A Systematic Review and Meta-Analysis of the Prevalence and Impact of Pulmonary Bacterial Colonisation in Stable State Chronic Obstructive Pulmonary Disease (COPD)
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection, Data Extraction and Quality Assessment
2.3. Statistical Analysis
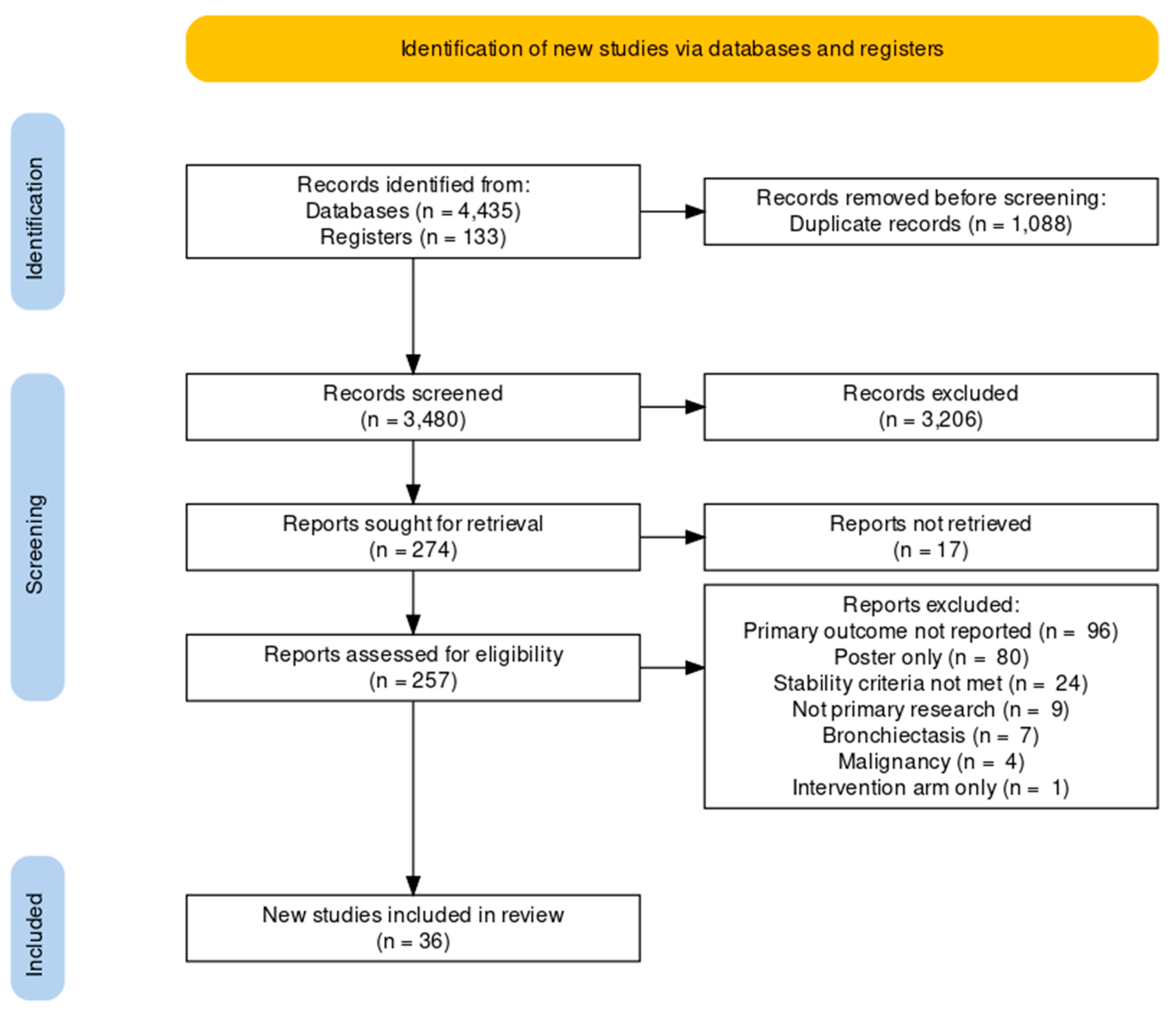
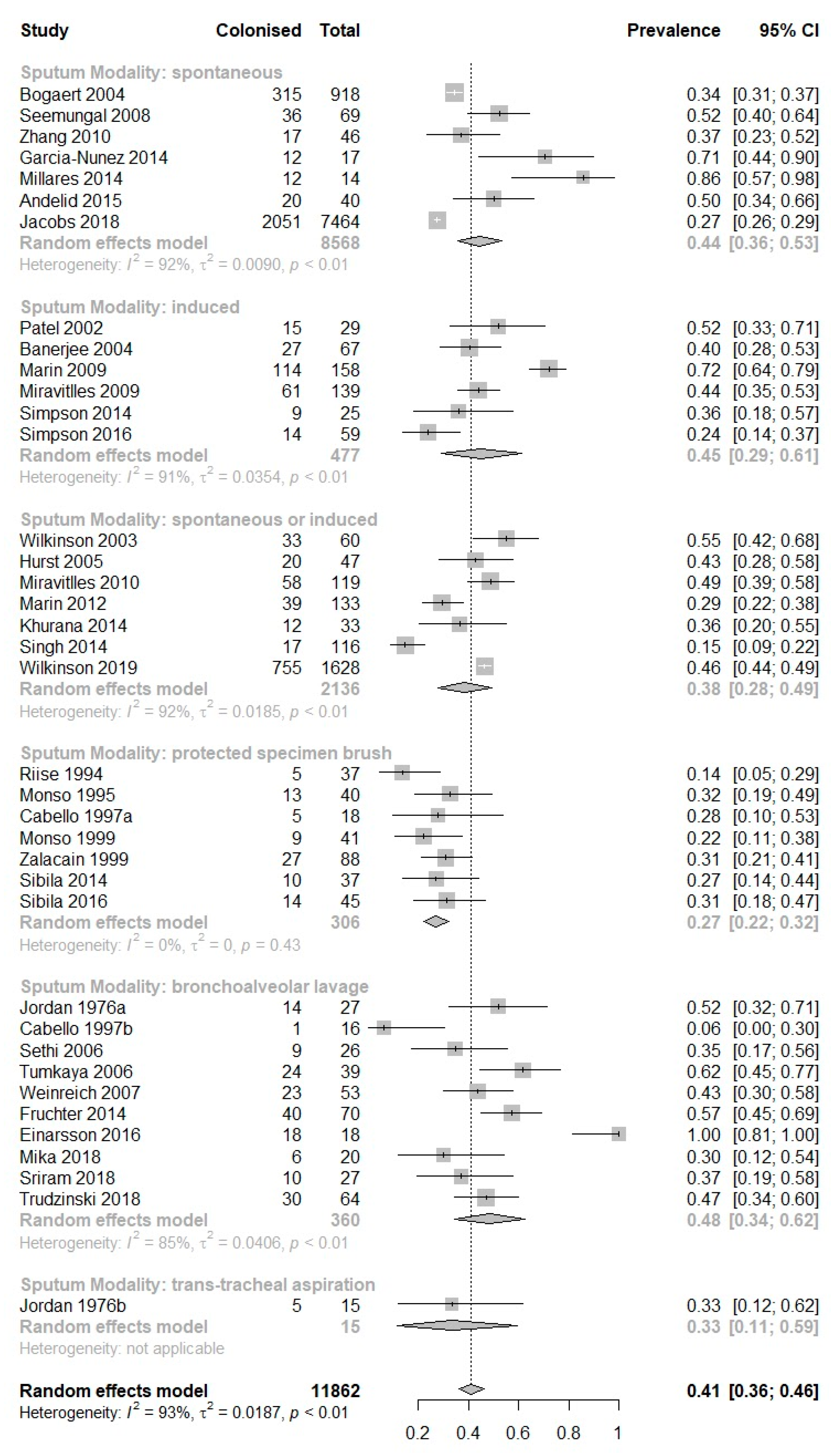
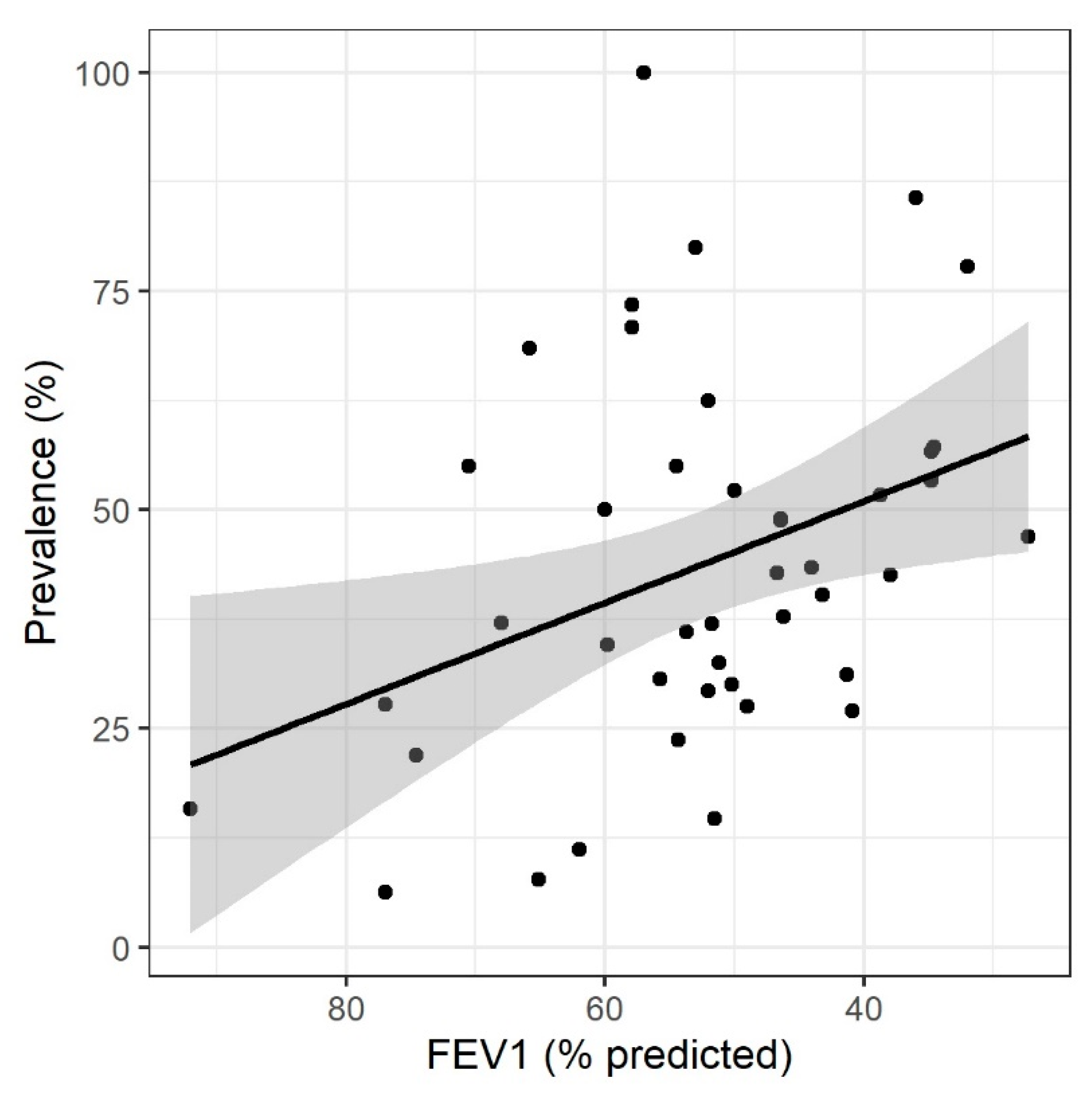
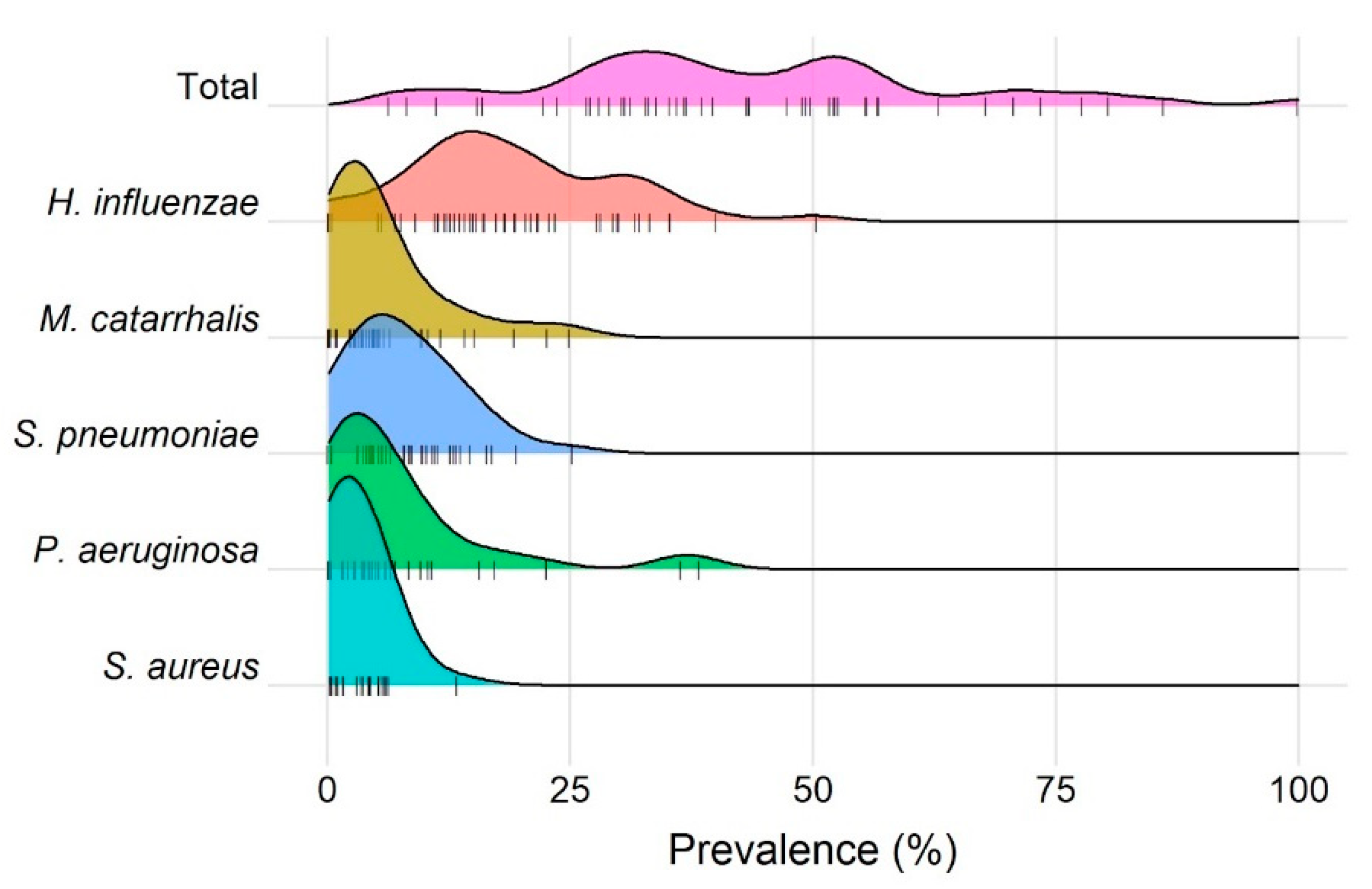
3. Results
| Study (Author, Year) | Country | Study Design | Population Description | Study Subgroup | No. Stable COPD Patients (% Female) | Age | Smoking, Pack-Years | FEV1, % Predicted | Stability Period Pre-Sampling |
|---|---|---|---|---|---|---|---|---|---|
| Andelid et al., 2015 [12] | Sweden | Prospective cohort | Smokers with obstructive disease and chronic bronchitis | - | 60 (24) | 62 {45–76} | 40 {14–156} | 60 {29–97} | 15 weeks |
| Banerjee et al., 2004 [13] | UK | Prospective cohort | Stable COPD outpatients | - | 67 (NA) | 66.7 (7.6) | 58.8 (25.1) | 43.2 (11.4) | 6 weeks |
| Bogaert et al., 2004 [14] | Netherlands | Prospective cohort | Stable COPD outpatients | - | 269 (NA) | {40–75} | NA | NA | Stable clinical condition |
| Cabello et al., 1997 [1] | Spain | Prospective cohort | Stable COPD outpatients with indication for bronchoscopy | PSB | 18 (17) | 60 (12) | NA | 77 (19) | 4 weeks |
| BAL | 18 (17) | 60 (12) | NA | 77 (19) | 4 weeks | ||||
| Einarsson et al., 2016 [15] | UK | Cross-sectional | COPD patients listed for bronchoscopy | - | 18 (22) | 60 {41–74} | NA | 57 {32–89} | 8 weeks |
| Fruchter et al., 2014 [16] | Israel | Prospective cohort | Severe COPD pre-BLVR | - | 70 (22) | 64 (8) | 28 (11) | 34.6 (7.3) | 90 days |
| Garcia-Nunez et al., 2014 [17] | Spain | Cross-sectional | Stable COPD outpatients | Moderate-to-severe disease | 17 (13) | 68 {62–69} | 75 {52–110} | 52.0 {41.5–69.0} | 4 weeks |
| Advanced disease | 17 (0) | 74 {68–77} | 55 {35–117} | 32.0 {29.5–35.0} | 4 weeks | ||||
| Hurst et al., 2005 [18] | UK | Prospective cohort | Stable COPD outpatients | Whole cohort | 47 (43) | 70.5 (7) | 46.1 (26.5) | 37.9 (13.6) | 12 weeks |
| Jacobs et al., 2018 [19] | USA | Prospective cohort | Stable COPD outpatients | - | 181 (NA) | 67 (9.2) | 79 (36) | 49 (18) | Stable clinical condition |
| Jordan et al., 1976 [20] | USA | Cross-sectional | Chronic bronchitis patients | BAL | 19 (NA) | NA | NA | NA | Stable clinical condition |
| Trans-tracheal aspiration | 19 (NA) | NA | NA | NA | Stable clinical condition | ||||
| Khurana et al., 2014 [21] | UK | Cross-sectional | Stable COPD outpatients | Non-persistent sputum | 52 (46) | 66.8 (6.5) | 35.3 {12.5–86} | 65.1 (16.3) | 6 weeks |
| Persistent sputum | 52 (54) | 65.7 (6.9) | 32.0 {18.5–122.2} | 54.5 (13.1) | 6 weeks | ||||
| Marin et al., 2009 [22] | Spain | Prospective cohort | Stable COPD outpatients | Baseline | 40 (3) | 66.5 (8.1) | NA | 57.9 (19.1) | 8 weeks |
| 9 month follow-up | 40 (3) | 66.5 (8.1) | NA | 57.9 (19.1) | 8 weeks | ||||
| Marin et al., 2012 [23] | Spain | Cross-sectional | COPD recruited on hospitalization for exacerbation | - | 133 (7) | 70 (9) | 67 {43–102} | 52 (16) | 12 weeks |
| Mika et al., 2018 [24] | Switzerland | Cross-sectional | COPD patients listed for bronchoscopy | - | 32 (31) | 65.7 (NA) | NA | 50.2 (24.9) | Stable clinical condition |
| Millares et al., 2014 [10] | Spain | Prospective cohort | COPD patients with >2 exacerbations per year | Whole cohort | 16 (0) | 71 (6) | 57 {57–110} | 36 {30–40} | >8 weeks |
