Multi-Level Analysis of Adipose Tissue Reveals the Relevance of Perivascular Subpopulations and an Increased Endothelial Permeability in Early-Stage Lipedema
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Experimental Set-Up
2.2. Histology
2.3. SVF Isolation
2.4. Isolation of Endothelial Cells and Pericytes by Fluorescence-Activated Cell Sorting (FACS)
2.5. ASC Cultivation
2.6. Cultivation of Human Primary EC
2.7. Immunofluorescence Staining of Endothelial Junctions
2.8. Convolutional Neural Network (CNN) Analysis of Endothelial Junction Imaging
2.9. Endothelial Permeability Assay and RNA Collection of hECs
2.10. RNA Isolation and Quantitative Real-Time RT-PCR
2.11. Protein Array Raybio C-Series
2.12. Statistics
3. Results
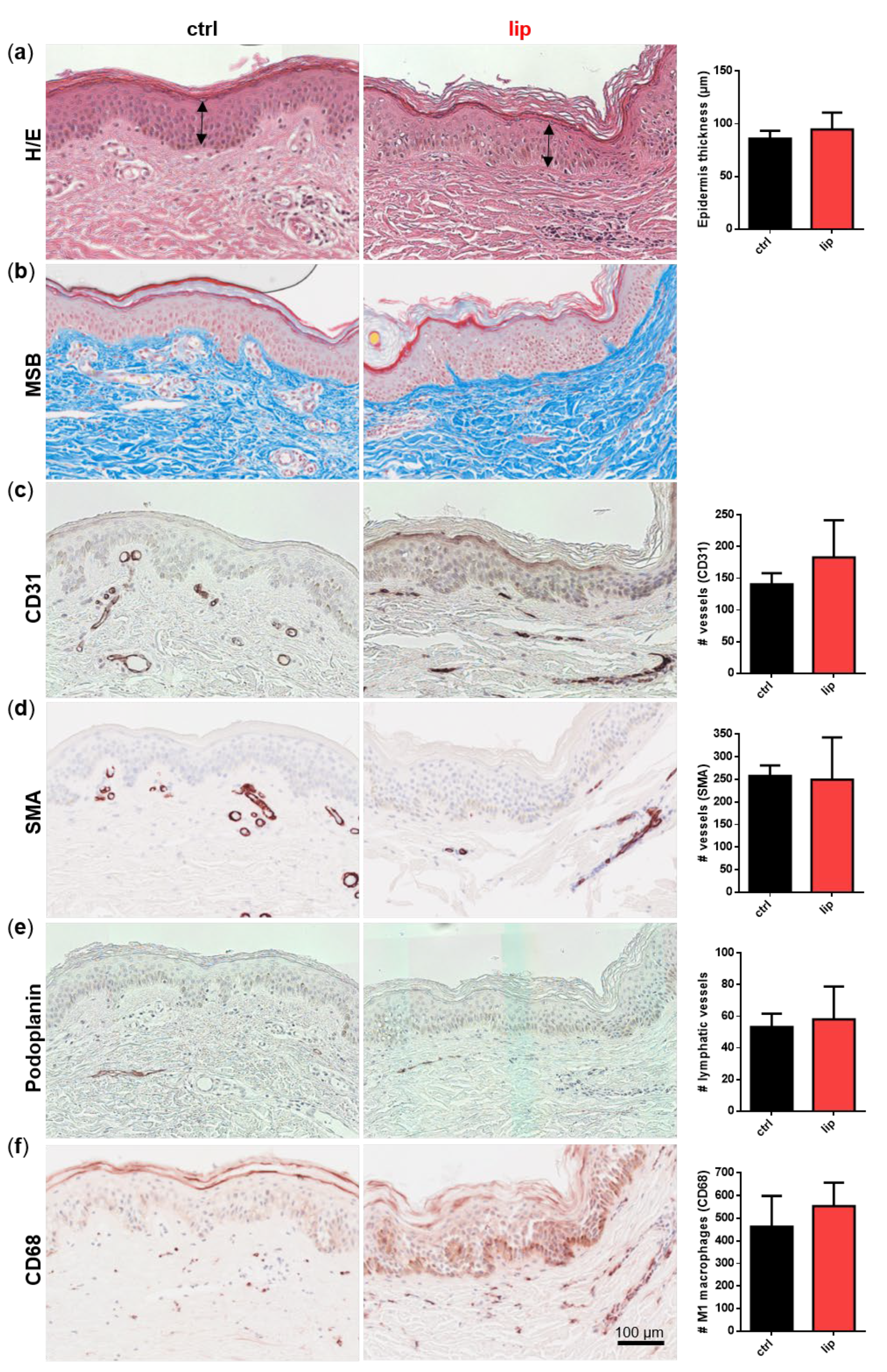
3.1. Skin Biopsies of Lipedema Patients Showed No Morphological Alterations
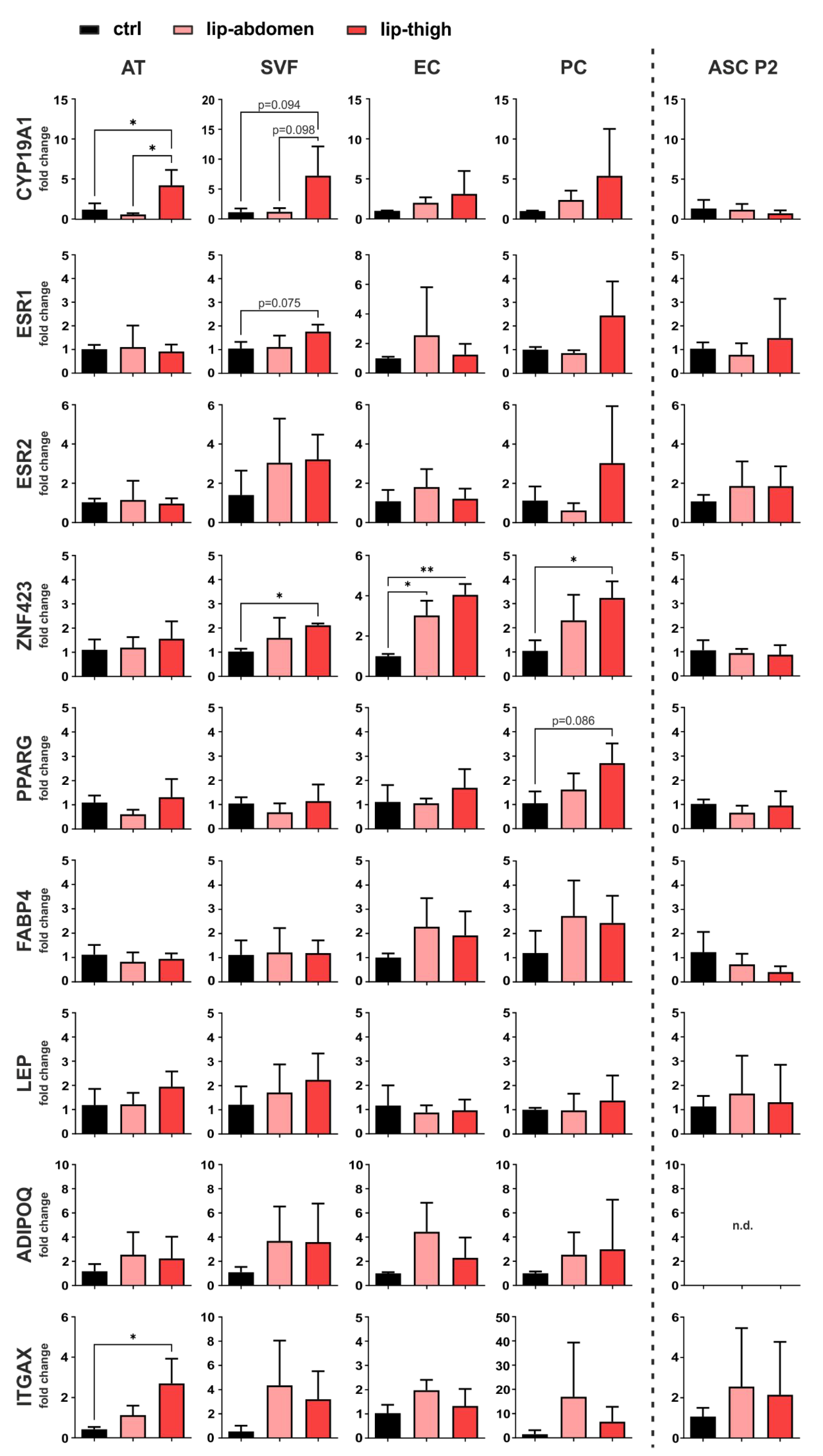
3.2. Gene Expression Analysis Revealed a Role of Local Estrogen Metabolism, Preadipocyte Determination and Immune Cell Infiltration in Lipedema
3.2.1. Estrogen-Related Genes
3.2.2. Adipogenic Marker Genes
3.2.3. Immune Cell Marker Gene
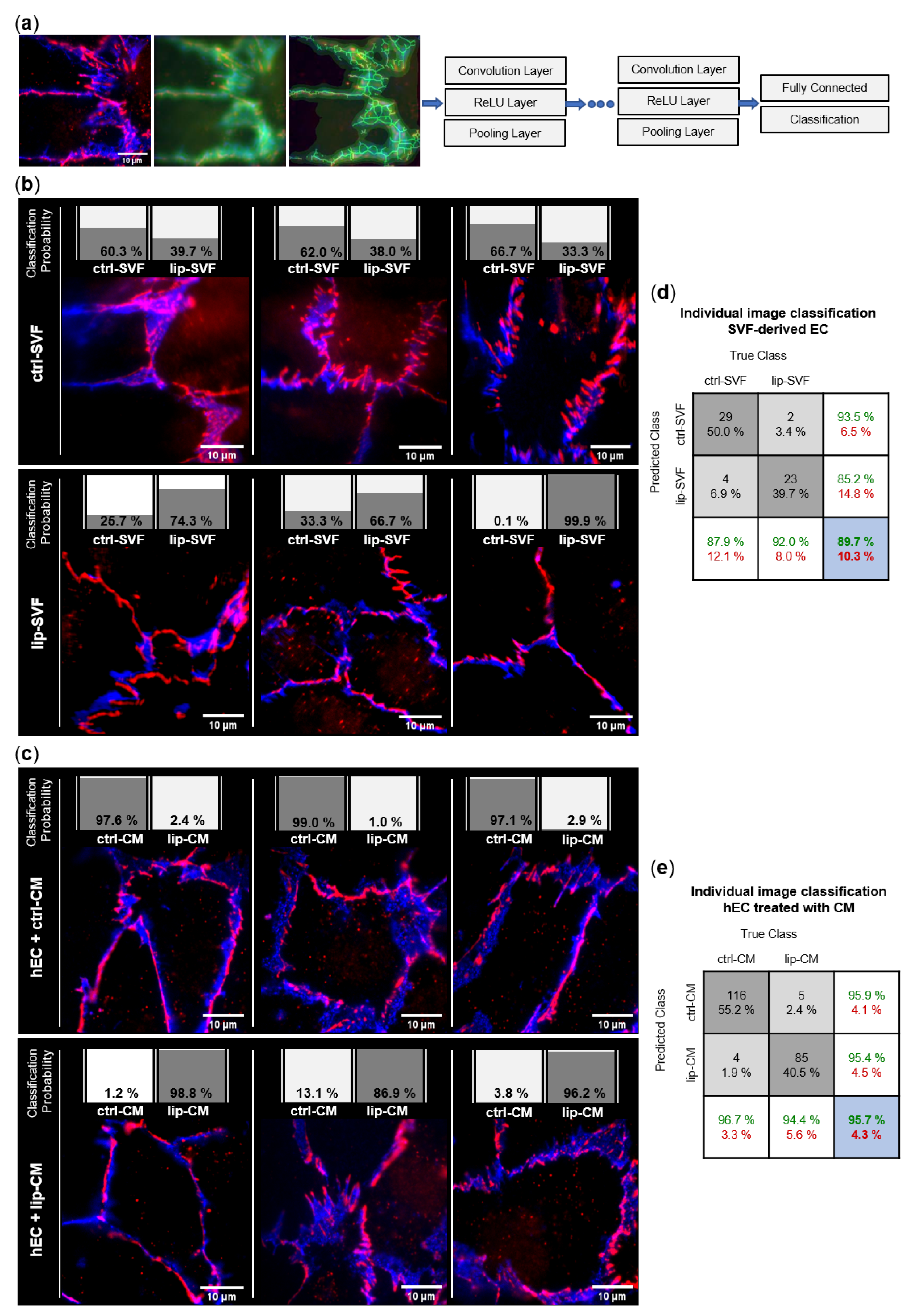
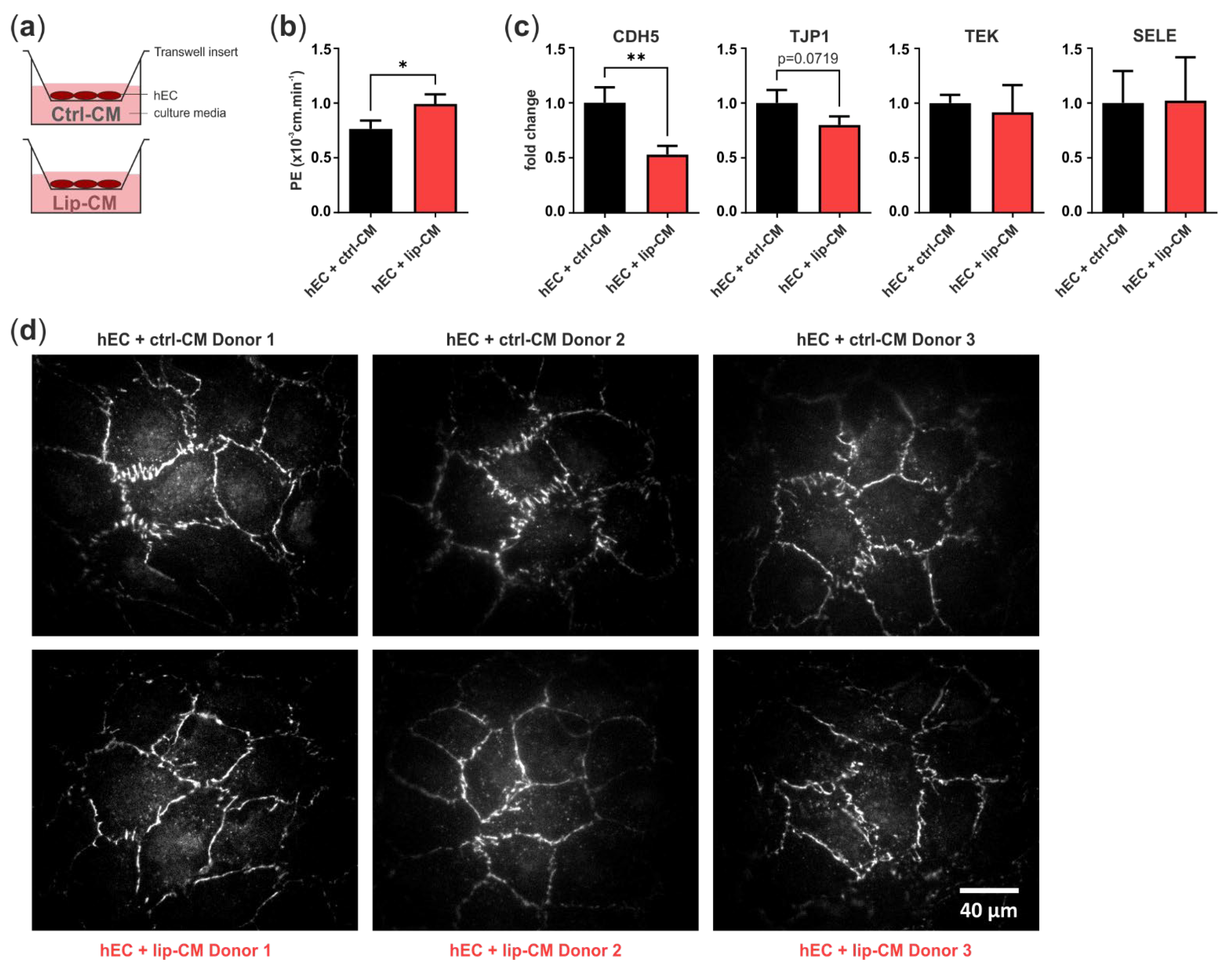
3.3. Lipedema Is Characterized by Altered Endothelial Cell Junctions and Higher Endothelial Permeability
3.3.1. Endothelial Junction Morphology
3.3.2. Endothelial Barrier Function
3.4. IL-8 Protein Secretion Is Down-Regulated in CM of Lipedema SVF Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wold, L.E.; Hines, E.A., Jr.; Allen, E.V. Lipedema of the legs; a syndrome characterized by fat legs and edema. Ann. Intern. Med. 1951, 34, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Dadras, M.; Mallinger, P.J.; Corterier, C.C.; Theodosiadi, S.; Ghods, M. Liposuction in the Treatment of Lipedema: A Longitudinal Study. Arch. Plast. Surg. 2017, 44, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandhofer, M.; Hanke, C.W.; Habbema, L.; Podda, M.; Rapprich, S.; Schmeller, W.; Herbst, K.; Anderhuber, F.; Pilsl, U.; Sattler, G.; et al. Prevention of Progression of Lipedema with Liposuction Using Tumescent Local Anesthesia: Results of an International Consensus Conference. Dermatol. Surg. 2020, 46, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Van De Pas, C.B.; Boonen, R.S.; Stevens, S.; Willemsen, S.; Valkema, R.; Neumann, M. Does tumescent liposuction damage the lymph vessels in lipoedema patients? Phlebol. J. Venous Dis. 2019, 35, 231–236. [Google Scholar] [CrossRef]
