Antineoplastic Properties by Proapoptotic Mechanisms Induction of Inula viscosa and Its Sesquiterpene Lactones Tomentosin and Inuviscolide
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Inula viscosa: Antineoplastic Activities and Molecular Mechanisms
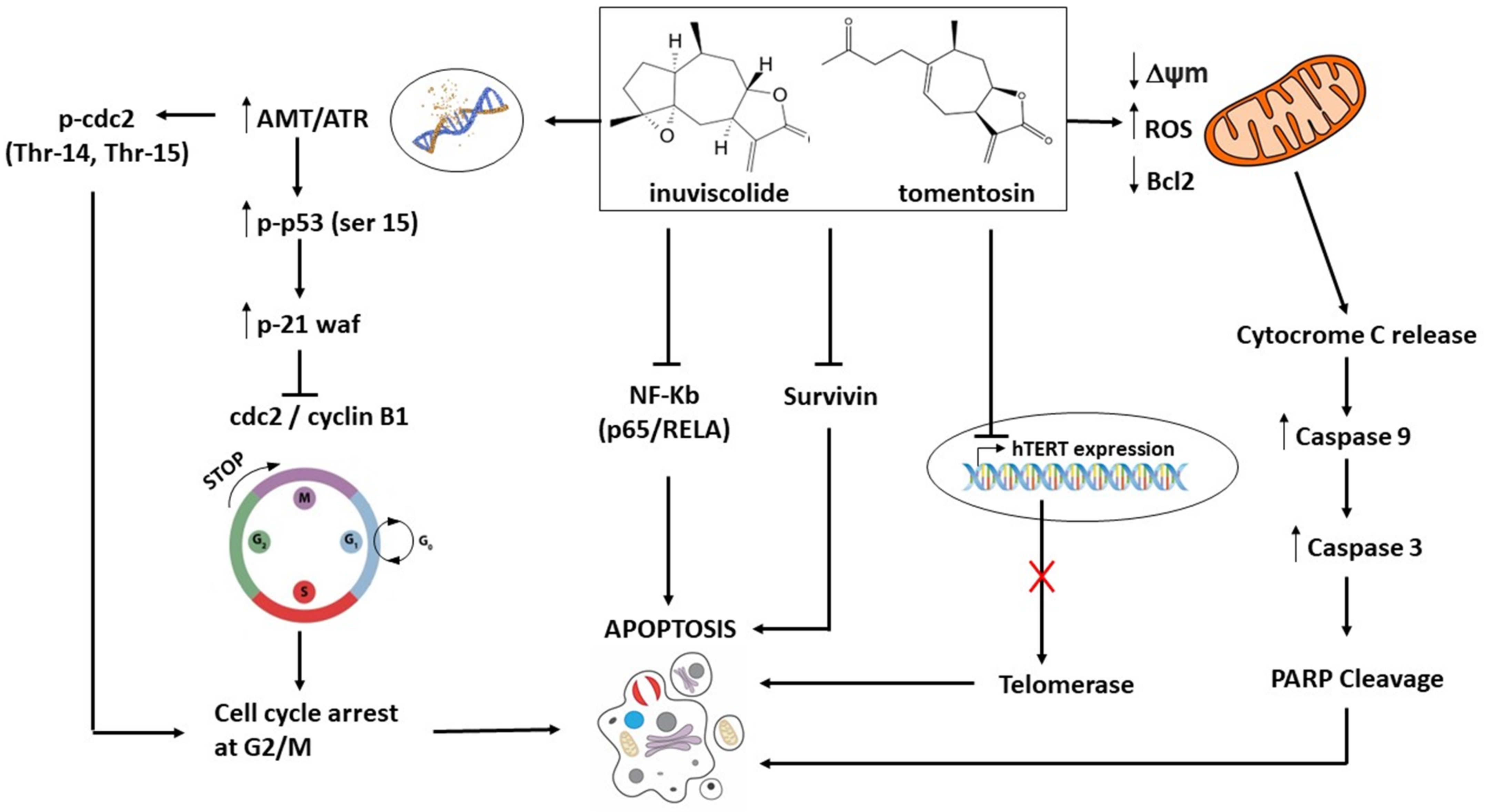
3.2. Sesquiterpene Lactones Tomentosin and Inuviscolide: Antineoplastic Activities and Molecular Mechanisms
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Çelik, T.A.; Aslantürk, Ö.S. Evaluation of cytotoxicity and genotoxicity of Inula viscosa leaf extracts with Allium test. J. Biomed. Biotechnol. 2010, 2010, 189252. [Google Scholar] [CrossRef]
- Wang, G.W.; Qin, J.J.; Cheng, X.R.; Shen, Y.H.; Shan, L.; Jin, H.Z.; Zhang, W.D. Inula sesquiterpenoids: Structural diversity, cytotoxicity and anti-tumor activity. Expert Opin. Investig. Drugs 2014, 23, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Barbetti, P.; Chiappini, I.; Fardella, G.; Menghini, A. A New Eudesmane Acid from Dittrichia (Inula) viscosa. Planta Med. 1985, 51, 471. [Google Scholar] [CrossRef] [PubMed]
- Lauro, L.; Rolih, C. Observations and research on an extract of Inula viscosa Ait. Boll. Soc. Ital. Biol Sper. 1990, 66, 829–834. [Google Scholar] [PubMed]
- Lev, E.; Amar, Z. Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J. Ethnopharmacol. 2000, 72, 191–205. [Google Scholar] [CrossRef]
- Al-Qura’n, S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009, 123, 45–50. [Google Scholar] [CrossRef]
- Yaniv, Z.; Dafni, A.; Friedman, J.; Palevitch, D. Plants used for the treatment of diabetes in Israel. J. Ethnopharmacol. 1987, 19, 145–151. [Google Scholar] [CrossRef]
- Hernández, V.; Recio, M.C.; Máñez, S.; Giner, R.M.; Ríos, J.L. Effects of naturally occurring dihydroflavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci. 2007, 81, 480–488. [Google Scholar] [CrossRef]
- Máñez, S.; Hernández, V.; Giner, R.M.; Ríos, J.L.; Recio, M.d.C. Inhibition of pro-inflammatory enzymes by inuviscolide, a sesquiterpene lactone from Inula viscosa. Fitoterapia 2007, 78, 329–331. [Google Scholar] [CrossRef]
- Khan, A.L.; Hussain, J.; Hamayun, M.; Gilani, S.A.; Ahmad, S.; Rehman, G.; Kim, Y.H.; Kang, S.M.; Lee, I.J. Secondary Metabolites from Inula britannica L. and Their Biological Activities. Molecules 2010, 15, 1562. [Google Scholar] [CrossRef]
- Kheyar-Kraouche, N.; da Silva, A.B.; Serra, A.T.; Bedjou, F.; Bronze, M.R. Characterization by liquid chromatography-mass spectrometry and antioxidant activity of an ethanolic extract of Inula viscosa leaves. J. Pharm. Biomed. Anal. 2018, 156, 297–306. [Google Scholar] [CrossRef]
