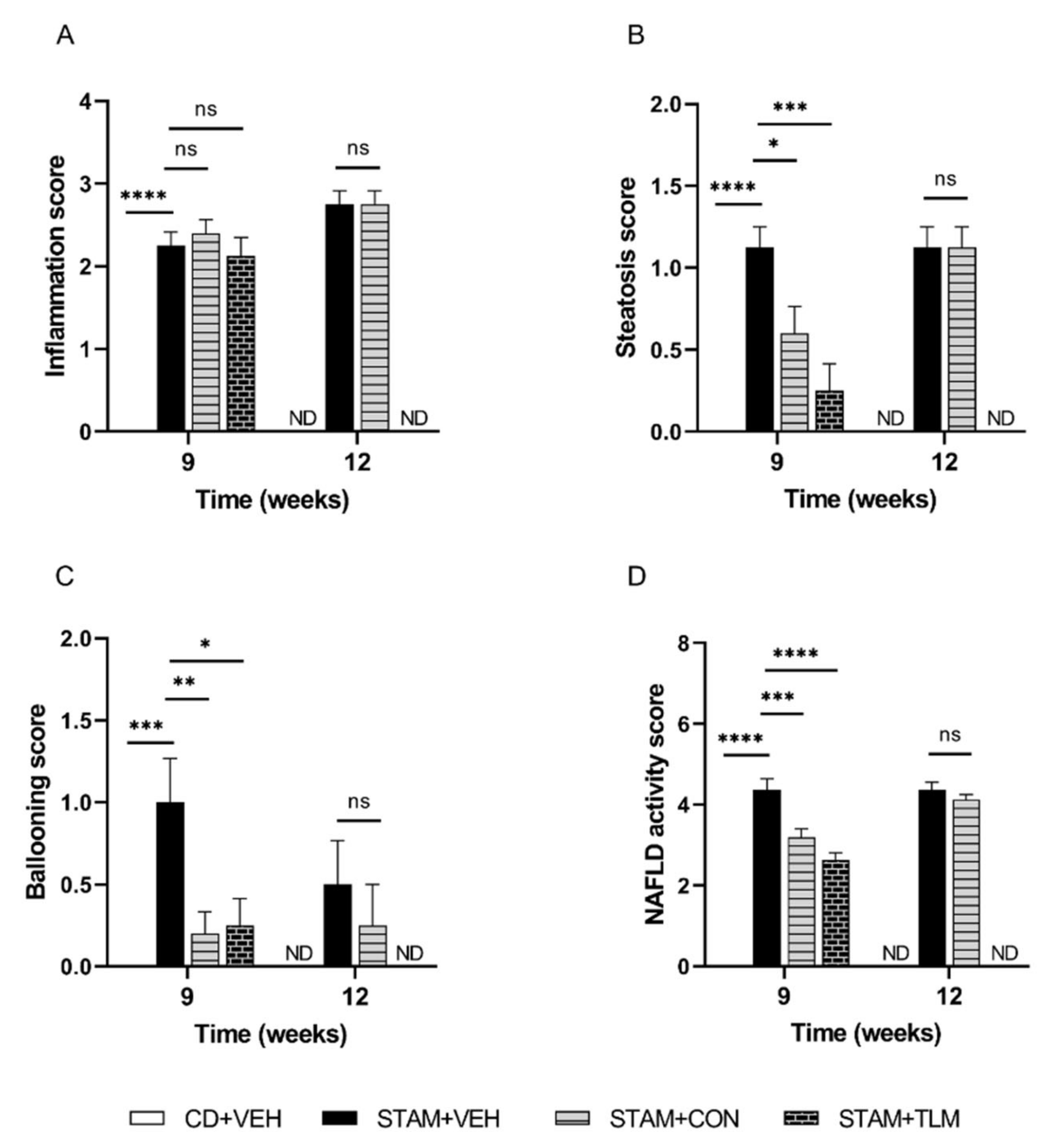
3.2.1. The Consortium of Nine Gut Commensals Improved NAS at Histopathology at 9 Weeks of Age
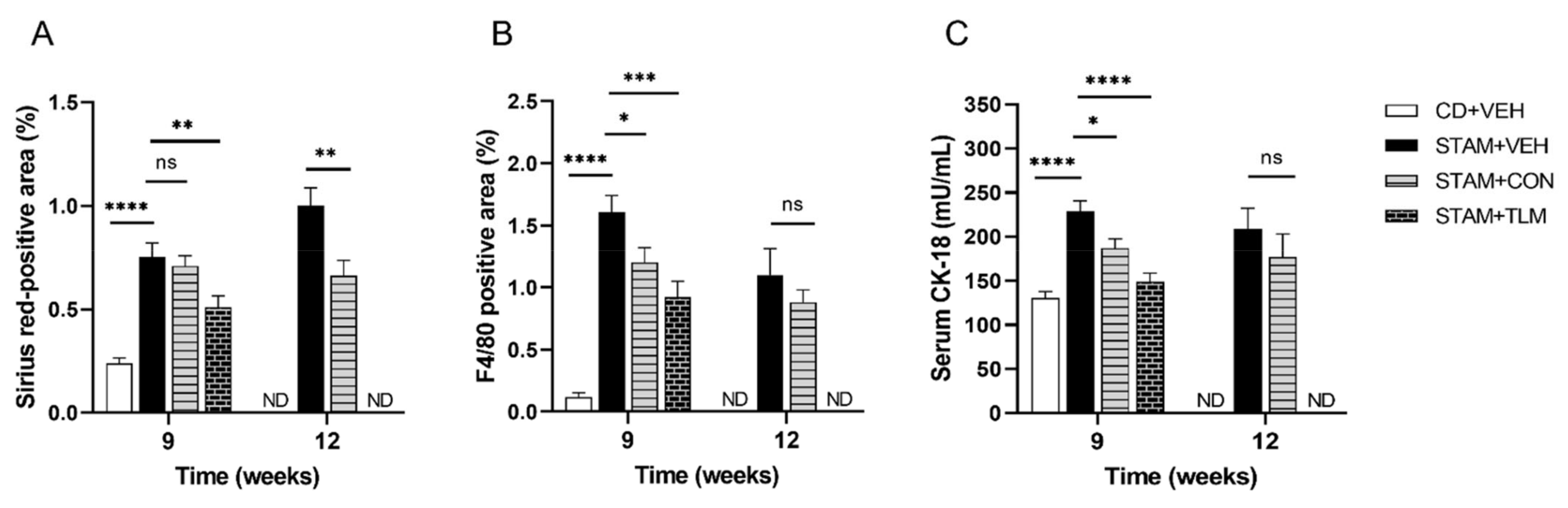
3.2.2. The Consortium of Nine Gut Commensals Improved Fibrosis, and Showed Reduced Hepatic Expression of F4/80 and Serum CK-18 Levels
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, Y.; Fan, J.G. Obesity, Fatty Liver and Liver Cancer. Hepatobiliary Pancreat. Dis. Int. 2005, 4, 173–177. [Google Scholar] [PubMed]
- Abdul-Hai, A.; Abdallah, A.; Malnick, S.D.H. Influence of Gut Bacteria on Development and Progression of Non-Alcoholic Fatty Liver Disease. World J. Hepatol. 2015, 7, 1679–1684. [Google Scholar] [CrossRef]
- Arslan, N. Obesity, Fatty Liver Disease and Intestinal Microbiota. World J. Gastroenterol. 2014, 20, 16452–16463. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The Role of the Gut Microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wu, W.K.; Wu, M.S. Microbiota-Associated Therapy for Non-Alcoholic Steatohepatitis-Induced Liver Cancer: A Review. Int. J. Mol. Sci. 2020, 21, 5999. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.B.; Gribble, F.M.; Reimann, F. Nutrient-Induced Cellular Mechanisms of Gut Hormone Secretion. Nutrients 2021, 13, 883. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.M.; Vieira, A.T.; Vinolo, M.A.R.; Oliveira, F.A.; Curi, R.; dos Santos Martins, F. The Central Role of the Gut Microbiota in Chronic Inflammatory Diseases. J. Immunol. Res. 2014, 2014, 689492. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.W.-S.; Tse, C.-H.; Lam, T.T.-Y.; Wong, G.L.-H.; Chim, A.M.-L.; Chu, W.C.-W.; Yeung, D.K.-W.; Law, P.T.-W.; Kwan, H.-S.; Yu, J.; et al. Molecular Characterization of the Fecal Microbiota in Patients with Nonalcoholic Steatohepatitis—A Longitudinal Study. PLoS ONE 2013, 8, e62885. [Google Scholar] [CrossRef] [Green Version]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Lin, A.; Kong, M.; Yao, X.; Yin, M.; Xia, H.; Ma, J.; Liu, H. Intestinal Microbiome and NAFLD: Molecular Insights and Therapeutic Perspectives. J. Gastroenterol. 2020, 55, 142–158. [Google Scholar] [CrossRef] [Green Version]
