Evidence of Renin–Angiotensin System Receptors in Deep Fascia: A Role in Extracellular Matrix Remodeling and Fibrogenesis?
Abstract
1. Introduction
2. Materials and Methods
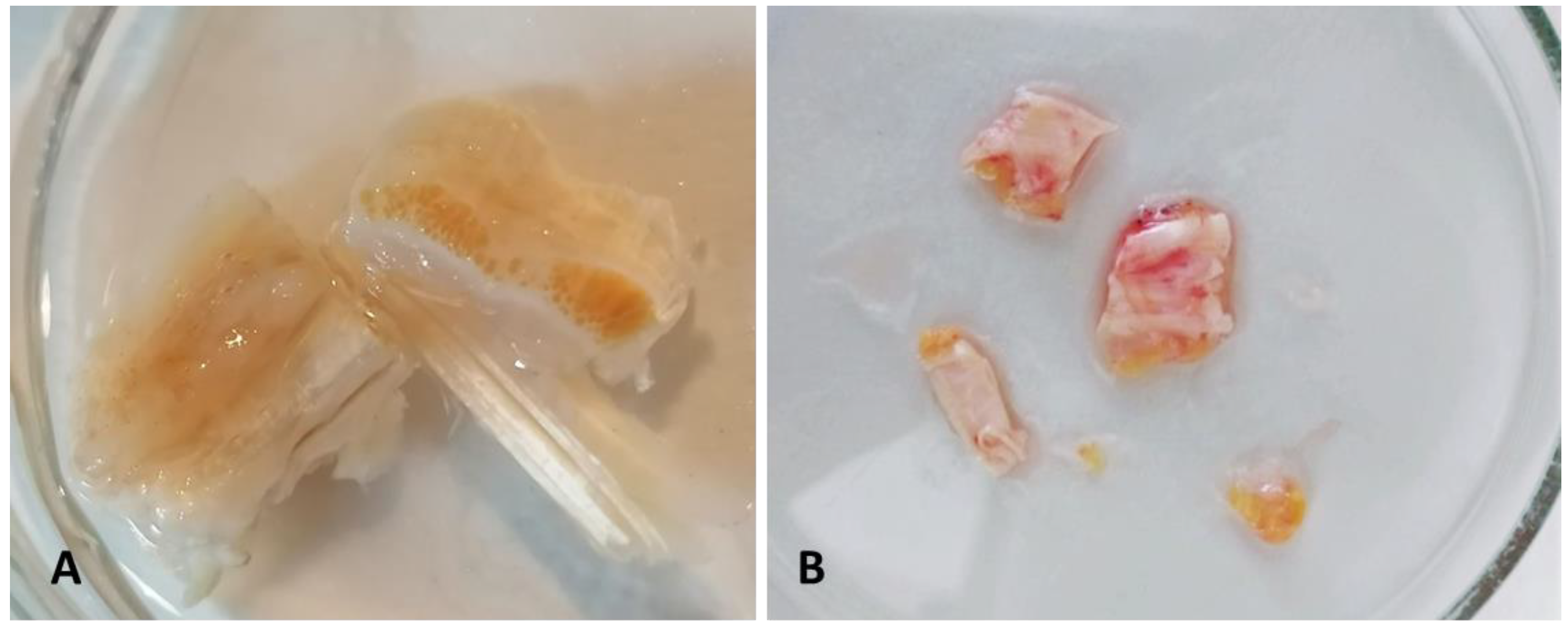
2.1. Patients and Tissues
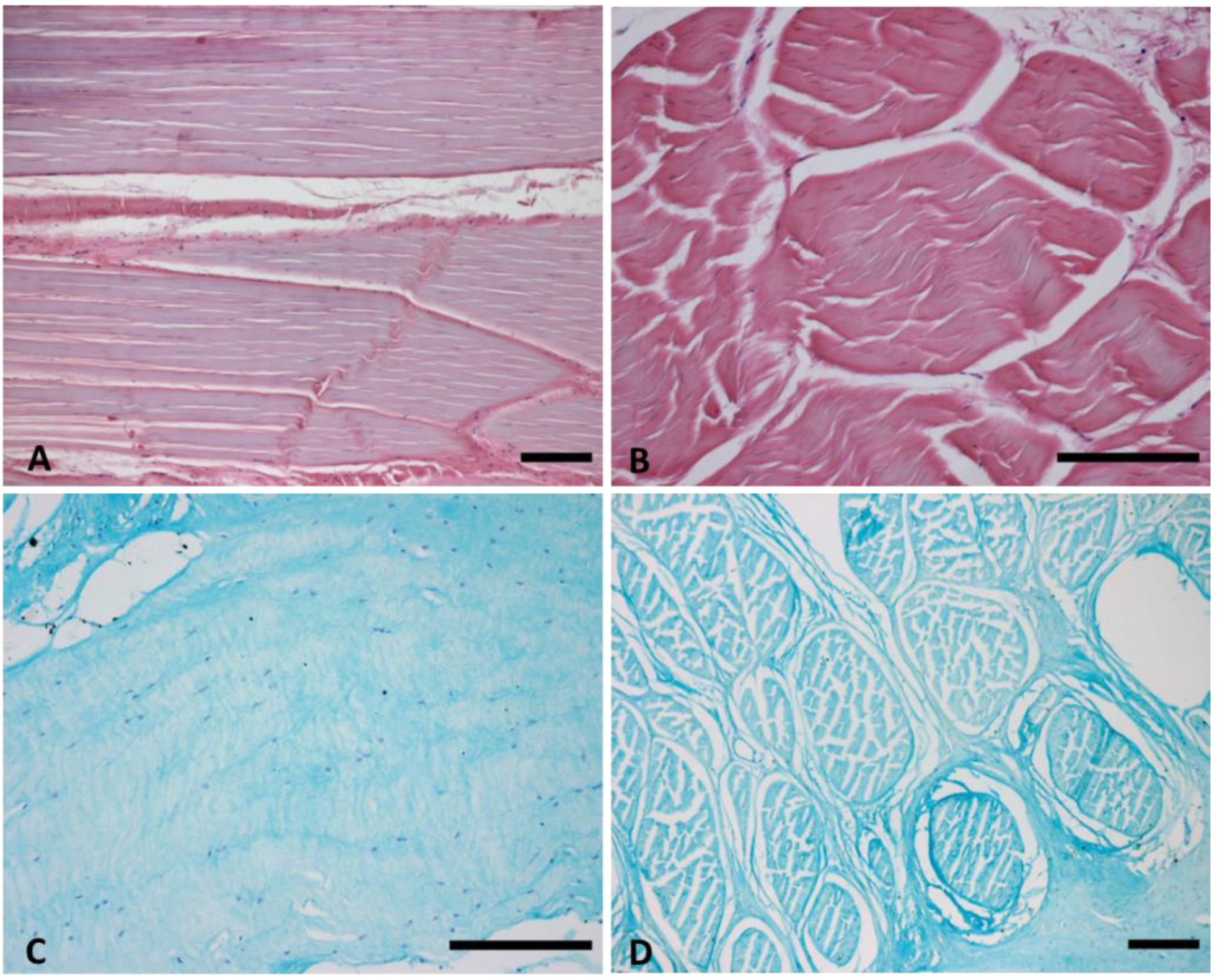
2.2. Histological Staining
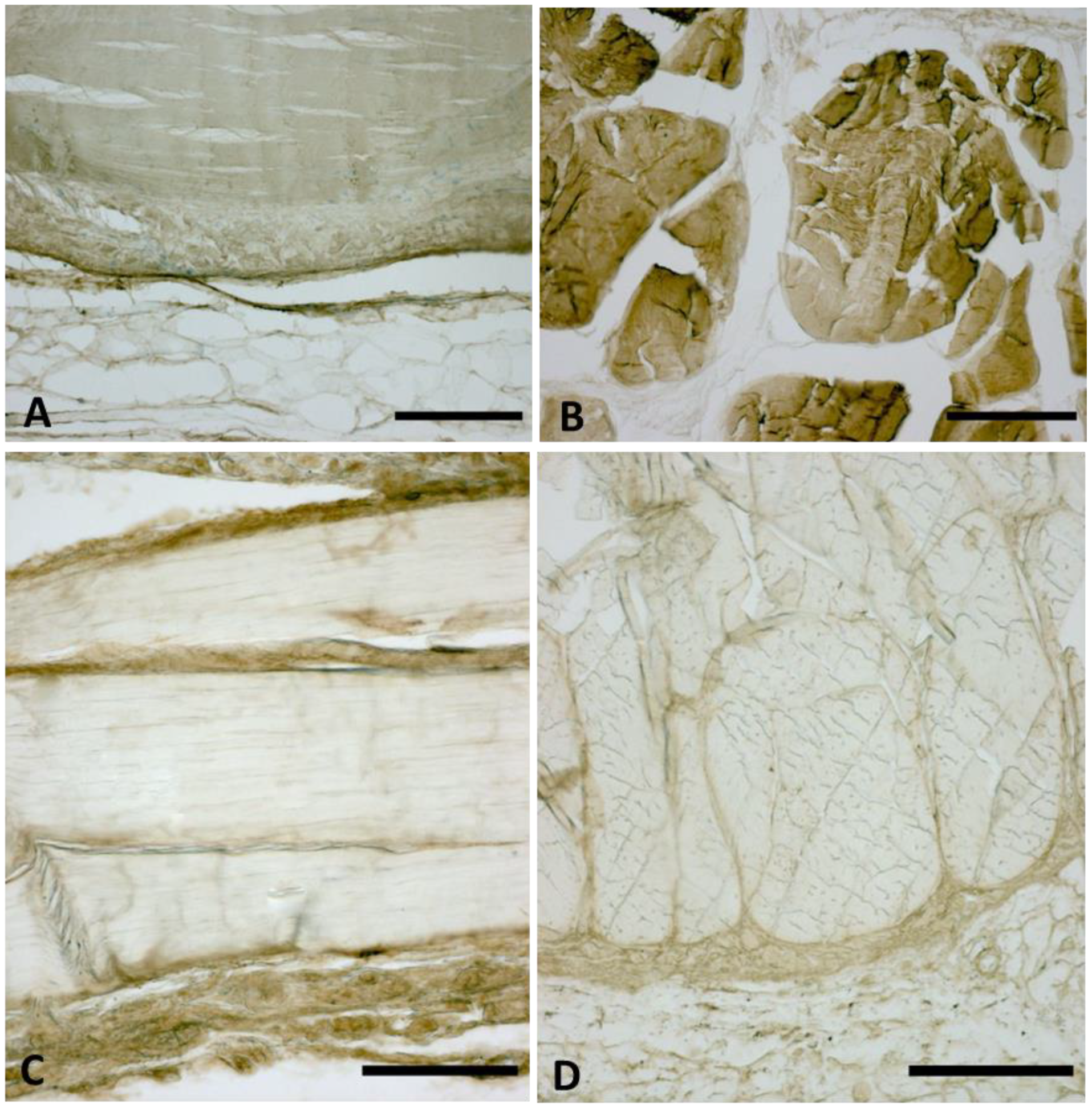
2.3. Immunocytochemistry to Detect Collagen Type I and III
2.4. Total Ribonucleic Acid (RNA) Extraction
2.5. Gene Expression Analysis
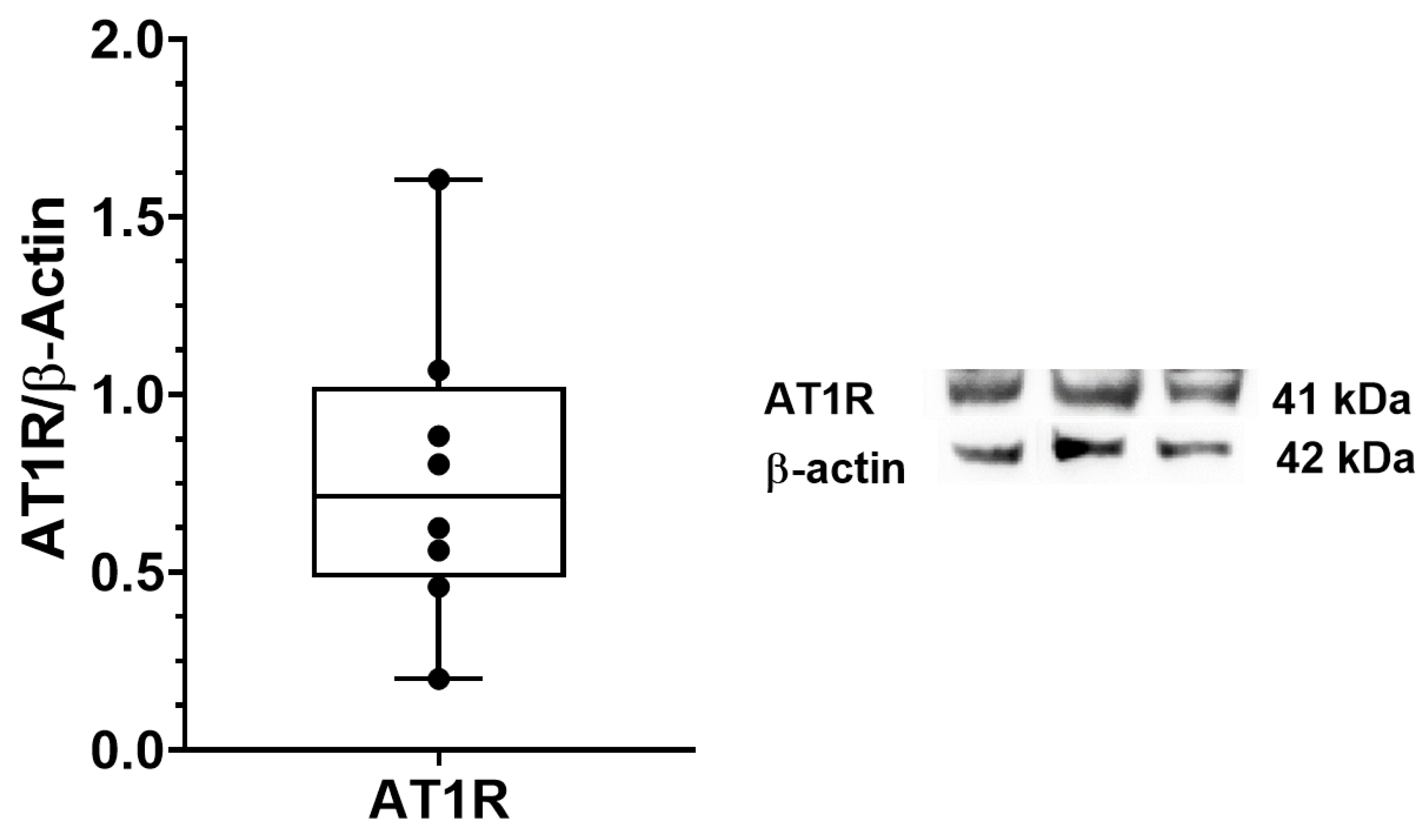
2.6. Immunoblotting
2.7. Statistical Analysis
3. Results
3.1. TL Fasciae Histological Description
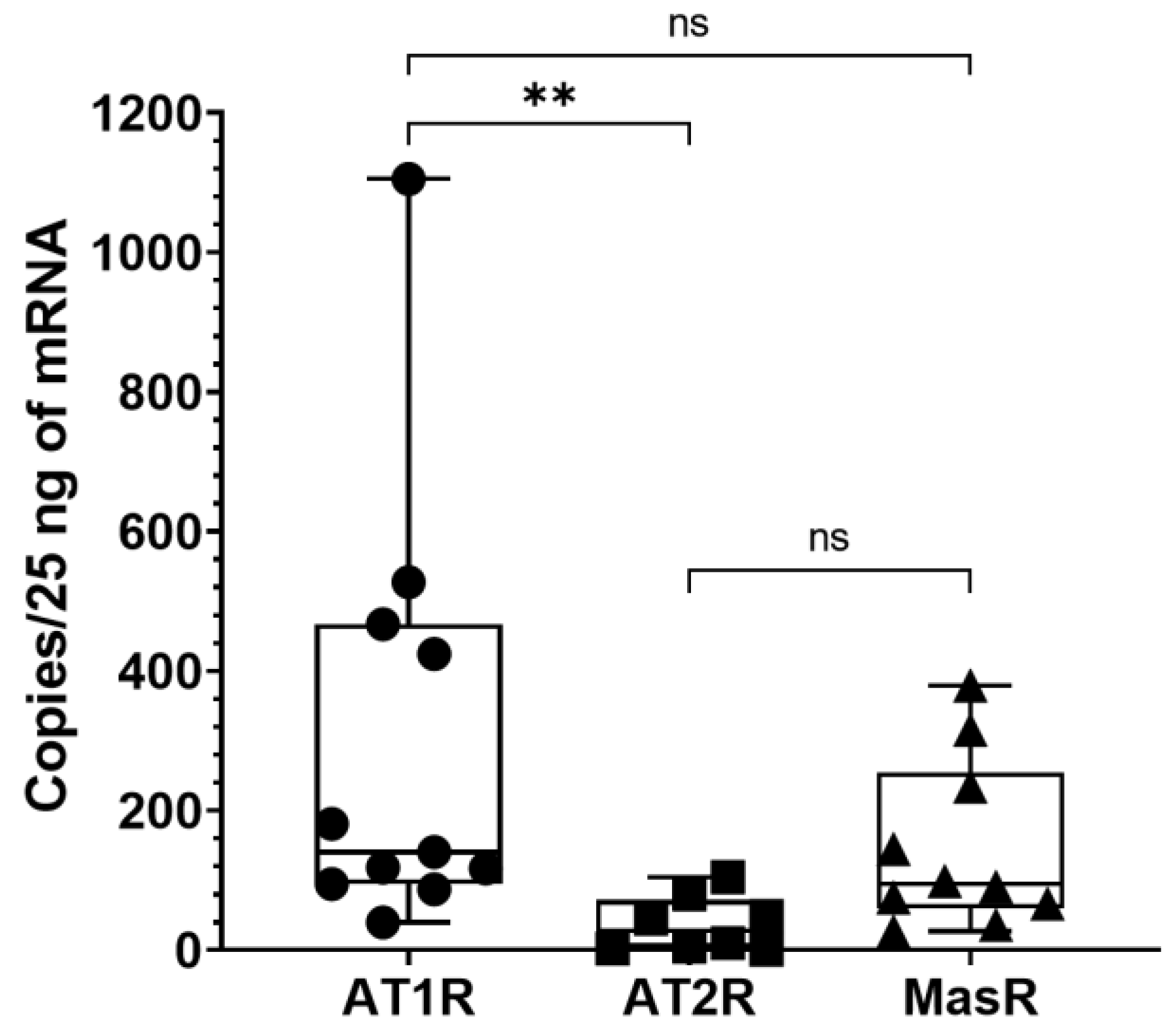
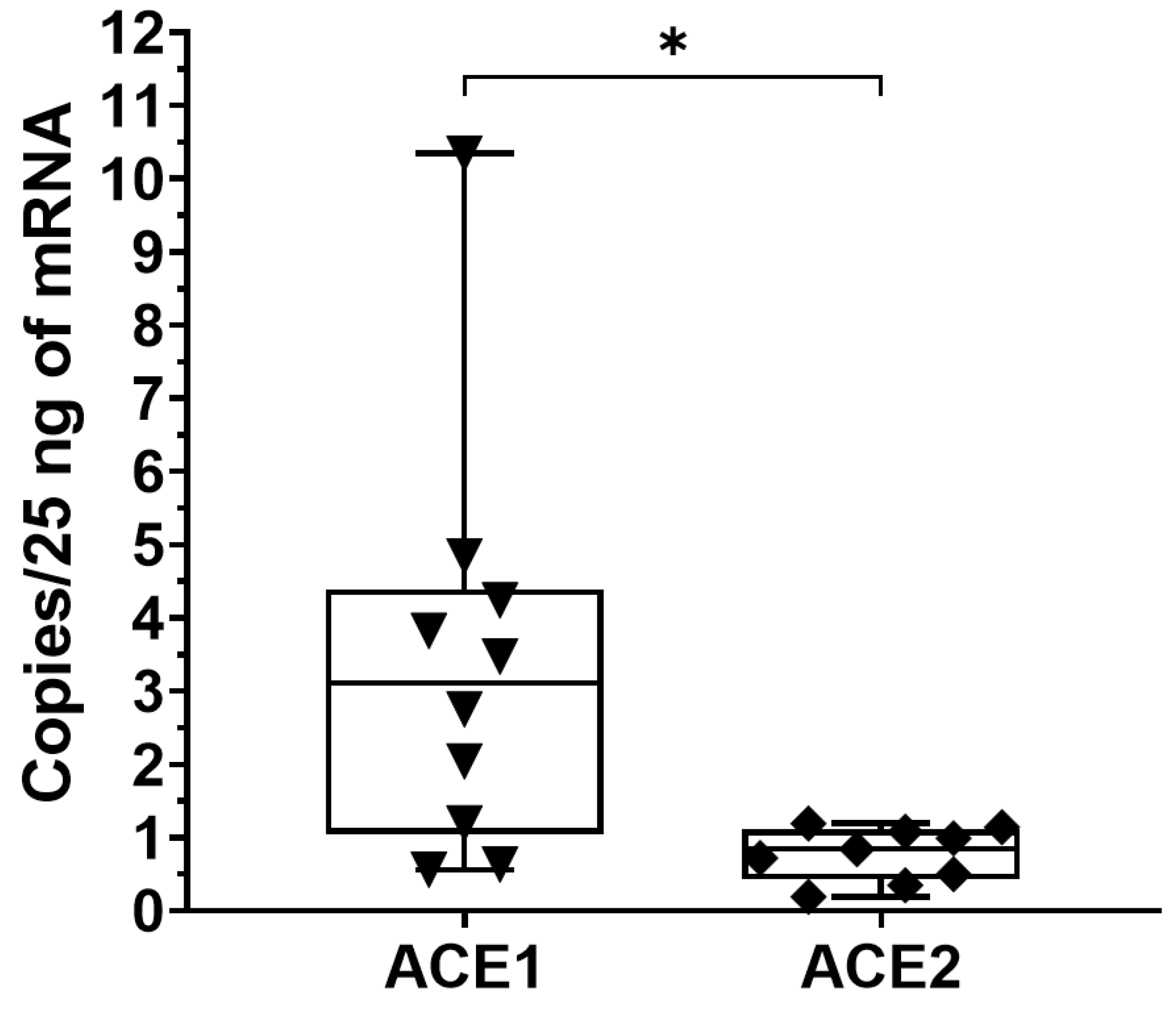
3.2. AT1R, AT2R and MasR Expression in Deep Fascia
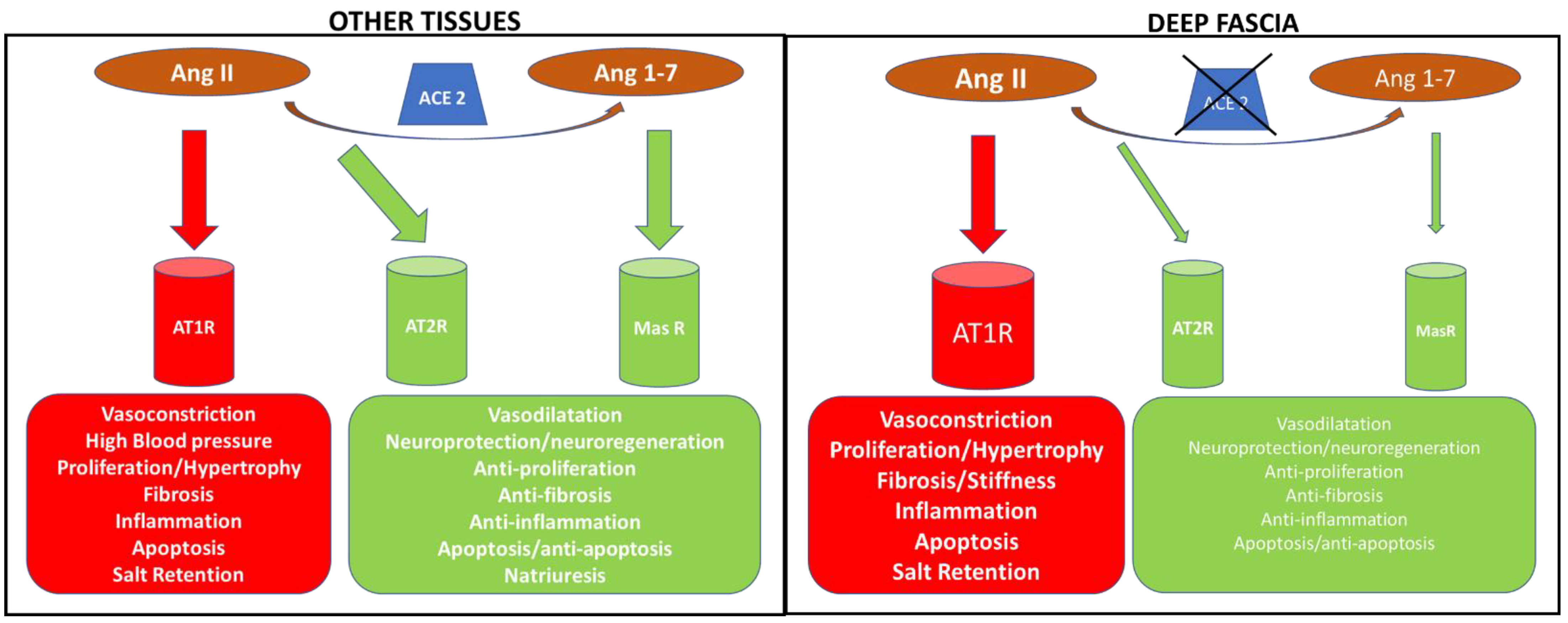
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schleip, R.; Gabbiani, G.; Wilke, J.; Naylor, I.; Hinz, B.; Zorn, A.; Jäger, H.; Breul, R.; Schreiner, S.; Klingler, W. Fascia Is Able to Actively Contract and May Thereby Influence Musculoskeletal Dynamics: A Histochemical and Mechanographic Investigation. Front Physiol. 2019, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Rodriguez, V.; Fede, C.; Pirri, C.; Petrelli, L.; Loro-Ferrer, J.F.; Rodriguez-Ruiz, D.; De Caro, R.; Stecco, C. Fascial Innervation: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 5674. [Google Scholar] [CrossRef] [PubMed]