| Miravitlles et al., 2009 [25] | Spain | Randomised control trial | COPD with sputum positive for PPM (p. aeruginosa excluded) | At randomisation | 119 (8) | 68 (9.1) | NA | 46.2 (14.1) | 16 weeks |
| Placebo 8 week follow-up | 119 (5) | 69 (10) | 43 (21) | 53 (16) | 16 weeks | ||||
| Miravitlles et al., 2010 [26] | Spain | Cross-sectional | Stable COPD outpatients | - | 119 (6) | 68 (9.1) | 40 (21.1) | 46.4 (14.1) | 12 weeks |
| Monso et al., 1995 [27] | Spain | Cross-sectional | COPD patients listed for bronchoscopy | - | 40 (0) | 61.1 (9.9) | NA | 51.2 (23) | 15 days |
| Monso et al., 1999 [28] | Spain | Cross-sectional | Stable chronic bronchitis | - | 41 (0) | 63.8 (9.1) | NA | 74.6 (23.7) | 15 days |
| Patel et al., 2002 [4] | UK | Prospective cohort | Stable COPD outpatients | - | 29 (28) | 66 {47–81} | 52.9 (42.2) | 38.7 (15.2) | 3 weeks |
| Riise et al., 1994 [29] | Sweden | Prospective cohort | Chronic bronchitis with and without COPD | Without COPD | 41 (NA) | 52 {36–68} | 36, 2 * | 92, 2 * | 4 weeks |
| With COPD | 41 (NA) | 57 {38–70} | 44, 4 * | 62, 2 * | 4 weeks | ||||
| Seemungal et al., 2008 [30] | UK | Randomised control trial | Stable COPD outpatients at baseline | - | 109 (37) | 67.2 (8.6) | 51.6 (33.9) | 50.0 (18.0) | 4 weeks |
| Sethi et al., 2006 [31] | USA | Prospective cohort | Ex-smokers with COPD | - | 26 (23) | 64.7 (1.7) | 66 (6.3) | 59.8 (4.1) | 4 weeks |
| Sibila et al., 2014 [32] | Spain | Cross-sectional | Stable COPD outpatients | - | 37 (24) | 67.9 (8.0) | 47.3 (12.7) | 40.9 (8.1) | 4 weeks |
| Sibila et al., 2016 [33] | Spain | Cross-sectional | Stable COPD outpatients | - | 45 (18) | 67.1 (8.5) | 54.3 (20.1) | 41.3 (10.2) | 4 weeks |
| Simpson et al., 2014 [34] | Australia | Randomised control trial | Stable COPD outpatients at randomisation | - | 30 (37) | 70.8 (7.6) | 46.1 (36.6) | 53.7 (13.7) | 4 weeks |
| Simpson et al., 2016 [35] | Australia | Cross-sectional | Stable COPD outpatients | - | 59 (51) | 69.7 (7.5) | 32.9 {17.0–53.8} | 54.3 (15.6) | Stable clinical condition |
| Singh et al., 2014 [36] | UK | Prospective cohort | Stable COPD outpatients | - | 99 (33) | 72.1 (8.9) | 48.4 {24.4–67.5} | 51.5 (21.6) | 4 weeks |
| Sriram et al., 2018 [37] | Australia | Cross-sectional | COPD patients listed for bronchoscopy | - | 27 (37) | 68 (9) | 43 (28) | 68 (25) | Excluded exacerbations |
| Trudzinski et al., 2018 [38] | Germany | Cross-sectional | COPD patients undergoing BLVR with EBV insertion | - | 64 (50) | 62.4 (8.7) | NA | 27.3 (9.5) | Excluded exacerbations |
| Tumkaya et al., 2006 [39] | Turkey | Prospective cohort | Stable COPD outpatients | Exacerbations (<3/year) | 39 (10) | 58.6 (7.7) | 46.2 (22.1) | 70.5 (12.0) | 4 weeks |
| Exacerbations (>3/year) | 39 (11) | 58.8 (7.7) | 50.26 (22.2) | 65.8 (12.8) | 4 weeks | ||||
| Weinreich et al., 2007 [40] | Denmark | Cross-sectional | COPD patients listed for bronchoscopy | - | 53 (49) | 67 {58–73} | 30 {21–45} | 44 {NA} | 4 weeks |
| Wilkinson et al., 2003 [41] | UK | Prospective cohort | Stable COPD outpatients | Baseline | 30 (27) | 66.4 (10.3) | 74.3 (66.5) | 34.8 (13.6) | 6 weeks |
| 12 month follow-up | 30 (27) | 66.4 (10.3) | 74.3 (66.5) | 34.8 (13.6) | 6 weeks | ||||
| Wilkinson et al., 2019 [11] | UK | Prospective cohort | Stable COPD outpatients | Year 1 | 127 (47) | 66.8 (8.6) | 47.0 {33.7–60.0} | 46.4 (15.2) | Stable clinical condition |
| Year 2 | 127 (44) | 66.7 (8.7) | 50.4 {34.0–60.0} | 46.7 (14.6) | Stable clinical condition | ||||
