Combination of Glycinamide and Ascorbic Acid Synergistically Promotes Collagen Production and Wound Healing in Human Dermal Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
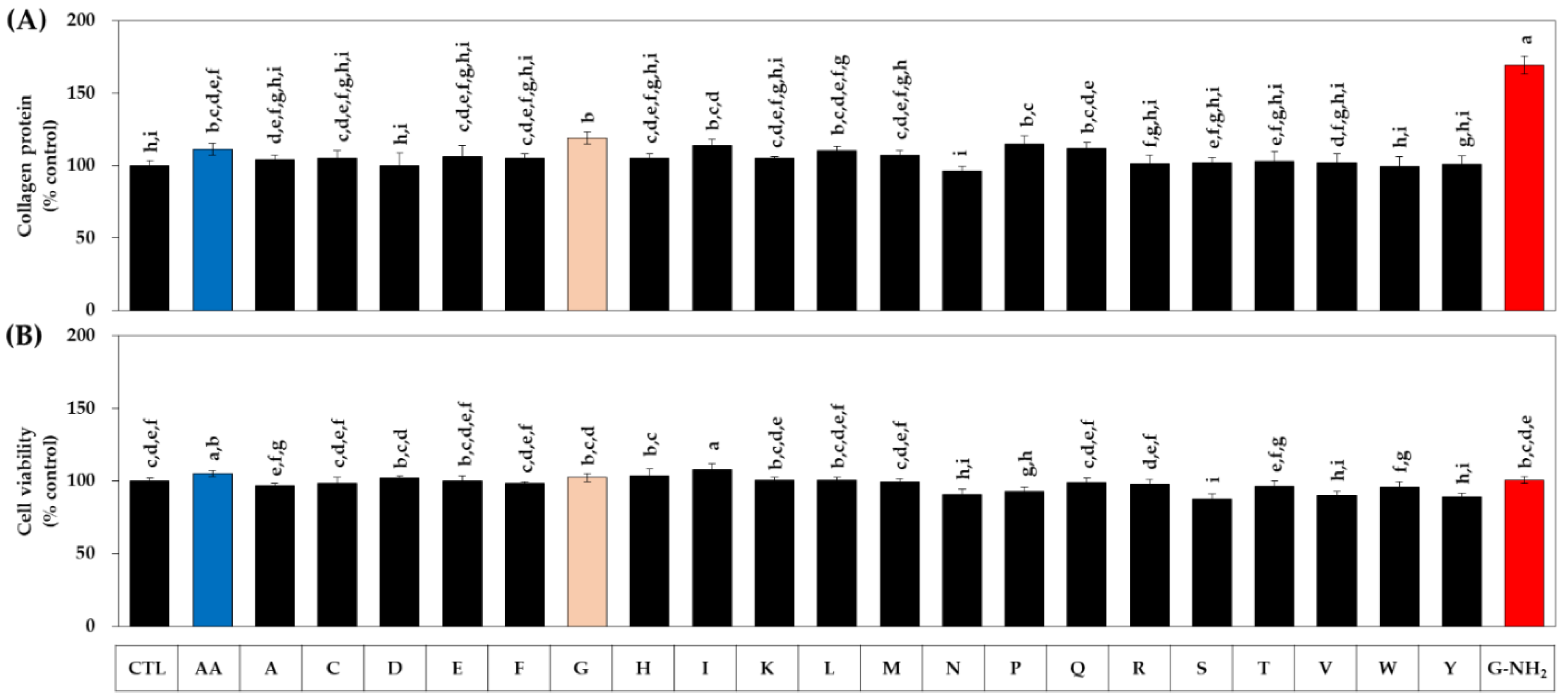
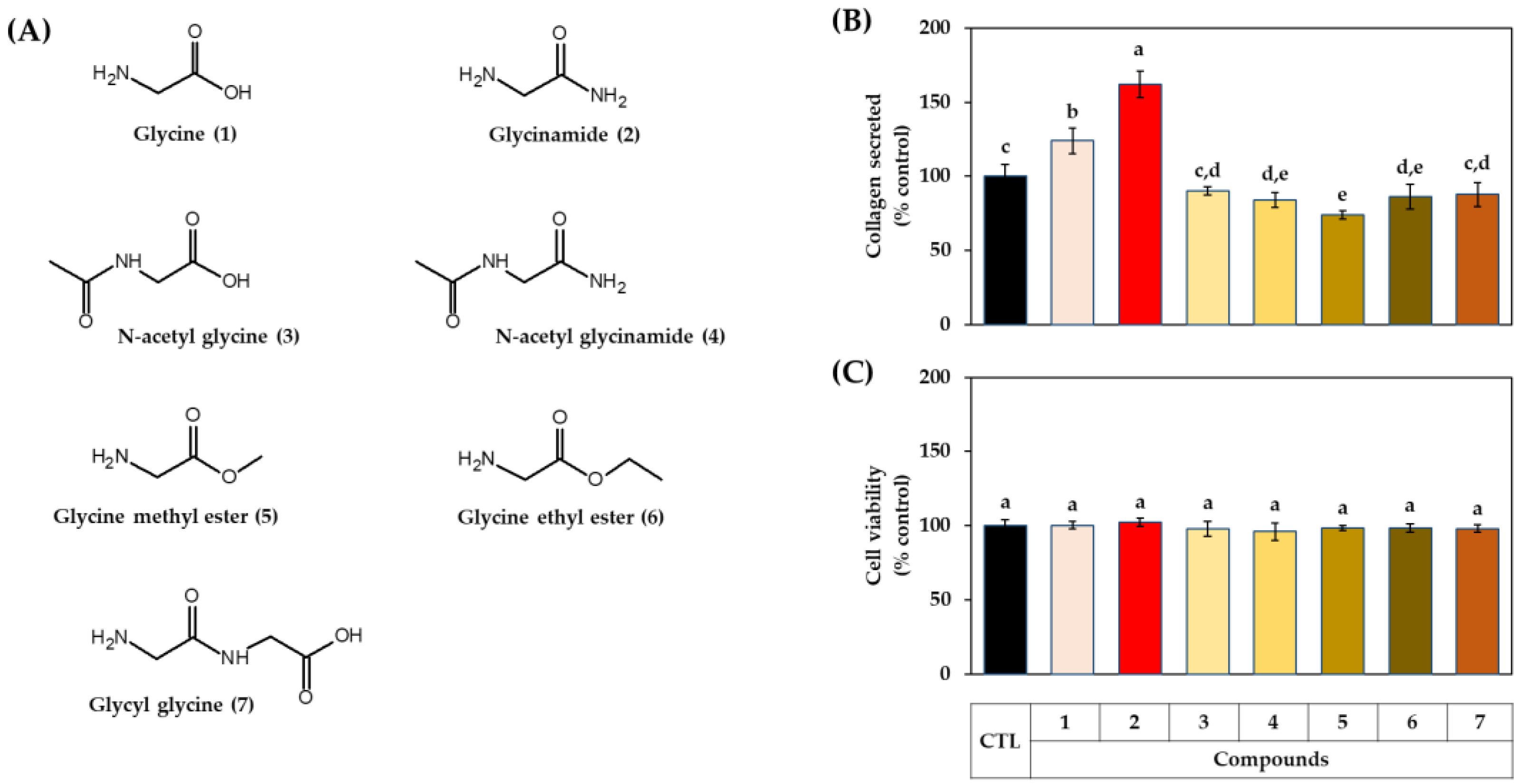
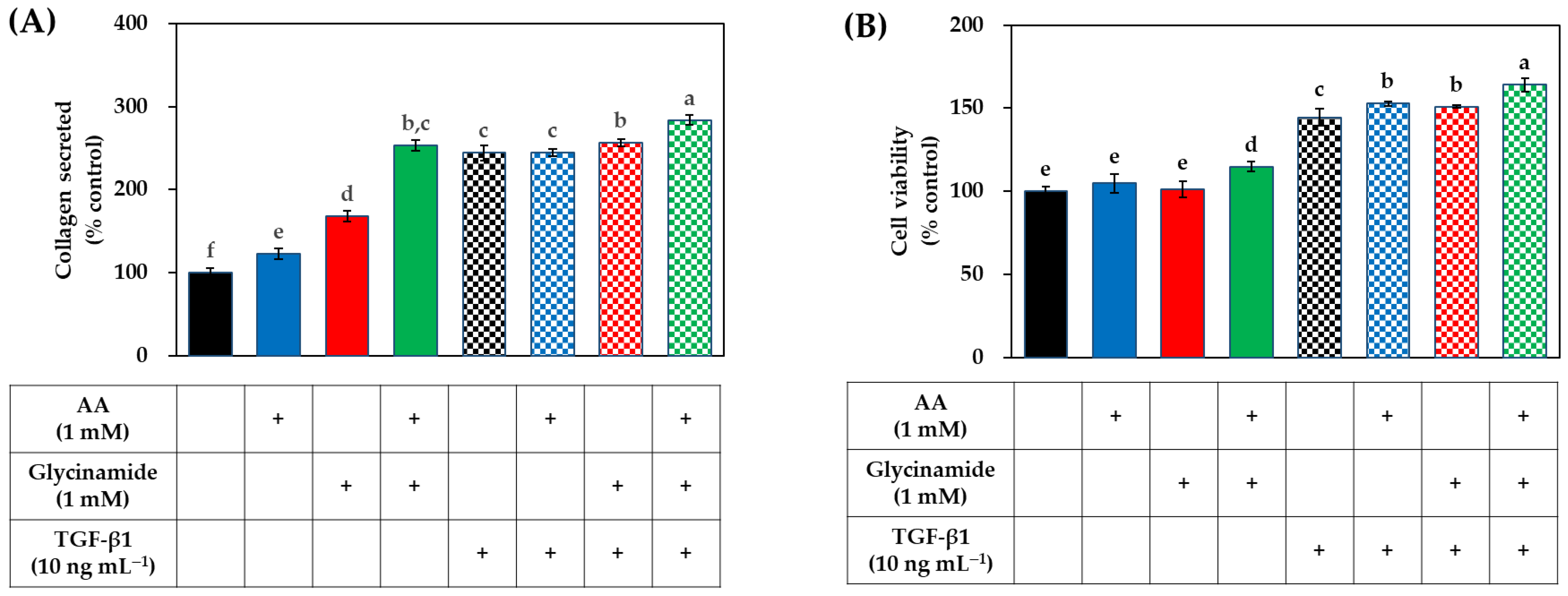
2.3. Cell Viability Assay
2.4. Measurement of Collagen Secreted
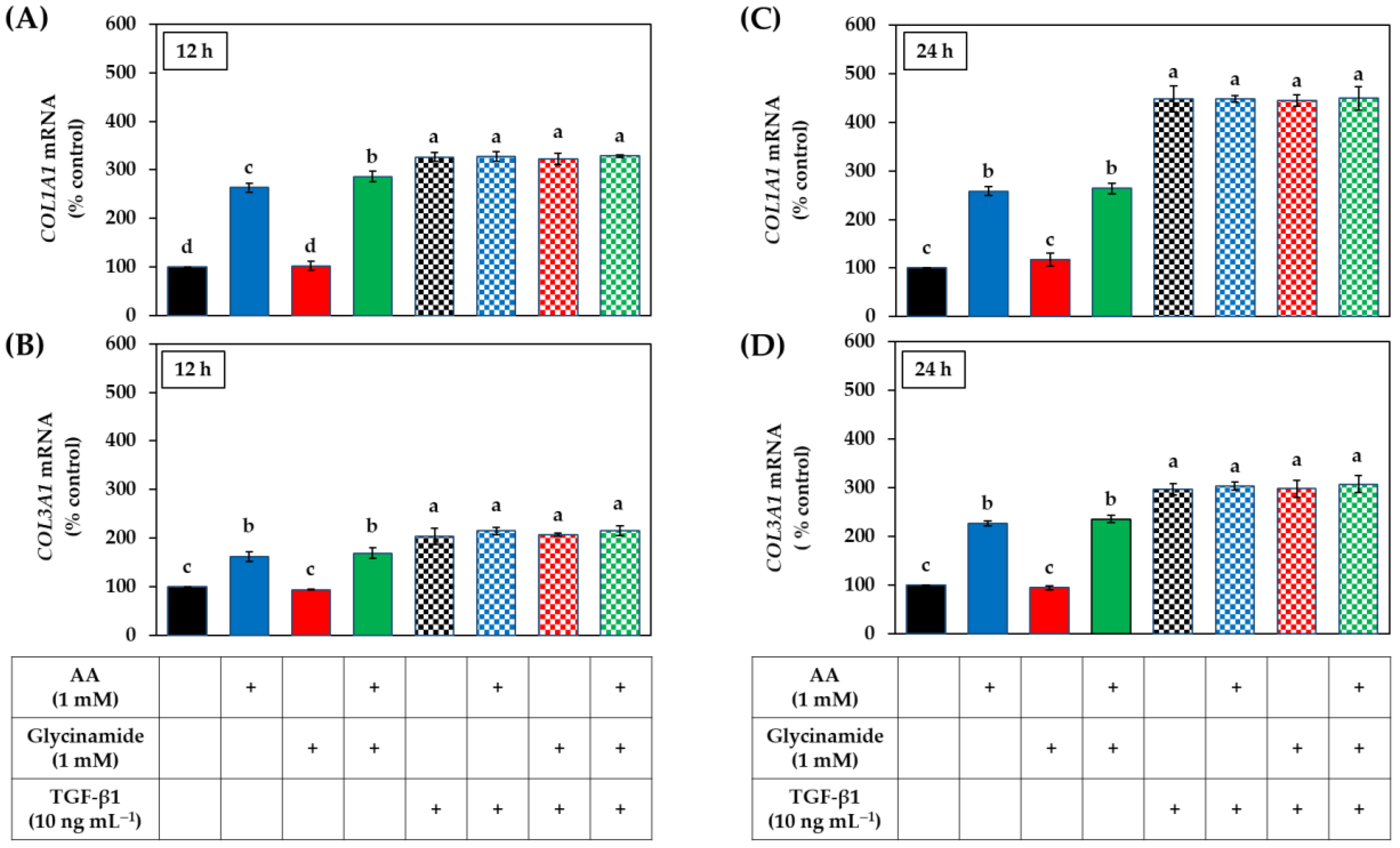
2.5. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR) Analysis
2.6. Western Blot Analysis
2.7. Wound Healing Assay
2.8. Statistical Analysis
3. Results
3.1. Effects of Various Amidated and Free Amino Acids on the Collagen Production of HDFs
3.2. Effects of Glycine Analogs on the Collagen Production of HDFs
3.3. Dose-dependent Effects of AA, Glycine, and Glycinamide on the Collagen Production of HDFs
3.4. Effects of AA, Glycinamide, and TGF-β1 on the Collagen Production of HDFs
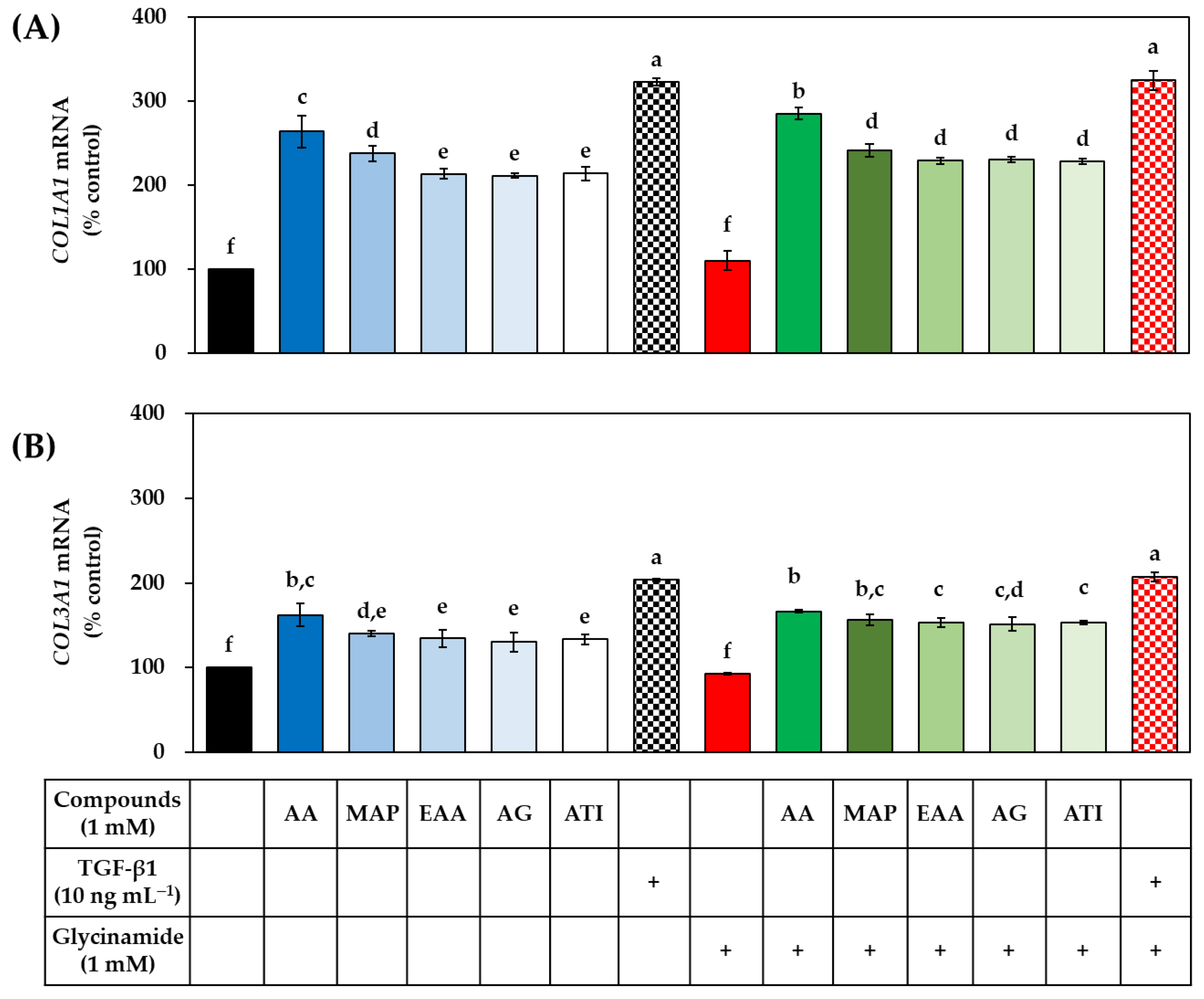
3.5. Effects of AA, Glycinamide, and TGF-β1 on the mRNA and Protein Levels of Collagens in HDFs
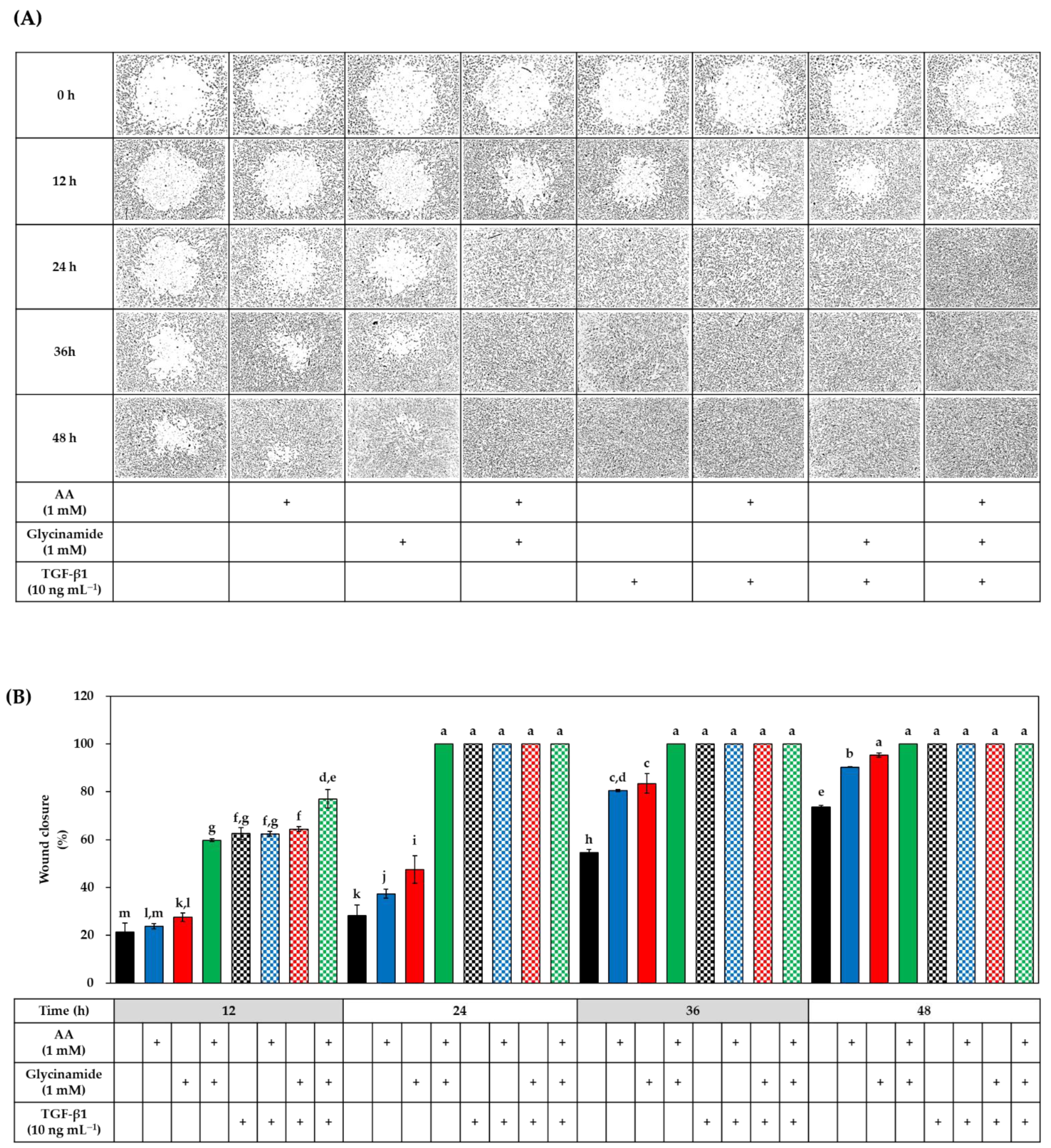
3.6. Effects of AA, Glycinamide, and TGF-β1 on the Wound Closure in HDFs
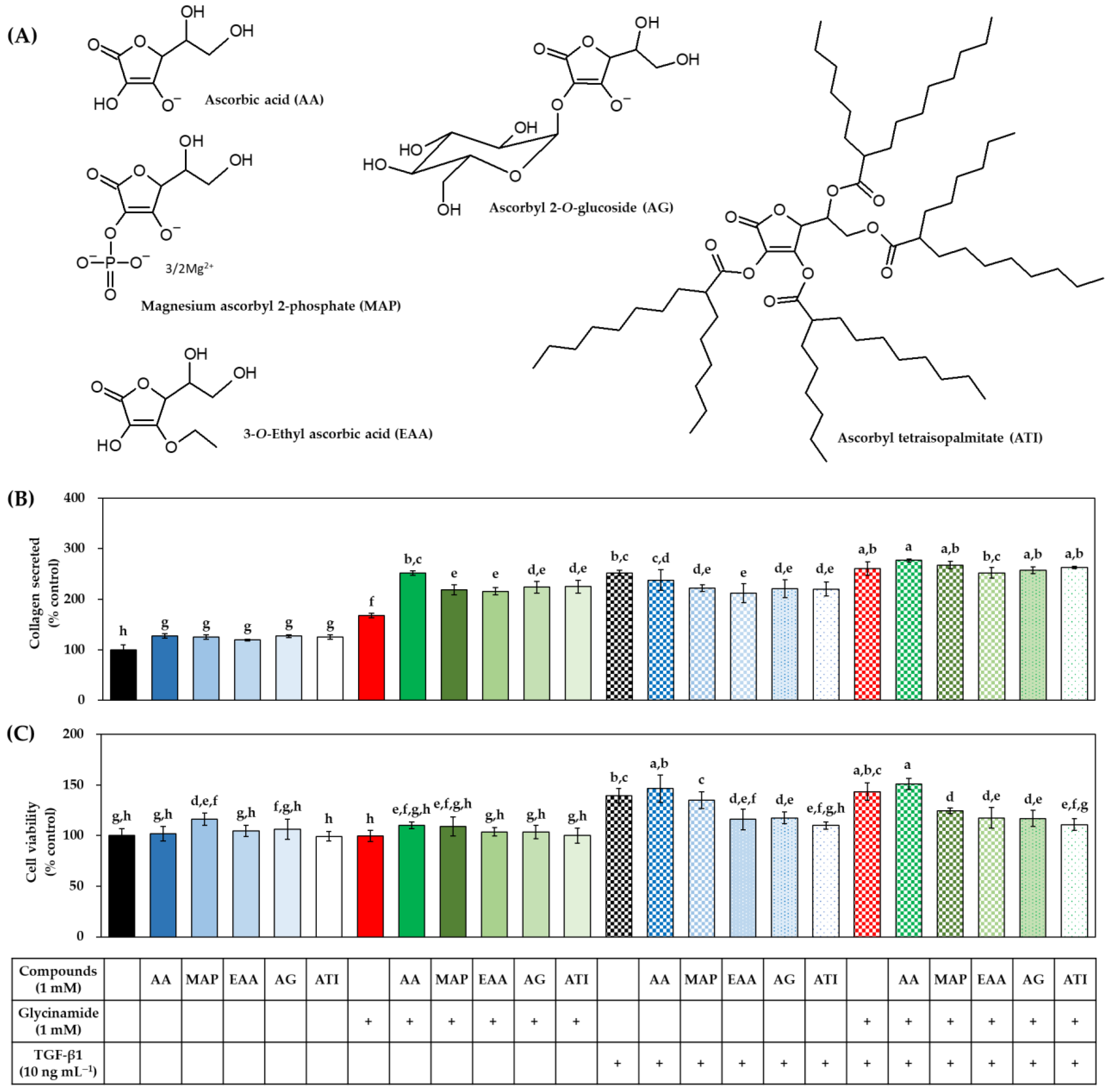
3.7. Effects of Various AA Analogs, Glycinamide, and TGF-β1 on the Collagen Production of HDFs
3.8. Effects of Various AA Analogs, Glycinamide, and TGF-β1 on the mRNA and Protein Levels of Collagens in HDFs
