Antimicrobial Activity of a 3D-Printed Polymethylmethacrylate Dental Resin Enhanced with Graphene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Graphene–PMMA Resin Preparation
2.2. Design and Fabrication
2.3. Characterization of Graphene–PMMA Resin
2.4. Surface Roughness Determination
2.5. Antimicrobial Activity of Graphene–PMMA Resin against C. albicans and S. mutans
2.5.1. Microbial Strains and Growth Conditions
2.5.2. Antimicrobial Activity of Graphene–PMMA Resin Specimens
2.5.3. Surface Adhesion Studies
2.6. Statistical Analysis
3. Results
3.1. Characterization of Graphene–PMMA Resin
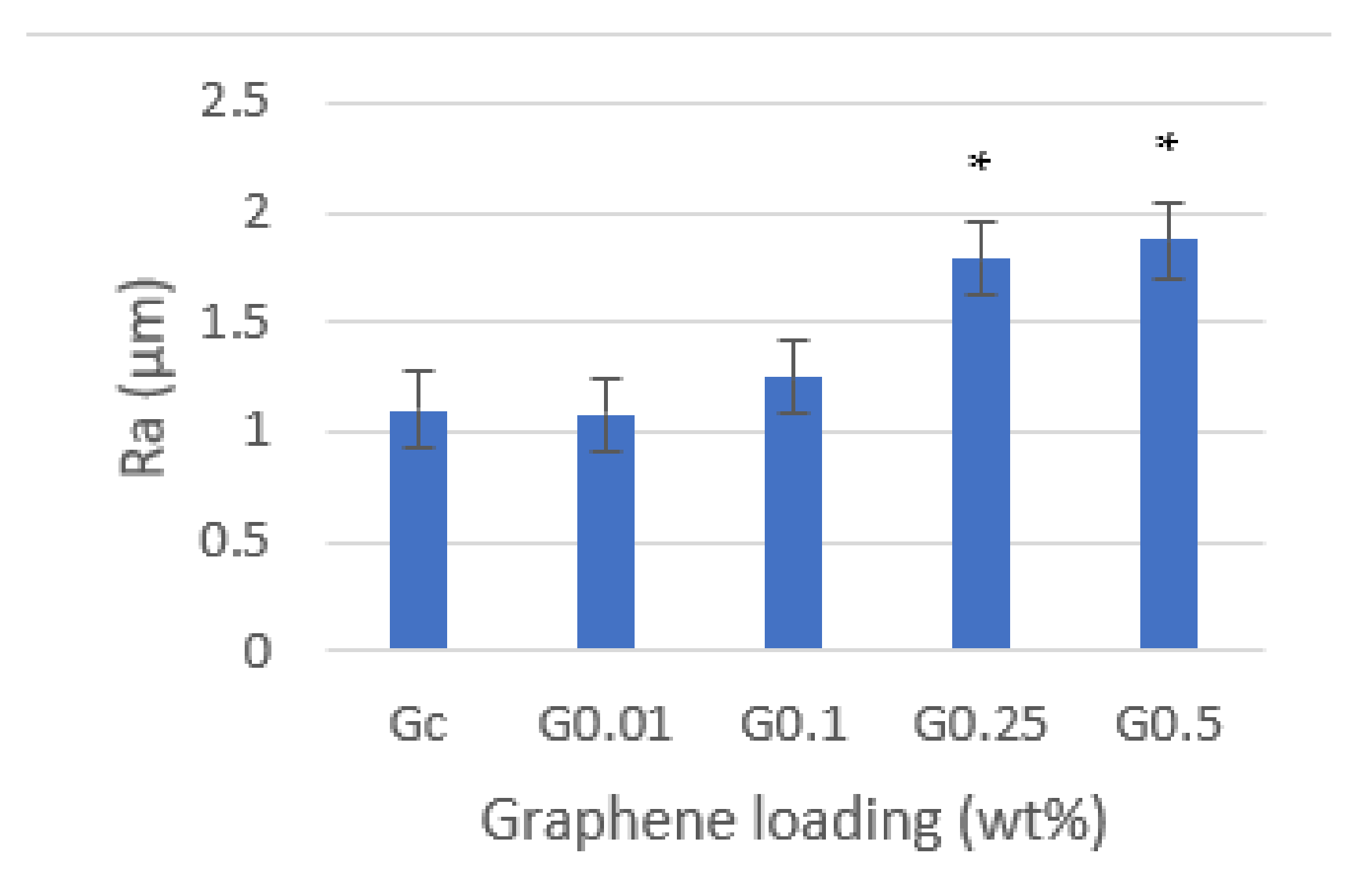
3.2. Surface Roughness
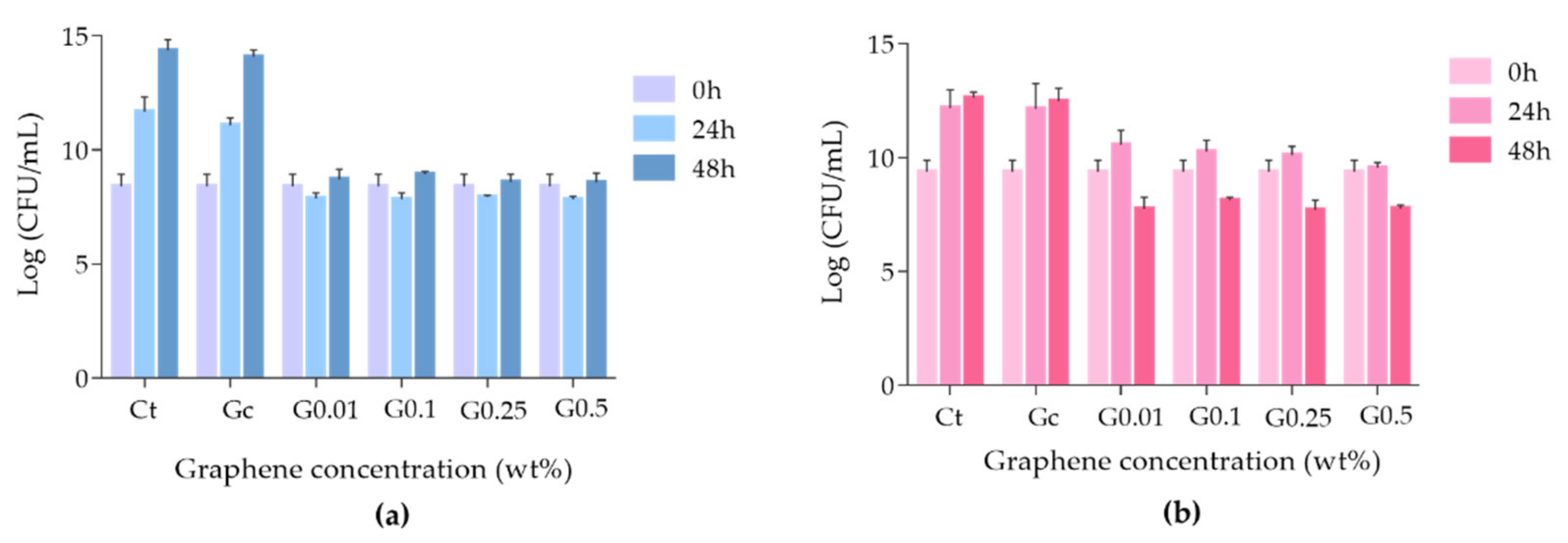
3.3. Antimicrobial Activity of Graphene–PMMA Resin against C. albicans and S. mutans
3.4. Surface Adhesion Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muller, F.; Naharro, M.; Carlson, G. What are the prevalence and incidence of tooth loss in the adult and elderly population in Europe? Clin. Oral. Implant. Res. 2007, 18, 2–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krausch-Hofmann, S.; Cuypers, L.; Ivanova, A.; Duyck, J. Predictors of Patient Satisfaction with Removable Denture Renewal: A Pilot Study. J. Prosthodont. 2018, 27, 509–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meirowitz, A.; Rahmanov, A.; Shlomo, E.; Zelikman, H.; Dolev, E.; Sterer, N. Effect of denture base fabrication technique on candida albicans adhesion In Vitro. Materials 2021, 14, 221. [Google Scholar] [CrossRef]
- Von Fraunhofer, J.A.; Loewy, Z.G. Factors involved in microbial colonization of oral prostheses. Gen. Dent. 2009, 57, 136–143. [Google Scholar]
- Kulak, Y.; Arikan, A.; Kazazoglu, E. Existence of Candida albicans and microorganisms in denture stomatitis patients. J. Oral Rehabil. 1997, 24, 788–790. [Google Scholar] [CrossRef]
- Redding, S.; Bhatt, B.; Rawls, H.; Siegel, G.; Scott, K.; Lopez-Ribot, J. Inhibition of Candida albicans biofilm formation on denture material. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 669–672. [Google Scholar] [CrossRef]
- Pasmore, M.; Todd, P.; Pfiefer, B.; Rhodes, M.; Bowman, C.N. Effect of polymer surface properties on the reversibility of attachment of Pseudomonas aeruginosa in the early stages of biofilm development. Biofouling 2002, 18, 65–71. [Google Scholar] [CrossRef]
- Terada, A.; Yuasa, A.; Kushimoto, T.; Tsuneda, S.; Katakai, A.; Tamada, M. Bacterial adhesion to and viability on positively charged polymer surfaces. Microbiology 2006, 152, 3575–3583. [Google Scholar] [CrossRef] [Green Version]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Quirynen, M.; Marechal, M.; Busscher, H.J.; Weerkamp, A.H.; Darius, P.L.; van Steenberghe, D. The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J. Clin. Periodontol. 1990, 17, 138–144. [Google Scholar] [CrossRef]