| Zalacain et al., 1999 [42] | Spain | Cross-sectional | Stable COPD outpatients | - | 88 (0) | 66.1 (7.2) | 53.6 (14.9) | 55.7 (12.9) | 4 weeks |
| Zhang et al., 2010 [43] | China | Prospective cohort | Stable COPD outpatients | - | 46 (17) | 70.9 (5.6) | NA | 51.8 (12.3) | 6 weeks |
| Study (Author, Year) | Study Subgroup | Sampling Modality | No. of Patients Producing Sputum | No. of Sputum Samples Produced | Prevalence of PPM Positive Sputum, Percent (95% CI) | Prevalence of H. influenzae in Sputum, Percent (95% CI) | Prevalence of M. catarrhalis in Sputum, Percent (95% CI) | Prevalence of S. pneumoniae in Sputum, Percent (95% CI) | Prevalence of P. aeruginosa in Sputum, Percent (95% CI) | Prevalence of S. aureus in Sputum, Percent (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Andelid et al., 2015 [12] | - | Spontaneous | 40 | 40 | 50 (34–66) | 13 (4–27) | 3 (0–13) | 5 (1–17) | 3 (0–13) | 0 (0–9) |
| Banerjee et al., 2004 [13] | - | Induced | 67 | 67 | 40 (29–53) | 21 (12–33) | 15 (7–26) | 13 (6–24) | 2 (0–8) | 2 (0–8) |
| Bogaert et al., 2004 [14] | - | Spontaneous | 269 | 918 | 34 (31–38) | 19 (17–22) | 19 (17–22) | 13 (11–15) | NA | NA |
| Cabello et al., 1997 [1] | PSB | PSB | 18 | 18 | 28 (10–54) | 11 (1–35) | 0 (0–19) | 11 (1–35) | 0 (0–19) | 6 (0–27) |
| BAL | BAL | 16 | 16 | 6 (0–30) | 0 (0–20.6) | 0 (0–21) | 6 (0–30) | 0 (0–21) | 0 (0–21) | |
| Einarsson et al., 2016 [15] | - | BAL | 18 | 18 | 100 (82–100) | 28 (10–54) | 0 (0–19) | 17 (4–41) | 6 (0–27) | 6 (0–27) |
| Fruchter et al., 2014 [16] | - | BAL | 70 | 70 | 57 (45–69) | 7.1 (2–16) | 1 (0–8) | 4 (1–12) | 17 (9–28) | 13 (6–23) |
| Garcia-Nunez et al., 2014 [17] | Moderate-to-severe disease | Spontaneous | 8 | 8 | 63 (25–92) | 13 (0–53) | 25 (3–65) | 13 (0–53) | 38 (8–76) | NA |
| Advanced disease | Spontaneous | 9 | 9 | 78 (40–97) | 33 (8–70) | 0 (0–34) | 11 (0–48) | 22 (3–60) | NA | |
| Hurst et al., 2005 [18] | Whole cohort | Spontaneous or induced | 47 | 47 | 43 (28–58) | 19 (9–33) | 6 (1–18) | 6 (1–18) | 2 (0–11) | NA |
| Jacobs et al., 2018 [19] | - | Spontaneous | 181 | 7464 | 28 (27–29) | 14 (13–15) | 6 (5–6) | 6 (5–6) | 8 (7–8) | NA |
| Jordan et al., 1976 [20] | BAL | BAL | 19 | 27 | 52 (32–71) | 22 (9–42) | 0 (0–13) | NA | 11 (2–29) | 4 (0–19) |
| Trans-tracheal aspiration | Trans-tracheal aspiration | 11 | 15 | 33 (12–62) | 20 (4–48) | 0 (0–22) | NA | 0 (0–22) | 0 (0–22) | |
| Khurana et al., 2014 [21] | Non-persistent sputum | Spontaneous or induced | 13 | 13 | 8 (0–36) | 8 (0–36) | 0 (0–25) | 0 (0–25) | 0 (0–25) | 0 (0–25) |
| Persistent sputum | Spontaneous or induced | 20 | 20 | 55 (32–77) | 35 (15–59) | 5 (0–25) | 15 (3–38) | 0 (0–17) | 5 (0–25) | |
| Marin et al., 2009 [22] | Baseline | Induced | 40 | 79 | 73 (62–83) | 35 (25–47) | 5 (1–13) | 0 (0–5) | NA | NA |
| 9 month follow-up | Induced | 40 | 79 | 71 (60–81) | 32 (22–43) | 3 (0–9) | 0 (0–5) | NA | NA | |
| Marin et al., 2012 [23] | - | Spontaneous or induced | 133 | 133 | 2 (22–38) | 17 (11–24) | 5 (2–10) | 4 (1–9) | 6 (3–12) | NA |
| Mika et al., 2018 [24] | - | BAL | 20 | 20 | 30 (12–54) | 15 (3–38) | 10 (1–32) | 10 (1–32) | NA | NA |
| Millares et al., 2014 [10] | Whole cohort | Spontaneous | 14 | 14 | 86 (57–98) | 29 (8–58) | 14 (2–43) | 14 (2–43) | 36 (13–65) | 0 (0–23) |
| Miravitlles et al., 2009 [25] | At randomisation | Induced | 119 | 119 | 38 (29–47) | 16 (10–24) | 3 (1–8) | 3 (1–7) | 4 (1–10) | 0 (0–3) |
| Placebo 8 week follow-up | Induced | 20 | 20 | 80 (56–94) | 50 (27–73) | 5 (0–25) | 0 (0–17) | 0 (0–17) | 0 (0–17) | |