- Torre, D.E.; Pirri, C.; Contristano, M.; Behr, A.U.; De Caro, R.; Stecco, C. Ultrasound-Guided PECS II + Serratus Plane Fascial Blocks Are Associated with Reduced Opioid Consumption and Lengths of Stay for Minimally Invasive Cardiac Surgery: An Observational Retrospective Study. Life 2022, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, F.; Gaudreault, N.; Venne, G. The deep fascia and its role in chronic pain and pathological conditions: A review. Clin. Anat. 2022, 35, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Pirri, C.; Fan, C.; Petrelli, L.; Guidolin, D.; De Caro, R.; Stecco, C. A Closer Look at the Cellular and Molecular Components of the Deep/Muscular Fasciae. Int. J. Mol. Sci. 2021, 22, 1411. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, V.; Barooah, P.S.; Nag, T.C.; Chaudhuri, G.R.; Bhattacharya, S. Detail microscopic analysis of deep fascia of lower limb and its surgical implication. Indian J. Plast. Surg. 2010, 43, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, V.; Watts, R.K.; Reddy, G.R. Live demonstration of microcirculation in the deep fascia and its implication. Plast Reconstr Surg. 2005, 115, 458–463. [Google Scholar] [CrossRef]
- Stecco, C. Functional Atlas of the Human Fascial System, 1st ed.; Elsevier: Edinburgh, UK, 2015; p. 4. [Google Scholar]
