Rapamycin Suppresses Penile NADPH Oxidase Activity to Preserve Erectile Function in Mice Fed a Western Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Dietary Intervention
2.2. Rapamycin Treatment
2.3. Metabolic Parameters and Anthropometrics
2.4. Erectile Function Assessment
2.5. Penile In Vivo ROS Measurement
2.6. Western Blotting
2.7. Data and Statistical Analysis
3. Results
3.1. Metabolic Parameters
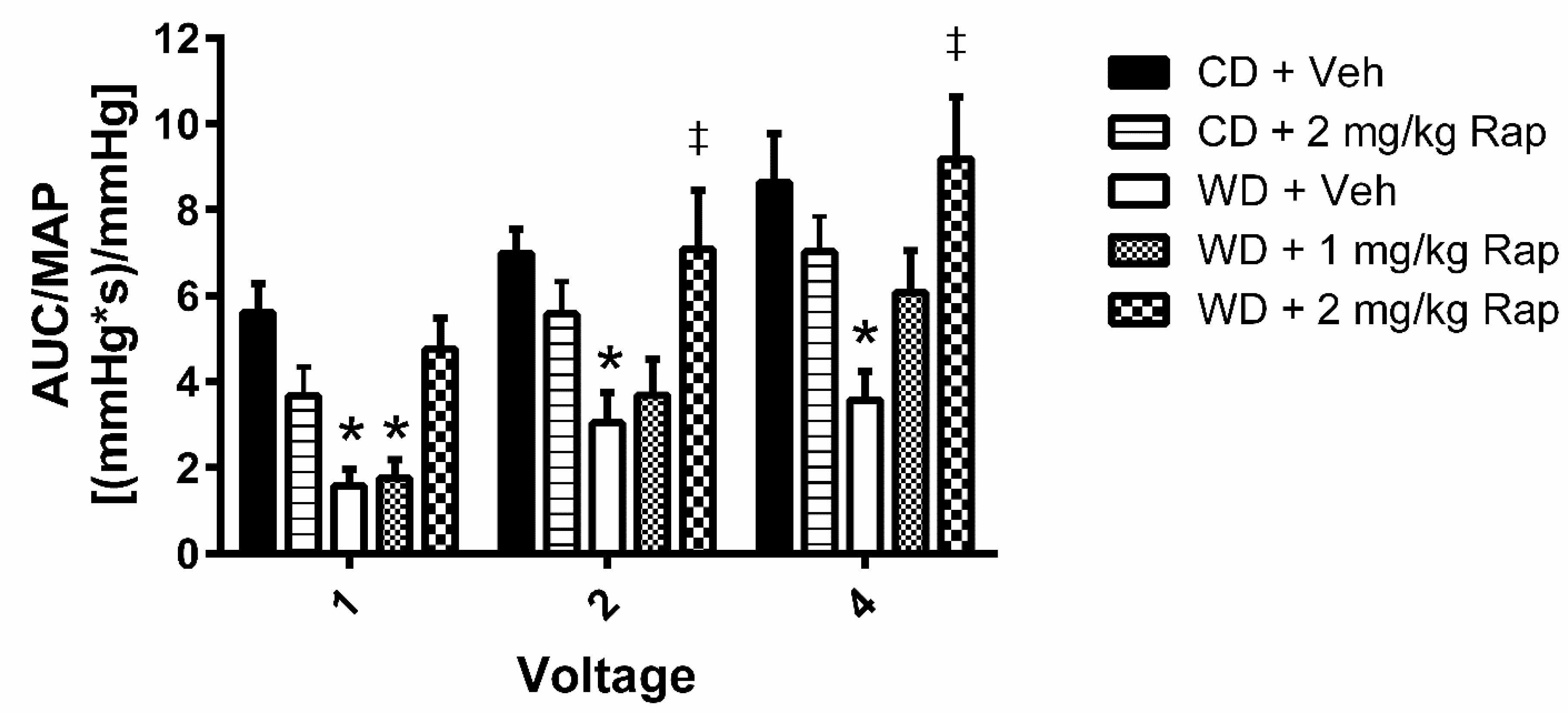
3.2. Voltage-Dependent Erectile Response
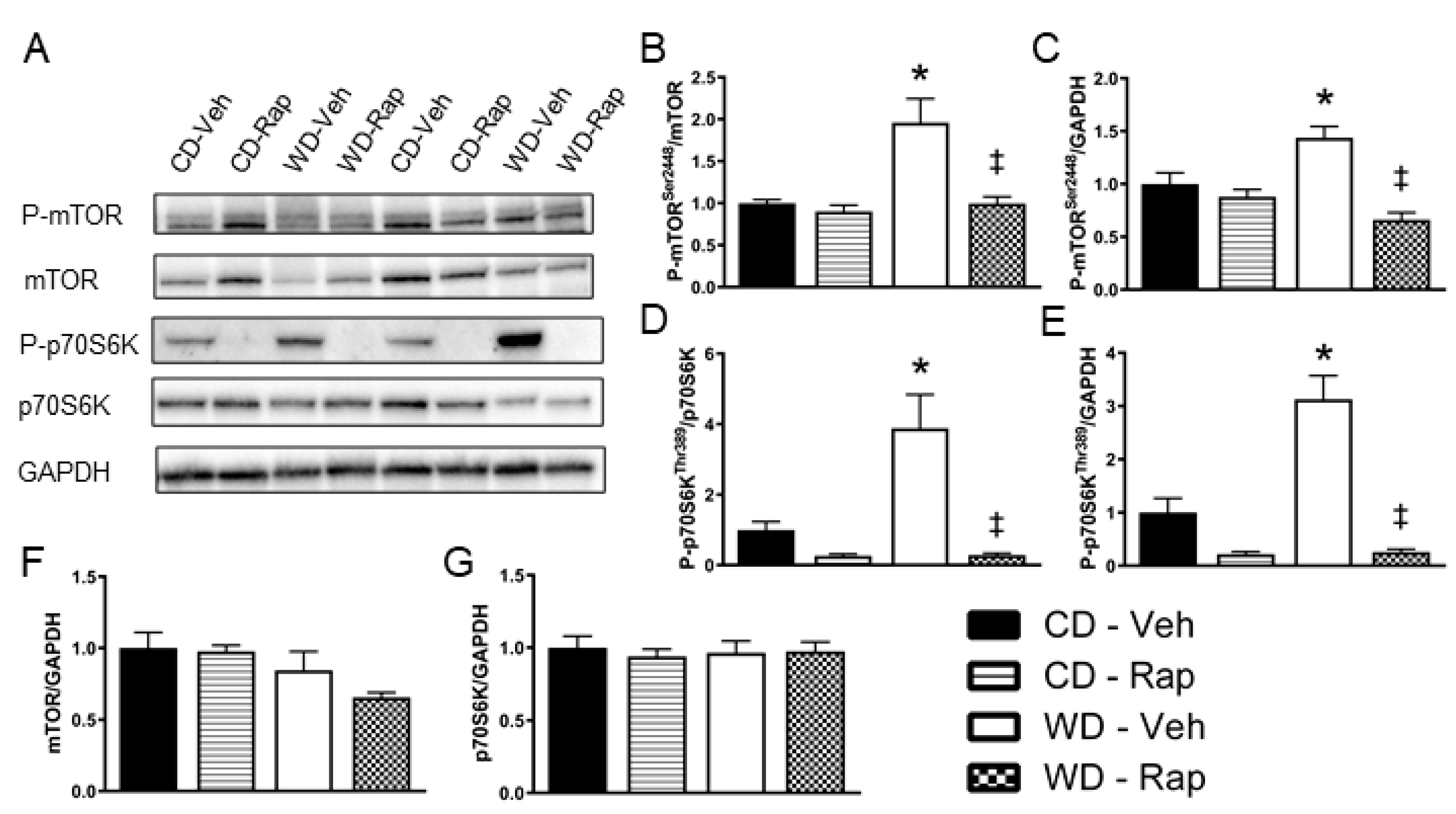
3.3. The Western Diet Activates mTOR Signaling in the Corpus Cavernosum
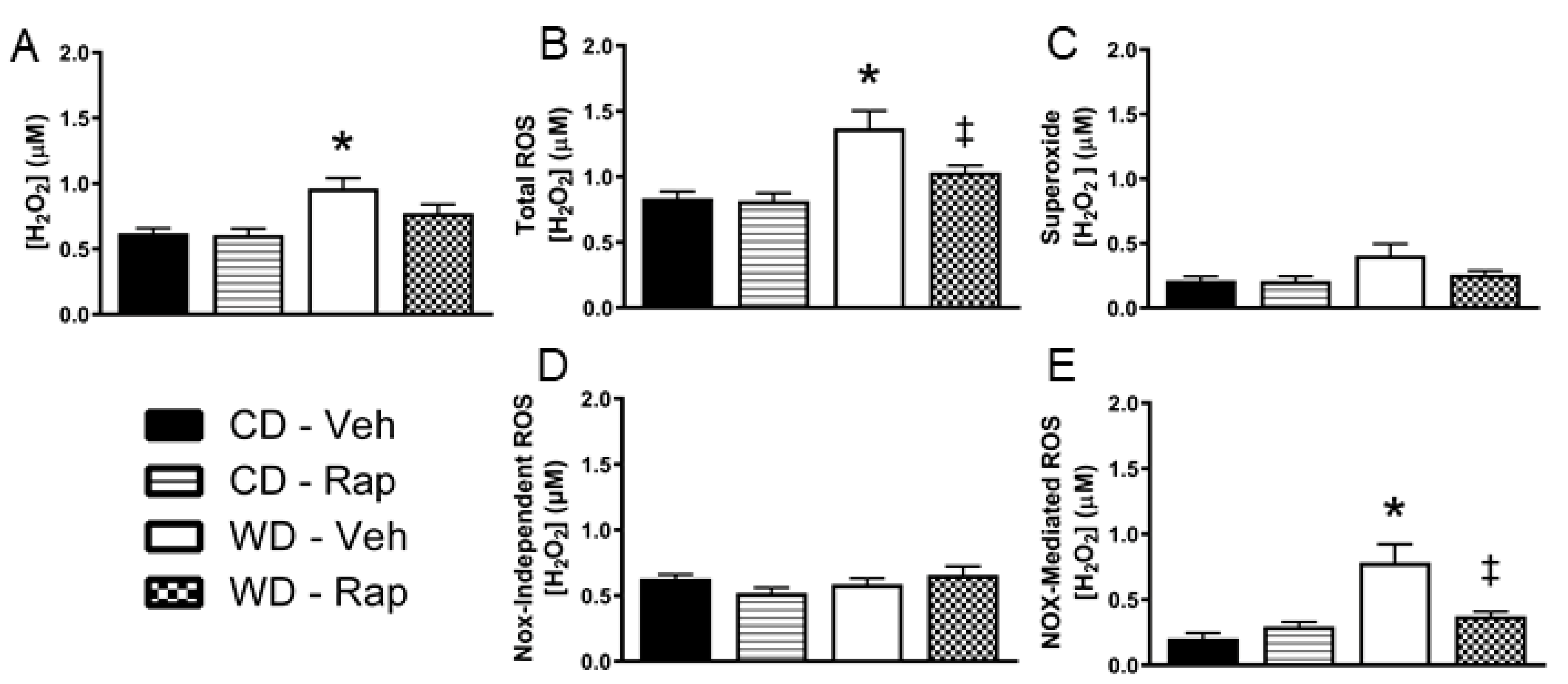
3.4. Excessive Western Diet-Mediated Penile ROS Is Suppressed by Rapamycin
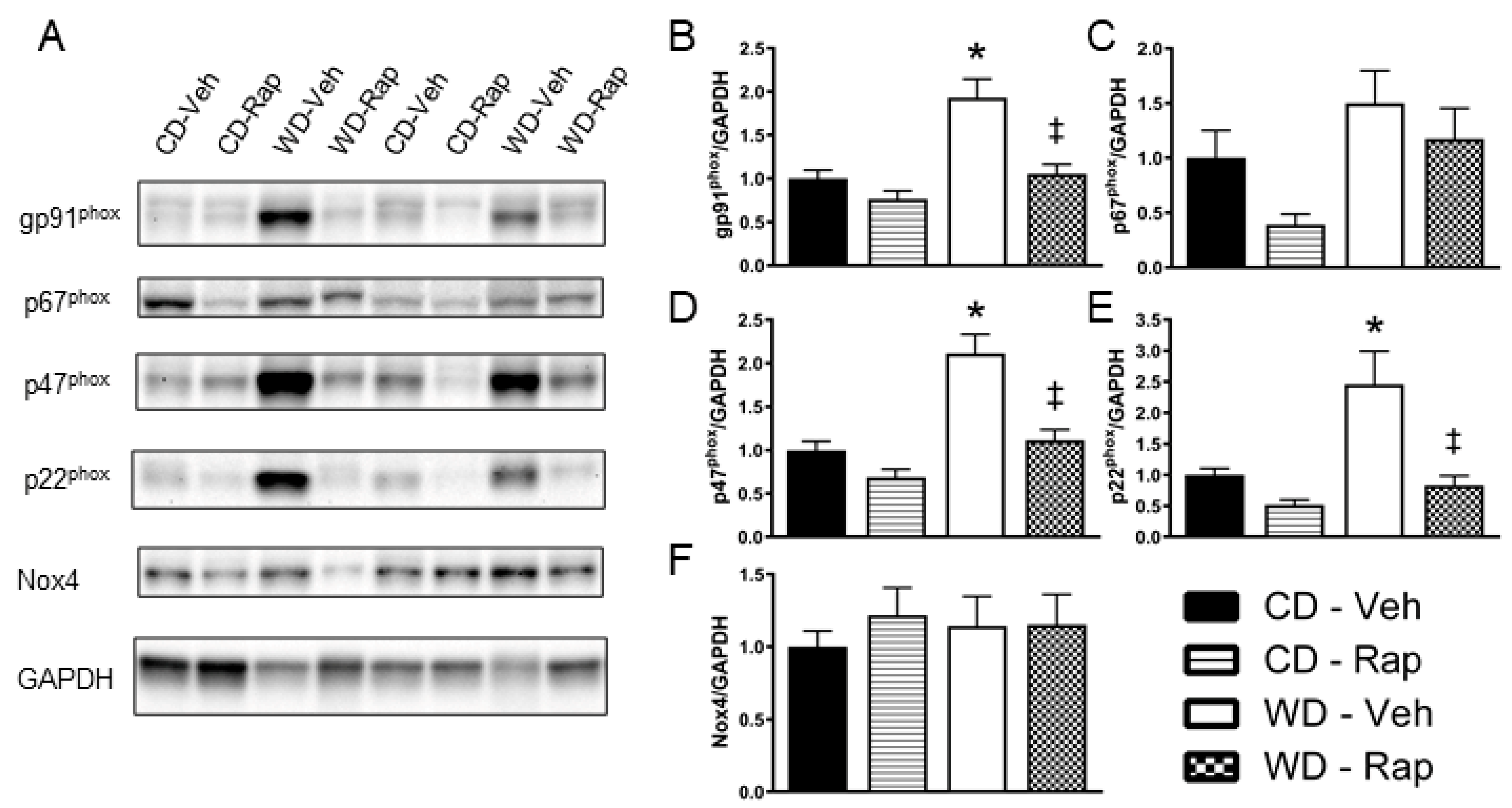
3.5. Western Diet-Induced Corpus Cavernosum NADPH Oxidase Protein Content Is Blunted by Rapamycin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, L.P.; Ng, S.W.; Popkin, B.M. Trends in US home food preparation and consumption: Analysis of national nutrition surveys and time use studies from 1965–1966 to 2007–2008. Nutr. J. 2013, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Duffey, K.J.; Popkin, B.M. High-fructose corn syrup: Is this what’s for dinner? Am. J. Clin. Nutr. 2008, 88, 1722S–1732S. