Brain Perfusion Alterations Induced by Standalone and Combined Non-Invasive Brain Stimulation over the Dorsolateral Prefrontal Cortex
Abstract
:1. Background
2. Materials and Methods
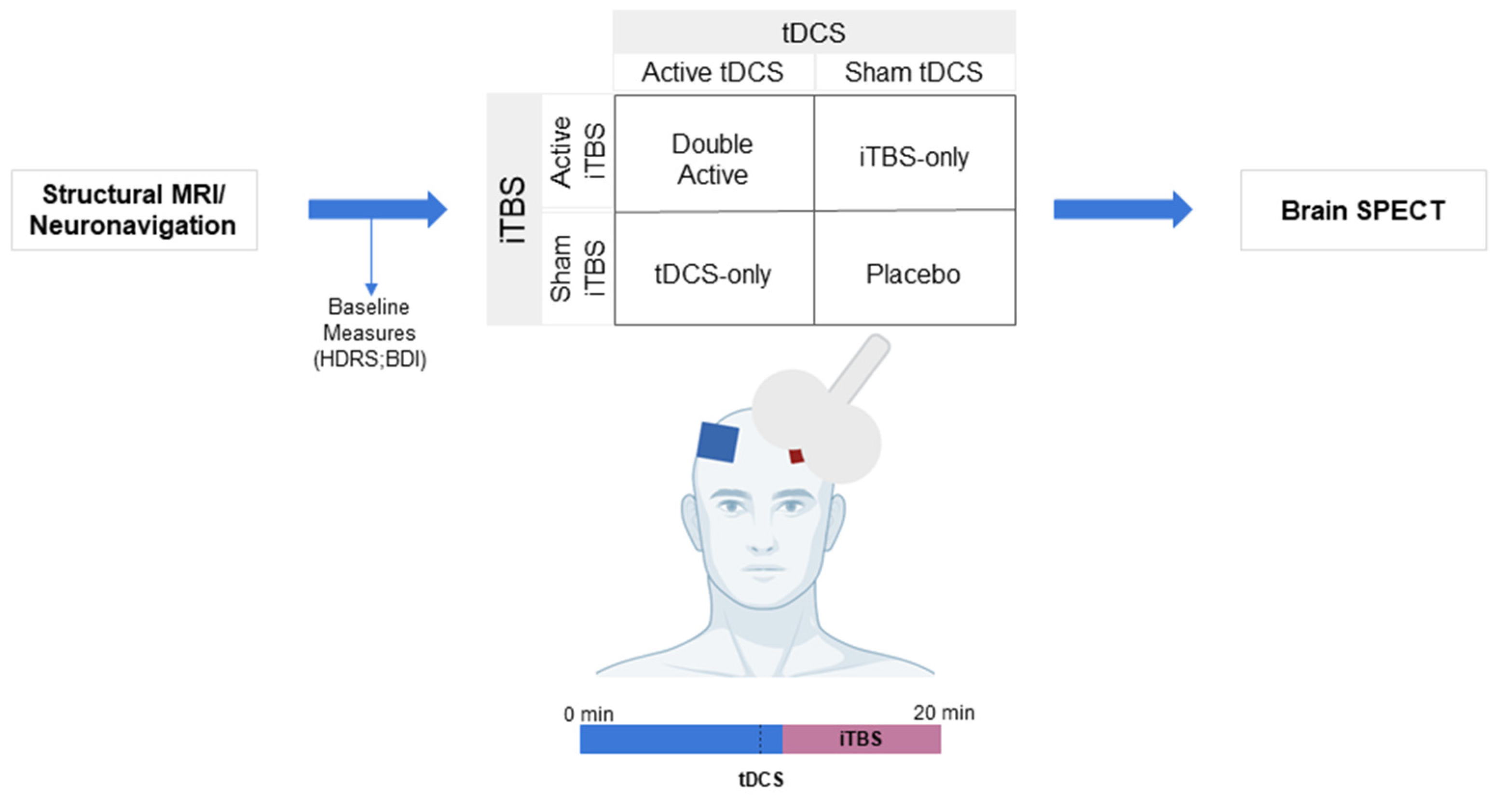
2.1. Design
2.2. Participants
2.3. Procedures
2.4. Interventions
2.5. Images Pre-Processing and Processing
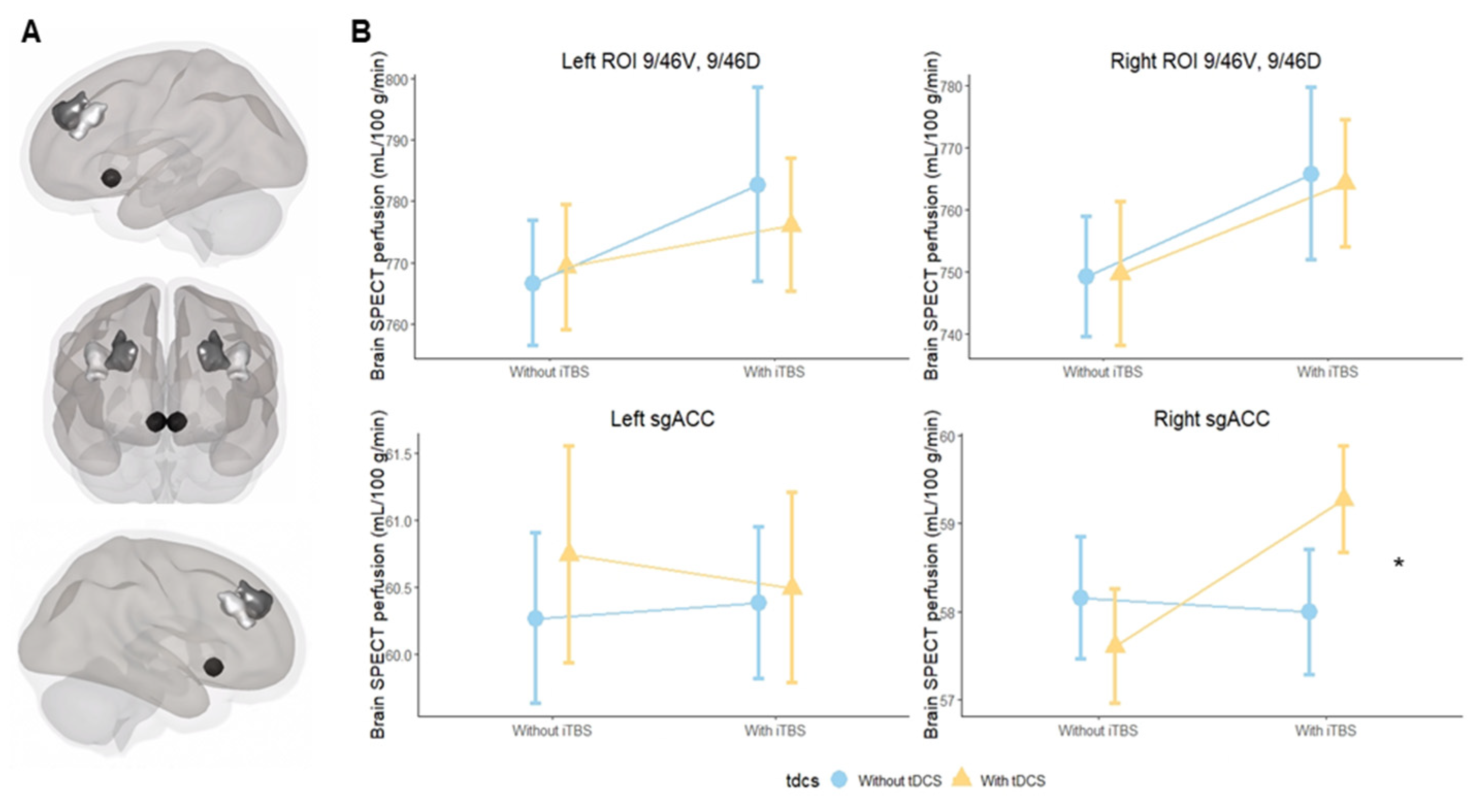
2.6. Regions of Interest
2.7. Statistical Analysis
3. Results
3.1. Primary Outcome
3.2. Secondary Outcomes for DLPFC Subregions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parkin, B.L.; Ekhtiari, H.; Walsh, V.F. Non-invasive Human Brain Stimulation in Cognitive Neuroscience: A Primer. Neuron 2015, 87, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Transcranial Magnetic Stimulation: A Primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta Burst Stimulation of the Human Motor Cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xu, L.; Xie, F.; Guo, X.; Zhang, J.; Yao, L.; Wu, X. The Altered Triple Networks Interaction in Depression under Resting State Based on Graph Theory. BioMed Res. Int. 2015, 2015, 386326. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, F.; Lanza, G.; Bella, R.; Pennisi, M. “Self-Neuroenhancement”: The Last Frontier of Noninvasive Brain Stimulation? J. Clin. Neurol. 2020, 16, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Bella, R.; Benussi, A.; Bologna, M.; Borroni, B.; Capone, F.; Chen, K.S.; Chen, R.; Chistyakov, A.V.; Classen, J.; et al. Diagnostic contribution and therapeutic perspectives of transcranial magnetic stimulation in dementia. Clin. Neurophysiol. 2021, 132, 2568–2607. [Google Scholar] [PubMed]
- Fox, M.D.; Buckner, R.L.; White, M.P.; Greicius, M.D.; Pascual-Leone, A. Efficacy of Transcranial Magnetic Stimulation Targets for Depression Is Related to Intrinsic Functional Connectivity with the Subgenual Cingulate. Biol. Psychiatry 2012, 72, 595–603. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Moffa, A.H.; Sampaio-Junior, B.; Borrione, L.; Moreno, M.L.; Fernandes, R.A.; Veronezi, B.P.; Nogueira, B.S.; Aparicio, L.V.; Razza, L.B.; et al. Trial of Electrical Direct-Current Therapy versus Escitalopram for Depression. N. Engl. J. Med. 2017, 376, 2523–2533. [Google Scholar] [CrossRef] [PubMed]
