Diagnostic Accuracy of Ultrasound in Predicting Extrathyroidal Extension and Its Relation to Body Mass Index in a North American Population
Abstract
:1. Introduction
2. Methods
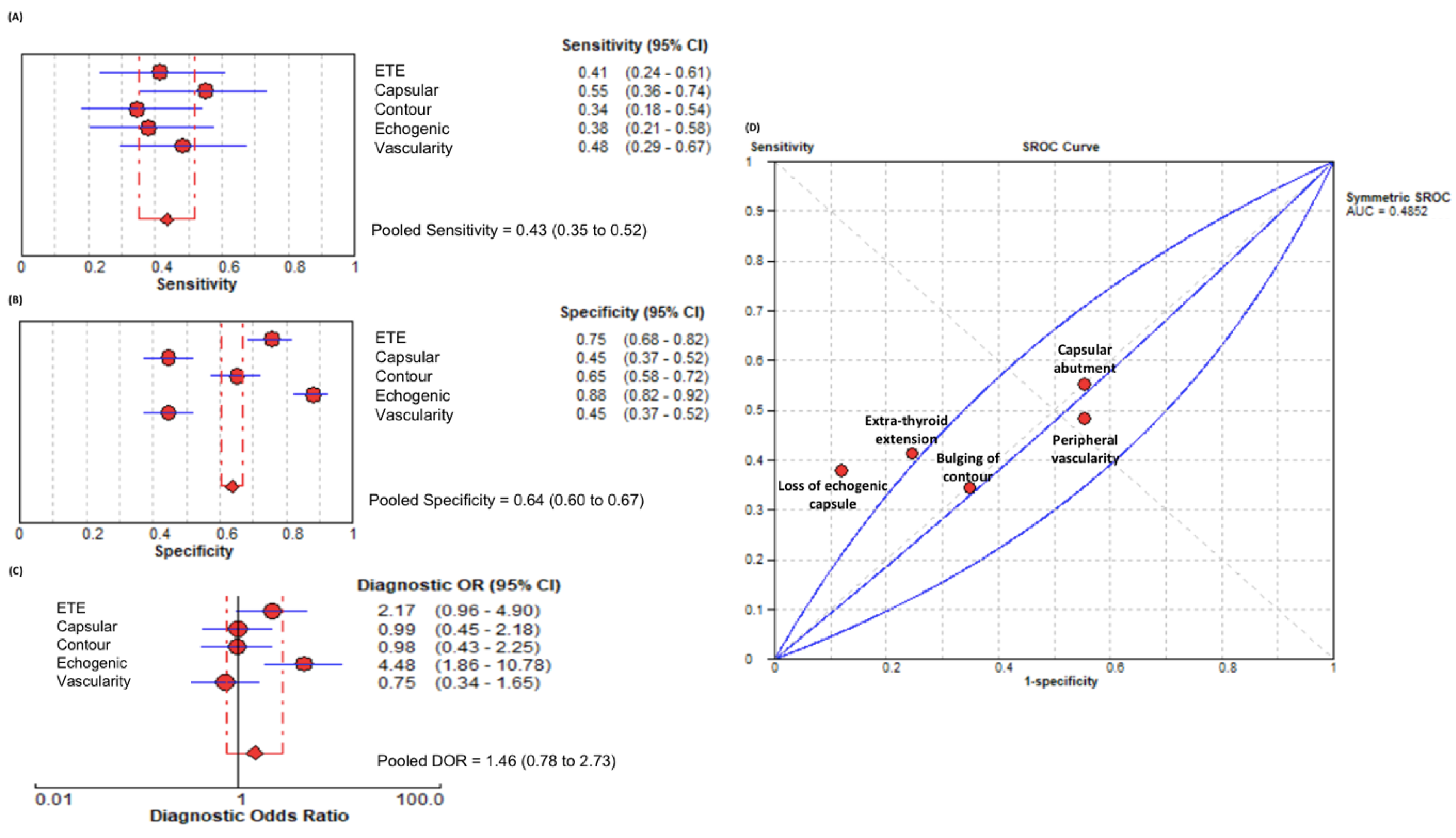
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, L.G.; Sikora, A.G.; Tosteson, T.D.; Davies, L. The Increasing Incidence of Thyroid Cancer: The Influence of Access to Care. Thyroid 2013, 23, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Kamaya, A.; Tahvildari, A.M.; Patel, B.N.; Willmann, J.K.; Jeffrey, R.B.; Desser, T.S. Sonographic Detection of Extracapsular Extension in Papillary Thyroid Cancer. J. Ultrasound Med. 2015, 34, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Malvezzi, M.; Bosetti, C.; Garavello, W.; Bertuccio, P.; Levi, F.; Negri, E. Thyroid Cancer Mortality and Incidence: A Global Overview. Int. J. Cancer 2015, 136, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Kim, S.J.; Ko, K.R.; Chung, K.-W.; Lee, J.-H. Predictive Factors for Extrathyroidal Extension of Papillary Thyroid Carcinoma Based on Preoperative Sonography. J. Ultrasound Med. 2014, 33, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Orlov, S.; Orlov, D.; Shaytzag, M.; Dowar, M.; Tabatabaie, V.; Dwek, P.; Yip, J.; Hu, C.; Freeman, J.L.; Walfish, P.G.; et al. Influence of Age and Primary Tumor Size on the Risk for Residual/Recurrent Well-differentiated Thyroid Carcinoma. Head Neck J. Sci. Spec. Head Neck 2009, 31, 782–788. [Google Scholar] [CrossRef]
- Krämer, J.A.; Schmid, K.W.; Dralle, H.; Dietlein, M.; Schicha, H.; Lerch, H.; Gerss, J.; Frankewitsch, T.; Schober, O.; Riemann, B.; et al. Primary Tumour Size Is a Prognostic Parameter in Patients Suffering from Differentiated Thyroid Carcinoma with Extrathyroidal Growth: Results of the MSDS Trial. Eur. J. Endocrinol. 2010, 163, 637. [Google Scholar] [CrossRef]
- Wada, N.; Nakayama, H.; Suganuma, N.; Masudo, Y.; Rino, Y.; Masuda, M.; Imada, T. Prognostic Value of the Sixth Edition AJCC/UICC TNM Classification for Differentiated Thyroid Carcinoma with Extrathyroid Extension. J. Clin. Endocrinol. Metab. 2007, 92, 215–218. [Google Scholar] [CrossRef]
- Liu, L.; Oh, C.; Heo, J.H.; Park, H.S.; Lee, K.; Chang, J.W.; Jung, S.-N.; Koo, B.S. Clinical Significance of Extrathyroidal Extension According to Primary Tumor Size in Papillary Thyroid Carcinoma. Eur. J. Surg. Oncol. 2018, 44, 1754–1759. [Google Scholar] [CrossRef]