- Sandhofer, M.; Hofer, V.; Sandhofer, M.; Sonani, M.; Moosbauer, W.; Barsch, M. High Volume Liposuction in Tumescence Anesthesia in Lipedema Patients: A Retrospective Analysis. J. Drugs Dermatol. 2021, 20, 326–334. [Google Scholar] [CrossRef]
- Rapprich, S.; Koller, J.; Sattler, G.; Wörle, B.; Sommer, B.; Bechara, F.G.; Koenen, W.; Kunte, C.; Grablowitz, D.; Hoffmann, K.; et al. Liposuction—A surgical procedure in dermatology. JDDG J. Dtsch. Dermatol. Ges. 2011, 10, 111–113. [Google Scholar] [CrossRef]
- Rapprich, S.; Dingler, A.; Podda, M. Liposuction is an effective treatment for lipedema-results of a study with 25 patients. JDDG J. Dtsch. Dermatol. Ges. 2010, 9, 33–40. [Google Scholar] [CrossRef]
- Sandhofer, M.; Schauer, P.; Anderhuber, F. Lipödem. Ästhetische Chir. 2017, 10, 61–70. [Google Scholar] [CrossRef]
- Sandhofer, M.; Sandhofer, M.; Moosbauer, W.; Linska, M.; Hofer, V.; Schauer, P. Das Lipödem—Wenn das subkutane Fett rebelliert. Kosmet. Med. 2016, 1, 3–14. [Google Scholar]
- Forner-Cordero, I.; Forner-Cordero, A.; Szolnoky, G. Update in the management of lipedema. Int. Angiol. 2021, 40, 345–357. [Google Scholar] [CrossRef]
- Romeijn, J.R.M.; de Rooij, M.J.M.; Janssen, L.; Martens, H. Exploration of Patient Characteristics and Quality of Life in Patients with Lipoedema Using a Survey. Dermatol. Ther. 2018, 8, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Katzer, K.; Hill, J.L.; McIver, K.B.; Foster, M.T. Lipedema and the Potential Role of Estrogen in Excessive Adipose Tissue Accumulation. Int. J. Mol. Sci. 2021, 22, 11720. [Google Scholar] [CrossRef]
- Langendoen, S.I.; Habbema, L.; Nijsten, T.E.C.; Neumann, H.A.M. Lipoedema: From clinical presentation to therapy. A review of the literature. Br. J. Dermatol. 2009, 161, 980–986. [Google Scholar] [CrossRef]
- Kruppa, P.; Georgiou, I.; Biermann, N.; Prantl, L.; Klein-Weigel, P.; Ghods, M. Lipedema—Pathogenesis, Diagnosis, and Treatment Options. Dtsch. Arztebl. Int. 2020, 117, 396–403. [Google Scholar] [CrossRef]
- Reich-Schupke, S.; Schmeller, W.; Brauer, W.J.; Cornely, M.E.; Faerber, G.; Ludwig, M.; Lulay, G.; Miller, A.; Rapprich, S.; Richter, D.F.; et al. S1 guidelines: Lipedema. J. Dtsch. Dermatol. Ges. 2017, 15, 758–767. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Joffin, N.; Scherer, P.E. The MMP14–caveolin axis and its potential relevance for lipoedema. Nat. Rev. Endocrinol. 2020, 16, 669–674. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Cromer, W.; Allen, M.; Ussery, C.; Badowski, M.; Harris, D.; Herbst, K.L. Dilated Blood and Lymphatic Microvessels, Angiogenesis, Increased Macrophages, and Adipocyte Hypertrophy in Lipedema Thigh Skin and Fat Tissue. J. Obes. 2019, 2019, 8747461. [Google Scholar] [CrossRef]