- Bestmann, S.; Feredoes, E. Combined neurostimulation and neuroimaging in cognitive neuroscience: Past, present, and future. Ann. N. Y. Acad. Sci. 2013, 1296, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.C.; Jovellar, D.B.; Song, Y.; Zrenner, C.; Belardinelli, P.; Siebner, H.R.; Ziemann, U. Recording brain responses to TMS of primary motor cortex by EEG—Utility of an optimized sham procedure. NeuroImage 2021, 245, 118708. [Google Scholar] [CrossRef] [PubMed]
- Mizutani-Tiebel, Y.; Tik, M.; Chang, K.-Y.; Padberg, F.; Soldini, A.; Wilkinson, Z.; Voon, C.C.; Bulubas, L.; Windischberger, C.; Keeser, D. Concurrent TMS-fMRI: Technical Challenges, Developments, and Overview of Previous Studies. Front. Psychiatry 2022, 13, 825205. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Tuominen, L.; Zayed, V.; Pascual-Leone, A.; Joutsa, J. The study of noninvasive brain stimulation using molecular brain imaging: A systematic review. NeuroImage 2020, 219, 117023. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.; Machado, L. Using tDCS priming to improve brain function: Can metaplasticity provide the key to boosting outcomes? Neurosci. Biobehav. Rev. 2017, 83, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Keeser, D.; Meindl, T.; Bor, J.; Palm, U.; Pogarell, O.; Mulert, C.; Brunelin, J.; Möller, H.-J.; Reiser, M.; Padberg, F. Prefrontal Transcranial Direct Current Stimulation Changes Connectivity of Resting-State Networks during fMRI. J. Neurosci. 2011, 31, 15284–15293. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, S.J.; Raschke, F.; Auer, D.P.; Liddle, P.F.; Lankappa, S.T.; Palaniyappan, L. Targeted transcranial theta-burst stimulation alters fronto-insular network and prefrontal GABA. NeuroImage 2017, 146, 395–403. [Google Scholar] [CrossRef]
- Razza, L.B.; Buchpiguel, C.A.; De Smet, S.; Klein, I.; Baeken, C.; Galhardoni, R.; Vanderhasselt, M.-A.; Brunoni, A.R. Combined effects of theta-burst stimulation with transcranial direct current stimulation of the prefrontal cortex: Study protocol of a randomized, double-blinded, sham-controlled trial using 99mTc-ECD SPECT. Trends Psychiatry Psychother. 2021, 43, 293–301. