Endothelial Progenitor Cells in Neurovascular Disorders—A Comprehensive Overview of the Current State of Knowledge
Abstract
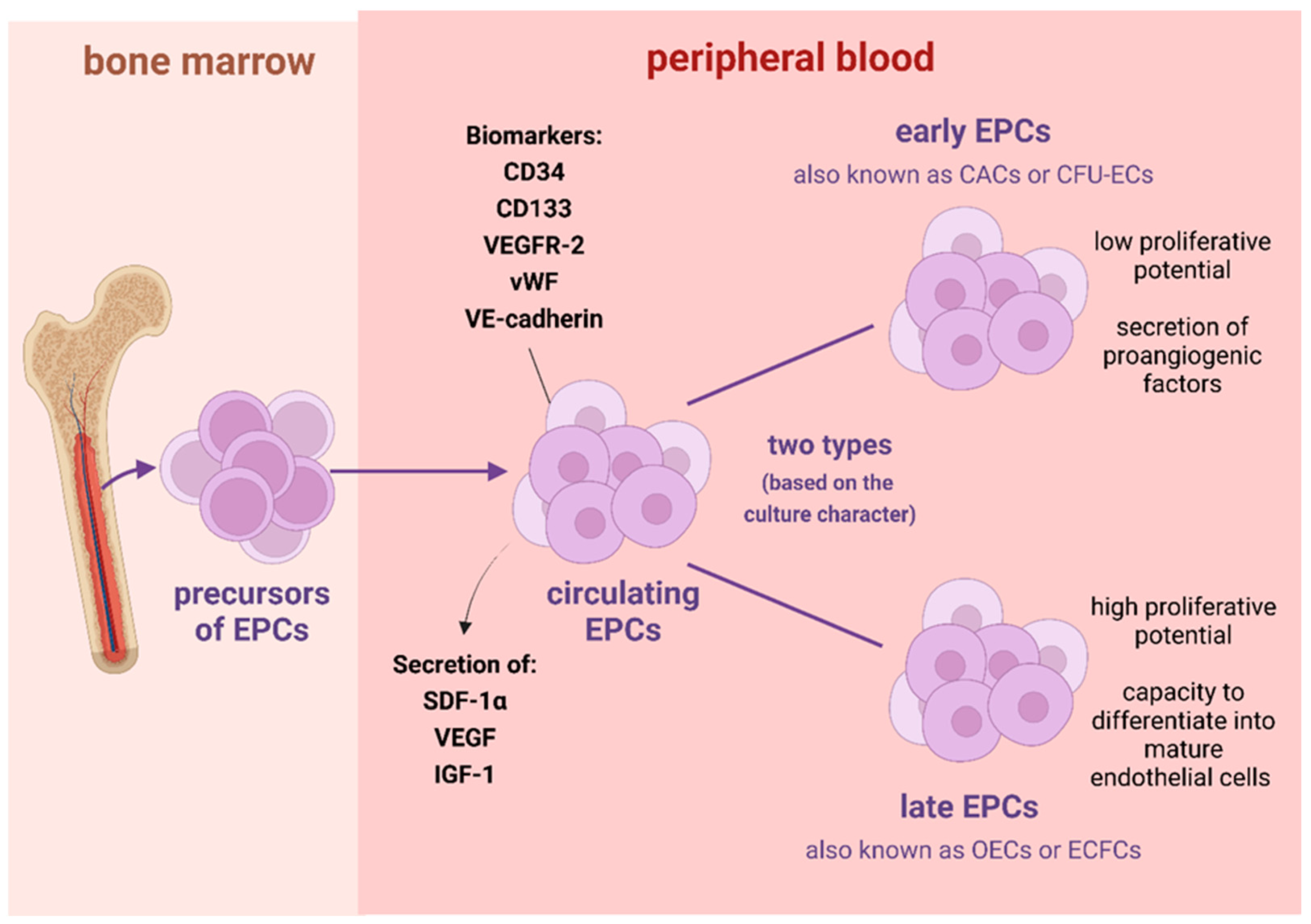
:1. Introduction
2. Alzheimer’s Disease
3. Cerebral Small Vessel Disease
4. Ischemic Stroke
5. Migraine
6. Conclusions
Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations (In Alphabetical Order)
| AD | Alzheimer’s disease |
| APP | amyloid precursor protein |
| APP/PS1 | amyloid precursor protein/presenilin 1 |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| BMECs | brain microvascular endothelial cells |
| BM-EPCs | bone marrow-derived endothelial progenitor cells |
| CACs | circulatory angiogenic cells |
| CBF | cerebral blood flow |
| CFU-ECs | colony-forming unit endothelial cells |
| cGMP | cyclic guanosine monophosphate |
| CM | conditioned media |
| CSVD | cerebral small vessel disease |
| ECFCs | endothelial colony-forming cells |
| EMPs | endothelial microparticles |
| EPCs | endothelial progenitor cells |
| EPC-CM | endothelial progenitor cells-derived conditioned media |
| EPO | erythropoietin |
| eNOS | endothelial nitric oxide synthase |
| eEPCs | embryonic endothelial progenitor cells |
| e-EPCs | early endothelial progenitor cells |
| FGF | fibroblast growth factor |
| GFP | green fluorescent protein |
| IGF-1 | insulin-like growth factor 1 |
| IL-1β | interleukin-1β |
| IL-10 | interleukin-10 |
| IS | ischemic stroke |
| l-EPCs | late EPCs |
| NGF | nerve growth factor |
| NO | nitric oxide |
| OEC-CM | outgrowth endothelial cell-derived conditioned media |
| OECs | outgrowth endothelial cells |
| PBSCs | peripheral blood hematopoietic stem cells |
| SDF-1α | stromal cell-derived factor-1α |
| SSS | Scandinavia Stroke Scale |
| TNF-α | tumor necrosis factor-α |
| TTH | tension type headache |
| VD | vascular dementia |
| VE-cadherin | vascular endothelial cadherin |
| VEGF | vascular endothelial growth factor |
| VEGFR-2 | vascular endothelial growth factor receptor-2 |
| vWF | von Willebrand factor |
References
- Hristov, M.; Erl, W.; Weber, P.C. Endothelial progenitor cells: Isolation and characterization. Trends Cardiovasc. Med. 2003, 13, 201–206. [Google Scholar] [CrossRef]
- Yoder, M.C. Human endothelial progenitor cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006692. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Mead, L.E.; Moore, D.B.; Woodard, W.; Fenoglio, A.; Yoder, M.C. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 2005, 105, 2783–2786. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.H.; Li, Z.M.; Du, Z.M.; Zhang, A.X.; Rana, J.S.; Liu, D.H.; Yang, D.Y.; Wu, G.F. Expanded human cord blood-derived endothelial progenitor cells salvage infarcted myocardium in rats with acute myocardial infarction. Clin. Exp. Pharm. Physiol. 2010, 37, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Imitola, J.; Raddassi, K.; Park, K.I.; Mueller, F.J.; Nieto, M.; Teng, Y.D.; Frenkel, D.; Li, J.; Sidman, R.L.; Walsh, C.A.; et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 18117–18122. [Google Scholar] [CrossRef] [Green Version]
- He, X.Y.; Chen, Z.Z.; Cai, Y.Q.; Xu, G.; Shang, J.H.; Kou, S.B.; Li, M.; Zhang, H.T.; Duan, C.Z.; Zhang, S.Z.; et al. Expression of cytokines in rat brain with focal cerebral ischemia after grafting with bone marrow stromal cells and endothelial progenitor cells. Cytotherapy 2011, 13, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Rouhl, R.P.; van Oostenbrugge, R.J.; Damoiseaux, J.; Tervaert, J.W.; Lodder, J. Endothelial progenitor cell research in stroke: A potential shift in pathophysiological and therapeutical concepts. Stroke 2008, 39, 2158–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadini, G.P.; Losordo, D.; Dimmeler, S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ. Res. 2012, 110, 624–637. [Google Scholar] [CrossRef] [Green Version]