- Felmerer, G.; Stylianaki, A.; Hägerling, R.; Wang, A.; Ströbel, P.; Hollmén, M.; Lindenblatt, N.; Gousopoulos, E. Adipose Tissue Hypertrophy, An Aberrant Biochemical Profile and Distinct Gene Expression in Lipedema. J. Surg. Res. 2020, 253, 294–303. [Google Scholar] [CrossRef]
- Felmerer, G.; Stylianaki, A.; Hollmén, M.; Ströbel, P.; Stepniewski, A.; Wang, A.; Frueh, F.S.; Kim, B.-S.; Giovanoli, P.; Lindenblatt, N.; et al. Increased levels of VEGF-C and macrophage infiltration in lipedema patients without changes in lymphatic vascular morphology. Sci. Rep. 2020, 10, 10947. [Google Scholar] [CrossRef]
- Szél, E.; Kemény, L.; Groma, G.; Szolnoky, G. Pathophysiological dilemmas of lipedema. Med. Hypotheses 2014, 83, 599–606. [Google Scholar] [CrossRef]
- Allen, M.; Schwartz, M.; Herbst, K.L. Interstitial Fluid in Lipedema and Control Skin. Women’s Health Rep. 2020, 1, 480–487. [Google Scholar] [CrossRef]
- Bilancini, S.; Lucchi, M.; Tucci, S.; Eleuteri, P. Functional Lymphatic Alterations in Patients Suffering from Lipedema. Angiology 1995, 46, 333–339. [Google Scholar] [CrossRef]
- Amann-Vesti, B.R.; Franzeck, U.K.; Bollinger, A. Microlymphatic aneurysms in patients with lipedema. Lymphology 2001, 34, 170–175. [Google Scholar]
- Suga, H.; Araki, J.; Aoi, N.; Kato, H.; Higashino, T.; Yoshimura, K. Adipose tissue remodeling in lipedema: Adipocyte death and concurrent regeneration. J. Cutan. Pathol. 2009, 36, 1293–1298. [Google Scholar] [CrossRef]
- Wolf, S.; Deuel, J.; Hollmén, M.; Felmerer, G.; Kim, B.-S.; Vasella, M.; Grünherz, L.; Giovanoli, P.; Lindenblatt, N.; Gousopoulos, E. A Distinct Cytokine Profile and Stromal Vascular Fraction Metabolic Status without Significant Changes in the Lipid Composition Characterizes Lipedema. Int. J. Mol. Sci. 2021, 22, 3313. [Google Scholar] [CrossRef]
- Ishaq, M.; Bandara, N.; Morgan, S.; Nowell, C.; Mehdi, A.M.; Lyu, R.; McCarthy, D.; Anderson, D.; Creek, D.J.; Achen, M.G.; et al. Key signaling networks are dysregulated in patients with the adipose tissue disorder, lipedema. Int. J. Obes. 2021, 46, 502–514. [Google Scholar] [CrossRef]