- Rozenblat, S.; Grossman, S.; Bergman, M.; Gottlieb, H.; Cohen, Y.; Dovrat, S. Induction of G2/M arrest and apoptosis by sesquiterpene lactones in human melanoma cell lines. Biochem. Pharmacol. 2008, 75, 369–382. [Google Scholar] [CrossRef]
- Benbacer, L.; Merghoub, N.; El Btaouri, H.; Gmouh, S.; Attaleb, M.; Morjani, H.; Amzazi, S.; El Mzibri, M. Antiproliferative Effect and Induction of Apoptosis by Inula viscosa L. and Retama monosperma L. Extracts in Human Cervical Cancer Cells. In Topics on Cervical Cancer with an Advocacy for Prevention; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Messaoudi, M.; Chahmi, N.; El-Mzibri, M.; Gmouh, S.; Amzazi, S.; Benbacer, L.; El-Hassouni, M. Cytotoxic Effect and Chemical Composition of Inula viscosa from Three Different Regions of Morocco. Eur. J. Med. Plants 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Jafari, N.; Zargar, S.J.; Delnavazi, M.-R.; Yassa, N. Cell Cycle Arrest and Apoptosis Induction of Phloroacetophenone Glycosides and Caffeoylquinic Acid Derivatives in Gastric Adenocarcinoma (AGS) Cells. Anticancer Agents Med. Chem. 2018, 18, 610–616. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019, 12, 55. [Google Scholar] [CrossRef]
- Kuo, P.C.; Liu, H.F.; Chao, J.I. Survivin and p53 modulate quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma cells. J. Biol. Chem. 2004, 279, 55875–55885. [Google Scholar] [CrossRef]
- Ong, C.S.; Tran, E.; Nguyen, T.T.T.; Ong, C.K.; Lee, S.K.; Lee, J.J.; Ng, C.P.; Leong, C.; Huynh, H. Quercetin-induced growth inhibition and cell death in nasopharyngeal carcinoma cells are associated with increase in Bad and hypophosphorylated retinoblastoma expressions. Oncol. Rep. 2004, 11, 727–733. [Google Scholar] [CrossRef]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef]
- Li, Q.; Ren, F.Q.; Yang, C.L.; Zhou, L.M.; Liu, Y.Y.; Xiao, J.; Zhu, L.; Wang, Z.G. Anti-proliferation effects of isorhamnetin on lung cancer cells in vitro and in vivo. Asian Pac. J. Cancer Prev. 2015, 16, 3035–3042. [Google Scholar] [CrossRef]
- Xiang, P.; Guo, X.; Han, Y.Y.; Gao, J.M.; Tang, J.J. Cytotoxic and Pro-apoptotic Activities of Sesquiterpene Lactones from Inula britannica. Nat. Prod. Commun. 2016, 11, 7–10. [Google Scholar] [CrossRef]
- Quintana, J.; Estévez, F. Recent Advances on Cytotoxic Sesquiterpene Lactones. Curr. Pharm. Des. 2019, 24, 4355–4361. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Lee, J.; Nam, M.J.; Choi, Y.S.; Park, S.H. Tomentosin displays anti-carcinogenic effect in human osteosarcoma MG-63 cells via the induction of intracellular reactive oxygen species. Int. J. Mol. Sci. 2019, 20, 1508. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, X.; Liu, Y.; Gong, N.; Yao, W.; Chen, P.; Qin, J.; Jin, H.; Li, J.; Chu, R.; et al. Japonicone A Suppresses Growth of Burkitt Lymphoma Cells through Its Effect on NF-κB. Clin. Cancer Res. 2013, 19, 2917–2928. [Google Scholar] [CrossRef]