- Langevin, H.M.; Cornbrooks, C.J.; Taatjes, D.J. Fibroblasts form a body-wide cellular network. Histochem. Cell. Biol. 2004, 122, 7–15. [Google Scholar] [CrossRef]
- Benjamin, M. The fascia of the limbs and back—A review. J. Anat. 2009, 214, 1–18. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Fede, C.; Pirri, C.; Fan, C.; Albertin, G.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Sensitivity of the fasciae to sex hormone levels: Modulation of collagen-I, collagen-III and fibrillin production. PLoS ONE 2019, 14, e0223195. [Google Scholar] [CrossRef]
- Fede, C.; Pirri, C.; Petrelli, L.; Guidolin, D.; Fan, C.; De Caro, R.; Stecco, C. Sensitivity of the Fasciae to the Endocannabinoid System: Production of Hyaluronan-Rich Vesicles and Potential Peripheral Effects of Cannabinoids in Fascial Tissue. Int. J. Mol. Sci. 2020, 21, 2936. [Google Scholar] [CrossRef]
- Pirri, C.; Fede, C.; Petrelli, L.; De Rose, E.; Biz, C.; Guidolin, D.; De Caro, R.; Stecco, C. Immediate Effects of Extracorporeal Shock Wave Therapy in Fascial Fibroblasts: An In Vitro Study. Biomedicines 2022, 10, 1732. [Google Scholar] [CrossRef]
- Stecco, A.; Cowman, M.; Pirri, N.; Raghavan, P.; Pirri, C. Densification: Hyaluronan Aggregation in Different Human Organs. Bioengineering 2022, 9, 159. [Google Scholar] [CrossRef]
- Brecher, P. Angiotensin II and cardiac fibrosis. Trends Cardiovasc. Med. 1996, 6, 193–198. [Google Scholar] [CrossRef]