| Miravitlles et al., 2010 [26] | - | Spontaneous or induced | 119 | 119 | 49 (40–58) | 18 (11–26) | 3 (1–8) | 3 (1–8) | 4 (1–10) | 1 (0–5) |
| Monso et al., 1995 [27] | - | PSB | 40 | 40 | 33 (19–49) | 15 (6–30) | 3 (0–13) | 8 (2–20) | 3 (0–13) | 3 (0–13) |
| Monso et al., 1999 [28] | - | PSB | 41 | 41 | 22 (11–38) | 12 (4–26) | NA | 5 (1–17) | NA | NA |
| Patel et al., 2002 [4] | - | Induced | 29 | 29 | 52 (33–71) | 28 (13–47) | 10 (2–27) | NA | 10 (2–27) | NA |
| Riise et al., 1994 [29] | Without COPD | PSB | 19 | 19 | 16 (3–40) | 11 (1–33) | 0 (0–18) | 5 (0–26) | NA | 0 (0–18) |
| With COPD | PSB | 18 | 18 | 11 (1–35) | 0 (0–19) | 0 (0–19) | 11 (1–35) | NA | 0 (0–19) | |
| Seemungal et al., 2008 [30] | - | Spontaneous | 69 | 69 | 52 (40–64) | 32 (21–44) | 4 (1–12) | 9 (3–18) | NA | NA |
| Sethi et al., 2006 [31] | - | BAL | 26 | 26 | 35 (17–56) | 12 (3–30) | 0 (0–13) | 4 (0–20) | 4 (0–20) | 4 (0–20) |
| Sibila et al., 2014 [32] | - | PSB | 37 | 37 | 27 (14–44) | 14 (5–29) | 5 (1–18) | 5 (1–18) | 0 (0–10) | 0 (0–10) |
| Sibila et al., 2016 [33] | - | PSB | 45 | 45 | 31 (18–47) | 18 (8–32) | 4 (1–15) | 4 (1–15) | 0 (0–8) | NA |
| Simpson et al., 2014 [34] | - | Induced | 25 | 25 | 36 (18–58) | 40 (0–20) | 4 (0–20) | 8 (1–26) | 16 (5–36) | 4 (0–20) |
| Simpson et al., 2016 [35] | - | Induced | 59 | 59 | 24 (14–37) | 5 (1–14) | 12 (5–23) | NA | 7 (2–17) | 3 (0–12) |
| Singh et al., 2014 [36] | - | Spontaneous or induced | 99 | 116 | 11 (9–22) | 6 (3–12) | 1 (0–5) | 4 (1–10) | 2 (0–6) | 1 (0–5) |
| Sriram et al., 2018 [37] | - | BAL | 27 | 27 | 37 (19–58) | 22 (9–42) | NA | 4 (0–19) | 7 (1–24) | 4 (0–19) |
| Trudzinski et al., 2018 [38] | - | BAL | 64 | 64 | 47 (34–60) | 9 (4–19) | 2 (0–8) | 6 (2–15) | 5 (1–13) | 6 (2–15) |
| Tumkaya et al., 2006 [39] | Exacerbations (<3/year) | BAL | 20 | 20 | 55 (32–77) | 0 (0–17) | 0 (0–17) | 10 (1–32) | NA | 0 (0–17) |
| Exacerbations (>3/year) | BAL | 19 | 19 | 69 (44–78) | 11 (1–33) | 5 (0–26) | 0 (0–18) | NA | 5 (0–26) | |
| Weinreich et al., 2007 [40] | - | BAL | 53 | 53 | 43 (30–58) | 23 (12–36) | 4 (1–13) | 25 (14–38) | 4 (1–13) | 4 (1–13) |
| Wilkinson et al., 2003 [41] | Baseline | Spontaneous or induced | 30 | 30 | 53 (34–72) | 30 (15–49) | 10 (2–27) | 10 (2–27) | 10 (2–27) | NA |
| 12 month follow-up | Spontaneous or induced | 30 | 30 | 57 (37–75) | 23 (10–42) | 23 (10–42) | 0 (0–12) | 10 (2–27) | NA | |
| Wilkinson et al., 2019 [11] | Year 1 | Spontaneous or induced | 127 | 952 | 49 (46–52) | 30 (27–33) | 5 (4–7) | 19 (16–21) | 5 (4–7) | 4 (3–6) |
| Year 2 | Spontaneous or induced | 103 | 676 | 43 (39–47) | 23 (19–26) | 3 (2–5) | 16 (13–19) | 5 (3–7) | 6 (5–9) | |
| Zalacain et al., 1999 [42] | - | PSB | 88 | 88 | 31 (21–41) | 16 (9–25) | 5 (1–11) | 8 (3–16) | 0 (0–4) | 1 (0–6) |
| Zhang et al., 2010 [43] | - | Spontaneous | 46 | 46 | 37 (23–53) | 15 (6–29) | 2 (0–12) | 9 (2–21) | 4 (1–15) | 2 (0–12) |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cabello, H.; Torres, A.; Celis, R.; El-Ebiary, M.; De La Bellacasa, J.P.; Xaubet, A.; Gonzalez, J.; Agusti, C.; Soler, N. Bacterial colonization of distal airways in healthy subjects and chronic lung disease: A bronchoscopic study. Eur. Respir. J. 1997, 10, 1137. [Google Scholar] [CrossRef] [PubMed]