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Wischnewski, M.; Mantell, K.E.; Opitz, A. Identifying regions in prefrontal cortex related to working memory improvement: A novel meta-analytic method using electric field modeling. Neurosci. Biobehav. Rev. 2021, 130, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Rorden, C.; Brett, M. Stereotaxic Display of Brain Lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, C.C.; Kinahan, P.; Greer, P.; Nichols, T.; Comtat, C.; Cantwell, M.N.; Lin, M.P.; Price, J.C. Comparative evaluation of MR-based partial-volume correction schemes for PET. J. Nucl. Med. 1999, 40, 2053–2065. [Google Scholar] [PubMed]
- Suen, P.J.C.; Doll, S.; Batistuzzo, M.C.; Busatto, G.; Razza, L.B.; Padberg, F.; Mezger, E.; Bulubas, L.; Keeser, D.; Deng, Z.-D.; et al. Association between tDCS computational modeling and clinical outcomes in depression: Data from the ELECT-TDCS trial. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 271, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Bulubas, L.; Padberg, F.; Bueno, P.V.; Souza-Duran, F.L.; Busatto, G.; Amaro, E.; Benseñor, I.M.; Lotufo, P.A.; Goerigk, S.; Gattaz, W.; et al. Antidepressant effects of tDCS are associated with prefrontal gray matter volumes at baseline: Evidence from the ELECT-TDCS trial. Brain Stimul. 2019, 12, 1197–1204. [Google Scholar] [CrossRef]
- Sallet, J.; Mars, R.; Noonan, M.P.; Neubert, F.-X.; Jbabdi, S.; O’Reilly, J.; Filippini, N.; Thomas, A.; Rushworth, M. The Organization of Dorsal Frontal Cortex in Humans and Macaques. J. Neurosci. 2013, 33, 12255–12274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. 2013. Available online: https://cran.microsoft.com/snapshot/2014-09-08/web/packages/dplR/vignettes/xdate-dplR.pdf (accessed on 22 February 2021).
- Nieto-Castanon, A. Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press: Boston, MA, USA, 2020. [Google Scholar] [CrossRef]
- Cole, E.J.; Stimpson, K.H.; Bentzley, B.S.; Gulser, M.; Cherian, K.; Tischler, C.; Nejad, R.; Pankow, H.; Choi, E.; Aaron, H.; et al. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am. J. Psychiatry 2020, 177, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Stöhrmann, P.; Godbersen, G.M.; Reed, M.B.; Unterholzner, J.; Klöbl, M.; Baldinger-Melich, P.; Vanicek, T.; Hahn, A.; Lanzenberger, R.; Kasper, S.; et al. Effects of bilateral sequential theta-burst stimulation on functional connectivity in treatment-resistant depression: First results. medRxiv 2022. [Google Scholar] [CrossRef]
- Sathappan, A.V.; Luber, B.M.; Lisanby, S.H. The Dynamic Duo: Combining noninvasive brain stimulation with cognitive interventions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 89, 347–360. [Google Scholar] [CrossRef]