- Hur, J.; Yoon, C.H.; Kim, H.S.; Choi, J.H.; Kang, H.J.; Hwang, K.K.; Oh, B.H.; Lee, M.M.; Park, Y.B. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arter. Thromb. Vasc. Biol. 2004, 24, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, S.; Yang, Z.; Wyler von Ballmoos, M.; Voelzmann, J.; Diehm, N.; Baumgartner, I.; Kalka, C. Novel cell-free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. PLoS ONE 2009, 4, e5643. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.R.; Thompson, K.A.; Isaac, K.; Vecchiarelli, J.; Zhang, Q.; Stewart, D.J.; Kutryk, M.J. Nitric oxide synthase gene transfer restores activity of circulating angiogenic cells from patients with coronary artery disease. Mol. Ther. 2011, 19, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Chang, S.J.; Chueh, Y.N.; Huang, T.S.; Huang, P.H.; Cheng, S.M.; Tsai, T.N.; Chen, J.W.; Wang, H.W. Distinct angiogenesis roles and surface markers of early and late endothelial progenitor cells revealed by functional group analyses. BMC Genom. 2013, 14, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, R.J.; O’Neill, C.L.; Sweeney, M.; Guduric-Fuchs, J.; Gardiner, T.A.; Simpson, D.A.; Stitt, A.W. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med. Genom. 2010, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Sieveking, D.P.; Buckle, A.; Celermajer, D.S.; Ng, M.K. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: Insights from a novel human angiogenesis assay. J. Am. Coll. Cardiol. 2008, 51, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, F.; Van Hauwermeiren, F.; De Smedt, M.; Raedt, R.; Plasschaert, F.; De Buyzere, M.L.; Gillebert, T.C.; Plum, J.; Vandekerckhove, B. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1572–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glynn, J.J.; Hinds, M.T. Endothelial outgrowth cells: Function and performance in vascular grafts. Tissue Eng. Part B Rev. 2014, 20, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Martin-Ramirez, J.; Hofman, M.; van den Biggelaar, M.; Hebbel, R.P.; Voorberg, J. Establishment of outgrowth endothelial cells from peripheral blood. Nat. Protoc. 2012, 7, 1709–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bou Khzam, L.; Bouchereau, O.; Boulahya, R.; Hachem, A.; Zaid, Y.; Abou-Saleh, H.; Merhi, Y. Early outgrowth cells versus endothelial colony forming cells functions in platelet aggregation. J. Transl. Med. 2015, 13, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinisch, A.; Hofmann, N.A.; Obenauf, A.C.; Kashofer, K.; Rohde, E.; Schallmoser, K.; Flicker, K.; Lanzer, G.; Linkesch, W.; Speicher, M.R.; et al. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood 2009, 113, 6716–6725. [Google Scholar] [CrossRef] [Green Version]
- Yoder, M.C.; Mead, L.E.; Prater, D.; Krier, T.R.; Mroueh, K.N.; Li, F.; Krasich, R.; Temm, C.J.; Prchal, J.T.; Ingram, D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007, 109, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Hristov, M.; Erl, W.; Weber, P.C. Endothelial progenitor cells: Mobilization, differentiation, and homing. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1185–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Custodia, A.; Ouro, A.; Romaus-Sanjurjo, D.; Pías-Peleteiro, J.M.; de Vries, H.E.; Castillo, J.; Sobrino, T. Endothelial Progenitor Cells and Vascular Alterations in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 13, 811210. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Yu, S.; Feng, J. Advances in the Role of Endothelial Cells in Cerebral Small Vessel Disease. Front. Neurol. 2022, 13, 861714. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, M.; Altamura, C.; Vernieri, F. The Role of Endothelial Dysfunction in the Pathophysiology and Cerebrovascular Effects of Migraine: A Narrative Review. J. Clin. Neurol. 2021, 17, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Daidone, M.; Pinto, A. Endothelial Dysfunction and Inflammation in Ischemic Stroke Pathogenesis. Curr. Pharm. Des. 2020, 26, 4209–4219. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.B.; Arnold, S.E.; Raible, K.; Brettschneider, J.; Xie, S.X.; Grossman, M.; Monsell, S.E.; Kukull, W.A.; Trojanowski, J.Q. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 2013, 136 Pt 9, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Parodi-Rullán, R.; Sone, J.Y.; Fossati, S. Endothelial Mitochondrial Dysfunction in Cerebral Amyloid Angiopathy and Alzheimer’s Disease. J. Alzheimers. Dis. 2019, 72, 1019–1039. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, R.A.; Bosetti, F.; Emr, M.; Gladman, J.T.; Koenig, J.I.; Moy, C.S.; Pahigiannis, K.; Waddy, S.P.; Koroshetz, W. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cell Mol. Neurobiol. 2016, 36, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Maler, J.M.; Spitzer, P.; Lewczuk, P.; Kornhuber, J.; Herrmann, M.; Wiltfang, J. Decreased circulating CD34+ stem cells in early Alzheimer’s disease: Evidence for a deficient hematopoietic brain support? Mol. Psychiatry 2006, 11, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.D.; Zhang, Y.; Liu, L.; Sun, N.; Zhang, M.Y.; Zhang, J.N. Endothelial progenitor cells with Alzheimer’s disease. Chin. Med. J. 2011, 124, 901–906. [Google Scholar] [PubMed]