- Merghoub, N.; El Btaouri, H.; Benbacer, L.; Gmouh, S.; Trentesaux, C.; Brassart, B.; Attaleb, M.; Madoulet, C.; Wenner, T.; Amzazi, S.; et al. Tomentosin Induces Telomere Shortening and Caspase-Dependant Apoptosis in Cervical Cancer Cells. J. Cell. Biochem. 2017, 118, 1689–1698. [Google Scholar] [CrossRef]
- Cardellina, J.H.; Fuller, R.W.; Gamble, W.R.; Westergaard, C.; Boswell, J.; Munro, M.H.G.; Currens, M.; Boyd, M.R. Evolving Strategies for the Selection, Dereplication and Prioritization of Antitumor and HIV-Inhibitory Natural Products Extracts. In Bioassay Methods in Natural Product Research and Drug Development; Springer: Berlin/Heidelberg, Germany, 1999; pp. 25–35. [Google Scholar] [CrossRef]
- Merghoub, N.; El Btaouri, H.; Benbacer, L.; Gmouh, S.; Trentesaux, C.; Brassart, B.; Terryn, C.; Attaleb, M.; Madoulet, C.; Benjouad, A.; et al. Inula viscosa extracts induces telomere shortening and apoptosis in cancer cells and overcome drug resistance. Nutr. Cancer 2016, 68, 131–143. [Google Scholar] [CrossRef]
- Chahmi, N.; Anissi, J.; Jennan, S.; Farah, A.; Sendide, K.; El Hassouni, M. Antioxidant activities and total phenol content of Inula viscosa extracts selected from three regions of Morocco. Asian Pac. J. Trop. Biomed. 2015, 5, 228–233. [Google Scholar] [CrossRef]
- Talib, W.H.; Mahasneh, A.M. Antiproliferative Activity of Plant Extracts Used against Cancer in Traditional Medicine. Sci. Pharm. 2010, 78, 33–46. [Google Scholar] [CrossRef]
- Hepokur, C.; Budak, Y.; Karayel, H.B.; Selvì, B.; Yaylim, İ. Investigation of Cytotoxic Effects of Inula viscosa Extract. Cumhur. Sci. J. 2019, 40, 578–582. [Google Scholar] [CrossRef][Green Version]
- Bar-Shalom, R.; Bergman, M.; Grossman, S.; Azzam, N.; Sharvit, L.; Fares, F. Inula viscosa Extract Inhibits Growth of Colorectal Cancer Cells in vitro and in vivo Through Induction of Apoptosis. Front. Oncol. 2019, 9, 227. [Google Scholar] [CrossRef]
- Virdis, P.; Migheli, R.; Galleri, G.; Fancello, S.; Cadoni, M.P.L.; Pintore, G.; Petretto, G.L.; Marchesi, I.; Fiorentino, F.P.; Di Francesco, A.; et al. Antiproliferative and proapoptotic effects of Inula viscosa extract on Burkitt lymphoma cell line. Tumor Biol. 2020, 42, 1010428319901061. [Google Scholar] [CrossRef]
- Colak, D.K.; Egeli, U.; Eryilmaz, I.E.; Aybastier, O.; Malyer, H.; Cecener, G.; Tunca, B. The Anticancer Effect of Inula viscosa Methanol Extract by miRNAs’ Re-regulation: An in vitro Study on Human Malignant Melanoma Cells. Nutr. Cancer 2021, 74, 211–224. [Google Scholar] [CrossRef]
- Meyer-Schwesinger, C. The ubiquitin–proteasome system in kidney physiology and disease. Nat. Rev. Nephrol. 2019, 15, 393–411. [Google Scholar] [CrossRef]