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Ding, E.L.; Malik, V.S. Convergence of obesity and high glycemic diet on compounding diabetes and cardiovascular risks in modernizing China: An emerging public health dilemma. Glob. Health 2008, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Blondin, S.A.; Mueller, M.P.; Bakun, P.J.; Choumenkovitch, S.F.; Tucker, K.L.; Economos, C.D. Cross-sectional associations between empirically-derived dietary patterns and indicators of disease risk among university students. Nutrients 2016, 8, 3. [Google Scholar] [CrossRef]
- Morgan, E.H.; Graham, M.L.; Folta, S.C.; Seguin, R.A. A qualitative study of factors related to cardiometabolic risk in rural men. BMC Public Health 2016, 16, 305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Bleich, S.N.; Gortmaker, S.L. Increasing caloric contribution from sugar- sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics 2008, 121, e1604–e1614. [Google Scholar] [CrossRef]
- Esposito, K.; Ciotola, M.; Giugliano, F.; De Sio, M.; Giugliano, G.; D’Armiento, M.; Giugliano, D. Mediterranean diet improves erectile function in subjects with the metabolic syndrome. Int. J. Impot. Res. 2006, 18, 405–410. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, F.; Maiorino, M.I.; Giugliano, D. Dietary Factors, Mediterranean Diet and Erectile Dysfunction. J. Sex. Med. 2010, 7, 2338–2345. [Google Scholar] [CrossRef]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obes. Rev. 2020, 21, e13126. [Google Scholar] [CrossRef]