- Desai, H.; Eschberger, K.; Wrona, C.; Grove, L.; Agrawal, A.; Grant, B.; Yin, J.; Parameswaran, G.I.; Murphy, T.; Sethi, S. Bacterial Colonization Increases Daily Symptoms in Patients with Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2014, 11, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, J.; Tabor, D.E.; Silver, J.S.; Nair, V.; Tovchigrechko, A.; Griffin, M.P.; Esser, M.T.; Sellman, B.R.; Jin, H. Microbial burden and viral exacerbations in a longitudinal multicenter COPD cohort. Respir. Res. 2020, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.S.; Seemungal, T.A.R.; Wilks, M.; Lloyd-Owen, S.J.; Donaldson, G.C.; Wedzicha, J.A. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002, 57, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Tangedal, S.; Aanerud, M.; Grønseth, R.; Drengenes, C.; Wiker, H.G.; Bakke, P.S.; Eagan, T.M. Comparing microbiota profiles in induced and spontaneous sputum samples in COPD patients. Respir. Res. 2017, 18, 164. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Freeman, C.M.; McCloskey, L.; Falkowski, N.R.; Huffnagle, G.B.; Curtis, J.L. Bacterial Topography of the Healthy Human Lower Respiratory Tract. mBio 2017, 8, e02287-16. [Google Scholar] [CrossRef]
- Herath, S.C.; Normansell, R.; Maisey, S.; Poole, P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2018, 10, CD009764. [Google Scholar] [CrossRef]
- The University of Adelaide. Critical Appraisal Checklist for Prevalence Studies. 2020. Available online: https://jbi.global/critical-appraisal-tools (accessed on 18 August 2021).
- Barker, T.H.; Migliavaca, C.B.; Stein, C.; Colpani, V.; Falavigna, M.; Aromataris, E.; Munn, Z. Conducting proportional meta-analysis in different types of systematic reviews: A guide for synthesisers of evidence. BMC Med. Res. Methodol. 2021, 21, 189. [Google Scholar] [CrossRef]
- Millares, L.; Ferrari, R.; Gallego, M.; Garcia-Nuñez, M.; Pérez-Brocal, V.; Espasa, M.; Pomares, X.; Monton, C.; Moya, A.; Monsó, E. Bronchial microbiome of severe COPD patients colonised by Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1101–1111. [Google Scholar] [CrossRef]
- Wilkinson, T.M.; Aris, E.; Bourne, S.C.; Clarke, S.C.; Peeters, M.; Pascal, T.G.; Taddei, L.; Tuck, A.C.; Kim, V.L.; Ostridge, K.K.; et al. Drivers of year-to-year variation in exacerbation frequency of COPD: Analysis of the AERIS cohort. ERJ Open Res. 2019, 5, 00248-2018. [Google Scholar] [CrossRef]
- Andelid, K.; Tengvall, S.; Andersson, A.; Levänen, B.; Christenson, K.; Jirholt, P.; Åhrén, C.; Qvarfordt, I.; Ekberg-Jansson, A.; Lindén, A. Systemic cytokine signaling via IL-17 in smokers with obstructive pulmonary disease: A link to bacterial colonization? Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 689–702. [Google Scholar]
- Banerjee, D.; Khair, O.A.; Honeybourne, D. Impact of sputum bacteria on airway inflammation and health status in clinical stable COPD. Eur. Respir. J. 2004, 23, 685. [Google Scholar] [CrossRef]
- Bogaert, D.; van der Valk, P.; Ramdin, R.; Sluijter, M.; Monninkhof, E.; Hendrix, R.; de Groot, R.; Hermans, P.W. Host-pathogen interaction during pneumococcal infection in patients with chronic obstructive pulmonary disease. Infect. Immun. 2004, 72, 818–823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Einarsson, G.G.; Comer, D.M.; McIlreavey, L.; Parkhill, J.; Ennis, M.; Tunney, M.M.; Elborn, J.S. Community dynamics and the lower airway microbiota in stable chronic obstructive pulmonary disease, smokers and healthy non-smokers. Thorax 2016, 71, 795. [Google Scholar] [CrossRef] [PubMed]