- Koper-Lenkiewicz, O.M.; Kamińska, J.; Reszeć, J.; Dymicka-Piekarska, V.; Ostrowska, H.; Karpińska, M.; Matowicka-Karna, J.; Tylicka, M. Elevated plasma 20S proteasome chymotrypsin-like activity is correlated with IL-8 levels and associated with an increased risk of death in glial brain tumor patients. PLoS ONE 2020, 15, e0238406. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Proteasome expression and activity in cancer and cancer stem cells. Tumor Biol. 2017, 39, 1010428317692248. [Google Scholar] [CrossRef]
- Berryman, K.; Buhimschi, C.S.; Zhao, G.; Axe, M.; Locke, M.; Buhimschi, I.A. Proteasome Levels and Activity in Pregnancies Complicated by Severe Preeclampsia and Hemolysis, Elevated Liver Enzymes, and Thrombocytopenia (HELLP) Syndrome. Hypertens 2019, 73, 1308–1318. [Google Scholar] [CrossRef]
- El Yaagoubi, O.M.; Lahmadi, A.; Bouyahya, A.; Filali, H.; Samaki, H.; El Antri, S.; Aboudkhil, S. Antitumor effect of Inula viscosa extracts on DMBA-induced skin carcinoma are mediated by proteasome inhibition. BioMed Res. Int. 2021, 2021, 6687589. [Google Scholar] [CrossRef]
- Belayachi, L.; Aceves-Luquero, C.; Merghoub, N.; Bakri, Y.; de Mattos, S.F.; Amzazi, S.; Villalonga, P. Screening of North African Medicinal Plant Extracts for Cytotoxic Activity Against Tumor Cell Lines. Eur. J. Med. Plants 2013, 3, 310–332. [Google Scholar] [CrossRef]
- Schmidt, T.J. Toxic activities of sesquiterpene lactones: Structural and biochemical aspects. Curr. Org. Chem. 1999, 3, 577–608. [Google Scholar]
- Woynarowski, J.M.; Konopa, J. Inhibition of DNA Biosynthesis in HeLa Cells by Cytotoxic and Antitumor Sesquiterpene Lactones. Mol. Pharmacol. 1981, 19, 97–102. [Google Scholar]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Grossman, D.; McNiff, J.M.; Li, F.; Altieri, D.C. Expression and targeting of the apoptosis inhibitor, survivin, in human melanoma. J. Investig. Dermatol. 1999, 113, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Wang, Q.; Wang, R.; Deng, D.; Xue, L.; Shao, N.; Zhang, Y.; Xia, X.; Zhi, F.; Yang, Y. Tubeimoside-1 induces glioma apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome C/Caspase-3 pathway. OncoTargets Ther. 2015, 8, 303. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhang, J.; Sun, L.L.; Li, B.H.; Gao, H.L.; Xie, T.; Zhang, N.; Ye, Z.M. Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: An in vitro and in vivo study. Cell Death Dis. 2015, 6, e1604. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, Y.H.; Zheng, X.W.; Liu, Y.J.; Zhang, H.; Fang, L.; Zhang, Y.W.; Yang, C.; Islam, K.; Wang, C.; et al. Phenylarsine oxide (PAO) induces apoptosis in HepG2 cells via ROS-mediated mitochondria and ER-stress dependent signaling pathways. Metallomics 2017, 9, 1756–1764. [Google Scholar] [CrossRef]