- Guo, G.; Morrissey, J.; McCracken, R.; Tolley, T.; Liapis, H.; Klahr, S. Contributions of angiotensin II and tumor necrosis factor alpha to the development of renal fibrosis. Am. J. Physiol. Renal. Physiol. 2001, 280, F777–F785. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, J.; Wang, H.; Gao, P.; Singh, M.; Shen, K.; Fang, N. Angiotensin II downregulates catalase expression and activity in vascular adventitial fibroblasts through an AT1R/ERK1/2-dependent pathway. Mol. Cell Biochem. 2011, 358, 21–29. [Google Scholar] [CrossRef]
- Morales, M.G.; Vazquez, Y.; Acuna, M.J.; Rivera, J.C.; Simon, F.; Salas, J.D.; Ruf, J.Á.; Brandan, E.; Cabello-Verrugio, C. Angiotensin IIinduced pro-fibrotic effects require p38MAPK activity and transforming growth factor beta 1 expression in skeletal muscle cells. Int. J. Biochem. Cell Biol. 2012, 44, 1993–2002. [Google Scholar] [CrossRef]
- Isobe, A.; Takeda, T.; Sakata, M.; Miyake, A.; Yamamoto, T.; Minekawa, R.; Nishimoto, F.; Oskamoto, Y.; Walker, C.L.; Kimura, T. Dual repressive effect of angiotensin II-type 1 receptor blocker telmisartan on angiotensin II-induced and estradiol-induced uterine leiomyoma cell proliferation. Hum. Reprod. 2008, 23, 440–446. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Kawano, H.; Do, Y.S.; Kawano, Y.; Starnes, V.; Barr, M.; Law, R.E.; Hsueh, W.A. Angiotensin II has multiple profibrotic effects in human cardiac fibroblasts. Circulation 2000, 101, 1130–1137. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Takagi, K.; Hara, M.; Fukasawa, C.; Sugiura, T.; Nishimagi, E.; Harigai, M.; Kamatani, N. Angiotensin II in the lesional skin of systemic sclerosis patients contributes to tissue fibrosis via angiotensin II type 1 receptors. Arthritis. Rheum. 2004, 50, 216–226. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, Y.; Fu, S.; Lu, Z.; Ye, W.; Xiao, Y. Angiotensin II as a morphogenic cytokine stimulating fibrogenesis of human tenon's capsule fibroblasts. Investig. Ophthalmol. Vis. Sci. 2015, 56, 855–864. [Google Scholar] [CrossRef]
- Costa, L.B.; Perez, L.G.; Palmeira, V.A.; Macedo e Cordeiro, T.; Ribeiro, V.T.; Lanza, K.; Simões e Silva, A.C. Insights on SARS-CoV-2 Molecular Interactions with the Renin-Angiotensin System. Front. Cell Dev. Biol. 2020, 8, 559841. [Google Scholar] [CrossRef]
- Danser, A.H. Cardiac angiotensin II: Does it have a function? Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1304–H1306. [Google Scholar] [CrossRef][Green Version]
- Kaparianos, A.; Argyropoulou, E. Local Renin-Angiotensin II Systems, Angiotensin-Converting Enzyme and its Homologue ACE2: Their Potential Role in the Pathogenesis of Chronic Obstructive Pulmonary Diseases, Pulmonary Hypertension and Acute Respiratory Distress Syndrome. Curr. Med. Chem. 2011, 18, 3506–3515. [Google Scholar] [CrossRef]