- Reichart, P. Oral mucosal lesions in a representative cross-sectional study of aging Germans. Community Dent. Oral Epidemiol. 2000, 28, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Thomas, C.; Willcox, M.; Harty, D.; Knox, K. Candida-associated denture stomatitis. Aetiology and management: A review. Part I. Factors influencing distribution of Candida species in the oral cavity. Aust. Dent. J. 1998, 43, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Tomsett, K.; Wickers, B.; López-Ribot, J.; Redding, S. Denture stomatitis: A role for Candida biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 53–59. [Google Scholar] [CrossRef]
- Figueiral, M.H.; Azul, A.M.; Pinto, E.; Fonseca, P.; Branco, F.M.; Scully, C. Denture-related stomatitis: Identification of aetiological and predisposing factors—A large cohort. J. Oral Rehabil. 2007, 34, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Gendreau, L.; Loewy, Z. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Ribeiro, I.C.; Ferreira, N.J.; Fortes, C.E.; Fonseca, P.A.; Figueiral, M.H. Correlation between enzyme production, germ tube formation and susceptibility to fluconazol in Candida species isolated from patients with denture-related stomatitis and control individuals. J. Oral Pathol. Med. 2008, 37, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Muttagi, S.; Subramanya, J. Effect of incorporating seed oils on the antifungal property, surface roughness, wettability, weight change, and glucose sorption of a soft liner. J. Prosthet. Dent. 2017, 117, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Linossier, A.; Vargas, A.; Villegas, R.; Chimenos, E. Quantitative relationship between salivary level of Streptococcus mutans and Candida albicans in children with Down’s syndrome. Med. Oral 2002, 7, 284–292. [Google Scholar]
- Chow, C.; Matear, D.; Lawrence, H. Efficacy of antifungal agents in tissue conditioners in treating candidiasis. Gerodontology 1999, 16, 110–118. [Google Scholar] [CrossRef]
- Iqbal, Z.; Zafar, M. Role of antifungal medicaments added to tissue conditioners: A systematic review. J. Prosthodont. Res. 2016, 60, 231–239. [Google Scholar] [CrossRef]
- Gad, M.; Fouda, S.; Al-Harbi, F.; Näpänkangas, R.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat Mater 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, N.; Eom, H.-J.; Choi, J. A systems toxicology approach to the surface functionality control of graphene-cell interactions. Biomaterials 2014, 35, 1109–1127. [Google Scholar] [CrossRef]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing dental pathogens using antibacterial graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef]