3.9. Effects of Various AA Analogs, Glycinamide, and TGF-β1 on the Wound Closure in HDFs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.A.B.A.; Sikarwar, A.S. Collagen: New Dimension in Cosmetic and Healthcare. Int. J. Biochem. Res. Rev. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Sun, B. The mechanics of fibrillar collagen extracellular matrix. Cell Rep. Phys. Sci. 2021, 2, 100515. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, S.V.; Gupta, S.; Bansal, H.; Singla, K.; Yadav, N.S. Collagen in Health and Disease. J. Orofac. Res. 2012, 2, 153–159. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.H.-C. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Tzaphlidou, M. The role of collagen and elastin in aged skin: An image processing approach. Micron 2004, 35, 173–177. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imokawa, G.; Ishida, K. Biological Mechanisms Underlying the Ultraviolet Radiation-Induced Formation of Skin Wrinkling and Sagging I: Reduced Skin Elasticity, Highly Associated with Enhanced Dermal Elastase Activity, Triggers Wrinkling and Sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.; Yan-Hua, R.; Fang-Gang, N.; Guo-An, Z. The content and ratio of type I and III collagen in skin differ with age and injury. Afr. J. Biotechnol. 2011, 10, 2524–2529. [Google Scholar]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, C.L.; Tajima, S.; Pinnell, S.R. Ascorbic acid and transforming growth factor-beta 1 increase collagen biosynthesis via different mechanisms: Coordinate regulation of pro alpha 1(I) and Pro alpha 1(III) collagens. Arch. Biochem. Biophys. 1992, 295, 397–403. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Park, S.; Seok, J.K.; Liu, K.-H.; Boo, Y.C. Ascorbyl coumarates as multifunctional cosmeceutical agents that inhibit melanogenesis and enhance collagen synthesis. Arch. Dermatol. Res. 2015, 307, 635–643. [Google Scholar] [CrossRef]