- Priglinger, E.; Strohmeier, K.; Weigl, M.; Lindner, C.; Auer, D.; Gimona, M.; Barsch, M.; Jacak, J.; Redl, H.; Grillari, J.; et al. SVF-derived extracellular vesicles carry characteristic miRNAs in lipedema. Sci. Rep. 2020, 10, 7211. [Google Scholar] [CrossRef]
- Priglinger, E.; Wurzer, C.; Steffenhagen, C.; Maier, J.; Hofer, V.; Peterbauer, A.; Nuernberger, S.; Redl, H.; Wolbank, S.; Sandhofer, M. The adipose tissue–derived stromal vascular fraction cells from lipedema patients: Are they different? Cytotherapy 2017, 19, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Jacak, J. Real-time 3D single-molecule localization microscopy analysis using lookup tables. Biomed. Opt. Express 2021, 12, 4955. [Google Scholar] [CrossRef] [PubMed]
- Mayr, S.; Hauser, F.; Puthukodan, S.; Axmann, M.; Göhring, J.; Jacak, J. Statistical analysis of 3D localisation microscopy images for quantification of membrane protein distributions in a platelet clot model. PLoS Comput. Biol. 2020, 16, e1007902. [Google Scholar] [CrossRef]
- Cecchelli, R.; Aday, S.; Sevin, E.; Almeida, C.; Culot, M.; Dehouck, L.; Coisne, C.; Engelhardt, B.; Dehouck, M.-P.; Ferreira, L. A Stable and Reproducible Human Blood-Brain Barrier Model Derived from Hematopoietic Stem Cells. PLoS ONE 2014, 9, e99733. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.K.; Arany, Z.; Seale, P.; Mepani, R.J.; Ye, L.; Conroe, H.M.; Roby, Y.A.; Kulaga, H.; Reed, R.R.; Spiegelman, B.M. Transcriptional control of preadipocyte determination by Zfp423. Nature 2010, 464, 619–623. [Google Scholar] [CrossRef] [Green Version]
- Furuhashi, M.; Saitoh, S.; Shimamoto, K.; Miura, T. Fatty Acid-Binding Protein 4 (FABP4): Pathophysiological Insights and Potent Clinical Biomarker of Metabolic and Cardiovascular Diseases. Clin. Med. Insights Cardiol. 2014, 8 (Suppl. S3), 22–33. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghadban, S.; Diaz, Z.T.; Singer, H.J.; Mert, K.B.; Bunnell, B.A. Increase in Leptin and PPAR-γ Gene Expression in Lipedema Adipocytes Differentiated in vitro from Adipose-Derived Stem Cells. Cells 2020, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- ElShal, M.; Khan, S.S.; Raghavachari, N.; Takahashi, Y.; Barb, J.; Bailey, J.J.; Munson, P.J.; Solomon, M.A.; Danner, R.L.; McCoy, J.P. A unique population of effector memory lymphocytes identified by CD146 having a distinct immunophenotypic and genomic profile. BMC Immunol. 2007, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Liu, J.; Chen, G.; Du, J.; Cai, H.; Chen, X.; Ye, G.; Luo, Y.; Luo, Y.; Zhang, L.; et al. CD146 is a Novel ANGPTL2 Receptor that Promotes Obesity by Manipulating Lipid Metabolism and Energy Expenditure. Adv. Sci. 2021, 8, 2004032. [Google Scholar] [CrossRef]