- García-Lezana, T.; Raurell, I.; Bravo, M.; Torres-Arauz, M.; Salcedo, M.T.; Santiago, A.; Schoenenberger, A.; Manichanh, C.; Genescà, J.; Martell, M.; et al. Restoration of a Healthy Intestinal Microbiota Normalizes Portal Hypertension in a Rat Model of Nonalcoholic Steatohepatitis. Hepatology 2018, 67, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Jojima, T.; Tomotsune, T.; Iijima, T.; Akimoto, K.; Suzuki, K.; Aso, Y. Empagliflozin (an SGLT2 Inhibitor), Alone or in Combination with Linagliptin (a DPP-4 Inhibitor), Prevents Steatohepatitis in a Novel Mouse Model of Non-Alcoholic Steatohepatitis and Diabetes. Diabetol. Metab. Syndr. 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, M.; Shibazaki, Y.; Wakamatsu, K.; Honda, Y.; Kawauchi, Y.; Suzuki, K.; Arumugam, S.; Watanabe, K.; Ichida, T.; Asakura, H.; et al. A Murine Model for Non-Alcoholic Steatohepatitis Showing Evidence of Association between Diabetes and Hepatocellular Carcinoma. Med. Mol. Morphol. 2013, 46, 141–152. [Google Scholar] [CrossRef]
- Park, J.G.; Mok, J.S.; Han, Y.I.; Park, T.S.; Kang, K.W.; Choi, C.S.; Park, H.D.; Park, J. Connectivity Mapping of Angiotensin-PPAR Interactions Involved in the Amelioration of Non-Alcoholic Steatohepatitis by Telmisartan. Sci. Rep. 2019, 9, 4003. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Bravo, M.; Raurell, I.; Hide, D.; Fernández-Iglesias, A.; Gil, M.; Barberá, A.; Salcedo, M.T.; Augustin, S.; Genescà, J.; Martell, M. Restoration of Liver Sinusoidal Cell Phenotypes by Statins Improves Portal Hypertension and Histology in Rats with NASH. Sci. Rep. 2019, 9, 20183. [Google Scholar] [CrossRef] [PubMed]
- Marrone, G.; Russo, L.; Rosado, E.; Hide, D.; García-Cardeña, G.; García-Pagán, J.C.; Bosch, J.; Gracia-Sancho, J. The Transcription Factor KLF2 Mediates Hepatic Endothelial Protection and Paracrine Endothelial-Stellate Cell Deactivation Induced by Statins. J. Hepatol. 2013, 58, 98–103. [Google Scholar] [CrossRef]
- Marrone, G.; Maeso-Díaz, R.; García-Cardena, G.; Abraldes, J.G.; García-Pagán, J.C.; Bosch, J.; Gracia-Sancho, J. KLF2 Exerts Antifibrotic and Vasoprotective Effects in Cirrhotic Rat Livers: Behind the Molecular Mechanisms of Statins. Gut 2015, 64, 1434–1443. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Reibe, S.; Shalapour, S.; Ooi, G.J.; Watt, M.J.; Karin, M. Preclinical Models for Studying NASH-Driven HCC: How Useful Are They? Cell Metab. 2019, 29, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, M.; Tanaka, N.; Kawakubo, M.; Sheena, Y.; Horiuchi, A.; Komatsu, M.; Nagaya, T.; Joshita, S.; Umemura, T.; Ichijo, T.; et al. Serum Fragmented Cytokeratin 18 Levels Reflect the Histologic Activity Score of Nonalcoholic Fatty Liver Disease More Accurately than Serum Alanine Aminotransferase Levels. J. Clin. Gastroenterol. 2010, 44, 440–447. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased Intestinal Permeability and Tight Junction Alterations in Nonalcoholic Fatty Liver Disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Hou, H.; Zhang, W.; Liu, T.; Li, Y.; Wang, S.; Wang, B.; Cao, H. Microbial Metabolites: Critical Regulators in NAFLD. Front. Microbiol. 2020, 11, 567654. [Google Scholar] [CrossRef] [PubMed]