- Lee, S.T.; Chu, K.; Jung, K.H.; Park, H.K.; Kim, D.H.; Bahn, J.J.; Kim, J.H.; Oh, M.J.; Lee, S.K.; Kim, M.; et al. Reduced circulating angiogenic cells in Alzheimer disease. Neurology 2009, 72, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Breining, A.; Silvestre, J.S.; Dieudonné, B.; Vilar, J.; Faucounau, V.; Verny, M.; Néri, C.; Boulanger, C.M.; Boddaert, J. Biomarkers of vascular dysfunction and cognitive decline in patients with Alzheimer’s disease: No evidence for association in elderly subjects. Aging Clin. Exp. Res. 2016, 28, 1133–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haiyuan, L.; Xue, X.; Min, L.; Lingyu, W.; Xianlin, G.; Hancong, S.; Qiulei, C.; Jia, X. Study of quantity and function of endothelial progenitor cells in peripheral blood of patients with Alzheimer’s disease. J. New Med. 2020, 51, 590–594. [Google Scholar]
- Bigalke, B.; Schreitmüller, B.; Sopova, K.; Paul, A.; Stransky, E.; Gawaz, M.; Stellos, K.; Laske, C. Adipocytokines and CD34 progenitor cells in Alzheimer’s disease. PLoS ONE 2011, 6, e20286. [Google Scholar] [CrossRef] [PubMed]
- Stellos, K.; Panagiota, V.; Sachsenmaier, S.; Trunk, T.; Straten, G.; Leyhe, T.; Seizer, P.; Geisler, T.; Gawaz, M.; Laske, C. Increased circulating progenitor cells in Alzheimer’s disease patients with moderate to severe dementia: Evidence for vascular repair and tissue regeneration? J. Alzheimers Dis. 2010, 19, 591–600. [Google Scholar] [CrossRef] [Green Version]
- Reskiawan, A.; Kadir, R.; Alwjwaj, M.; Ahmad Othman, O.; Rakkar, K.; Sprigg, N.; Bath, P.M.; Bayraktutan, U. Inhibition of oxidative stress delays senescence and augments functional capacity of endothelial progenitor cells. Brain Res. 2022, 1787, 147925. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Chu, K.; Jung, K.H.; Jeon, D.; Bahn, J.J.; Kim, J.H.; Kun Lee, S.; Kim, M.; Roh, J.K. Dysfunctional characteristics of circulating angiogenic cells in Alzheimer’s disease. J. Alzheimers Dis. 2010, 19, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.M.; Arab, H.H.; Rizk, S.M.; El-Maraghy, S.A. Bone Marrow-Derived Endothelial Progenitor Cells Protect Against Scopolamine-Induced Alzheimer-Like Pathological Aberrations. Mol. Neurobiol. 2016, 53, 1403–1418. [Google Scholar] [CrossRef]
- Yuan, X.; Mei, B.; Zhang, L.; Zhang, C.; Zheng, M.; Liang, H.; Wang, W.; Zheng, J.; Ding, L.; Zheng, K. Enhanced penetration of exogenous EPCs into brains of APP/PS1 transgenic mice. Am. J. Transl. Res. 2016, 8, 1460–1470. [Google Scholar] [PubMed]
- Zhang, S.; Zhi, Y.; Li, F.; Huang, S.; Gao, H.; Han, Z.; Ge, X.; Li, D.; Chen, F.; Kong, X.; et al. Transplantation of in vitro cultured endothelial progenitor cells repairs the blood-brain barrier and improves cognitive function of APP/PS1 transgenic AD mice. J. Neurol. Sci. 2018, 387, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Rajani, R.M.; Quick, S.; Ruigrok, S.R.; Graham, D.; Harris, S.E.; Verhaaren, B.F.J.; Fornage, M.; Seshadri, S.; Atanur, S.S.; Dominiczak, A.F.; et al. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci. Transl. Med. 2018, 10, eaam9507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Rouhl, R.P.; van Oostenbrugge, R.J.; Damoiseaux, J.G.; Debrus-Palmans, L.L.; Theunissen, R.O.; Knottnerus, I.L.; Staals, J.E.; Delanghe, J.R.; Tervaert, J.W.; Lodder, J. Haptoglobin phenotype may alter endothelial progenitor cell cluster formation in cerebral small vessel disease. Curr. Neurovasc. Res. 2009, 6, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Rouhl, R.P.; Mertens, A.E.; van Oostenbrugge, R.J.; Damoiseaux, J.G.; Debrus-Palmans, L.L.; Henskens, L.H.; Kroon, A.A.; de Leeuw, P.W.; Lodder, J.; Tervaert, J.W. Angiogenic T-cells and putative endothelial progenitor cells in hypertension-related cerebral small vessel disease. Stroke 2012, 43, 256–258. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, A.; Gaubert, A.; Marshall, A.; Meier, I.B.; Yew, B.; Ho, J.K.; Blanken, A.E.; Dutt, S.; Sible, I.J.; Li, Y.; et al. Increased Levels of Circulating Angiogenic Cells and Signaling Proteins in Older Adults With Cerebral Small Vessel Disease. Front. Aging Neurosci. 2021, 13, 711784. [Google Scholar] [CrossRef]