- Fruchter, O.; Rosengarten, D.; Goldberg, E.; Ben-Zvi, H.; Tor, R.; Kramer, M.R. Airway bacterial colonization and serum C-reactive protein are associated with chronic obstructive pulmonary disease exacerbation following bronchoscopic lung volume reduction. Clin. Respir. J. 2014, 10, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Nuñez, M.; Millares, L.; Pomares, X.; Ferrari, R.; Pérez-Brocal, V.; Gallego, M.; Espasa, M.; Moya, A.; Monsó, E. Severity-related changes of bronchial microbiome in chronic obstructive pulmonary disease. J. Clin. Microbiol. 2014, 52, 4217–4223. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.R.; Wilkinson, T.M.A.; Perera, W.R.; Donaldson, G.C.; Wedzicha, J.A. Relationships Among Bacteria, Upper Airway, Lower Airway, and Systemic Inflammation in COPD. Chest 2005, 127, 1219–1226. [Google Scholar] [CrossRef]
- Jacobs, D.M.; Ochs-Balcom, H.M.; Zhao, J.; Murphy, T.F.; Sethi, S. Lower Airway Bacterial Colonization Patterns and Species-Specific Interactions in Chronic Obstructive Pulmonary Disease. J. Clin. Microbiol. 2018, 56, e00330-18. [Google Scholar] [CrossRef] [PubMed]
- Jordan, G.W.; Wong, G.A.; Hoeprich, P.D. Bacteriology of the Lower Respiratory Tract as Determined by Fiber-Optic Bronchoscopy and Transtracheal Aspiration. J. Infect. Dis. 1976, 134, 428–435. [Google Scholar] [CrossRef]
- Khurana, S.; Ravi, A.; Sutula, J.; Milone, R.; Williamson, R.; Plumb, J.; Vestbo, J.; Singh, D. Clinical characteristics and airway inflammation profile of COPD persistent sputum producers. Respir. Med. 2014, 108, 1761–1770. [Google Scholar] [CrossRef]
- Marin, A.; Monsó, E.; Garcia-Nunez, M.; Sauleda, J.; Noguera, A.; Pons, J.; Agustí, A.; Morera, J. Variability and effects of bronchial colonisation in patients with moderate COPD. Eur. Respir. J. 2010, 35, 295. [Google Scholar] [CrossRef]
- Marin, A.; Garcia-Aymerich, J.; Sauleda, J.; Belda, J.; Millares, L.; García-Núñez, M.; Serra, I.; Benet, M.; Agustí, A.; Antó, J.M.; et al. Effect of Bronchial Colonisation on Airway and Systemic Inflammation in Stable COPD. COPD J. Chronic Obstr. Pulm. Dis. 2012, 9, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Mika, M.; Nita, I.; Morf, L.; Qi, W.; Beyeler, S.; Bernasconi, E.; Marsland, B.J.; Ott, S.R.; von Garnier, C.; Hilty, M. Microbial and host immune factors as drivers of COPD. ERJ Open Res. 2018, 4, 00015-2018. [Google Scholar] [CrossRef]
- Miravitlles, M.; Marín, A.; Monsó, E.; Vilà, S.; de la Roza, C.; Hervás, R.; Esquinas, C.; García, M.; Millares, L.; Morera, J.; et al. Efficacy of moxifloxacin in the treatment of bronchial colonisation in COPD. Eur. Respir. J. 2009, 34, 1066. [Google Scholar] [CrossRef]
- Miravitlles, M.; Marín, A.; Monsó, E.; Vilà, S.; de la Roza, C.; Hervás, R.; Esquinas, C.; García, M.; Millares, L.; Morera, J.; et al. Colour of sputum is a marker for bacterial colonisation in chronic obstructive pulmonary disease. Respir. Res. 2010, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Monso, E.; Ruiz, J.; Rosell, A.; Manterola, J.; Fiz, J.; Morera, J.; Ausina, V. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am. J. Respir. Crit. Care Med. 1995, 152, 1316–1320. [Google Scholar] [CrossRef]
- Monso, E.; Rosell, A.; Bonet, G.; Manterola, J.; Cardona, P.J.; Ruiz, J.; Morera, J. Risk factors for lower airway bacterial colonization in chronic bronchitis. Eur. Respir. J. 1999, 13, 338. [Google Scholar] [CrossRef] [PubMed]
- Riise, G.C.; Larsson, S.; Larsson, P.; Jeansson, S.; Andersson, B.A. The intrabronchial microbial flora in chronic bronchitis patients: A target for N-acetylcysteine therapy? Eur. Respir. J. 1994, 7, 94. [Google Scholar] [CrossRef]
- Seemungal, T.A.R.; Wilkinson, T.M.A.; Hurst, J.R.; Perera, W.R.; Sapsford, R.J.; Wedzicha, J.A. Long-term Erythromycin Therapy Is Associated with Decreased Chronic Obstructive Pulmonary Disease Exacerbations. Am. J. Respir. Crit. Care Med. 2008, 178, 1139–1147. [Google Scholar] [CrossRef]