- Davidowitz, G.; Kotick, P. The use of CAD/CAM in dentistry. Dent. Clin. North. Am. 2011, 55, 559–570. [Google Scholar] [CrossRef]
- Revilla-Leon, M.; Ozcan, M. Additive manufacturing technologies used for processing polymers: Current status and potential application in prosthetic dentistry. J. Prosthodont. 2019, 28, 146–158. [Google Scholar] [CrossRef] [Green Version]
- De Armentia, S.L.; Fernández-Villamarín, S.; Ballesteros, Y.; Del Real, J.C.; Dunne, N.; Paz, E. 3D Printing of a Graphene-Modified Photopolymer Using Stereolithography for Biomedical Applications: A Study of the Polymerization Reaction. Int. J. Bioprint. 2022, 8, 503. [Google Scholar] [CrossRef]
- Hrubanova, K.; Krzyzanek, V.; Nebesarova, J.; Ruzicka, F.; Pilat, Z.; Samek, O. Monitoring Candida parapsilosis and Staphylococcus epidermidis Biofilms by a Combination of Scanning Electron Microscopy and Raman Spectroscopy. Sensors 2018, 18, 4089. [Google Scholar] [CrossRef] [Green Version]
- Torres, P.M.C.; Marote, A.; Cerqueira, A.R.; Calado, A.J.; Abrantes, J.C.C.; Olhero, S.; da Cruz E Silva, O.A.B.; Vieira, S.I.; Ferreira, J.M.F. Injectable MnSr-doped brushite bone cements with improved biological performance. J. Mater. Chem. B 2017, 5, 2775–2787. [Google Scholar] [CrossRef]
- Lee, J.H.; Jo, J.K.; Kim, D.A.; Patel, K.D.; Kim, H.W.; Lee, H.H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. PMMA: An essential material in medicine and dentistry. J. Long Term Eff. Med. Implant. 2005, 15, 629–639. [Google Scholar] [CrossRef]
- Scarano, A.; Orsini, T.; Di Carlo, F.; Valbonetti, L.; Lorusso, F. Graphene-Doped Poly (Methyl-Methacrylate) (Pmma) Implants: A Micro-CT and Histomorphometrical Study in Rabbits. Int. J. Mol. Sci. 2021, 22, 1441. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, X.; Qi, Z.; Wang, L.; Aldalbahi, A.; Shi, J.; Song, S.; Fan, C.; Lv, M.; Tang, Z. The inhibition effect of graphene oxide nanosheets on the development of Streptococcus mutans biofilms. Part. Part. Syst. Charact. 2017, 34, 1700001. [Google Scholar] [CrossRef]
- Srimaneepong, V.; Skallevold, H.E.; Khurshid, Z.; Zafar, M.S.; Rokaya, D.; Sapkota, J. Graphene for Antimicrobial and Coating Application. Int. J. Mol. Sci. 2022, 23, 499. [Google Scholar] [CrossRef]
- Yousefi, M.; Dadashpour, M.; Hejazi, M.; Hasanzadeh, M.; Behnam, B.; de la Guardia, M.; Shadjou, N.; Mokhtarzadeh, A. Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 568–581. [Google Scholar] [CrossRef]
- Zhao, M.; Shan, T.; Wu, Q.; Gu, L. The antibacterial effect of graphene oxide on Streptococcus mutans. J. Nanosci. Nanotechnol. 2019, 20, 2095–2103. [Google Scholar] [CrossRef]