- Yu, S.H.; Lee, C.M.; Ha, S.H.; Lee, J.; Jang, K.Y.; Park, S.H. Induction of cell cycle arrest and apoptosis by tomentosin in hepatocellular carcinoma HepG2 and Huh7 cells. Hum. Exp. Toxicol. 2021, 40, 231–244. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, H.; Dong, X.; Yang, Z.; Chang, W. Tomentosin induces apoptotic pathway by blocking inflammatory mediators via modulation of cell proteins in AGS gastric cancer cell line. J. Biochem. Mol. Toxicol. 2020, 34, e22501. [Google Scholar] [CrossRef]
- Virdis, P.; Marchesi, I.; Fiorentino, F.P.; Migheli, R.; Sanna, L.; Bordoni, V.; Pintore, G.; Galleri, G.; Muroni, M.R.; Bagella, L.; et al. Tomentosin a sesquiterpene lactone induces antiproliferative and proapoptotic effects in human Burkitt lymphoma by deregulation of anti- and pro-apoptotic genes. Life 2021, 11, 1128. [Google Scholar] [CrossRef]
- Virdis, P.; Migheli, R.; Bordoni, V.; Fiorentino, F.P.; Sanna, L.; Marchesi, I.; Pintore, G.; Galleri, G.; Muroni, M.R.; Bagella, L.; et al. Clarifying the molecular mechanism of tomentosin-induced antiproliferative and proapoptotic effects in human multiple myeloma via gene expression profile and genetic interaction network analysis. Int. J. Mol. Med. 2021, 48, 213. [Google Scholar] [CrossRef]
- Yang, L.; Xie, J.; Almoallim, H.S.; Alharbi, S.A.; Chen, Y. Tomentosin inhibits cell proliferation and induces apoptosis in MOLT-4 leukemia cancer cells through the inhibition of mTOR/PI3K/Akt signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22719. [Google Scholar] [CrossRef]
- Güçlü, E.; Çınar Ayan, İ.; Dursun, H.G.; Vural, H. Tomentosin induces apoptosis in pancreatic cancer cells through increasing reactive oxygen species and decreasing mitochondrial membrane potential. Toxicol. In Vitro 2022, 84, 105458. [Google Scholar] [CrossRef]
- Fan, T.; Sun, G.; Sun, X.; Zhao, L.; Zhong, R.; Peng, Y. Tumor Energy Metabolism and Potential of 3-Bromopyruvate as an Inhibitor of Aerobic Glycolysis: Implications in Tumor Treatment. Cancers 2019, 11, 317. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, G.; Sun, X.; Li, F.; Zhao, L.; Zhong, R.; Peng, Y. The Potential of Lonidamine in Combination with Chemotherapy and Physical Therapy in Cancer Treatment. Cancers 2020, 12, 3332. [Google Scholar] [CrossRef]
| Type of Cancer | Treatment | Model | Biological and Molecular Effects | Ref. |
|---|---|---|---|---|
| Cervical cancer | Hexane extract from 15.6 to 500 μg/mL for 48 or 72 h | SiHa and HeLa cell lines | Cell growth inhibition and apoptosis induction. Decrease ΔΨm and intracellular ROS production | [13] |