- Sangiorgi, G.; Cereda, A.; Benedetto, D.; Bonanni, M.; Chiricolo, G.; Cota, L.; Martuscelli, E.; Greco, F. Anatomy, pathophysiology, molecular mechanisms, and clinical management of erectile dysfunction in patients affected by coronary artery disease: A review. Biomedicines 2021, 9, 432. [Google Scholar] [CrossRef]
- La Favor, J.D.; Anderson, E.J.; Hickner, R.C.; Wingard, C.J. Erectile Dysfunction Precedes Coronary Artery Endothelial Dysfunction in Rats Fed a High-Fat, High-Sucrose, Western Pattern Diet. J. Sex. Med. 2013, 10, 694–703. [Google Scholar] [CrossRef] [PubMed]
- La Favor, J.D.; Anderson, E.J.; Dawkins, J.T.; Hickner, R.C.; Wingard, C.J. Exercise prevents Western diet-associated erectile dysfunction and coronary artery endothelial dysfunction: Response to acute apocynin and sepiapterin treatment. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R423–R434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. MTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef]
- André, C.; Cota, D. Coupling nutrient sensing to metabolic homoeostasis: The role of the mammalian target of rapamycin complex 1 pathway. Proc. Nutr. Soc. 2012, 71, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Kim, H.; Ahn, J.; Jeon, T.-I.; Lee, D.H.; Ha, T.Y. Fisetin regulates obesity by targeting mTORC1 signaling. J. Nutr. Biochem. 2013, 24, 1547–1554. [Google Scholar] [CrossRef]