- Antony, A.; Lian, Z.; Perrard, X.D.; Perrard, J.; Liu, H.; Cox, A.R.; Saha, P.; Hennighausen, L.; Hartig, S.M.; Ballantyne, C.M.; et al. Deficiency of Stat1 in CD11c+ Cells Alters Adipose Tissue Inflammation and Improves Metabolic Dysfunctions in Mice Fed a High-Fat Diet. Diabetes 2020, 70, 720–732. [Google Scholar] [CrossRef]
- Cho, K.W.; Zamarron, B.F.; Muir, L.A.; Singer, K.; Porsche, C.E.; DelProposto, J.B.; Geletka, L.; Meyer, K.A.; O’Rourke, R.W.; Lumeng, C.N. Adipose Tissue Dendritic Cells Are Independent Contributors to Obesity-Induced Inflammation and Insulin Resistance. J. Immunol. 2016, 197, 3650–3661. [Google Scholar] [CrossRef]
- Gupta, R.K.; Mepani, R.J.; Kleiner, S.; Lo, J.C.; Khandekar, M.J.; Cohen, P.; Frontini, A.; Bhowmick, D.C.; Ye, L.; Cinti, S.; et al. Zfp423 Expression Identifies Committed Preadipocytes and Localizes to Adipose Endothelial and Perivascular Cells. Cell Metab. 2012, 15, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Yun, U.J.; Song, N.; Yang, D.K.; Kwon, S.; Kim, K.; Kim, S.; Jo, D.; Park, W.J.; Park, K.W.; Kang, H. miR-195a Inhibits Adipocyte Differentiation by Targeting the Preadipogenic Determinator Zfp423. J. Cell. Biochem. 2015, 116, 2589–2597. [Google Scholar] [CrossRef]
- Addison, W.N.; Fu, M.M.; Yang, H.X.; Lin, Z.; Nagano, K.; Gori, F.; Baron, R. Direct Transcriptional Repression of Zfp423 by Zfp521 Mediates a Bone Morphogenic Protein-Dependent Osteoblast versus Adipocyte Lineage Commitment Switch. Mol. Cell. Biol. 2014, 34, 3076–3085. [Google Scholar] [CrossRef] [Green Version]
- Shao, M.; Hepler, C.; Vishvanath, L.; MacPherson, K.A.; Busbuso, N.C.; Gupta, R.K. Fetal development of subcutaneous white adipose tissue is dependent on Zfp423. Mol. Metab. 2016, 6, 111–124. [Google Scholar] [CrossRef]
- Yang, Q.-Y.; Liang, J.-F.; Rogers, C.J.; Zhao, J.-X.; Zhu, M.-J.; Du, M. Maternal Obesity Induces Epigenetic Modifications to Facilitate Zfp423 Expression and Enhance Adipogenic Differentiation in Fetal Mice. Diabetes 2013, 62, 3727–3735. [Google Scholar] [CrossRef] [Green Version]
