A Touchscreen Device for Behavioral Testing in Pigs
Abstract
1. Introduction
2. Materials and Methods
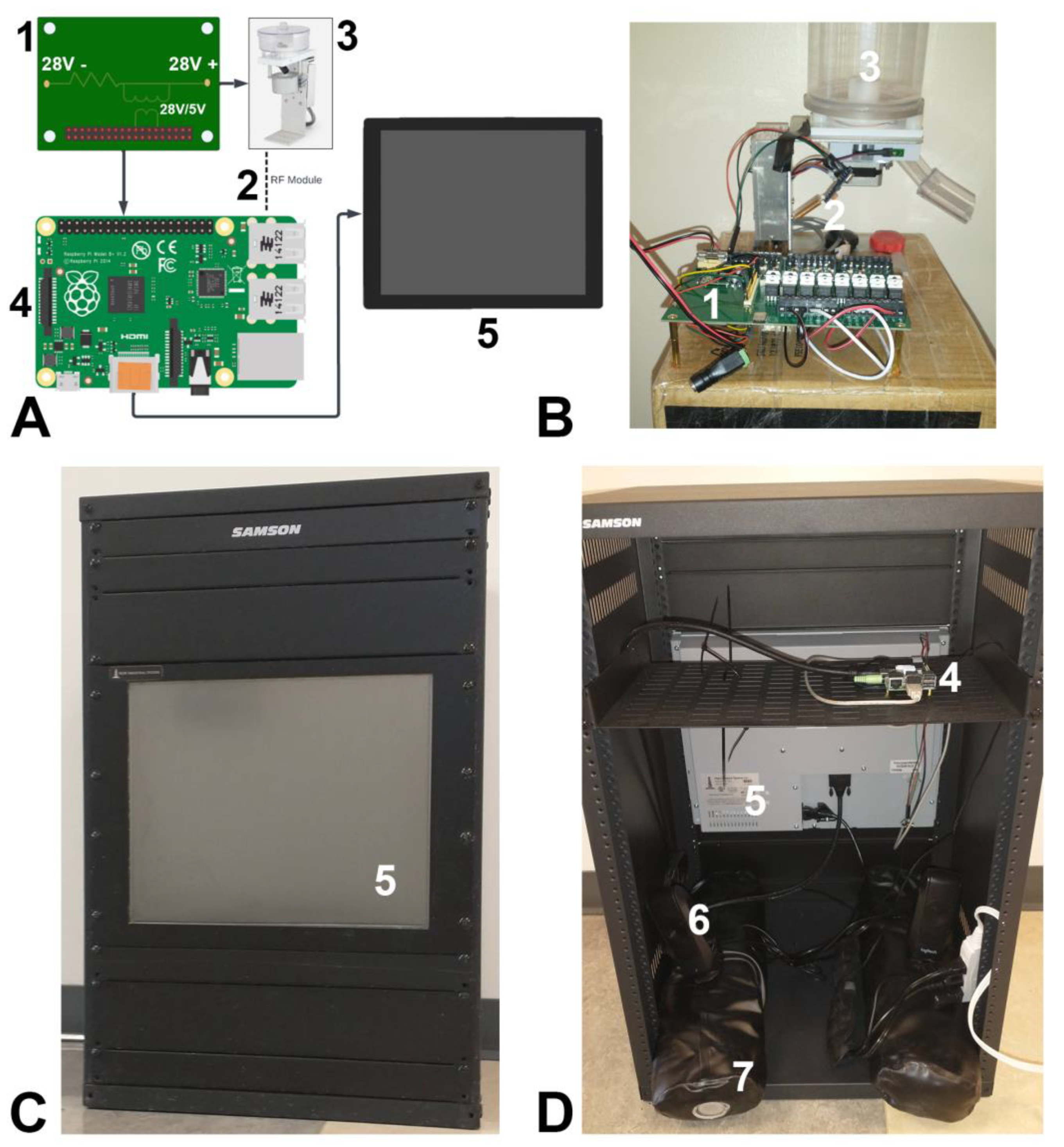
2.1. Touchscreen Design Overview
2.2. Physical Components and Construction
2.2.1. Frame
2.2.2. Screen
2.2.3. Circuit Board Interface
2.2.4. RF Transmitter/Receiver
2.2.5. Raspberry Pi
2.2.6. Peripheral Components
2.2.7. Alternative Materials
2.3. Software Components
2.3.1. Peripheral Device Control
2.3.2. Graphical User Interface (GUI)
2.3.3. Data Recording
2.3.4. Behavioral Testing Programs
2.3.5. Data Transfer To/From Raspberry Pi
2.4. Evaluation of Device Durability
2.5. Subjects
2.6. Behavioral Training
2.6.1. Reinforcers
2.6.2. Pre-Training of Pigs
2.6.3. Response Shaping
2.7. Behavioral Assessment of Pigs
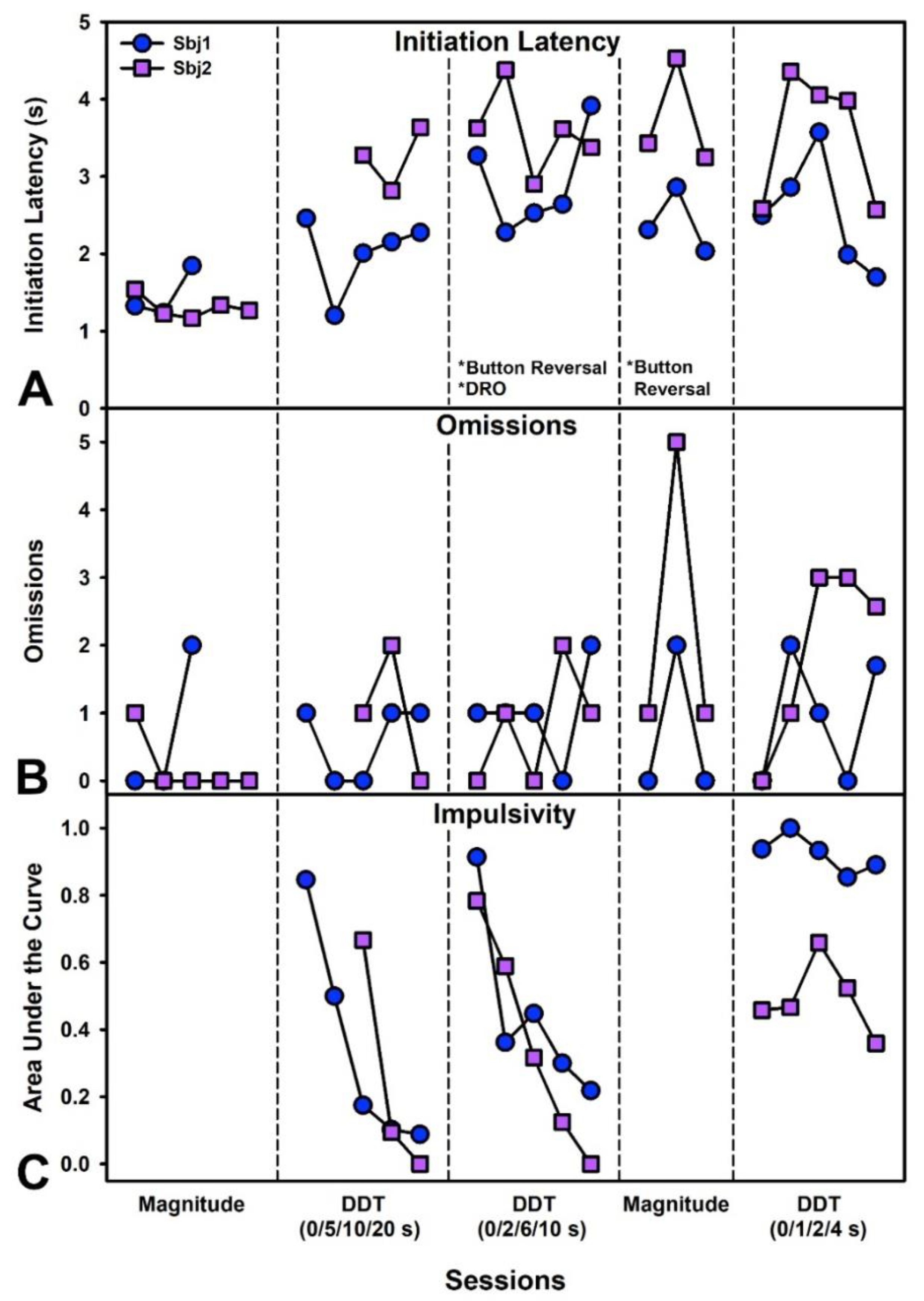
2.7.1. Experiment 1—Delay Discounting Task
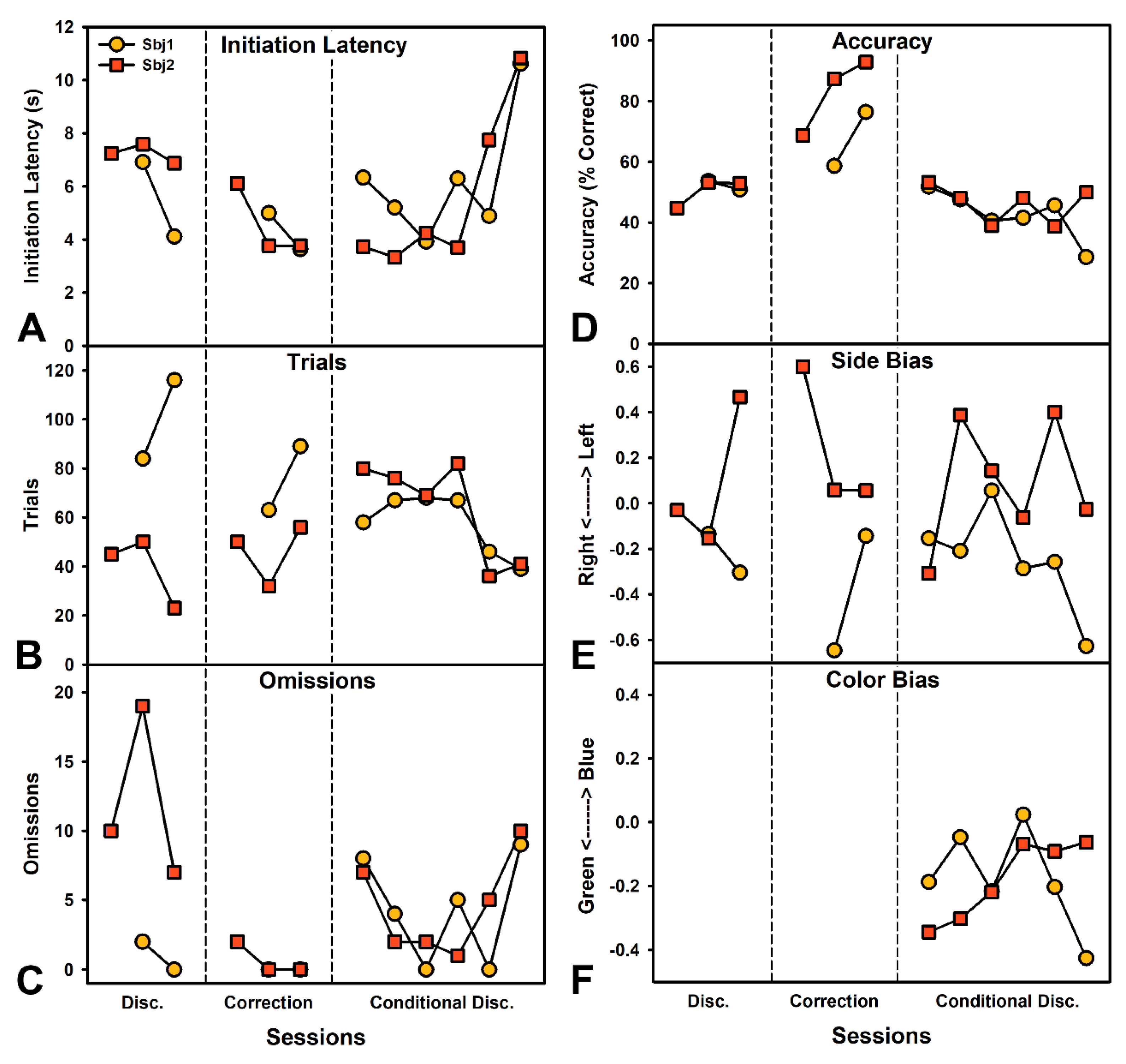
2.7.2. Experiment 2—Visual Discrimination
3. Results
3.1. Device Durability
3.2. Behavioral Topology of Pigs on the Touchscreen
3.2.1. Capacitive vs. Resistive Touchscreen
3.2.2. Presses on Touchscreen
3.2.3. Rooting
3.2.4. Responsiveness to Tones
3.2.5. Frustration and Sensitivity to Reinforcer Magnitude
3.2.6. Competing Exploration
3.3. Device Components & Evaluation
3.3.1. First Touchscreen Device
3.3.2. Second Touchscreen Device
3.3.3. Extension to Rodents or Other Animals
3.4. Pre-Training and Response Shaping
3.5. Experiment 1—Delay Discounting Task
3.6. Experiment 2—Color Discrimination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Pillay, P.; Manger, P.R. Order-specific quantitative patterns of cortical gyrification. Eur. J. Neurosci. 2007, 25, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- MacManus, D.B.; Pierrat, B.; Murphy, J.G.; Gilchrist, M.D. Region and species dependent mechanical properties of adolescent and young adult brain tissue. Sci. Rep. 2017, 7, 13729. [Google Scholar] [CrossRef] [PubMed]
