A Single Session of Bifrontal tDCS Can Improve Facial Emotion Recognition in Major Depressive Disorder: An Exploratory Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Stimulation Procedure
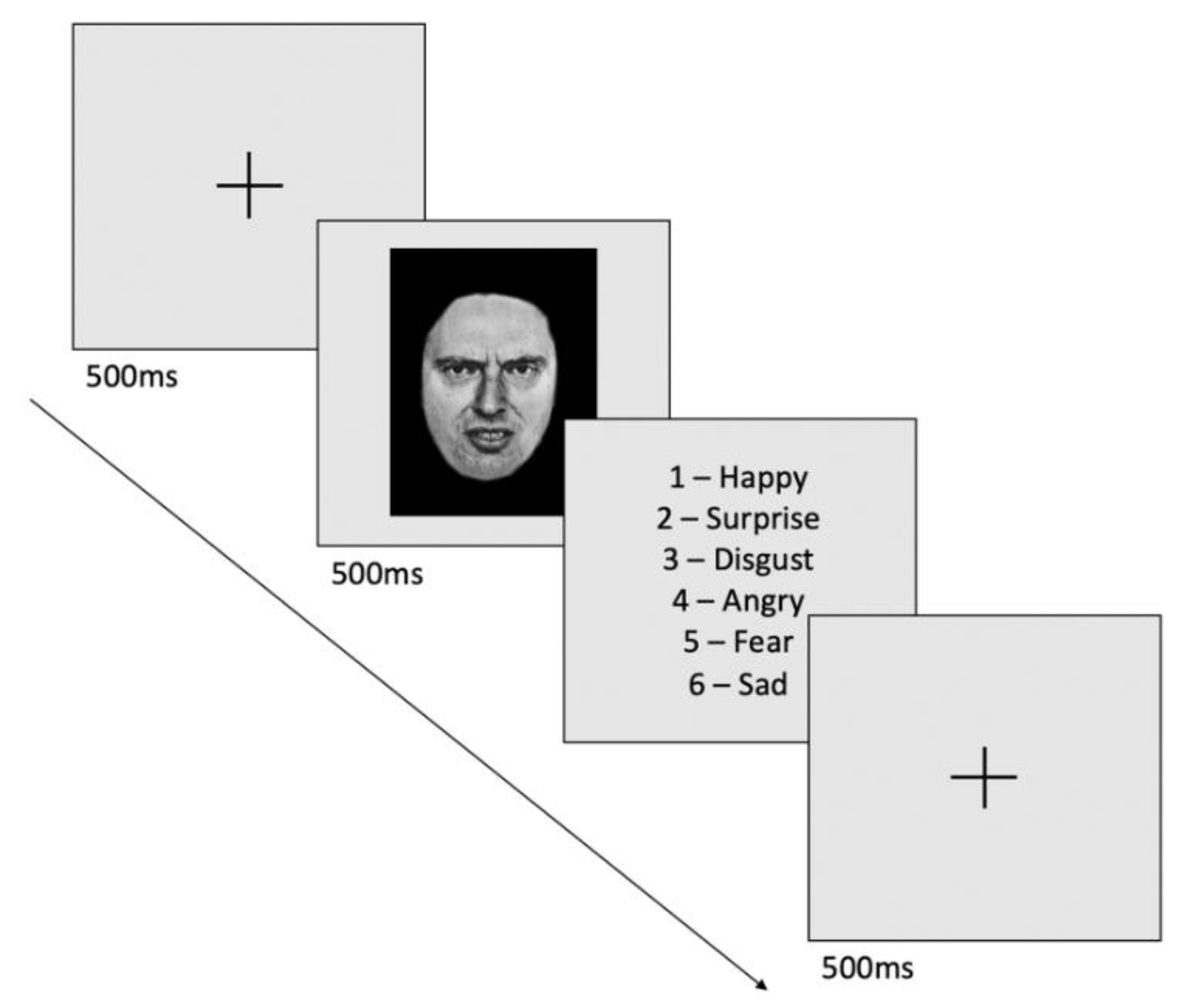
2.3. Facial Emotion Recognition Task
2.4. Statistical Analysis
3. Results
3.1. Clinical and Sociodemographic Characteristics
3.2. Effects of tDCS on Facial Emotion Recognition
3.2.1. Within-Group Analysis
3.2.2. Between-Group Analysis
3.2.3. Exploratory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/254610/W?sequence=1 (accessed on 7 February 2022).