- Longo, M.; Raciti, G.A.; Zatterale, F.; Parrillo, L.; Desiderio, A.; Spinelli, R.; Hammarstedt, A.; Hedjazifar, S.; Hoffmann, J.M.; Nigro, C.; et al. Epigenetic modifications of the Zfp/ZNF423 gene control murine adipogenic commitment and are dysregulated in human hypertrophic obesity. Diabetologia 2017, 61, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.; Peshavaria, M.; Monga, N.; Jetton, T.L.; Leahy, J.L. Physiologic and Pharmacologic Modulation of Glucose-Dependent Insulinotropic Polypeptide (GIP) Receptor Expression in β-Cells by Peroxisome Proliferator–Activated Receptor (PPAR)-γ Signaling: Possible mechanism for the GIP resistance in type 2 diabetes. Diabetes 2010, 59, 1445–1450. [Google Scholar] [CrossRef] [Green Version]
- Bauer, A.-T.; Von Lukowicz, D.; Lossagk, K.; Hopfner, U.; Kirsch, M.; Moog, P.; Bauer, H.; Machens, H.-G.; Schmauss, D. Adipose Stem Cells from Lipedema and Control Adipose Tissue Respond Differently to Adipogenic Stimulation In Vitro. Plast. Reconstr. Surg. 2019, 144, 623–632. [Google Scholar] [CrossRef]
- Ingle, J.N.; Liu, M.; Wickerham, D.L.; Schaid, D.J.; Wang, L.; Mushiroda, T.; Kubo, M.; Costantino, J.P.; Vogel, V.G.; Paik, S.; et al. Selective Estrogen Receptor Modulators and Pharmacogenomic Variation in ZNF423 Regulation of BRCA1 Expression: Individualized Breast Cancer Prevention. Cancer Discov. 2013, 3, 812–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Ho, M.-F.; Schaid, D.J.; Scherer, S.E.; Kalari, K.; Liu, M.; Biernacka, J.; Yee, V.; Evans, J.; Carlson, E.; et al. Breast cancer chemoprevention pharmacogenomics: Deep sequencing and functional genomics of the ZNF423 and CTSO genes. NPJ Breast Cancer 2017, 3, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szolnoky, G.; Nemes, A.; Gavallér, H.; Forster, T.; Kemény, L. Lipedema is associated with increased aortic stiffness. Lymphology 2012, 45, 71–79. [Google Scholar] [PubMed]
- Herbst, K.L. Subcutaneous Adipose Tissue Diseases: Dercum Disease, Lipedema, Familial Multiple Lipomatosis, and Mad-elung Disease. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Alexander, J.S.; Elrod, J.W. Extracellular matrix, junctional integrity and matrix metalloproteinase interactions in endothelial permeability regulation. J. Anat. 2002, 200, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huang, R.; Chen, L.; Li, S.; Shi, Q.; Jordan, C.; Huang, R.-P. Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int. J. Cancer 2004, 109, 507–515. [Google Scholar] [CrossRef]