- Meroni, M.; Longo, M.; Dongiovanni, P. The Role of Probiotics in Nonalcoholic Fatty Liver Disease: A New Insight into Therapeutic Strategies. Nutrients 2019, 11, 2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimzadeh Leylabadlo, H.; Ghotaslou, R.; Samadi Kafil, H.; Feizabadi, M.M.; Moaddab, S.Y.; Farajnia, S.; Sheykhsaran, E.; Sanaie, S.; Shanehbandi, D.; Bannazadeh Baghi, H. Non-Alcoholic Fatty Liver Diseases: From Role of Gut Microbiota to Microbial-Based Therapies. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 613–627. [Google Scholar] [CrossRef]
- Philips, C.A.; Pande, A.; Shasthry, S.M.; Jamwal, K.D.; Khillan, V.; Chandel, S.S.; Kumar, G.; Sharma, M.K.; Maiwall, R.; Jindal, A.; et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin. Gastroenterol. Hepatol. 2017, 15, 600–602. [Google Scholar] [CrossRef]
- Li, Z.; Yang, S.; Lin, H.; Huang, J.; Watkins, P.A.; Moser, A.B.; Desimone, C.; Song, X.; Diehl, A.M. Probiotics and Antibodies to TNF Inhibit Inflammatory Activity and Improve Nonalcoholic Fatty Liver Disease. Hepatology 2003, 37, 343–350. [Google Scholar] [CrossRef]
- Spooner, H.C.; Derrick, S.A.; Maj, M.; Manjarín, R.; Hernandez, G.V.; Tailor, D.S.; Bastani, P.S.; Fanter, R.K.; Fiorotto, M.L.; Burrin, D.G.; et al. High-Fructose, High-Fat Diet Alters Muscle Composition and Fuel Utilization in a Juvenile Iberian Pig Model of Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 4195. [Google Scholar] [CrossRef]
- Xue, L.; He, J.; Gao, N.; Lu, X.; Li, M.; Wu, X.; Liu, Z.; Jin, Y.; Liu, J.; Xu, J.; et al. Probiotics May Delay the Progression of Nonalcoholic Fatty Liver Disease by Restoring the Gut Microbiota Structure and Improving Intestinal Endotoxemia. Sci. Rep. 2017, 7, 45176. [Google Scholar] [CrossRef]
- Campollo, O.; Sprengers, D.; McIntyre, N. The BCAA/AAA Ratio of Plasma Amino Acids in Three Different Groups of Cirrhotics. Rev. Investig. Clin. Organo Hosp. Enferm. Nutr. 1992, 44, 513–518. [Google Scholar]
- Iwao, M.; Gotoh, K.; Arakawa, M.; Endo, M.; Honda, K.; Seike, M.; Murakami, K.; Shibata, H. Supplementation of Branched-Chain Amino Acids Decreases Fat Accumulation in the Liver through Intestinal Microbiota-Mediated Production of Acetic Acid. Sci. Rep. 2020, 10, 18768. [Google Scholar] [CrossRef]