- Disner, S.G.; Beevers, C.G.; Haigh, E.A.P.; Beck, A.T. Neural Mechanisms of the Cognitive Model of Depression. Nat. Rev. Neurosci. 2011, 12, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Fossati, P. Is Major Depression a Cognitive Disorder? Rev. Neurol. 2018, 174, 212–215. [Google Scholar] [CrossRef]
- Gotlib, I.H.; Joormann, J. Cognition and Depression: Current Status and Future Directions. Annu. Rev. Clin. Psychol. 2010, 6, 285–312. [Google Scholar] [CrossRef]
- Kohler, C.G.; Hoffman, L.J.; Eastman, L.B.; Healey, K.; Moberg, P.J. Facial Emotion Perception in Depression and Bipolar Disorder: A Quantitative Review. Psychiatry Res. 2011, 188, 303–309. [Google Scholar] [CrossRef]
- Dalili, M.N.; Penton-Voak, I.S.; Harmer, C.J.; Munafò, M.R. Meta-Analysis of Emotion Recognition Deficits in Major Depressive Disorder. Psychol. Med. 2015, 45, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Delle-Vigne, D.; Wang, W.; Kornreich, C.; Verbanck, P.; Campanella, S. Emotional Facial Expression Processing in Depression: Data from Behavioral and Event-Related Potential Studies. Neurophysiol. Clin./Clin. Neurophysiol. 2014, 44, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.L.; Drevets, W.C.; Rauch, S.L.; Lane, R. Neurobiology of Emotion Perception I: The Neural Basis of Normal Emotion Perception. Biol. Psychiatry 2003, 54, 504–514. [Google Scholar] [CrossRef]
- Grimm, S.; Beck, J.; Schuepbach, D.; Hell, D.; Boesiger, P.; Bermpohl, F.; Niehaus, L.; Boeker, H.; Northoff, G. Imbalance between Left and Right Dorsolateral Prefrontal Cortex in Major Depression Is Linked to Negative Emotional Judgment: An FMRI Study in Severe Major Depressive Disorder. Biol. Psychiatry 2008, 63, 369–376. [Google Scholar] [CrossRef]
- George, M.S.; Wassermann, E.M.; Kimbrell, T.A.; Little, J.T.; Williams, W.E.; Danielson, A.L.; Greenberg, B.D.; Hallett, M.; Post, R.M. Mood Improvement Following Daily Left Prefrontal Repetitive Transcranial Magnetic Stimulation in Patients With Depression: A Placebo-Controlled Crossover Trial. AJP 1997, 154, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.C.; Vakhtin, A.; Clark, V.P.; Abbott, C.C.; Quinn, D.K. Revisiting Hemispheric Asymmetry in Mood Regulation: Implications for RTMS for Major Depressive Disorder. Brain Sci. 2022, 12, 112. [Google Scholar] [CrossRef]
- O’Reardon, J.P.; Solvason, H.B.; Janicak, P.G.; Sampson, S.; Isenberg, K.E.; Nahas, Z.; McDonald, W.M.; Avery, D.; Fitzgerald, P.B.; Loo, C.; et al. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol. Psychiatry 2007, 62, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Brunelin, J.; Jalenques, I.; Trojak, B.; Attal, J.; Szekely, D.; Gay, A.; Januel, D.; Haffen, E.; Schott-Pethelaz, A.-M.; Brault, C.; et al. The Efficacy and Safety of Low Frequency Repetitive Transcranial Magnetic Stimulation for Treatment-Resistant Depression: The Results From a Large Multicenter French RCT. Brain Stimul. 2014, 7, 855–863. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Valiengo, L.; Baccaro, A.; Zanão, T.A.; de Oliveira, J.F.; Goulart, A.; Boggio, P.S.; Lotufo, P.A.; Benseñor, I.M.; Fregni, F. The Sertraline vs Electrical Current Therapy for Treating Depression Clinical Study: Results From a Factorial, Randomized, Controlled Trial. JAMA Psychiatry 2013, 70, 383. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; El-Hagrassy, M.M.; Pacheco-Barrios, K.; Carvalho, S.; Leite, J.; Simis, M.; Brunelin, J.; Nakamura-Palacios, E.M.; Marangolo, P.; Venkatasubramanian, G.; et al. Evidence-Based Guidelines and Secondary Meta-Analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric Disorders. Int. J. Neuropsychopharmacol. 2021, 24, 256–313. [Google Scholar] [CrossRef] [PubMed]
- Moffa, A.H.; Martin, D.; Alonzo, A.; Bennabi, D.; Blumberger, D.M.; Benseñor, I.M.; Daskalakis, Z.; Fregni, F.; Haffen, E.; Lisanby, S.H.; et al. Efficacy and Acceptability of Transcranial Direct Current Stimulation (TDCS) for Major Depressive Disorder: An Individual Patient Data Meta-Analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 99, 109836. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Moffa, A.H.; Sampaio-Junior, B.; Borrione, L.; Moreno, M.L.; Fernandes, R.A.; Veronezi, B.P.; Nogueira, B.S.; Aparicio, L.V.M.; Razza, L.B.; et al. Trial of Electrical Direct-Current Therapy versus Escitalopram for Depression. N. Engl. J. Med. 2017, 376, 2523–2533. [Google Scholar] [CrossRef] [PubMed]
- Mondino, M.; Bennabi, D.; Poulet, E.; Galvao, F.; Brunelin, J.; Haffen, E. Can Transcranial Direct Current Stimulation (TDCS) Alleviate Symptoms and Improve Cognition in Psychiatric Disorders? World J. Biol. Psychiatry 2014, 15, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Psomiades, M.; Fonteneau, C.; Suaud-Chagny, M.-F.; Haesebaert, F.; Brunelin, J. Neurostimulation du cortex préfrontal dorsolatéral: Quels effets sur la symptomatologie, l’humeur et les émotions dans la dépression et la schizophrénie? St. Ment. Québec 2016, 41, 223–239. [Google Scholar] [CrossRef]
- Brennan, S.; McLoughlin, D.M.; O’Connell, R.; Bogue, J.; O’Connor, S.; McHugh, C.; Glennon, M. Anodal Transcranial Direct Current Stimulation of the Left Dorsolateral Prefrontal Cortex Enhances Emotion Recognition in Depressed Patients and Controls. J. Clin. Exp. Neuropsychol. 2017, 39, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Silvanto, J.; Pascual-Leone, A. State-Dependency of Transcranial Magnetic Stimulation. Brain Topogr 2008, 21, 1–10. [Google Scholar] [CrossRef]
- Kohler, C.G.; Turner, T.H.; Bilker, W.B.; Brensinger, C.M.; Siegel, S.J.; Kanes, S.J.; Gur, R.E.; Gur, R.C. Facial Emotion Recognition in Schizophrenia: Intensity Effects and Error Pattern. AJP 2003, 160, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Surguladze, S.A.; Young, A.W.; Senior, C.; Brébion, G.; Travis, M.J.; Phillips, M.L. Recognition Accuracy and Response Bias to Happy and Sad Facial Expressions in Patients With Major Depression. Neuropsychology 2004, 18, 212–218. [Google Scholar] [CrossRef]
- de Moraes, R.; Pereira da Cruz, R.; Manso Melchiades, A.; Arantes Tiraboschi, G.; Rodrigues da Silva, I.C.; de Souza, W.C. Increased Sensitivity for Happy Faces in Depressed Patients Following 15 Hz Repetitive Transcranial Magnetic Stimulation (RTMS) over the Left Dorsolateral Prefrontal Cortex. Psychol. Neurosci. 2020, 13, 19–31. [Google Scholar] [CrossRef]