- Kinder, H.A.; Baker, E.W.; West, F.D. The pig as a preclinical traumatic brain injury model: Current models, functional outcome measures, and translational detection strategies. Neural Regen. Res. 2019, 14, 413. [Google Scholar] [PubMed]
- Baxa, M.; Hruska-Plochan, M.; Juhas, S.; Vodicka, P.; Pavlok, A.; Juhasova, J.; Motlik, J. A transgenic minipig model of Huntington’s disease. J. Huntingt. Dis. 2013, 2, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.E.; Johansen, M.G.; Schmidt, M.; Liu, Y.; Li, R.; Callesen, H.; Jørgensen, A.L. Expression of the Alzheimer’s disease mutations AβPP695sw and PSEN1M146I in double-transgenic Göttingen minipigs. J. Alzheimer’s Dis. 2016, 53, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Schook, L.B.; Collares, T.V.; Hu, W.; Liang, Y.; Rodrigues, F.M.; Rund, L.A.; Schachtschneider, K.M.; Seixas, F.K.; Singh, K.; Wells, K.D.; et al. A genetic porcine model of cancer. PLoS ONE 2015, 10, e0128864. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Astiz, S.; Parraguez, V.H.; Garcia-Contreras, C.; Vazquez-Gomez, M. Empowering translational research in fetal growth restriction: Sheep and swine animal models. Curr. Pharm. Biotechnol. 2016, 17, 848–855. [Google Scholar] [CrossRef]
- Mallard, C.; Vexler, Z.S. Modeling ischemia in the immature brain: How translational are animal models? Stroke 2015, 46, 3006–3011. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Dennahy, I.S.; Bhatti, U.F.; Halaweish, I.; Xiong, Y.; Chang, P.; Nikolian, V.C.; Chtraklin, K.; Brown, J.; Zhang, Y. Mesenchymal stem cell-derived exosomes provide neuroprotection and improve long-term neurologic outcomes in a swine model of traumatic brain injury and hemorrhagic shock. J. Neurotrauma 2019, 36, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Friess, S.H.; Ralston, J.; Smith, C.; Propert, K.J.; Rapp, P.E.; Margulies, S.S. Improved behavior, motor, and cognition assessments in neonatal piglets. J. Neurotrauma 2013, 30, 1770–1779. [Google Scholar] [CrossRef]