- Raghunath, A.; Panneerselvam, L.; Sundarraj, K.; Perumal, E. Heat Shock Proteins and Endoplasmic Reticulum Stress. In Heat Shock Proteins and Stress; Asea, A.A.A., Kaur, P., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 39–78. ISBN 978-3-319-90725-3. [Google Scholar]
- Zhang, X.Q.; Xu, C.F.; Yu, C.H.; Chen, W.X.; Li, Y.M. Role of Endoplasmic Reticulum Stress in the Pathogenesis of Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2014, 20, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Seelig, S.; Liaw, C.; Towle, H.C.; Oppenheimer, J.H. Thyroid Hormone Attenuates and Augments Hepatic Gene Expression at a Pretranslational Level. Proc. Natl. Acad. Sci. USA 1981, 78, 4733–4737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, B.A.; Moncur, J.T.; Huntington, J.T.; Kinlaw, W.B. “Spot 14” Protein: A Metabolic Integrator in Normal and Neoplastic Cells. Thyroid 1998, 8, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B.; Bell, A.; Lepar, G.; Hu, D. Insulin Rapidly Induces Rat Liver S14 Gene Transcription. Mol. Endocrinol. 1990, 4, 1655–1660. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.T.; Tseng, P.H.; Tseng, F.Y.; Chi, Y.C.; Han, D.S.; Yang, W.S. The Serum Level of a Novel Lipogenic Protein Spot 14 Was Reduced in Metabolic Syndrome. PLoS ONE 2019, 14, e0212341. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.J.; Vazquez-Martin, A.; Moreno-Navarrete, J.-M.; Bassols, J.; Rodriguez-Hermosa, J.; Gironés, J.; Ricart, W.; Peral, B.; Tinahones, F.J.; Fruhbeck, G.; et al. Thyroid Hormone Responsive Spot 14 Increases during Differentiation of Human Adipocytes and Its Expression Is Down-Regulated in Obese Subjects. Int. J. Obes. 2010, 34, 487–499. [Google Scholar] [CrossRef] [Green Version]
- Cannito, S.; Novo, E.; Parola, M. Therapeutic Pro-Fibrogenic Signaling Pathways in Fibroblasts. Adv. Drug Deliv. Rev. 2017, 121, 57–84. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Liu, C.; Chen, Y.W.; Fan, J.G. Sodium Butyrate Attenuates High-Fat Diet-Induced Steatohepatitis in Mice by Improving Gut Microbiota and Gastrointestinal Barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [Green Version]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing a Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoendel, M.; Jeraldo, P.; Greenwood-Quaintance, K.E.; Yao, J.; Chia, N.; Hanssen, A.D.; Abdel, M.P.; Patel, R. Comparison of Three Commercial Tools for Metagenomic Shotgun Sequencing Analysis. J. Clin. Microbiol. 2020, 58, e00981-19. [Google Scholar] [CrossRef]