- Youngwirth, L.M.; Adam, M.A.; Scheri, R.P.; Roman, S.A.; Sosa, J.A. Extrathyroidal Extension Is Associated with Compromised Survival in Patients with Thyroid Cancer. Thyroid 2017, 27, 626–631. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Chen, S.; Hu, D.; Wang, M.; Zhou, L.; Zhou, W.; Chen, D.; Feng, H.; Wei, W.; et al. Minimal Extrathyroidal Extension Affects the Prognosis of Differentiated Thyroid Cancer: Is There a Need for Change in the AJCC Classification System? PLoS ONE 2019, 14, e0218171. [Google Scholar] [CrossRef] [Green Version]
- Al-Qurayshi, Z.; Nilubol, N.; Tufano, R.P.; Kandil, E. Wolf in Sheep’s Clothing: Papillary Thyroid Microcarcinoma in the US. J. Am. Coll. Surg. 2020, 230, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-S.; Chang, J.Y.; Kim, K.S.; Shong, M. Oncogenes, Mitochondrial Metabolism, and Quality Control in Differentiated Thyroid Cancer. Korean J. Intern. Med. 2017, 32, 780. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, S.; Rodriguez, J.; Soria, T.; Perez-Flores, D.; Pinero, A.; Moreno, J.; Parrilla, P. Extrathyroid Spread in Papillary Carcinoma of the Thyroid: Clinicopathological and Prognostic Study. Otolaryngol. Neck Surg. 2001, 124, 261–265. [Google Scholar] [CrossRef]
- Yamamura, N.; Fukushima, S.; Nakao, K.; Nakahara, M.; Kurozumi, K.; Imabun, S.; Tsujimoto, M. Relation between Ultrasonographic and Histologic Findings of Tracheal Invasion by Differentiated Thyroid Cancer. World J. Surg. 2002, 26, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Vellguth, K.; Brüning, J.; Tautz, L.; Degener, F.; Wamala, I.; Sündermann, S.; Kertzscher, U.; Kuehne, T.; Hennemuth, A.; Falk, V.; et al. User-Dependent Variability in Mitral Valve Segmentation and Its Impact on CFD-Computed Hemodynamic Parameters. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1687–1696. [Google Scholar] [CrossRef]
- Buerger, A.M.; Clark, K.R. Point-of-Care Ultrasound: A Trend in Health Care. Radiol. Technol. 2017, 89, 127–138. [Google Scholar] [PubMed]
- Simmons, O.; Fetzer, D.T.; Yokoo, T.; Marrero, J.A.; Yopp, A.; Kono, Y.; Parikh, N.; Browning, T.; Singal, A.G. Predictors of Adequate Ultrasound Quality for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. Aliment. Pharmacol. Ther. 2017, 45, 169–177. [Google Scholar] [CrossRef]