| Cervical cancer | Hexane and methanol extract from 5 to 80 μg/mL for 72 h | SiHa and HeLa cell lines | Cell growth inhibition and apoptosis induction. Inhibition of telomerase activity and induction of telomere shortening | [28] |
| Breast cancer | Ethyl acetate and ethanolic extract from 15.6 to 500 μg/mL for 72 h | MCF-7 and MDA-MB231 cell lines | Cytotoxic effect | [14] |
| Colorectal cancer | Aqueous extract from 100 to 300 μg/mL for 24–72 h | HCT116 and colo320 cell lines | Reduction in cell viability. Apoptosis induction through the intrinsic mitochondrial pathway in well-differentiated cells and through both, the intrinsic and extrinsic pathways in poorly differentiated cells | [32] |
| Aqueous extract 150 or 300 mg/kg | C57BL/6 mice transplanted with MC38 cells | Inhibition of tumor growth | ||
| Burkitt lymphoma | Ethanolic extract: 5, 10, 20, 30, 40, 60, and 80 mg/mL for 24 and 48 h | Raji cell line | Cell cycle arrest in the G2/M phase, decreased cell viability and increased cell apoptosis. Downregulation of genes involved in cell cycle and proliferation (c-MYC, CCND1) and in the inhibition of cell apoptosis (BCL2, BCL2L1, BCL11A) | [33] |
| Malignant melanoma | Aqueous and methanolic extracts from 10 µg/mL to 140 µg/mL for 24–72 h | A2058 and MeWo cell lines | Antiproliferative effect by induction of apoptosis and cell cycle arrest, suppression of cell migration. Deregulation of oncogenic and oncosuppressive miRNAs | [34] |
| Skin carcinogenesis | Ethanolic extract 100 μL for 4 days | Swiss albino mice treated with DMBA/croton oil | Inhibition of the development of papilloma. I. viscosa extract induces inhibition on the subunits of the proteasome, as well as decrease in the concentration of proteasome and its catalytic activity in serum and intracellularly. | [39] |
| Type of Cancer | Treatment | Model | Biological and Molecular Effects | Ref. |
|---|---|---|---|---|
| Melanoma | Tomentosin and Inuviscolide 9–36 mM for 24 h | SK-28, 624 mel, and 1363 mel cell lines | Cell cycle arrest at the G2/M phase and apoptosis. Activation of ATM/R followed by phosphorylation of TP53 and CDC2 and p21waf1 overexpression. Decrease of Survivin and of NF-kB | [12] |
| Cervical cancer | Tomentosin 0–100 mM for 24, 48, 72, and 96 h | HeLa and SiHa cell lines | Cell cycle arrest and apoptosis. Increased ROS and decrease in mitochondrial membrane potential. Telomeric G-overhang shortening | [26] |
| Osteosarcoma | Tomentosin 0, 10, 20, and 40 µM for 24 and 48 h | MG-63 cell line | Decreased cell viability and migration, apoptosis, cell cycle arrest. Increase of ROS induces FOXO3 and p27 overexpression. Decrease of peroxiredoxin-1 | [24] |