- Cantone, M.; Lanza, G.; Ranieri, F.; Opie, G.M.; Terranova, C. Editorial: Non-invasive Brain Stimulation in the Study and Modulation of Metaplasticity in Neurological Disorders. Front. Neurol. 2021, 12, 721906. [Google Scholar] [CrossRef] [PubMed]
- Mutz, J.; Vipulananthan, V.; Carter, B.; Hurlemann, R.; Fu, C.H.Y.; Young, A.H. Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: Systematic review and network meta-analysis. BMJ 2019, 364, l1079. [Google Scholar] [CrossRef] [PubMed]
- Iimori, T.; Nakajima, S.; Miyazaki, T.; Tarumi, R.; Ogyu, K.; Wada, M.; Tsugawa, S.; Masuda, F.; Daskalakis, Z.J.; Blumberger, D.M.; et al. Effectiveness of the prefrontal repetitive transcranial magnetic stimulation on cognitive profiles in depression, schizophrenia, and Alzheimer’s disease: A systematic review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 88, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.-F.; Nitsche, M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef]
- Hassanzahraee, M.; Nitsche, M.A.; Zoghi, M.; Jaberzadeh, S. Determination of anodal tDCS intensity threshold for reversal of corticospinal excitability: An investigation for induction of counter-regulatory mechanisms. Sci. Rep. 2020, 10, 16108. [Google Scholar] [CrossRef]
- Hone-Blanchet, A.; Edden, R.A.; Fecteau, S. Online Effects of Transcranial Direct Current Stimulation in Real Time on Human Prefrontal and Striatal Metabolites. Biol. Psychiatry 2015, 80, 432–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezger, E.; Rauchmann, B.-S.; Brunoni, A.R.; Bulubas, L.; Thielscher, A.; Werle, J.; Mortazavi, M.; Karali, T.; Stöcklein, S.; Ertl-Wagner, B.; et al. Effects of bifrontal transcranial direct current stimulation on brain glutamate levels and resting state connectivity: Multimodal MRI data for the cathodal stimulation site. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 271, 111–122. [Google Scholar] [CrossRef]
- Weller, S.; Nitsche, M.A.; Plewnia, C. Enhancing cognitive control training with transcranial direct current stimulation: A systematic parameter study. Brain Stimul. 2020, 13, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- McCalley, D.M.; Lench, D.H.; Doolittle, J.D.; Imperatore, J.P.; Hoffman, M.; Hanlon, C.A. Determining the optimal pulse number for theta burst induced change in cortical excitability. Sci. Rep. 2021, 11, 8726. [Google Scholar] [CrossRef] [PubMed]