- Campbell, D.J. Clinical relevance of local Renin Angiotensin systems. Front Endocrinol. (Lausanne) 2014, 5, 113. [Google Scholar] [CrossRef]
- Nejat, R.; Sadr, A.S. Are losartan and imatinib effective against SARS-CoV-2 pathogenesis? A pathophysiologic-based in silico study. Silico Pharmacol. 2021, 9, 1. [Google Scholar] [CrossRef]
- Thomas, W.G.; Mendelsohn, F.A. Angiotensin receptors: Form and function and distribution. Int. J. Biochem. Cell Biol. 2003, 35, 774–779. [Google Scholar] [CrossRef]
- Tang, C.H.; Chen, T.H.; Wang, C.C.; Hong, C.Y.; Huang, K.C.; Sue, Y.M. Renin-angiotensin system blockade in heart failure patients on long-term haemodialysis in Taiwan. Eur. J. Heart Fail. 2013, 15, 1194–1202. [Google Scholar] [CrossRef]
- Stecco, C.; Pirri, C.; Fede, C.; Yucesoy, C.A.; De Caro, R.; Stecco, A. Fascial or Muscle Stretching? A Narrative Review. Appl. Sci. 2021, 11, 307. [Google Scholar] [CrossRef]
- Pavan, P.; Monti, E.; Bondí, M.; Fan, C.; Stecco, C.; Narici, M.; Reggiani, C.; Marcucci, L. Alterations of Extracellular Matrix Mechanical Properties Contribute to Age-Related Functional Impairment of Human Skeletal Muscles. Int. J. Mol. Sci. 2020, 21, 3992. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D.A. Mechanisms of fibrogenesis. Exp. Biol. Med. 2008, 233, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.S.; Vinh, A.; McCarthy, C.A.; Gaspari, T.A.; Widdop, R.E. AT2 receptors: Functional relevance in cardiovascular disease. Pharmacol. Ther. 2008, 120, 292–316. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Egido, J. Angiotensin II modulates cell growth-related events and synthesis of matrix proteins in renal interstitial fibroblasts. Kidney Int. 1997, 52, 1497–1510. [Google Scholar] [CrossRef]
| Forward | Reverse | |
|---|---|---|
| AGT | TCCAGCCTCACTATGCCTCT | GCGGTCATTGCTCAATTTTT |
| AT1R | ATGATTCCAGCGCCTGAC | GGTCCAGACGTCCTGTCACT |
| AT2R | GGTTTCTAGCATATACATCTTCAACCT | TTGCCCATAGAGGAAGAGTAGC |
| MasR | TTCGCTATGCCCATGAGACT | TGGTGTAGGTTCCCAAAGGT |
| ACE1 | AGGAGCAGAACCAGCAGAAC | TCAGCCTCATCAGTCACCAG |
| ACE2 | AAAGTGGTGGGAGATGAAGC | GAGATGCGGGGTCACAGTAT |
| AT1R | AT2R | MasR | |
|---|---|---|---|
| Number of values | 11 | 8 | 10 |
| Minimum | 39.77 | 0.63 | 26.88 |
| 25% Percentile | 94.8 | 2.74 | 59.16 |
| Median | 140.6 | 27.59 | 95.09 |
| 75% Percentile | 466.9 | 72.45 | 254.7 |
| Maximum | 1105 | 104.8 | 379.3 |
| Range | 1065 | 104.1 | 352.4 |
| Mean | 300.2 | 37.09 | 147 |
| Std. Deviation | 317 | 39.56 | 121.9 |
| Std. Error of Mean | 95.57 | 13.99 | 38.55 |
| ACE1 | ACE2 | |
|---|---|---|
| Number of values | 10 | 9 |
| Minimum | 0.56 | 0.19 |
| 25% Percentile | 1.05 | 0.42 |
| Median | 3.11 | 0.84 |
| 75% Percentile | 4.39 | 1.11 |
| Maximum | 10.34 | 1.19 |
| Range | 9.78 | 1 |
| Mean | 3.38 | 0.77 |
| Std. Deviation | 2.86 | 0.36 |