- Radhi, A.; Mohamad, D.; Abdul Rahman, F.S.; Abdullah, A.M.; Hasan, H. Mechanism and factors influence of graphene-based nanomaterials antimicrobial activities and application in dentistry. J. Mater. Res. Technol. 2021, 11, 1290–1307. [Google Scholar] [CrossRef]
- Cui, F.; Li, T.; Wang, D.; Yi, S.; Li, J.; Li, X. Recent advances in carbon-based nanomaterials for combating bacterial biofilm-associated infections. J. Hazard. Mater. 2022, 431, 128597. [Google Scholar] [CrossRef]
- Gamal, R.; Gomaa, Y.F.; Said, A.M. Incorporating nano graphene oxide to poly-methyl methacrylate—Antibacterial effect and thermal expansion. J. Mod. Res. 2019, 1, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.R.; Harshit, P.; Lena, C.; Mohan, E. The effect of graphene–poly(methyl methacrylate) fibres on microbial growth. Interface Focus 2018, 8, 20170058. [Google Scholar] [CrossRef] [Green Version]
- Rayannavar, S.; Shaha, B.S.; Shankargouda, S.; Nelogi, S. Antifungal efficacy of tissue conditioner incorporated with nano graphene oxide: An in-vitro study. IJDSIR. 2020, 3, 601–607. [Google Scholar]
- De Campos, M.R.; Botelho, A.L.; dos Reis, A.C. Antimicrobial incorporation on 3D-printed polymers used as potential dental materials and biomaterials: A systematic review of the state of the art. Polym. Bull. 2022, 79, 1–28. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodríguez Hernandez, J. Antimicrobial Polymers for Additive Manufacturing. Int. J. Mol. Sci. 2019, 20, 1210. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado, H.; Gomes, A.T.P.C.; Duarte, A.S.; Ferreira, J.M.F.; Fernandes, C.; Figueiral, M.H.; Mesquita, P. Antimicrobial Activity of a 3D-Printed Polymethylmethacrylate Dental Resin Enhanced with Graphene. Biomedicines 2022, 10, 2607. https://doi.org/10.3390/biomedicines10102607
Salgado H, Gomes ATPC, Duarte AS, Ferreira JMF, Fernandes C, Figueiral MH, Mesquita P. Antimicrobial Activity of a 3D-Printed Polymethylmethacrylate Dental Resin Enhanced with Graphene. Biomedicines. 2022; 10(10):2607. https://doi.org/10.3390/biomedicines10102607
Chicago/Turabian StyleSalgado, Helena, Ana T. P. C. Gomes, Ana S. Duarte, José M. F. Ferreira, Carlos Fernandes, Maria Helena Figueiral, and Pedro Mesquita. 2022. "Antimicrobial Activity of a 3D-Printed Polymethylmethacrylate Dental Resin Enhanced with Graphene" Biomedicines 10, no. 10: 2607. https://doi.org/10.3390/biomedicines10102607
APA StyleSalgado, H., Gomes, A. T. P. C., Duarte, A. S., Ferreira, J. M. F., Fernandes, C., Figueiral, M. H., & Mesquita, P. (2022). Antimicrobial Activity of a 3D-Printed Polymethylmethacrylate Dental Resin Enhanced with Graphene. Biomedicines, 10(10), 2607. https://doi.org/10.3390/biomedicines10102607