- Anderson, I.M.; Shippen, C.; Juhasz, G.; Chase, D.; Thomas, E.; Downey, D.; Toth, Z.G.; Lloyd-Williams, K.; Elliott, R.; Deakin, J.F.W. State-Dependent Alteration in Face Emotion Recognition in Depression. Br. J. Psychiatry 2011, 198, 302–308. [Google Scholar] [CrossRef]
- Rassovsky, Y.; Dunn, W.; Wynn, J.; Wu, A.D.; Iacoboni, M.; Hellemann, G.; Green, M.F. The Effect of Transcranial Direct Current Stimulation on Social Cognition in Schizophrenia: A Preliminary Study. Schizophr. Res. 2015, 165, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Keeser, D.; Meindl, T.; Bor, J.; Palm, U.; Pogarell, O.; Mulert, C.; Brunelin, J.; Moller, H.-J.; Reiser, M.; Padberg, F. Prefrontal Transcranial Direct Current Stimulation Changes Connectivity of Resting-State Networks during FMRI. J. Neurosci. 2011, 31, 15284–15293. [Google Scholar] [CrossRef]
- Fonteneau, C.; Redoute, J.; Haesebaert, F.; Le Bars, D.; Costes, N.; Suaud-Chagny, M.-F.; Brunelin, J. Frontal Transcranial Direct Current Stimulation Induces Dopamine Release in the Ventral Striatum in Human. Cereb. Cortex 2018, 28, 2636–2646. [Google Scholar] [CrossRef]
- Dondé, C.; Brevet-Aeby, C.; Poulet, E.; Mondino, M.; Brunelin, J. Potential Impact of Bifrontal Transcranial Random Noise Stimulation (TRNS) on the Semantic Stroop Effect and Its Resting-State EEG Correlates. Neurophysiol. Clin. 2019, 49, 243–248. [Google Scholar] [CrossRef]
- Kesler-West, M.L.; Andersen, A.H.; Smith, C.D.; Avison, M.J.; Davis, C.E.; Kryscio, R.J.; Blonder, L.X. Neural Substrates of Facial Emotion Processing Using FMRI. Cogn. Brain Res. 2001, 11, 213–226. [Google Scholar] [CrossRef]
- Weightman, M.J.; Air, T.M.; Baune, B.T. A Review of the Role of Social Cognition in Major Depressive Disorder. Front. Psychiatry 2014, 5, 179. [Google Scholar] [CrossRef]
- Solomon, D.A.; Leon, A.C.; Endicott, J.; Mueller, T.I.; Coryell, W.; Shea, M.T.; Keller, M.B. Psychosocial Impairment and Recurrence of Major Depression. Compr. Psychiatry 2004, 45, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, R.M.A.; Dunner, D.L.; Keitner, G.; Klein, D.N.; Koran, L.M.; Kornstein, S.G.; Markowitz, J.C.; Miller, I.; Nemeroff, C.B.; Ninan, P.T.; et al. Does Psychosocial Functioning Improve Independent of Depressive Symptoms? A Comparison of Nefazodone, Psychotherapy, and Their Combination. Biol. Psychiatry 2002, 51, 123–133. [Google Scholar] [CrossRef]
- Moirand, R.; Imbert, L.; Haesebaert, F.; Chesnoy, G.; Bediou, B.; Poulet, E.; Brunelin, J. Ten Sessions of 30 Min TDCS over 5 Days to Achieve Remission in Depression: A Randomized Pilot Study. JCM 2022, 11, 782. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.M.; Teng, J.Z.; Lo, T.Y.; Alonzo, A.; Goh, T.; Iacoviello, B.M.; Hoch, M.M.; Loo, C.K. Clinical Pilot Study of Transcranial Direct Current Stimulation Combined with Cognitive Emotional Training for Medication Resistant Depression. J. Affect. Disord. 2018, 232, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Shariat Bagheri, M.M.; Ahmadi, M. Clinical and Demographic Predictors of Response to Anodal TDCS Treatment in Major Depression Disorder (MDD). J. Psychiatr. Res. 2021, 138, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Mondino, M.; Szekely, D.; Bubrovszky, M.; Bulteau, S.; Downar, J.; Poulet, E.; Brunelin, J. Predicting Treatment Response to 1 Hz RTMS Using Early Self-Rated Clinical Changes in Major Depression. Brain Stimul. 2020, 13, 1603–1605. [Google Scholar] [CrossRef]