- Franzosa, E.A.; McIver, L.J.; Rahnavard, G.; Thompson, L.R.; Schirmer, M.; Weingart, G.; Lipson, K.S.; Knight, R.; Caporaso, J.G.; Segata, N.; et al. Species-Level Functional Profiling of Metagenomes and Metatranscriptomes. Nat. Methods 2018, 15, 962–968. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. MixOmics: An R Package for ’omics Feature Selection and Multiple Data Integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, S.; Huber, W. Differential Expression Analysis for Sequence Count Data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Martens, M.; Ammar, A.; Riutta, A.; Waagmeester, A.; Slenter, D.N.; Hanspers, K.; A Miller, R.; Digles, D.; Lopes, E.N.; Ehrhart, F.; et al. WikiPathways: Connecting Communities. Nucleic Acids Res. 2021, 49, D613–D621. [Google Scholar] [CrossRef]
- Karp, P.D.; Riley, M.; Paley, S.M.; Pellegrini-Toole, A. The MetaCyc Database. Nucleic Acids Res. 2002, 30, 59–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, D. A View from the Web BioCarta. Biotech. Softw. Internet Rep. 2001, 2, 117–120. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The Harmonizome: A Collection of Processed Datasets Gathered to Serve and Mine Knowledge about Genes and Proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef] [PubMed]
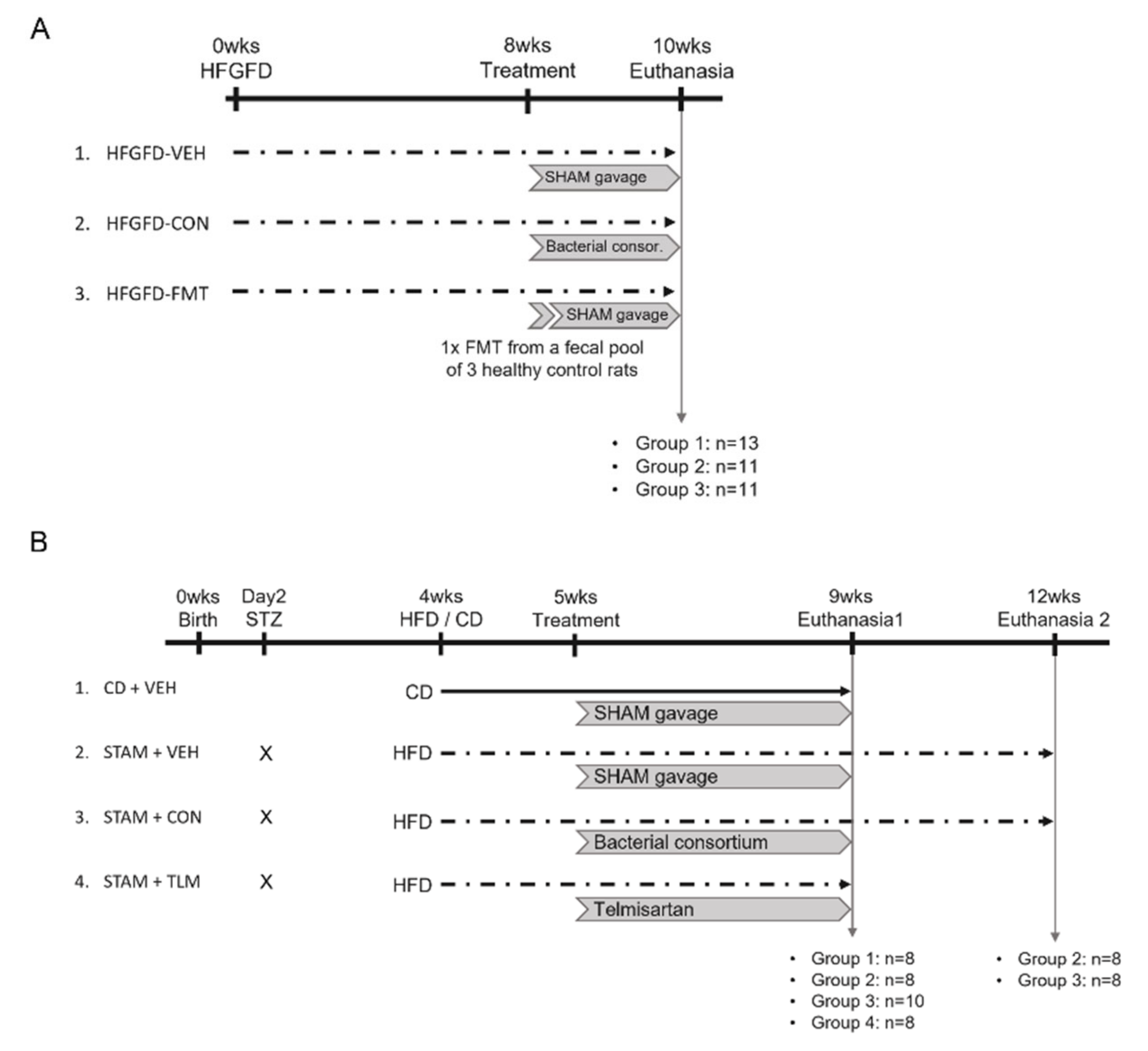
| HFGFD-VEH n = 13 | HFGFD-CON n = 11 | HFGFD-FMT n = 11 | |
|---|---|---|---|
| Body weight pre-HFGFD (g) | 254.46 ± 2.59 | 256 ± 2.1 | 256 ± 3.32 |
| Body weight pre-trt. (g) | 531.7 ± 10.3 | 543.6 ± 10.29 | 515.1 ± 13.48 |
| Body weight gain with trt. (g) | 22.08 ± 3.18 | 2.91 ± 3.55 * | −5 ± 3.47 * |
| Body weight post-trt. (g) | 552.33 ± 11.77 | 546.6 ± 11.53 | 507 ± 12.39 * |
| Glucose (mg/dL) | 182.45 ± 14.94 | 166.9 ± 14.89 | 171.36 ± 10.47 |
| Insulin (ng/mL) | 15.36 ± 3.6 | 9.24 ± 1.17 | 11.21 ± 1.13 |
| HOMA-IR | 7.89 ± 2.3 | 3.7 ± 0.54 | 4.68 ± 0.45 |
| Albumin (g/dL) | 2.9 ± 0.04 | 3.03 ± 0.05 | 3.13 ± 0.06* |
| Bilirubin (mg/dL) | 0.09 ± 0.01 | 0.09 ± 0.02 | 0.11 ± 0.01 |
| AST (IU/L) | 205.56 ± 34.05 | 234.29 ± 45.72 | 172 ± 63.52 |
| ALT (IU/L) | 66.15 ± 4.36 | 67.1 ± 9.29 | 47.5 ± 31.31 * |
| TG (mg/dL) | 27.92 ± 1.35 | 33 ± 1.91 * | 33.54 ± 2.96 * |
| Total cholesterol (mg/dL) | 75.54 ± 4.75 | 86.78 ± 3.89 | 78.91 ± 2.99 |
| Cholesterol HDL (mg/dL) | 41.93 ± 2.43 | 46.78 ± 1.97 | 45.54 ± 2.48 |