| Gastric cancer | Tomentosin from 5 to 30 μM for 24 h | AGS cell line | Apoptosis via mitochondria-mediated signaling pathway induced by increase of ROS. PCNA and Cyclin D1 downregulation. BAX overexpression and BCL-2 downregulation. Inhibition of inflammation | [51] |
| Hepatocellular carcinoma | Tomentosin 0,10, 20 and 40 μM for 24 and 48 h | HepG2 and Huh7 cell lines | G2/M phase cell cycle arrest through p27 overexpression regulated by upregulation of FOXO3 | [50] |
| Leukemia | Tomentosin 0–25 μM for up to 48 h | MOLT-4 cell line | Apoptosis via mitochondria-mediated signaling pathway induced by increase of ROS. Suppression of NF-κB and proinflammatory cytokines. mTOR/PI3K/AKT pathway activation | [54] |
| Burkitt lymphoma | Tomentosin 50, 25, 12.5, 6.25, 3.125, 1.56 and 0.75 μM for 24 h | Raji cell line | Cell proliferation inhibition and cell apoptosis induction. Induction of apoptosis by upregulation of the PERK/eIF2a/ATF4/DDIT3 pathway | [52] |
| Multiple myeloma | Tomentosin 50, 25, 12.5, 6.25, 3.125, 1.56 and 0.75 μM for 24 h | RPMI-8226 cell line | Cell proliferation inhibition and cell apoptosis induction. Downregulation of anti-apoptotic genes such as BCL2A1 and CDKN1A and upregulation of the proapoptotic PMAIP1 gene | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migheli, R.; Virdis, P.; Galleri, G.; Arru, C.; Lostia, G.; Coradduzza, D.; Muroni, M.R.; Pintore, G.; Podda, L.; Fozza, C.; et al. Antineoplastic Properties by Proapoptotic Mechanisms Induction of Inula viscosa and Its Sesquiterpene Lactones Tomentosin and Inuviscolide. Biomedicines 2022, 10, 2739. https://doi.org/10.3390/biomedicines10112739
Migheli R, Virdis P, Galleri G, Arru C, Lostia G, Coradduzza D, Muroni MR, Pintore G, Podda L, Fozza C, et al. Antineoplastic Properties by Proapoptotic Mechanisms Induction of Inula viscosa and Its Sesquiterpene Lactones Tomentosin and Inuviscolide. Biomedicines. 2022; 10(11):2739. https://doi.org/10.3390/biomedicines10112739
Chicago/Turabian StyleMigheli, Rossana, Patrizia Virdis, Grazia Galleri, Caterina Arru, Giada Lostia, Donatella Coradduzza, Maria Rosaria Muroni, Giorgio Pintore, Luigi Podda, Claudio Fozza, and et al. 2022. "Antineoplastic Properties by Proapoptotic Mechanisms Induction of Inula viscosa and Its Sesquiterpene Lactones Tomentosin and Inuviscolide" Biomedicines 10, no. 11: 2739. https://doi.org/10.3390/biomedicines10112739
APA StyleMigheli, R., Virdis, P., Galleri, G., Arru, C., Lostia, G., Coradduzza, D., Muroni, M. R., Pintore, G., Podda, L., Fozza, C., & De Miglio, M. R. (2022). Antineoplastic Properties by Proapoptotic Mechanisms Induction of Inula viscosa and Its Sesquiterpene Lactones Tomentosin and Inuviscolide. Biomedicines, 10(11), 2739. https://doi.org/10.3390/biomedicines10112739