- Murad, S.; Tajima, S.; Johnson, G.R.; Sivarajah, S.A.; Pinnell, S.R. Collagen Synthesis in Cultured Human Skin Fibroblasts: Effect of Ascorbic Acid and Its Analogs. J. Investig. Dermatol. 1983, 81, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Maione-Silva, L.; De Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci. Rep. 2019, 9, 522. [Google Scholar] [CrossRef] [Green Version]
- Geesin, J.C.; Darr, D.; Kaufman, R.; Murad, S.; Pinnell, S.R. Ascorbic Acid Specifically Increases Type I and Type III Procollagen Messenger RNA Levels in Human Skin Fibroblasts. J. Investig. Dermatol. 1988, 90, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Tajima, S.; Pinnell, S.R. Ascorbic acid preferentially enhances type I and III collagen gene transcription in human skin fibroblasts. J. Dermatol. Sci. 1996, 11, 250–253. [Google Scholar] [CrossRef]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-dependent regulation of collagen metabolism. Cell. Mol. Life Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellon, G.; Monboisse, J.; Randoux, A.; Borel, J. Effects of preformed proline and proline amino acid precursors (including glutamine) on collagen synthesis in human fibroblast cultures. Biochim. Biophys. Acta 1987, 930, 39–47. [Google Scholar] [CrossRef]
- Kay, E.J.; Koulouras, G.; Zanivan, S. Regulation of Extracellular Matrix Production in Activated Fibroblasts: Roles of Amino Acid Metabolism in Collagen Synthesis. Front. Oncol. 2021, 11, 719922. [Google Scholar] [CrossRef]