| Cholesterol LDL (mg/dL) | 25.27 ± 2.52 | 34.75 ± 3.4 | 27 ± 1.96 |
| HFGFD-VEH n = 13 | HFGFD-CON n = 11 | HFGFD-FMT n = 11 | |
|---|---|---|---|
| MAP (mmHg) | 113.05 ± 4.53 | 119.95 ± 4.21 | 117.34 ± 5.14 |
| PP (mmHg) | 10.32 ± 0.22 | 9.58 ± 0.19 * | 9.21 ± 0.12 * |
| SMABF (mL/[min × 100 g]) | 2.73 ± 0.24 | 2.71 ± 0.21 | 2.82 ± 0.17 |
| SMAR (mmHg/mL × min × 100 g) | 40.78 ± 3.25 | 40.79 ± 4.36 | 39.35 ± 2.36 |
| IHVR (mmHg/mL × min × 100 g) | 7.58 ± 1.27 | 4.20 ± 0.35 * | 3.71 ± 0.21 * |
| Rat Gene | Human Ortholog | CON/VEH Log2FC | CON/VEH pFDR | FMT/VEH Log2FC | FMT/VEH pFDR | Protein |
|---|---|---|---|---|---|---|
| Hspa1b | HSPA1B, HSPA1A | −3.48 | 0.002 | −4.36 | 0.000 | Heat shock 70 kDa protein 1B, and 1A |
| AABR07048992.1 | HSPA8 | −1.49 | 0.000 | −1.50 | 0.000 | Heat shock cognate 71 kDa protein |
| Hsph1 | HSPH1 | −0.74 | 0.060 | −0.93 | 0.005 | Heat shock protein 105 kDa |
| Pir | PIR | −0.74 | 0.084 | - | - | Pirin |
| Sdf2l1 | SDF2L1 | −0.71 | 0.066 | - | - | Stromal cell-derived factor 2-like protein 1 |
| Dnajb9 | DNAJB9 | −0.71 | 0.016 | −0.69 | 0.012 | DNAJ homolog subfamily B member 9 |
| LOC680121 | HSPA8 | −0.64 | 0.003 | −0.48 | 0.024 | Heat shock cognate 71 kDa protein |
| AABR07012795.1 | PRDX1 | −0.62 | 0.067 | - | - | Peroxiredoxin−1 |
| Hspa5 | HSPA5 | −0.61 | 0.058 | −0.62 | 0.031 | Endoplasmic reticulum chaperone BiP |
| Sult2a1 | SULT2A1 | −0.55 | 0.073 | - | - | Sulfotransferase 2A1 |
| Dusp6 | DUSP6 | −0.51 | 0.084 | - | - | Dual specificity protein phosphatase 6 |
| Calr | CALR | −0.40 | 0.030 | −0.38 | 0.022 | Calreticulin |
| Pnrc1 | PNRC1 | −0.40 | 0.081 | - | - | Proline-rich nuclear receptor coactivator 1 |
| Cdc42 | CDC42 | −0.36 | 0.000 | −0.21 | 0.013 | Cell division control protein 42 homolog |
| NEWGENE_620180 | SLC40A1 | −0.35 | 0.021 | −0.43 | 0.002 | Solute carrier family 40 member 1 |
| Prdx1 | PRDX1 | −0.35 | 0.001 | - | - | Peroxiredoxin-1 |
| Litaf | LITAF | −0.34 | 0.000 | - | - | Lipopolysaccharide-induced tumor necrosis factor-alpha factor |
| Atp2a2 | ATP2A2 | −0.32 | 0.065 | −0.40 | 0.007 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 |
| Slc10a1 | SLC10A1 | −0.30 | 0.081 | −0.28 | 0.074 | Sodium/bile acid cotransporter |
| Pdia3 | PDIA3 | −0.29 | 0.084 | −0.29 | 0.051 | Protein disulfide isomerase A3 |
| Idh1 | IDH1 | −0.28 | 0.050 | −0.32 | 0.008 | Isocitrate dehydrogenase |
| Slc39a8 | SLC39A8 | −0.27 | 0.017 | −0.19 | 0.093 | Metal cation symporter ZIP8 |
| Apoe | APOE | −0.26 | 0.067 | −0.23 | 0.067 | Apolipoprotein E |