- Chamorro-Jorganes, A.; Araldi, E.; Suárez, Y. MicroRNAs as pharmacological targets in endothelial cell function and dysfunction. Pharmacol. Res. 2013, 75, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Aspalter, I.M.; Gordon, E.; Dubrac, A.; Ragab, A.; Narloch, J.; Vizán, P.; Geudens, I.; Collins, R.T.; Franco, C.A.; Abrahams, C.L.; et al. Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat. Commun. 2015, 6, 7264. [Google Scholar] [CrossRef] [Green Version]
- Mouillesseaux, K.P.; Wiley, D.S.; Saunders, L.; Wylie, L.A.; Kushner, E.J.; Chong, D.C.; Citrin, K.M.; Barber, A.T.; Park, Y.; Kim, J.-D.; et al. Notch regulates BMP responsiveness and lateral branching in vessel networks via SMAD6. Nat. Commun. 2016, 7, 13247. [Google Scholar] [CrossRef]
- Xu, Y.; Ouyang, L.; He, L.; Qu, Y.; Han, Y.; Duan, D. Inhibition of exosomal miR-24-3p in diabetes restores angiogenesis and facilitates wound repair via targeting PIK3R3. J. Cell. Mol. Med. 2020, 24, 13789–13803. [Google Scholar] [CrossRef]
- Muluhngwi, P.; Klinge, C. Identification and Roles of miR-29b-1-3p and miR29a-3p-Regulated and Non-Regulated lncRNAs in Endocrine-Sensitive and Resistant Breast Cancer Cells. Cancers 2021, 13, 3530. [Google Scholar] [CrossRef]
- Cao, Z.-G.; Li, J.-J.; Yao, L.; Huang, Y.-N.; Liu, Y.-R.; Chuan-Gui, S.; Song, C.-G.; Shao, Z.-M. High expression of microRNA-454 is associated with poor prognosis in triple-negative breast cancer. Oncotarget 2016, 7, 64900–64909. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Ouyang, X.; Zuo, L.; Xiao, Y.; Sun, Y.; Chang, C.; Qin, X.; Yeh, S. R-2HG downregulates ERα to inhibit cholangiocarcinoma via the FTO/m6A-methylated ERα/miR16-5p/YAP1 signal pathway. Mol. Ther. Oncolytics 2021, 23, 65–81. [Google Scholar] [CrossRef]


| Protein Name | ctrl (A.U. Mean ± SD) | lip (A.U. Mean ± SD) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| IL-8 | 1004 | ± | 639 | 191 | ± | 100 | 0.049 |
| TIMP-1 | 404 | ± | 204 | 856 | ± | 455 | 0.176 |
| TIMP-2 | 287 | ± | 164 | 525 | ± | 309 | 0.287 |
| IL-12p40 | 415 | ± | 364 | 631 | ± | 243 | 0.386 |
| IL-11 | 336 | ± | 135 | 414 | ± | 95 | 0.408 |
| IL-6 | 621 | ± | 99 | 4149 | ± | 6814 | 0.422 |
| MCP-1 | 2717 | ± | 348 | 2998 | ± | 702 | 0.558 |
| bFGF | 249 | ± | 190 | 319 | ± | 168 | 0.627 |
| IL-13 | 105 | ± | 154 | 63 | ± | 75 | 0.648 |
| VEGF-D | 169 | ± | 127 | 217 | ± | 148 | 0.671 |
| IGF-1 | 350 | ± | 310 | 277 | ± | 132 | 0.684 |
| MMP-1 | 5711 | ± | 3794 | 7004 | ± | 4061 | 0.686 |
| uPAR | 285 | ± | 273 | 357 | ± | 198 | 0.700 |