- Krupsky, M.; Kuang, P.-P.; Goldstein, R.H. Regulation of Type I Collagen mRNA by Amino Acid Deprivation in Human Lung Fibroblasts. J. Biol. Chem. 1997, 272, 13864–13868. [Google Scholar] [CrossRef] [Green Version]
- Karna, E.; Miltyk, W.; Wołczyński, S.; Pałka, J. The potential mechanism for glutamine-induced collagen biosynthesis in cultured human skin fibroblasts. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 130, 23–32. [Google Scholar] [CrossRef]
- Szoka, L.; Karna, E.; Hlebowicz-Sarat, K.; Karaszewski, J.; Palka, J.A. Exogenous proline stimulates type I collagen and HIF-1α expression and the process is attenuated by glutamine in human skin fibroblasts. Mol. Cell. Biochem. 2017, 435, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Lugo, P.D.P.; Lupiáñez, J.A.; Meléndez-Hevia, E. High glycine concentration increases collagen synthesis by articular chondrocytes in vitro: Acute glycine deficiency could be an important cause of osteoarthritis. Amino Acids 2018, 50, 1357–1365. [Google Scholar] [CrossRef] [Green Version]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Ohto-Fujita, E.; Konno, T.; Shimizu, M.; Ishihara, K.; Sugitate, T.; Miyake, J.; Yoshimura, K.; Taniwaki, K.; Sakurai, T.; Hasebe, Y.; et al. Hydrolyzed eggshell membrane immobilized on phosphorylcholine polymer supplies extracellular matrix environment for human dermal fibroblasts. Cell Tissue Res. 2011, 345, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.W.; Song, H.; Hong, S.S.; Boo, Y.C. Marine Alga Ecklonia cava Extract and Dieckol Attenuate Prostaglandin E2 Production in HaCaT Keratinocytes Exposed to Airborne Particulate Matter. Antioxidants 2019, 8, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.