| Maoa | MAOA | −0.25 | 0.049 | −0.24 | 0.042 | Amine oxidase |
| Ctsb | CTSB | −0.25 | 0.048 | −0.34 | 0.001 | Cathepsin B |
| Psmb4 | PSMB4 | −0.24 | 0.040 | - | - | Proteasome subunit beta type-4 |
| Ccnd3 | CCND3 | −0.24 | 0.051 | - | - | G1/S-specific cyclin-D3 |
| Dpp4 | DPP4 | −0.23 | 0.050 | −0.35 | 0.000 | Dipeptidyl peptidase 4 |
| Aldoa | ALDOA | −0.23 | 0.067 | - | - | Fructose bisphosphate aldolase A |
| Ctsd | CTSD | −0.22 | 0.069 | −0.29 | 0.005 | Cathepsin D |
| Rac1 | RAC1 | −0.21 | 0.000 | −0.24 | 0.000 | Ras-related C3 botulinum toxin substrate 1 |
| Enpp1 | ENPP1 | −0.20 | 0.057 | −0.26 | 0.004 | Ectonucleotide pyrophosphatase/phosphodiesterase 1 |
| Calm1 | CALM1 | −0.19 | 0.064 | - | - | Calmodulin−1 |
| Sdc2 | SDC2 | −0.19 | 0.064 | −0.33 | 0.000 | Syndecan-2 |
| Akt1 | AKT1 | 0.20 | 0.056 | - | - | RAC-alpha serine/threonine-protein kinase |
| Abcg3l3 | ABCG2 | 0.36 | 0.078 | - | - | Broad substrate specificity ATP-binding cassette transporter ABCG2 |
| Atm | ATM | 0.39 | 0.006 | - | - | Serine-protein kinase ATM |
| Abcg3l1 | ABCG2 | 0.42 | 0.019 | - | - | Broad substrate specificity ATP-binding cassette transporter ABCG2 |
| Inhba | INHBA | 0.48 | 0.099 | - | - | Inhibin beta A chain |
| Abcc5 | ABCC5 | 0.49 | 0.097 | - | - | ATP-binding cassette sub-family C member 5 |
| VEGFA | VEGFA | 0.57 | 0.003 | 0.50 | 0.007 | Vascular endothelial growth factor A |
| Egf | EGF | 0.64 | 0.030 | 0.56 | 0.042 | Pro-epidermal growth factor |
| LOC108348190 | EGF | 0.72 | 0.046 | - | - | Pro-epidermal growth factor |
| Thrsp | THRSP | 1.07 | 0.049 | 0.93 | 0.067 | Thyroid hormone-inducible hepatic protein |
| Pathway ID | N | q-Value | Human Orthologs | Description |
|---|---|---|---|---|
| WP3888↓ | 21 | 0.090 | RPS11, TPP1, ALDOA, PPP1CA, CTNND1, SDCBP, DNAJA1, CDC42, SSR4, DNAJB9, RAP1B, RHOA, PFN1, YWHAE, PSMD11, PAK2, SDF2L1, CALR, ATP6V0D1, HSPA1A, RAC1 | VEGFA-VEGFR2 Signaling pathway |
| WP4656↑ | 8 | 0.067 | CSPP1, CEP104, DVL1, OFD1, ATM, BBS4, PCM1, CEP120 | Joubert Syndrome |
| WP428↓ | 7 | 0.091 | RHOA, RAC1, WNT11, CCND3, PPP3R1, GPC4, NLK | Wnt Signaling |
| P00016↓ | 6 | 0.031 | ARPC4, CDC42, ARPC5, PFN1, PAK2, RAC1 | Cytoskeletal regulation by Rho GTPase |
| pid_21478↓ | 5 | 0.007 | ARPC4, CDC42, PIR, ACTR3, RAC1 | y branching of actin filaments |
| pid_5967↓ | 5 | 0.007 | ARPC4, CDC42, RHOA, ACTR3, RAC1 | Role of pi3k subunit p85 in regulation of actin organization and cell migration |
| R-HSA-390450↓ | 3 | 0.030 | TCP1, CCT4, CCT8 | Folding of actin by CCT/TriC |