- Sethi, S.; Maloney, J.; Grove, L.; Wrona, C.; Berenson, C.S. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006, 173, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Sibila, O.; Garcia-Bellmunt, L.; Giner, J.; Merino, J.L.; Suarez-Cuartin, G.; Torrego, A.; Solanes, I.; Castillo, D.; Valera, J.L.; Cosio, B.G.; et al. Identification of airway bacterial colonization by an electronic nose in Chronic Obstructive Pulmonary Disease. Respir. Med. 2014, 108, 1608–1614. [Google Scholar] [CrossRef]
- Sibila, O.; Garcia-Bellmunt, L.; Giner, J.; Rodrigo-Troyano, A.; Suarez-Cuartin, G.; Torrego, A.; Castillo, D.; Solanes, I.; Mateus, E.F.; Vidal, S.; et al. Airway Mucin 2 Is Decreased in Patients with Severe Chronic Obstructive Pulmonary Disease with Bacterial Colonization. Ann. Am. Thorac. Soc. 2016, 13, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; Powell, H.; Baines, K.J.; Milne, D.; Coxson, H.O.; Hansbro, P.M.; Gibson, P.G. The effect of azithromycin in adults with stable neutrophilic COPD: A double blind randomised, placebo controlled trial. PLoS ONE 2014, 9, e105609. [Google Scholar] [CrossRef]
- Simpson, J.L.; Baines, K.J.; Horvat, J.C.; Essilfie, A.T.; Brown, A.C.; Tooze, M.; McDonald, V.M.; Gibson, P.G.; Hansbro, P.M. COPD is characterized by increased detection of Haemophilus influenzae, Streptococcus pneumoniae and a deficiency of Bacillus species. Respirology 2016, 21, 697–704. [Google Scholar] [CrossRef]
- Singh, R.; Mackay, A.J.; Patel, A.R.; Garcha, D.S.; Kowlessar, B.S.; Brill, S.E.; Donnelly, L.E.; Barnes, P.J.; Donaldson, G.C.; Wedzicha, J.A. Inflammatory thresholds and the species-specific effects of colonising bacteria in stable chronic obstructive pulmonary disease. Respir. Res. 2014, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.B.; Cox, A.J.; Sivakumaran, P.; Singh, M.; Watts, A.M.; West, N.P.; Cripps, A.W. Non-typeable Haemophilus Influenzae detection in the lower airways of patients with lung cancer and chronic obstructive pulmonary disease. Multidiscip. Respir. Med. 2018, 13, 11. [Google Scholar] [CrossRef]
- Trudzinski, F.C.; Seiler, F.; Wilkens, H.; Metz, C.; Kamp, A.; Bals, R.; Gärtner, B.; Lepper, P.M.; Becker, S.L. Microbiological airway colonization in COPD patients with severe emphysema undergoing endoscopic lung volume reduction. Int. J. Chronic Obstr. Pulm. Dis. 2017, 13, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Tumkaya, M.; Atis, S.; Ozge, C.; Delialioglu, N.; Polat, G.; Kanik, A. Relationship between airway colonization, inflammation and exacerbation frequency in COPD. Respir. Med. 2007, 101, 729–737. [Google Scholar] [CrossRef]
- Weinreich, U.M.; Korsgaard, J. Bacterial colonisation of lower airways in health and chronic lung disease. Clin. Respir. J. 2008, 2, 116–122. [Google Scholar] [CrossRef]
- Wilkinson, T.M.A.; Patel, I.S.; Wilks, M.; Donaldson, G.C.; Wedzicha, J.A. Airway Bacterial Load and FEV1 Decline in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2003, 167, 1090–1095. [Google Scholar] [CrossRef]
- Zalacain, R.; Sobradillo, V.; Amilibia, J.; Barron, J.; Achotegui, V.; Pijoan, J.I.; Llorente, J.L. Predisposing factors to bacterial colonization in chronic obstructive pulmonary disease. Eur. Respir. J. 1999, 13, 343. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Q.; Zhang, X.Y.; Ding, X.; Zhu, D.; Zhou, X. Relevance of lower airway bacterial colonization, airway inflammation, and pulmonary function in the stable stage of chronic obstructive pulmonary disease. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1487–1493. [Google Scholar] [CrossRef]