- Paladini, D. Sonography in Obese and Overweight Pregnant Women: Clinical, Medicolegal and Technical Issues. Ultrasound Obstet.Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2009, 33, 720–729. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, H.S.; Kim, E.; Moon, H.J.; Kwak, J.Y. The Influence of Body Mass Index on the Diagnostic Performance of Pre-operative Staging Ultrasound in Papillary Thyroid Carcinoma. Clin. Endocrinol. 2015, 83, 550–555. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Esfeh, J.M.; Hajifathalian, K.; Ansari-Gilani, K. Sensitivity of Ultrasound in Detecting Hepatocellular Carcinoma in Obese Patients Compared to Explant Pathology as the Gold Standard. Clin. Mol. Hepatol. 2020, 26, 54. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128–129 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Kim, E.-K.; Youk, J.H.; Kim, M.J.; Son, E.J.; Choi, S.H.; Oh, K.K. Extrathyroid Extension of Well-Differentiated Papillary Thyroid Microcarcinoma on US. Thyroid 2008, 18, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Offi, C.; Romano, R.M.; De Palma, M.; Ruggiero, R.; Candela, G.; Puziello, A.; Docimo, L.; Grasso, M.; Docimo, G. Transcutaneous Laryngeal Ultrasonography: A Reliable, Non-Invasive and Inexpensive Preoperative Method in the Evaluation of Vocal Cords Motility—A Prospective Multicentric Analysis on a Large Series and a Literature Review. Updat. Surg. 2020, 72, 885–892. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 204) | ETE Absent (n = 155) | ETE Present (n = 49) | p-Value | |
|---|---|---|---|---|
| Age (years) | 50.4 ± 15.1 | 50.4 ± 15.2 | 49.4 ± 15.8 | 0.71 |
| Females | 169 (83%) | 144 (82%) | 27 (93%) | 0.14 |
| BMI (kg/m2) | 32.0 ± 8.9 | 32.0 ± 8.9 | 31.1 ± 8.4 | 0.24 |
| Non-obese | 42 (20.58%) | 36 (17.64%) | 6 (2.94%) | 0.35 |
| Obese | 162 (79.41%) | 126 (61.76%) | 36 (17.64%) | |
| Mean tumor size (cm) | 1.2 ± 1.3 | 1.2 ± 1.3 | 1.8 ± 1.4 | 0.04 |
| Multifocality | 84 (41.17%) | 68 (33.33%) | 16 (7.84%) | 0.09 |
| Sonographic Feature | Non-Obese | Obese | p-Value | |
|---|---|---|---|---|
| Sensitivity | ETE | 0.83 (0.36–1.00) | 0.30 (0.13–0.53) | 0.015 |
| Capsular abutment | 0.67 (0.22–0.96) | 0.52 (0.31–0.73) | 0.52 | |
| Bulging of contour | 0.50 (0.12–0.88) | 0.30 (0.13–0.53) | 0.37 | |
| Loss of echogenic capsule | 0.67 (0.22–0.96) | 0.30 (0.13–0.53) | 0.09 | |
| Peripheral vascularity | 0.83 (0.36–1.00) | 0.39 (0.20–0.62) | 0.05 | |
| Pooled sensitivity | 0.70 (0.51–0.85) | 0.37 (0.28–0.46) | 0.001 | |
| Specificity | ETE | 0.74 (0.57–0.88) | 0.76 (0.68–0.83) | 0.81 |
| Capsular abutment | 0.40 (0.24–0.58) | 0.46 (0.37–0.54) | 0.53 | |
| Bulging of contour | 0.71 (0.54–0.85) | 0.64 (0.55–0.72) | 0.07 | |
| Loss of echogenic capsule | 0.83 (0.66–0.93) | 0.89 (0.83–0.94) | 0.52 | |