| R-HSA-5625970↓ | 3 | 0.030 | CDC42, RHOA, RAC1 | RHO GTPases activate KTN1 |
| R-HSA-389960↓ | 3 | 0.053 | TCP1, CCT4, CCT8 | Formation of tubulin folding intermediates by CCT/TriC |
| CD + VEH n = 8 9 Weeks | STAM + VEH n = 8 9 Weeks | STAM + CON n = 10 9 Weeks | STAM + TLM n = 8 9 Weeks | STAM + VEH n = 8 12 Weeks | STAM + CON n = 8 12 Weeks | |
|---|---|---|---|---|---|---|
| ALT (IU/L) | 24.00 *** ± 1.27 | 39.38 ± 2.41 | 43.5 ± 3.28 | 35.00 ± 2.39 | 65.13 ± 14.04 | 60.63 ±12.81 |
| TG (mg/dL) | 79.63 * ± 10.46 | 500.9 ± 152.5 | 679.9 ± 91.35 | 465.6 ± 107.6 | 926.4 ± 251.6 | 1017 ± 248.8 |
| Total cholesterol (mg/dL) | 76.92 **** ± 3.51 | 125.7 ± 8.11 | 124.8 ± 4.32 | 132.7 ± 2.71 | 187.3 ± 41.99 | 186.9 ± 38.81 |
| Cholesterol HDL (mg/dL) | 57.84 ** ± 2.75 | 83.69 ± 7.13 | 75.55 ± 3.31 | 97.21 ± 4.73 | 72.24 ± 7.46 | 70.21 ± 6.84 |
| Cholesterol LDL (mg/dL) | 13.1 ± 0.66 | 14.68 ± 1.20 | 13.64 ± 0.74 | 18.78 * ± 1.10 | 24.35 ± 6.12 | 23.76 ± 5.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, I.; Barberá, A.; Raurell, I.; Estrella, F.; de Leeuw, M.; Bolca, S.; Gottardi, D.; Horscroft, N.; Possemiers, S.; Salcedo, M.T.; et al. A Nine-Strain Bacterial Consortium Improves Portal Hypertension and Insulin Signaling and Delays NAFLD Progression In Vivo. Biomedicines 2022, 10, 1191. https://doi.org/10.3390/biomedicines10051191
Pinheiro I, Barberá A, Raurell I, Estrella F, de Leeuw M, Bolca S, Gottardi D, Horscroft N, Possemiers S, Salcedo MT, et al. A Nine-Strain Bacterial Consortium Improves Portal Hypertension and Insulin Signaling and Delays NAFLD Progression In Vivo. Biomedicines. 2022; 10(5):1191. https://doi.org/10.3390/biomedicines10051191
Chicago/Turabian StylePinheiro, Iris, Aurora Barberá, Imma Raurell, Federico Estrella, Marcel de Leeuw, Selin Bolca, Davide Gottardi, Nigel Horscroft, Sam Possemiers, María Teresa Salcedo, and et al. 2022. "A Nine-Strain Bacterial Consortium Improves Portal Hypertension and Insulin Signaling and Delays NAFLD Progression In Vivo" Biomedicines 10, no. 5: 1191. https://doi.org/10.3390/biomedicines10051191
APA StylePinheiro, I., Barberá, A., Raurell, I., Estrella, F., de Leeuw, M., Bolca, S., Gottardi, D., Horscroft, N., Possemiers, S., Salcedo, M. T., Genescà, J., Martell, M., & Augustin, S. (2022). A Nine-Strain Bacterial Consortium Improves Portal Hypertension and Insulin Signaling and Delays NAFLD Progression In Vivo. Biomedicines, 10(5), 1191. https://doi.org/10.3390/biomedicines10051191