- Choi, S.H.; Cha, S.I.; Choi, K.J.; Lim, J.K.; Seo, H.; Yoo, S.S.; Lee, J.; Lee, S.Y.; Kim, C.H.; Park, J.Y. Clinical Characteristics of Community-Acquired Viridans Streptococcal Pneumonia. Tuberc. Respir. Dis. 2015, 78, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Evans, N.; Grant, B.J.B.; Murphy, T.F. New Strains of Bacteria and Exacerbations of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2002, 347, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Duim, B.; van Alphen, L.; Eijk, P.; Jansen, H.M.; Dankert, J. Antigenic drift of non-encapsulated Haemophilus influenzae major outer membrane protein P2 in patients with chronic bronchitis is caused by point mutations. Mol. Microbiol. 1994, 11, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Cole, P. The damaging role of bacteria in chronic lung infection. J. Antimicrob. Chemother. 1997, 40 (Suppl. S1), 5–10. [Google Scholar] [CrossRef]
- Wilson, R. Bacteria, antibiotics and COPD. Eur. Respir. J. 2001, 17, 995. [Google Scholar] [CrossRef]
- Matkovic, Z.; Miravitlles, M. Chronic bronchial infection in COPD. Is there an infective phenotype? Respir. Med. 2013, 107, 10–22. [Google Scholar] [CrossRef]
- Stockley, R.A.; O’Brien, C.; Pye, A.; Hill, S.L. Relationship of Sputum Color to Nature and Outpatient Management of Acute Exacerbations of COPD. Chest 2000, 117, 1638–1645. [Google Scholar] [CrossRef]

| Outcomes | Data Collection Points | |
|---|---|---|
| Demographics | Age; sex; alpha-1 antitrypsin status; stability period; smoking status and pack years | |
| Primary Outcome | Determine prevalence of bacterial colonisation in stable-state COPD | Number of patients that produced a sample; number of samples collected; number of positive cultures; individual bacteriology (number positive for individual PPMs) |
| Secondary Outcomes | Assess the relationship between sampling modality and colonisation | Sampling modality (spontaneous, induced, PSB, bronchoscopy, trans-tracheal aspiration) |
| Assess relationship between bacterial colonisation and disease phenotype | FEV1; FEV1 category by GOLD criteria; quality of life (SGRQ/CAT/mMRC); exacerbation frequency; hospitalisation rate; mortality rate | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armitage, M.N.; Spittle, D.A.; Turner, A.M. A Systematic Review and Meta-Analysis of the Prevalence and Impact of Pulmonary Bacterial Colonisation in Stable State Chronic Obstructive Pulmonary Disease (COPD). Biomedicines 2022, 10, 81. https://doi.org/10.3390/biomedicines10010081
Armitage MN, Spittle DA, Turner AM. A Systematic Review and Meta-Analysis of the Prevalence and Impact of Pulmonary Bacterial Colonisation in Stable State Chronic Obstructive Pulmonary Disease (COPD). Biomedicines. 2022; 10(1):81. https://doi.org/10.3390/biomedicines10010081
Chicago/Turabian StyleArmitage, Michael N., Daniella A. Spittle, and Alice M. Turner. 2022. "A Systematic Review and Meta-Analysis of the Prevalence and Impact of Pulmonary Bacterial Colonisation in Stable State Chronic Obstructive Pulmonary Disease (COPD)" Biomedicines 10, no. 1: 81. https://doi.org/10.3390/biomedicines10010081
APA StyleArmitage, M. N., Spittle, D. A., & Turner, A. M. (2022). A Systematic Review and Meta-Analysis of the Prevalence and Impact of Pulmonary Bacterial Colonisation in Stable State Chronic Obstructive Pulmonary Disease (COPD). Biomedicines, 10(1), 81. https://doi.org/10.3390/biomedicines10010081