- Baxa, M.; Levinska, B.; Skrivankova, M.; Pokorny, M.; Juhasova, J.; Klima, J.; Klempir, J.; Motlı, K.J.; Juhas, S.; Ellederova, Z. Longitudinal study revealing motor, cognitive and behavioral decline in a transgenic minipig model of Huntington’s disease. Dis. Models Mech. 2019, 13, dmm041293. [Google Scholar] [CrossRef]
- Søndergaard, L.V.; Ladewig, J.; Dagnæs-Hansen, F.; Herskin, M.S.; Holm, I.E. Object recognition as a measure of memory in 1–2 years old transgenic minipigs carrying the APPsw mutation for Alzheimer’s disease. Transgenic Res. 2012, 21, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Moustgaard, A.; Arnfred, S.M.; Lind, N.M.; Hemmingsen, R.; Hansen, A.K. Acquisition of visually guided conditional associative tasks in Göttingen minipigs. Behav. Process. 2005, 68, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.A.; Gopee, N.V.; Paule, M.G.; Howard, P.C. Female mini-pig performance of temporal response differentiation, incremental repeated acquisition, and progressive ratio operant tasks. Behav. Process. 2009, 80, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Moustgaard, A.; Arnfred, S.M.; Lind, N.M.; Hansen, A.K.; Hemmingsen, R. Discriminations, reversals, and extra-dimensional shifts in the Göttingen minipig. Behav. Process. 2004, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Zonderland, J.J.; Cornelissen, L.; Wolthuis-Fillerup, M.; Spoolder, H.A. Visual acuity of pigs at different light intensities. Appl. Anim. Behav. Sci. 2008, 111, 28–37. [Google Scholar] [CrossRef]