- Huang, Z.X.; Fang, J.; Zhou, C.H.; Zeng, J.; Yang, D.; Liu, Z. CD34+ cells and endothelial progenitor cell subpopulations are associated with cerebral small vessel disease burden. Biomark Med. 2021, 15, 191–200. [Google Scholar] [CrossRef]
- Heller, L.; Thinard, R.; Chevalier, M.; Arpag, S.; Jing, Y.; Greferath, R.; Heller, R.; Nicolau, C. Secretion of proteins and antibody fragments from transiently transfected endothelial progenitor cells. J. Cell Mol. Med. 2020, 24, 8772–8778. [Google Scholar] [CrossRef]
- Sargento-Freitas, J.; Aday, S.; Nunes, C.; Cordeiro, M.; Gouveia, A.; Silva, F.; Machado, C.; Rodrigues, B.; Santo, G.C.; Ferreira, C.; et al. Endothelial Progenitor Cells influence acute and subacute stroke hemodynamics. J. Neurol. Sci. 2018, 385, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; De Silva, T.M.; Chen, J.; Faraci, F.M. Cerebral Vascular Disease and Neurovascular Injury in Ischemic Stroke. Circ. Res. 2017, 120, 449–471. [Google Scholar] [CrossRef] [Green Version]
- Blum, A.; Vaispapir, V.; Keinan-Boker, L.; Soboh, S.; Yehuda, H.; Tamir, S. Endothelial dysfunction and procoagulant activity in acute ischemic stroke. J. Vasc. Interv. Neurol. 2012, 5, 33–39. [Google Scholar] [PubMed]
- Schmidt-Lucke, C.; Rössig, L.; Fichtlscherer, S.; Vasa, M.; Britten, M.; Kämper, U.; Dimmeler, S.; Zeiher, A.M. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation 2005, 111, 2981–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umemura, T.; Soga, J.; Hidaka, T.; Takemoto, H.; Nakamura, S.; Jitsuiki, D.; Nishioka, K.; Goto, C.; Teragawa, H.; Yoshizumi, M.; et al. Aging and hypertension are independent risk factors for reduced number of circulating endothelial progenitor cells. Am. J. Hypertens. 2008, 21, 1203–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golab-Janowska, M.; Paczkowska, E.; Machalinski, B.; Kotlega, D.; Meller, A.; Safranow, K.; Wankowicz, P.; Nowacki, P. Elevated Inflammatory Parameter Levels Negatively Impact Populations of Circulating Stem Cells (CD133+), Early Endothelial Progenitor Cells (CD133+/VEGFR2+), and Fibroblast Growth Factor in Stroke Patients. Curr. Neurovasc. Res. 2019, 16, 19–26. [Google Scholar] [CrossRef]
- Zhou, W.J.; Zhu, D.L.; Yang, G.Y.; Zhang, Y.; Wang, H.Y.; Ji, K.D.; Lu, Y.M.; Gao, P.J. Circulating endothelial progenitor cells in Chinese patients with acute stroke. Hypertens. Res. 2009, 32, 306–310. [Google Scholar] [CrossRef]
- Bogoslovsky, T.; Spatz, M.; Chaudhry, A.; Maric, D.; Luby, M.; Frank, J.; Warach, S. NINDS Natural History of Stroke Investigators. Stromal-derived factor-1[alpha] correlates with circulating endothelial progenitor cells and with acute lesion volume in stroke patients. Stroke 2011, 42, 618–625. [Google Scholar] [CrossRef] [Green Version]
- Kukumberg, M.; Zaw, A.M.; Wong, D.H.C.; Toh, C.M.; Chan, B.P.L.; Seet, R.C.S.; Wong, P.T.H.; Yim, E.K.F. Characterization and Functional Assessment of Endothelial Progenitor Cells in Ischemic Stroke Patients. Stem Cell Rev. Rep. 2021, 17, 952–967. [Google Scholar] [CrossRef]
- Loiola, R.A.; García-Gabilondo, M.; Grayston, A.; Bugno, P.; Kowalska, A.; Duban-Deweer, S.; Rizzi, E.; Hachani, J.; Sano, Y.; Shimizu, F.; et al. Secretome of endothelial progenitor cells from stroke patients promotes endothelial barrier tightness and protects against hypoxia-induced vascular leakage. Stem Cell Res. Ther. 2021, 12, 552. [Google Scholar] [CrossRef]
- Bogoslovsky, T.; Chaudhry, A.; Latour, L.; Maric, D.; Luby, M.; Spatz, M.; Frank, J.; Warach, S. Endothelial progenitor cells correlate with lesion volume and growth in acute stroke. Neurology 2010, 75, 2059–2062. [Google Scholar] [CrossRef] [Green Version]
- Sobrino, T.; Hurtado, O.; Moro, M.A.; Rodríguez-Yáñez, M.; Castellanos, M.; Brea, D.; Moldes, O.; Blanco, M.; Arenillas, J.F.; Leira, R.; et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke 2007, 38, 2759–2764. [Google Scholar] [CrossRef] [Green Version]
- Yip, H.K.; Chang, L.T.; Chang, W.N.; Lu, C.H.; Liou, C.W.; Lan, M.Y.; Liu, J.S.; Youssef, A.A.; Chang, H.W. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke 2008, 39, 69–74. [Google Scholar] [CrossRef]