-M.; Koh, J.-S.; Boo, Y.-C. Inhibition of melanogenesis by tyrosinase siRNA in human melanocytes. BMB Rep. 2009, 42, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Mun, G.I.; Park, S.; Kremerskothen, J.; Boo, Y.C. Expression of synaptopodin in endothelial cells exposed to laminar shear stress and its role in endothelial wound healing. FEBS Lett. 2014, 588, 1024–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–412. [Google Scholar]
- Tracy, L.E.; Minasian, R.A.; Caterson, E. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Heng, S.; Zhang, W.; Liu, Y.; Xia, T.; Ji, C.; Zhang, L.J. Dermal extracellular matrix molecules in skin development, homeostasis, wound regeneration and diseases. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Ye, Z.; Li, W.; Jiang, Z.; Wang, E.; Wang, J. An intermediate state in trans-differentiation with proliferation, metabolic, and epigenetic switching. iScience 2021, 24, 103057. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic Scarring and Keloids: Pathomechanisms and Current and Emerging Treatment Strategies. Mol. Med. 2010, 17, 113–125. [Google Scholar] [CrossRef]
- Friedman, D.W.; Boyd, C.D.; MacKenzie, J.W.; Norton, P.; Olson, R.M.; Deak, S.B. Regulation of Collagen Gene Expression in Keloids and Hypertrophic Scars. J. Surg. Res. 1993, 55, 214–222. [Google Scholar] [CrossRef]
- Geesin, J.C.; Gordon, J.S.; Berg, R.A. Magnesium Ascorbyl-2-Phosphate Is a More Stable Alternative to Ascorbic-Acid in the Stimulation of Collagen-Synthesis in Human Dermal Fibroblasts. J. Investig. Dermatol. 1992, 98, 623. [Google Scholar]