- Melotti, L.; Thomsen, L.R.; Toscano, M.J.; Mendl, M.; Held, S. Delay discounting task in pigs reveals response strategies related to dopamine metabolite. Physiol. Behav. 2013, 120, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.R.; Kornum, B.R.; Moustgaard, A.; Gade, A.; Lind, N.M.; Knudsen, G.M. A novel spatial delayed non-match to sample (DNMS) task in the Göttingen minipig. Behav. Brain Res. 2009, 196, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.; Dumont, J.R.; Dexter, T.D.; Prado, M.A.M.; Finger, E.; Bussey, T.J.; Saksida, L.M. Touchscreen cognitive testing: Cross-species translation and co-clinical trials in neurodegenerative and neuropsychiatric disease. Neurobiol. Learn. Mem. 2021, 182, 107443. [Google Scholar] [CrossRef]
- Buscher, N.; Ojeda, A.; Francoeur, M.; Hulyalkar, S.; Claros, C.; Tang, T.; Terry, A.; Gupta, A.; Fakhraei, L.; Ramanathan, D.S. Open-source raspberry Pi-based operant box for translational behavioral testing in rodents. J. Neurosci. Methods 2020, 342, 108761. [Google Scholar] [CrossRef]
- Escobar, R.; Gutiérrez, B.; Benavides, R. 3D-printed operant chambers for rats: Design, assembly, and innovations. Behav. Process. 2022, 199, 104647. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.; Özgür, S.; LaQua, M. Rfchat. Available online: https://github.com/mrpjevans/rfchat (accessed on 13 July 2022).
- Vanderveldt, A.; Oliveira, L.; Green, L. Delay discounting: Pigeon, rat, human—Does it matter? J. Exp. Psychol. Anim. Learn. Cogn. 2016, 42, 141. [Google Scholar] [CrossRef] [PubMed]
- Colleton, C.; Brewster, D.; Chester, A.; Clarke, D.O.; Heining, P.; Olaharski, A.; Graziano, M. The use of minipigs for preclinical safety assessment by the pharmaceutical industry: Results of an IQ DruSafe minipig survey. Toxicol. Pathol. 2016, 44, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Middleton, S. Porcine ophthalmology. Vet. Clin. North Am. Food Anim. Pract. 2010, 26, 557–572. [Google Scholar] [CrossRef]