| Left Hemisphere | Right Hemisphere | |||||||
|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | p-Value | Beta | 95% CI | p-Value | |||
| iTBS vs. tDCS | ||||||||
| 9/46D, 9/46V | −9.17 | −40.04 | 21.7 | 0.55 | −2 | −33.35 | 29.3 | 0.9 |
| sgACC | −0.4 | −2.4 | 1.7 | 0.7 | 19 | 1.69 | 36.1 | 0.03 |
| tDCS | ||||||||
| 9/46D, 9/46V | 2.58 | −19.25 | 24.4 | 0.8 | 0.5 | −21.7 | 22.7 | 1 |
| sgACC | 0.47 | −1 | 1.9 | 0.52 | −7.4 | −19.6 | 4.8 | 0.2 |
| iTBS | ||||||||
| 9/46D, 9/46V | 16.01 | −5.82 | 37.4 | 0.15 | 16.6 | −5.6 | 38.7 | 0.14 |
| sgACC | 0.11 | −1.35 | 1.5 | 0.9 | −7.3 | −19.5 | 4.9 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razza, L.B.; da Silva, P.H.R.; Busatto, G.F.; Duran, F.L.d.S.; Pereira, J.; De Smet, S.; Klein, I.; Zanão, T.A.; Luethi, M.S.; Baeken, C.; et al. Brain Perfusion Alterations Induced by Standalone and Combined Non-Invasive Brain Stimulation over the Dorsolateral Prefrontal Cortex. Biomedicines 2022, 10, 2410. https://doi.org/10.3390/biomedicines10102410
Razza LB, da Silva PHR, Busatto GF, Duran FLdS, Pereira J, De Smet S, Klein I, Zanão TA, Luethi MS, Baeken C, et al. Brain Perfusion Alterations Induced by Standalone and Combined Non-Invasive Brain Stimulation over the Dorsolateral Prefrontal Cortex. Biomedicines. 2022; 10(10):2410. https://doi.org/10.3390/biomedicines10102410
Chicago/Turabian StyleRazza, Lais Boralli, Pedro Henrique Rodrigues da Silva, Geraldo F. Busatto, Fábio Luis de Souza Duran, Juliana Pereira, Stefanie De Smet, Izio Klein, Tamires A. Zanão, Matthias S. Luethi, Chris Baeken, and et al. 2022. "Brain Perfusion Alterations Induced by Standalone and Combined Non-Invasive Brain Stimulation over the Dorsolateral Prefrontal Cortex" Biomedicines 10, no. 10: 2410. https://doi.org/10.3390/biomedicines10102410
APA StyleRazza, L. B., da Silva, P. H. R., Busatto, G. F., Duran, F. L. d. S., Pereira, J., De Smet, S., Klein, I., Zanão, T. A., Luethi, M. S., Baeken, C., Vanderhasselt, M.-A., Buchpiguel, C. A., & Brunoni, A. R. (2022). Brain Perfusion Alterations Induced by Standalone and Combined Non-Invasive Brain Stimulation over the Dorsolateral Prefrontal Cortex. Biomedicines, 10(10), 2410. https://doi.org/10.3390/biomedicines10102410