- Smaoui, S.; Ben Hlima, H.; Kadri, A. Application of l-Ascorbic Acid and its Derivatives (Sodium Ascorbyl Phosphate and Magnesium Ascorbyl Phosphate) in Topical Cosmetic Formulations: Stability Studies. J. Chem. Soc. Pak. 2013, 35, 1096–1102. [Google Scholar]
- Liao, W.C.; Huang, Y.-T.; Lu, L.-P.; Huang, W.-Y. Antioxidant Ability and Stability Studies of 3-O-Ethyl Ascorbic Acid, a Cosmetic Tyrosinase Inhibitor. J. Cosmet. Sci. 2018, 69, 233–243. [Google Scholar] [PubMed]
- Huang, S.-C.; Lin, C.-C.; Wen, K.-C. Simultaneous determination of magnesium ascorbyl phosphate, ascorbyl glucoside, kojic acid, arbutin and hydroquinone in skin whitening cosmetics by HPLC. J. Food Drug Anal. 2004, 12, 1. [Google Scholar] [CrossRef]
- Machado, N.; dos Santos, L.; Carvalho, B.; Singh, P.; Soto, C.T.; Azoia, N.; Cavaco-Paulo, A.; Martin, A.; Favero, P. Assessment of penetration of Ascorbyl Tetraisopalmitate into biological membranes by molecular dynamics. Comput. Biol. Med. 2016, 75, 151–159. [Google Scholar] [CrossRef] [Green Version]
- de Almeida, M.M.; Lima, C.R.R.D.; Quenca-Guillen, J.S.; Moscardini, E.; Mercuri, L.P.; Santoro, M.I.R.M.; Kedor-Hackmann, E.R.M. Stability evaluation of tocopheryl acetate and ascorbyl tetraisopalmitate in isolation and incorporated in cosmetic formulations using thermal analysis. Braz. J. Pharm. Sci. 2010, 46, 129–134. [Google Scholar] [CrossRef]