| Peripheral vascularity | 0.31 (0.17–0.49) | 0.48 (0.39–0.57) | 0.08 | |
| Pooled specificity | 0.60 (0.52–0.67) | 0.64 (0.61–0.68) | 0.32 | |
| PLR | ETE | 3.24 (1.66–6.32) | 1.25 (0.63–2.48) | 0.07 |
| Capsular abutment | 1.11 (0.59–2.08) | 0.96 (0.63–1.46) | 0.74 | |
| Bulging of contour | 1.75 (0.67–4.55) | 0.84 (0.43–1.61) | 0.24 | |
| Loss of echogenic capsule | 3.89 (1.55–9.78) | 2.84 (1.30–6.21) | 0.68 | |
| Peripheral vascularity | 1.22 (0.80–1.85) | 0.75 (0.44–1.28) | 0.29 | |
| Pooled PLR | 1.85 (1.12–3.07) | 1.09 (0.74–1.62) | 0.13 | |
| NLR | ETE | 0.22 (0.04–1.36) | 0.92 (0.69–1.22) | 0.029 |
| Capsular abutment | 0.83 (0.25–2.77) | 1.05 (0.66–1.66) | 0.71 | |
| Bulging of contour | 0.70 (0.31–1.60) | 1.09 (0.81–1.47) | 0.56 | |
| Loss of echogenic capsule | 0.40 (0.13–1.26) | 0.78 (0.59–1.03) | 0.15 | |
| Peripheral vascularity | 0.53 (0.08–3.39) | 1.27 (0.88–1.84) | <0.001 | |
| Pooled NLR | 0.57 (0.33–0.97) | 0.98 (0.83–1.16) | 0.29 | |
| DOR | ETE | 14.44 (1.4–140.7) | 1.36 (0.52–3.59) | 0.46 |
| Capsular abutment | 1.33 (0.21–8.29) | 0.92 (0.38–2.22) | 0.78 | |
| Bulging of contour | 2.50 (0.43–14.54) | 0.76 (0.30–1.98) | 0.38 | |
| Loss of echogenic capsule | 9.67 (1.43–65.38) | 3.65 (1.29–10.29) | 0.51 | |
| Peripheral vascularity | 2.29 (0.24–22.02) | 0.59 (0.24–1.45) | 0.54 | |
| Pooled DOR | 3.70 (1.53–8.94) | 1.12 (0.62–2.03) | 0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, M.; Attia, A.S.; Issa, P.P.; Christensen, B.R.; Sugumar, K.; Alnahla, A.; Hadedeya, D.; Shalaby, H.; Gupta, N.; Shama, M.; et al. Diagnostic Accuracy of Ultrasound in Predicting Extrathyroidal Extension and Its Relation to Body Mass Index in a North American Population. Biomedicines 2022, 10, 2408. https://doi.org/10.3390/biomedicines10102408
Omar M, Attia AS, Issa PP, Christensen BR, Sugumar K, Alnahla A, Hadedeya D, Shalaby H, Gupta N, Shama M, et al. Diagnostic Accuracy of Ultrasound in Predicting Extrathyroidal Extension and Its Relation to Body Mass Index in a North American Population. Biomedicines. 2022; 10(10):2408. https://doi.org/10.3390/biomedicines10102408
Chicago/Turabian StyleOmar, Mahmoud, Abdallah S. Attia, Peter P. Issa, Bryce R. Christensen, Kavin Sugumar, Ahmed Alnahla, Deena Hadedeya, Hosam Shalaby, Neel Gupta, Mohamed Shama, and et al. 2022. "Diagnostic Accuracy of Ultrasound in Predicting Extrathyroidal Extension and Its Relation to Body Mass Index in a North American Population" Biomedicines 10, no. 10: 2408. https://doi.org/10.3390/biomedicines10102408