- Choi, K.E.; Anh, V.T.Q.; Kim, J.T.; Yun, C.; Cha, S.; Ahn, J.; Goo, Y.S.; Kim, S.W. An experimental pig model with outer retinal degeneration induced by temporary intravitreal loading of N-methyl-N-nitrosourea during vitrectomy. Sci. Rep. 2021, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Barone, F.; Nannoni, E.; Elmi, A.; Lambertini, C.; Scorpio, D.G.; Ventrella, D.; Vitali, M.; Maya-Vetencourt, J.F.; Martelli, G.; Benfenati, F.; et al. Behavioral assessment of vision in pigs. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 350–356. [Google Scholar] [CrossRef]
- Vink, R. Large animal models of traumatic brain injury. J. Neurosci. Res. 2018, 96, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Rochat, L.; Beni, C.; Billieux, J.; Azouvi, P.; Annoni, J.-M.; Van der Linden, M. Assessment of impulsivity after moderate to severe traumatic brain injury. Neuropsychol. Rehabil. 2010, 20, 778–797. [Google Scholar] [CrossRef]
- Lee, J.K.; Santos, P.T.; Chen, M.W.; O’Brien, C.E.; Kulikowicz, E.; Adams, S.; Hardart, H.; Koehler, R.C.; Martin, L.J. Combining hypothermia and oleuropein subacutely protects subcortical white matter in a swine model of neonatal hypoxic-ischemic encephalopathy. J. Neuropathol. Exp. Neurol. 2021, 80, 182–198. [Google Scholar] [CrossRef] [PubMed]
- Srinivas Jois, R. Neurodevelopmental outcome of late-preterm infants: A pragmatic review. Aust. J. Gen. Pract. 2018, 47, 776–781. [Google Scholar] [CrossRef]
- Maxeiner, J.; Sharma, R.; Amrhein, C.; Gervais, F.; Duda, M.; Ward, J.; Mikkelsen, L.F.; Forster, R.; Malewicz, M.; Krishnan, J. Genomics Integrated Systems Transgenesis (GENISYST) for gain-of-function disease modelling in Göttingen Minipigs. J. Pharmacol. Toxicol. Methods 2021, 108, 106956. [Google Scholar] [CrossRef]