- Koopman, R.; Caldow, M.K.; Ham, D.J.; Lynch, G.S. Glycine metabolism in skeletal muscle: Implications for metabolic homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 237–242. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Kim, J.H.; Seok, J.K.; Kim, Y.M.; Boo, Y.C. Identification of small peptides and glycinamide that inhibit melanin synthesis using a positional scanning synthetic peptide combinatorial library. Br. J. Dermatol. 2019, 181, 128–137. [Google Scholar] [CrossRef]
- Boo, Y.C. Up- or Downregulation of Melanin Synthesis Using Amino Acids, Peptides, and Their Analogs. Biomedicines 2020, 8, 322. [Google Scholar] [CrossRef]
- Boo, Y.; Jo, D.; Oh, C.; Lee, S.; Kim, Y. The First Human Clinical Trial on the Skin Depigmentation Efficacy of Glycinamide Hydrochloride. Biomedicines 2020, 8, 257. [Google Scholar] [CrossRef]





| Free Amino Acids | Single-Letter Codes | Amidated Amino Acids | Single-Letter Codes |
|---|---|---|---|
| L-Alanine | A | L-Alaninamide HCl | A-NH2 |
| L-Cysteine | C | L-Cysteinamide HCl | C-NH2 |
| L-Aspartic acid | D | L-Aspartic acid α-amide HCl | D-NH2 |
| L-Glutamic acid | E | L-Glutamic acid α-amide | E-NH2 |
| L-Phenylalanine | F | L-Phenylalaninamide HCl | F-NH2 |
| Glycine | G | Glycinamide HCl | G-NH2 |
| L-Histidine | H | L-Histidinamide HCl | H-NH2 |
| L-Isoleucine | I | L-Isoleucinamide HCl | I-NH2 |
| L-Lysine | K | L-Lysinamide diHCl | K-NH2 |
| L-Leucine | L | L-Leucinamide HCl | L-NH2 |
| L-Methionine | M | L-Methioninamide HCl | M-NH2 |
| L-Asparagine | N | L-Asparaginamide HCl | N-NH2 |
| L-Proline | P | L-Prolinamide HCl | P-NH2 |
| L-Glutamine | Q | L-Glutaminamide HCl | Q-NH2 |
| L-Arginine | R | L-Argininamide diHCl | R-NH2 |
| L-Serine | S | L-Serinamide HCl | S-NH2 |
| L-Threonine | T | L-Threoninamide HCl | T-NH2 |
| L-Valine | V | L-Valinamide HCl | V-NH2 |