| Std. Error of Mean | 0.90 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirri, C.; Caroccia, B.; Angelini, A.; Petrelli, L.; Piazza, M.; Biz, C.; Ruggieri, P.; De Caro, R.; Stecco, C. Evidence of Renin–Angiotensin System Receptors in Deep Fascia: A Role in Extracellular Matrix Remodeling and Fibrogenesis? Biomedicines 2022, 10, 2608. https://doi.org/10.3390/biomedicines10102608
Pirri C, Caroccia B, Angelini A, Petrelli L, Piazza M, Biz C, Ruggieri P, De Caro R, Stecco C. Evidence of Renin–Angiotensin System Receptors in Deep Fascia: A Role in Extracellular Matrix Remodeling and Fibrogenesis? Biomedicines. 2022; 10(10):2608. https://doi.org/10.3390/biomedicines10102608
Chicago/Turabian StylePirri, Carmelo, Brasilina Caroccia, Andrea Angelini, Lucia Petrelli, Maria Piazza, Carlo Biz, Pietro Ruggieri, Raffaele De Caro, and Carla Stecco. 2022. "Evidence of Renin–Angiotensin System Receptors in Deep Fascia: A Role in Extracellular Matrix Remodeling and Fibrogenesis?" Biomedicines 10, no. 10: 2608. https://doi.org/10.3390/biomedicines10102608
APA StylePirri, C., Caroccia, B., Angelini, A., Petrelli, L., Piazza, M., Biz, C., Ruggieri, P., De Caro, R., & Stecco, C. (2022). Evidence of Renin–Angiotensin System Receptors in Deep Fascia: A Role in Extracellular Matrix Remodeling and Fibrogenesis? Biomedicines, 10(10), 2608. https://doi.org/10.3390/biomedicines10102608