- Rakkar, K.; Othman, O.; Sprigg, N.; Bath, P.; Bayraktutan, U. Endothelial progenitor cells, potential biomarkers for diagnosis and prognosis of ischemic stroke: Protocol for an observational case-control study. Neural. Regen. Res. 2020, 15, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadir, R.R.; Alwjwaj, M.; Othman, O.A.; Rakkar, K.; Bayraktutan, U. Outgrowth endothelial cells form a functional cerebral barrier and restore its integrity after damage. Neural. Regen. Res. 2020, 15, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Kadir, R.R.A.; Alwjwaj, M.; Rakkar, K.; Othman, O.A.; Sprigg, N.; Bath, P.M.; Bayraktutan, U. Outgrowth Endothelial Cell Conditioned Medium Negates TNF-α-Evoked Cerebral Barrier Damage: A Reverse Translational Research to Explore Mechanisms. Stem Cell Rev. Rep. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Shyu, W.C.; Lin, S.Z.; Chiang, M.F.; Su, C.Y.; Li, H. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J. Neurosci. 2006, 26, 3444–3453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, T.; Kikuta, K.; Imamura, H.; Takagi, Y.; Nishimura, M.; Arakawa, Y.; Hashimoto, N.; Nozaki, K. Administration of ex vivo-expanded bone marrow-derived endothelial progenitor cells attenuates focal cerebral ischemia-reperfusion injury in rats. Neurosurgery 2006, 59, 679–686 discussion 679–686. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shen, F.; Frenzel, T.; Zhu, W.; Ye, J.; Liu, J.; Chen, Y.; Su, H.; Young, W.L.; Yang, G.Y. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann. Neurol. 2010, 67, 488–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moubarik, C.; Guillet, B.; Youssef, B.; Codaccioni, J.L.; Piercecchi, M.D.; Sabatier, F.; Lionel, P.; Dou, L.; Foucault-Bertaud, A.; Velly, L.; et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev. Rep. 2011, 7, 208–220. [Google Scholar] [CrossRef]
- Rosell, A.; Morancho, A.; Navarro-Sobrino, M.; Martínez-Saez, E.; Hernández-Guillamon, M.; Lope-Piedrafita, S.; Barceló, V.; Borrás, F.; Penalba, A.; García-Bonilla, L.; et al. Factors secreted by endothelial progenitor cells enhance neurorepair responses after cerebral ischemia in mice. PLoS ONE 2013, 8, e73244. [Google Scholar] [CrossRef] [Green Version]
- Hecht, N.; Schneider, U.C.; Czabanka, M.; Vinci, M.; Hatzopoulos, A.K.; Vajkoczy, P.; Woitzik, J. Endothelial progenitor cells augment collateralization and hemodynamic rescue in a model of chronic cerebral ischemia. J. Cereb. Blood Flow Metab. 2014, 34, 1297–1305. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Peng, X.G.; Wang, L.S.; Li, Z.H.; Wang, Y.C.; Lu, C.Q.; Ding, J.; Li, P.C.; Zhao, Z.; Ju, S.H. Bone Marrow Endothelial Progenitor Cell Transplantation After Ischemic Stroke: An Investigation Into Its Possible Mechanism. CNS Neurosci. Ther. 2015, 21, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xie, X.; Yu, Q.; Feng, H.; Wang, M.; Li, Y.; Liu, Y. Constitutive Expression of Adiponectin in Endothelial Progenitor Cells Protects a Rat Model of Cerebral Ischemia. Neural. Plast. 2017, 2017, 6809745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, L.; Bennis, Y.; Guillet, B.; Velly, L.; Garrigue, P.; Sabatier, F.; Dignat-George, F.; Bruder, N.; Pisano, P. Therapeutic benefit of a combined strategy using erythropoietin and endothelial progenitor cells after transient focal cerebral ischemia in rats. Neurol. Res. 2013, 35, 937–947. [Google Scholar] [CrossRef]
- Xin, B.; Liu, C.L.; Yang, H.; Peng, C.; Dong, X.H.; Zhang, C.; Chen, A.F.; Xie, H.H. Prolonged Fasting Improves Endothelial Progenitor Cell-Mediated Ischemic Angiogenesis in Mice. Cell Physiol. Biochem. 2016, 40, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Guo, Y.; Tan, S.; Li, Z.; Xie, H.; Chen, P.; Wang, K.; He, Z.; He, P.; Ke, Y.; et al. Autologous Endothelial Progenitor Cells Transplantation for Acute Ischemic Stroke: A 4-Year Follow-Up Study. Stem Cells Transl. Med. 2019, 8, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Autologous Endothelial Progenitor Cells Transplantation for Chronic Ischemic Stroke—Full Text View—Clinicaltrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02605707 (accessed on 15 September 2022).