| L-Tryptophane | W | L-Tryptophanamide HCl | W-NH2 |
| L-Tyrosine | Y | L-Tyrosinamide HCl | Y-NH2 |
| Gene Name | GenBank Accession Number | Forward (F) and Reverse (R) Primer Sequences | References |
|---|---|---|---|
| Collagen I alpha 1 chain (COL1A1) | NM_000088.4 | F: 5′-GGGATTCCCTGGACCTAAAG-3′ R: 5′-GGAACACCTCGCTCTCCA-3′ | [30] |
| Collagen III alpha 1 chain (COL3A1) | NM_000090.4 | F: 5′-GGACCTCCTGGTGCTATAGGT-3′ R: 5′-CGGGTCTACCTGATTCTCCAT-3′ | [30] |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | NM_002046.3 | F: 5′- ATGGGGAAGGTGAAGGTCG -3′ R: 5′- GGGGTCATTGATGGCAACAA -3′ | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.E.; Boo, Y.C. Combination of Glycinamide and Ascorbic Acid Synergistically Promotes Collagen Production and Wound Healing in Human Dermal Fibroblasts. Biomedicines 2022, 10, 1029. https://doi.org/10.3390/biomedicines10051029
Lee JE, Boo YC. Combination of Glycinamide and Ascorbic Acid Synergistically Promotes Collagen Production and Wound Healing in Human Dermal Fibroblasts. Biomedicines. 2022; 10(5):1029. https://doi.org/10.3390/biomedicines10051029
Chicago/Turabian StyleLee, Ji Eun, and Yong Chool Boo. 2022. "Combination of Glycinamide and Ascorbic Acid Synergistically Promotes Collagen Production and Wound Healing in Human Dermal Fibroblasts" Biomedicines 10, no. 5: 1029. https://doi.org/10.3390/biomedicines10051029
APA StyleLee, J. E., & Boo, Y. C. (2022). Combination of Glycinamide and Ascorbic Acid Synergistically Promotes Collagen Production and Wound Healing in Human Dermal Fibroblasts. Biomedicines, 10(5), 1029. https://doi.org/10.3390/biomedicines10051029