- Khamzina, L.; Veilleux, A.; Bergeron, S.; Marette, A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: Possible involvement in obesity-linked insulin resistance. Endocrinology 2005, 146, 1473–1481. [Google Scholar] [CrossRef]
- Pajvani, U.B.; Qiang, L.; Kangsamaksin, T.; Kitajewski, J.; Ginsberg, H.N.; Accili, D. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat. Med. 2013, 19, 1054–1060. [Google Scholar] [CrossRef]
- Stemmer, K.; Perez-Tilve, D.; Ananthakrishnan, G.; Bort, A.; Seeley, R.J.; Tschöp, M.H.; Dietrich, D.R.; Pfluger, P.T. High-fat-diet-induced obesity causes an inflammatory and tumor-promoting microenvironment in the rat kidney. DMM Dis. Model. Mech. 2012, 5, 627–635. [Google Scholar] [CrossRef]
- Turdi, S.; Kandadi, M.R.; Zhao, J.; Huff, A.F.; Du, M.; Ren, J. Deficiency in AMP-activated protein kinase exaggerates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J. Mol. Cell. Cardiol. 2011, 50, 712–722. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Wu, Y.; Lu, X.; Chidiac, P.; Wang, G.; Feng, Q. Maternal diabetes up-regulates NOX2 and enhances myocardial ischaemia/reperfusion injury in adult offspring. J. Cell. Mol. Med. 2018, 22, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.B.; Sonowal, H.; Shukla, K.; Srivastava, S.K.; Ramana, K.V. Aldose reductase regulates hyperglycemia-induced huvec death via SIRT1/AMPK-α1/mTOR pathway. J. Mol. Endocrinol. 