- Diamandis, T.; Borlongan, C.V. One, two, three steps toward cell therapy for stroke. Stroke 2015, 46, 588–591. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhou, Y.; Zhao, H.; Peng, C. Migraine and the risk of stroke: An updated meta-analysis of prospective cohort studies. Neurol. Sci. 2017, 38, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Chu, K.; Jung, K.H.; Kim, D.H.; Kim, E.H.; Choe, V.N.; Kim, J.H.; Im, W.S.; Kang, L.; Park, J.E.; et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology 2008, 70, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Osorio, X.; Sobrino, T.; Brea, D.; Martínez, F.; Castillo, J.; Leira, R. Endothelial progenitor cells: A new key for endothelial dysfunction in migraine. Neurology 2012, 79, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Liman, T.G.; Neeb, L.; Rosinski, J.; Reuter, U.; Endres, M. Stromal Cell-Derived Factor-1 Alpha Is Decreased in Women with Migraine with Aura. Headache 2016, 56, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.R.; Borges-Canha, M.; Cardoso, R.; Neves, J.S.; Castro-Ferreira, R.; Leite-Moreira, A. Novel Biomarkers for Evaluation of Endothelial Dysfunction. Angiology 2020, 71, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Liman, T.G.; Bachelier-Walenta, K.; Neeb, L.; Rosinski, J.; Reuter, U.; Böhm, M.; Endres, M. Circulating endothelial microparticles in female migraineurs with aura. Cephalalgia 2015, 35, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oterino, A.; Toriello, M.; Palacio, E.; Quintanilla, V.G.; Ruiz-Lavilla, N.; Montes, S.; Vega, M.S.; Martinez-Nieto, R.; Castillo, J.; Pascual, J. Analysis of endothelial precursor cells in chronic migraine: A case-control study. Cephalalgia 2013, 33, 236–244. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Mean Age of Patients | Stage of AD | Results |
|---|---|---|---|
| Maler et al. (2006) [30] | Not specified (n = 23) | Early AD | Decreased counts of circulating CD34+ cells among AD patients (compared to healthy controls). The number of circulating CD34+ was significantly inversely correlated with Aβ 1-42 and Aβ42/40 ratio in cerebrospinal fluid. |
| Lee et al. (2009) [32] | 71.7 ± 7.8 (n = 55) | Newly diagnosed AD | No significant differences were found in the number of circulating EPCs between patients with AD and risk factor-matched controls. Patients with AD had lower CFU-ECs than risk factor-matched controls. A reduction in CFU-ECs was associated with lower cognitive function. |
| Stellos et al. (2010) [36] | Patients with mild AD = 73.8 ± 5.4 Patients with moderate to severe AD = 73.2 ± 9.3 (n = 45) | 17/45 (38%) of patients had mild AD 28/45 (62%) of patients had moderate to severe AD | Significantly increased numbers of circulating CD34+ and CD34+/CD133+ progenitor cells were found among individuals with moderate to severe AD compared to healthy controls. No such changes were detected in patients with mild AD. A negative correlation was found between the level of CD34+/CD133+ progenitor cells circulating in the blood and age, cognitive function and SDF-1α plasma level. |
| Bigalke et al. (2010) [35] | 74.3 ± 9.1 (n = 41) | Early AD | An increased number of circulating CD34+ cells was associated with the presence of AD and showed an inverse correlation with leptin plasma levels. |
| Kong et al. (2011) [31] | 71.4 ± 2.3 (n = 30) | Newly diagnosed AD | Decreased number of circulating EPCs in AD patients compared to healthy subjects. A correlation between lower number of circulating EPCs and lower cognitive function. |
| Breining et al. (2016) [33] | 83.2 ± 6.4 (n = 48) | Not specified | No significant differences were found in the number of circulating EPCs between AD patients and control groups. |
| Haiyuan et al. (2020) [34] | Patients with mild AD = 76.9 ± 12.0 Patients with moderate AD = 77.1 ± 12.3 Patients with severe AD = 81.3 ± 7.3 | 19/58 (33%) of patients had mild AD 21/58 (36%) of patients had moderate AD 18/58 (31%) of patients had severe AD | No significant differences were found in the number of circulating EPCs between four groups (three groups of AD patients according to AD severity and healthy control group). |
| Author (Year) | Subjects | EPCs Dosage | Results |
|---|---|---|---|
| Safar et al. (2016) [39] | Adult male Wistar rats with cognitive impairment induced by daily administration of scopolamine for 6 weeks | 2 × 106 of BM-EPCs administered intravenously to the rat tail vein 5 days after the last scopolamine dose | BM-EPCs migrated into the brain of rats, mitigated the accumulation of Aβ and associated histopathological alterations, dulled the increase in hippocampal Aβ and APP, restored the Aβ-degrading neprilysin and downregulated p-tau. They also boosted VEGF, NGF, and BDNF and suppressed the proinflammatory TNF-α and IL-1β. An application of BM-EPCs also resulted in a correction of perturbed neurotransmitter levels, including acetylcholine, dopamine, GABA and glutamate. Improvements in rats’ deficits in learning and memory were also observed. |