- Berthelsen, M.F.; Riedel, M.; Cai, H.; Skaarup, S.H.; Alstrup, A.K.O.; Dagnæs-Hansen, F.; Luo, Y.; Jensen, U.B.; Hager, H.; Liu, Y.; et al. The CRISPR/Cas9 minipig-A transgenic minipig to produce specific mutations in designated tissues. Cancers 2021, 13, 3024. [Google Scholar] [CrossRef]
| Item | Parts | Price | Vendor |
|---|---|---|---|
| Computer | $148 | ||
| Raspberry Pi 4B (2×) | $110 | SparkFun | |
| Power supply (2×) | $16 | SparkFun | |
| Misc. jumpers, etc. | $10 | SparkFun | |
| RF Transceivers | $12 | Amazon | |
| PCB | $39 | ||
| Board * | $19 | PCBWay | |
| Misc. components * | $20 | Digikey | |
| Peripherals | $799 | ||
| Pellet Dispenser | $729 | Med-Associates | |
| Sonalert | $70 | Med-Associates | |
| Desktop speakers | $0 | in lab | |
| Monitor | $855 | ||
| 19″ Rack-mounted Resistive | $835 | Hope Industrial | |
| Screen protector | $20 | Hope Industrial | |
| Frame | $238 | ||
| Samson SRK-16 ** | $165 | B&H Audio-Video | |
| 1U blank panel (4×) | $18 | B&H Audio-Video | |
| 2U blank panels (2×) | $12 | B&H Audio-Video | |
| rack shelf *** | $15 | B&H Audio-Video | |
| Sandbags (2×) | $28 | Amazon | |
| Total | $2079 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ao, W.; Grace, M.; Floyd, C.L.; Vonder Haar, C. A Touchscreen Device for Behavioral Testing in Pigs. Biomedicines 2022, 10, 2612. https://doi.org/10.3390/biomedicines10102612
Ao W, Grace M, Floyd CL, Vonder Haar C. A Touchscreen Device for Behavioral Testing in Pigs. Biomedicines. 2022; 10(10):2612. https://doi.org/10.3390/biomedicines10102612
Chicago/Turabian StyleAo, Will, Megan Grace, Candace L. Floyd, and Cole Vonder Haar. 2022. "A Touchscreen Device for Behavioral Testing in Pigs" Biomedicines 10, no. 10: 2612. https://doi.org/10.3390/biomedicines10102612
APA StyleAo, W., Grace, M., Floyd, C. L., & Vonder Haar, C. (2022). A Touchscreen Device for Behavioral Testing in Pigs. Biomedicines, 10(10), 2612. https://doi.org/10.3390/biomedicines10102612