| IGF-2 | 185 | ± | 192 | 231 | ± | 170 | 0.750 |
| TLR3 | 47 | ± | 60 | 64 | ± | 73 | 0.755 |
| HGF | 220 | ± | 214 | 167 | ± | 216 | 0.757 |
| PECAM-1 | 165 | ± | 170 | 134 | ± | 123 | 0.791 |
| MMP-9 | 203 | ± | 178 | 235 | ± | 146 | 0.803 |
| TGF-beta | 264 | ± | 321 | 322 | ± | 309 | 0.821 |
| VEGF-A | 197 | ± | 137 | 227 | ± | 181 | 0.825 |
| IFN-gamma | 276 | ± | 231 | 306 | ± | 133 | 0.834 |
| IL-10 | 127 | ± | 130 | 143 | ± | 106 | 0.860 |
| TLR4 | 90 | ± | 89 | 81 | ± | 61 | 0.880 |
| TPO | 116 | ± | 129 | 104 | ± | 105 | 0.899 |
| IL-4 | 220 | ± | 206 | 238 | ± | 168 | 0.902 |
| IL-1beta | 197 | ± | 163 | 206 | ± | 136 | 0.936 |
| Leptin | 68 | ± | 83 | 66 | ± | 72 | 0.972 |
| EGF | 8696 | ± | 3611 | 8709 | ± | 2820 | 0.996 |
| TNF-alpha | 186 | ± | 169 | 186 | ± | 156 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strohmeier, K.; Hofmann, M.; Jacak, J.; Narzt, M.-S.; Wahlmueller, M.; Mairhofer, M.; Schaedl, B.; Holnthoner, W.; Barsch, M.; Sandhofer, M.; et al. Multi-Level Analysis of Adipose Tissue Reveals the Relevance of Perivascular Subpopulations and an Increased Endothelial Permeability in Early-Stage Lipedema. Biomedicines 2022, 10, 1163. https://doi.org/10.3390/biomedicines10051163
Strohmeier K, Hofmann M, Jacak J, Narzt M-S, Wahlmueller M, Mairhofer M, Schaedl B, Holnthoner W, Barsch M, Sandhofer M, et al. Multi-Level Analysis of Adipose Tissue Reveals the Relevance of Perivascular Subpopulations and an Increased Endothelial Permeability in Early-Stage Lipedema. Biomedicines. 2022; 10(5):1163. https://doi.org/10.3390/biomedicines10051163
Chicago/Turabian StyleStrohmeier, Karin, Martina Hofmann, Jaroslaw Jacak, Marie-Sophie Narzt, Marlene Wahlmueller, Mario Mairhofer, Barbara Schaedl, Wolfgang Holnthoner, Martin Barsch, Matthias Sandhofer, and et al. 2022. "Multi-Level Analysis of Adipose Tissue Reveals the Relevance of Perivascular Subpopulations and an Increased Endothelial Permeability in Early-Stage Lipedema" Biomedicines 10, no. 5: 1163. https://doi.org/10.3390/biomedicines10051163
APA StyleStrohmeier, K., Hofmann, M., Jacak, J., Narzt, M.-S., Wahlmueller, M., Mairhofer, M., Schaedl, B., Holnthoner, W., Barsch, M., Sandhofer, M., Wolbank, S., & Priglinger, E. (2022). Multi-Level Analysis of Adipose Tissue Reveals the Relevance of Perivascular Subpopulations and an Increased Endothelial Permeability in Early-Stage Lipedema. Biomedicines, 10(5), 1163. https://doi.org/10.3390/biomedicines10051163