| Yuan et al. (2016) [40] | APP/PS1 transgenic mice | 2 × 106 of EPCs transfected with GFP adenoviral vectors administered intravenously into the tail vein | A penetration of exogenous EPCs into the brain was enhanced in the APP/PS1 transgenic mice compared to wild-type mice. |
| Zhang et al. (2018) [41] | APP/PS1 transgenic mice | 4 × 105 of EPCs transplanted into the hippocampus | A transplantation of EPCs enhanced the expression of BBB tight junction proteins, increased the microvessel density, decreased the Aβ plaque deposition and hippocampal cell apoptosis. Moreover, significant improvements were observed in memory functions and spatial learning in mice transplanted with EPCs. |
| Author (Year) | Mean Age of Patients | Manifestations of CSVD | Results |
|---|---|---|---|
| Rouhl et al. (2009) [44] | 64.0 (±11.4) n = 42 | Lacunar stroke (which occurred at least 2 years prior) | CSVD patients had lower EPC cluster counts than healthy controls. EPC cluster formation was inhibited by patient serum. |
| Rouhl et al. (2012) [45] | 65.2 (±9.3) n = 32 | Lesions in the white matter, microbleeds or asymptomatic lacunar strokes. All patients had hypertension. | CSVD individuals with hypertension exhibited lower levels of EPCs than healthy controls. |
| Kapoor et al. (2021) [46] | 69.8 (±7.3) n= 64 | CSVD burden determined by MRI markers: microbleeds, small lacunes, white matter hyperintesities. Patients were free of stroke and dementia. | Increased levels of EPCs and VEGF were related to greater CSVD burden. |
| Huang et al. (2021) [47] | n = 364 | Patients with confirmed CSVD | Patients with greater CSVD burden had decreased level of circulating CD34+ cells and significantly elevated levels of CD34+CD133+ and CD34+CD133+CD309+ cells compared to those with lower CSVD burden. |
| Author (Year)/ National Clinical Trial Identifier (Start Year) | Subjects | EPCs Dosage | Results |
|---|---|---|---|
| Shyu et al. (2006) [65] | Adult male rats after 90-min occlusion of middle cerebral artery | ∼2 × 105 of peripheral blood hematopoietic stem cells (PBSCs) (CD34+) were stereotaxically injected intracerebrally 7 days after ischemia | Implanted PBSCs differentiated into glial cells, neurons or endothelial vascular cells. Improvement in neurological behavior, increase in neuronal cortical activity, promotion of formation of new vessels, increase in the local cortical blood flow in the ischemic hemisphere. |
| Ohta et al. (2006) [66] | Adult male rats after 90 min occlusion of the middle cerebral artery | 2.5 × 105 of EPCs were administered into internal carotid artery right after ischemia | Administration of EPCs reduced infarct volume and functional neurological deficits. |
| Di Santo et al. (2009) [10] | Male athymic nude rats subjected to chronic hindlimb ischemia | 1 × 106 of EPCs or 250 µL of EPC-CM were administered intramuscularly at 5 sites into the ischemic hindlimb, 3 times within 7 days, 4 weeks after ischemia | Both EPCs and EPC-CM caused an increase in capillary density, enhanced vascular maturation and muscle viability, which was visible in significantly increased hindlimb blood flow and improved muscle performance. Moreover, EPC-CM stimulated the mobilization of the bone marrow-derived EPCs. |
| Fan et al. (2010) [67] | Adult mice after 1 h of transient middle cerebral artery occlusion | 1 × 106 of EPCs injected into jugular vein right after ischemia | A transplantation of EPCs significantly reduced infarct volume 3 days after ischemia and reduced cortex atrophy 4 weeks after ischemia. EPCs also improved neurobehavioral outcomes and increased angiogenesis in the peri-infarction zone. |
| Moubarik et al. (2011) [68] | Adult male rats after 60 min of the middle cerebral artery transient occlusion | 4 × 106 of endothelial colony-forming cells (ECFCs) injected into femoral vein 24 h after ischemia. | A transplantation of ECFCs was associated with a stimulation of neurogenesis, an increase in capillary density and a reduction in apoptotic cell number at the site of an infarct. |
| Rosell et al. (2013) [69] | Adult male mice subjected to the middle cerebral artery permanent distal occlusion | 104 to 2×105 of EPCs or cell-free conditioned media (CM) obtained from EPCs were administered randomly 30–32 h after ischemia. | A significant increase in the density of capillaries and an improvement in the post-ischemia forelimb strength were noted both among mice treated with EPCs and CM. An increase in axonal rewiring was observed among animals treated with EPCs, but not in those treated with CM. |