- Browning, M.; Kingslake, J.; Dourish, C.T.; Goodwin, G.M.; Harmer, C.J.; Dawson, G.R. Predicting Treatment Response to Antidepressant Medication Using Early Changes in Emotional Processing. Eur. Neuropsychopharmacol. 2019, 29, 66–75. [Google Scholar] [CrossRef]
- Tranter, R.; Bell, D.; Gutting, P.; Harmer, C.; Healy, D.; Anderson, I.M. The Effect of Serotonergic and Noradrenergic Antidepressants on Face Emotion Processing in Depressed Patients. J. Affect. Disord. 2009, 118, 87–93. [Google Scholar] [CrossRef]
- Fonteneau, C.; Mondino, M.; Arns, M.; Baeken, C.; Bikson, M.; Brunoni, A.R.; Burke, M.J.; Neuvonen, T.; Padberg, F.; Pascual-Leone, A.; et al. Sham TDCS: A Hidden Source of Variability? Reflections for Further Blinded, Controlled Trials. Brain Stimul. 2019, 12, 668–673. [Google Scholar] [CrossRef]

| Sham tDCS | Active tDCS | p | |
|---|---|---|---|
| n (male/female) | 17 (5/12) | 18 (9/9) | 0.36 |
| Age | 51.0 (±9.8) | 48.4 (±9.1) | 0.42 |
| Education level (years) | 15.2 (±3.1) | 13.6 (±2.6) | 0.11 |
| Current episode duration (months) | 16.6 (±14.9) | 23.6 (±14.9) | 0.14 |
| Illness duration (years) | 23.1 (±13.9) | 23.7 (±12.2) | 0.90 |
| MADRS10 (baseline score) | 27.2 (±5.0) | 26.9 (±4.8) | 0.86 |
| Antidepressant medication (n) | |||
| SSRIs | 11 | 10 | 0.73 |
| SNRIs | 5 | 6 | 1.00 |
| TCAs | 6 | 5 | 0.73 |
| MAOIs | 1 | 1 | 1.00 |
| Other medication (n) | |||
| benzodiazepine | 3 | 3 | 1.00 |
| antipsychotics | 2 | 3 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imbert, L.; Moirand, R.; Bediou, B.; Koenig, O.; Chesnoy, G.; Fakra, E.; Brunelin, J. A Single Session of Bifrontal tDCS Can Improve Facial Emotion Recognition in Major Depressive Disorder: An Exploratory Pilot Study. Biomedicines 2022, 10, 2397. https://doi.org/10.3390/biomedicines10102397
Imbert L, Moirand R, Bediou B, Koenig O, Chesnoy G, Fakra E, Brunelin J. A Single Session of Bifrontal tDCS Can Improve Facial Emotion Recognition in Major Depressive Disorder: An Exploratory Pilot Study. Biomedicines. 2022; 10(10):2397. https://doi.org/10.3390/biomedicines10102397
Chicago/Turabian StyleImbert, Laetitia, Rémi Moirand, Benoit Bediou, Olivier Koenig, Gabrielle Chesnoy, Eric Fakra, and Jérôme Brunelin. 2022. "A Single Session of Bifrontal tDCS Can Improve Facial Emotion Recognition in Major Depressive Disorder: An Exploratory Pilot Study" Biomedicines 10, no. 10: 2397. https://doi.org/10.3390/biomedicines10102397
APA StyleImbert, L., Moirand, R., Bediou, B., Koenig, O., Chesnoy, G., Fakra, E., & Brunelin, J. (2022). A Single Session of Bifrontal tDCS Can Improve Facial Emotion Recognition in Major Depressive Disorder: An Exploratory Pilot Study. Biomedicines, 10(10), 2397. https://doi.org/10.3390/biomedicines10102397