| Pellegrini et al. (2013) [73] | Adult male rats subjected to 1 h of transient middle cerebral artery occlusion | 5 × 106 of ECFCs intravenously and/or 2500 UI/kg/day for 3 days of EPO intraperitoneally 24 h after ischemia | The combination of ECFSs and EPO was more effective in increasing angiogenesis and neurogenesis and decreasing apoptosis compared to ECFCs or EPO alone. Also the ECFCs+EPO combination was the only treatment that resulted in a complete recovery of neurological function. |
| Hecht et al. (2014) [70] | Adult male rats subjected to a 3-vessel occlusion (chronic cerebral hypoperfusion) | 1 × 106 of embryonic EPCs (eEPCs) intravenously right after occlusion and at day 7 and day 14 after ischemia. | A treatment with eEPCs provided better functional recovery, which was reflected in significant increases in parenchymal capillary density and in vessel diameters in the anterior Circle of Willis, as well as higher number of leptomeningeal anastomoses. |
| Bai et al. (2015) [71] | Adult male mice subjected to a right middle cerebral artery occlusion induced by a photochemical reaction | 1 × 106 of EPCs injected into the ipsilateral internal carotid artery 24 h after ischemia. | In the EPC-treated mice, increased angiogenesis and neurogenesis, activation of eNOS and the expression of BDNF were increased, axonal growth was stimulated. A decrease was noted in infarct volume and neurological deficits. |
| Xin et al. (2016) [74] | Adult male mice subjected to a permanent left middle cerebral artery occlusion | 1 × 106 of EPCs injected into the tail vein right after cerebral ischemia + mice were subjected to prolonged fasting or periodic prolonged fasting after cerebral ischemia | Prolonged fasting significantly enhanced the EPC functions, angiogenesis and mitigated ischemic injury in the brain. |
| Zhang et al. (2017) [72] | Adult male rats subjected to 2 h of middle cerebral artery occlusion | 2 × 106 of EPCs or LV-APN-EPCs (EPCs transfected with the adiponectin gene) were injected intravenously into the tail vein after 2 h of reperfusion | Higher improvements in infarct area, microvessel density, behavioral function and cell apoptosis were observed in the LV-APN-EPCs group than in the EPCs group. |
| Fang et al. (2019) [75] | 18 adult patients with acute cerebral stroke in the middle cerebral artery territory | 2.5 × 106 cells/kg body weight of autologous EPCs administered intravenously 4–5 weeks after ischemia and additional 2.5 × 106 cells/kg body weight of EPCs 1 week after initial boosting | No toxicity events or allergic reactions were noted. Patients who received EPCs had fewer serious adverse events compared to the placebo group; however, there was no difference in mortality between the groups. No significant differences were observed in neurological or functional improvement, except for the higher SSS score among the EPCs group at a 3-month follow-up. |
| NCT02605707 (2015) [76] | 12 adult patients with chronic ischemic stroke (which occurred between 6 and 60 months prior) | Autologous EPCs administered intravenously | Not available |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudnicka-Drożak, E.; Drożak, P.; Mizerski, G.; Drożak, M. Endothelial Progenitor Cells in Neurovascular Disorders—A Comprehensive Overview of the Current State of Knowledge. Biomedicines 2022, 10, 2616. https://doi.org/10.3390/biomedicines10102616
Rudnicka-Drożak E, Drożak P, Mizerski G, Drożak M. Endothelial Progenitor Cells in Neurovascular Disorders—A Comprehensive Overview of the Current State of Knowledge. Biomedicines. 2022; 10(10):2616. https://doi.org/10.3390/biomedicines10102616
Chicago/Turabian StyleRudnicka-Drożak, Ewa, Paulina Drożak, Grzegorz Mizerski, and Martyna Drożak. 2022. "Endothelial Progenitor Cells in Neurovascular Disorders—A Comprehensive Overview of the Current State of Knowledge" Biomedicines 10, no. 10: 2616. https://doi.org/10.3390/biomedicines10102616
APA StyleRudnicka-Drożak, E., Drożak, P., Mizerski, G., & Drożak, M. (2022). Endothelial Progenitor Cells in Neurovascular Disorders—A Comprehensive Overview of the Current State of Knowledge. Biomedicines, 10(10), 2616. https://doi.org/10.3390/biomedicines10102616