2019, 63, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, H.; Wu, K.; Lee, S.; Li, R.; Liu, X. PTEN phosphorylation and nuclear export mediate free fatty acid-induced oxidative stress. Antioxid. Redox Signal. 2014, 20, 1382–1395. [Google Scholar] [CrossRef] [PubMed]
- Lagoda, G.; Sezen, S.F.; Burnett, A.L. FK506 and Rapamycin Neuroprotect Erection and Involve Different Immunophilins in a Rat Model of Cavernous Nerve Injury. J. Sex. Med. 2009, 6, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- La Favor, J.D.; Fu, Z.; Venkatraman, V.; Bivalacqua, T.J.; Van Eyk, J.E.; Burnett, A.L. Molecular Profile of Priapism Associated with Low Nitric Oxide Bioavailability. J. Proteome Res. 2018, 17, 1031–1040. [Google Scholar] [CrossRef]
- La Favor, J.D.; Anderson, E.J.; Hickner, R.C. Novel method for detection of reactive oxygen species in vivo in human skeletal muscle. Physiol. Res. 2014, 63, 387–392. [Google Scholar] [CrossRef]
- La Favor, J.D.; Burnett, A.L. A microdialysis method to measure in vivo hydrogen peroxide and superoxide in various rodent tissues. Methods 2016, 109, 131–140. [Google Scholar] [CrossRef]
- La Favor, J.D.; Dubis, G.S.; Yan, H.; White, J.D.; Nelson, M.A.M.; Anderson, E.J.; Hickner, R.C. Microvascular Endothelial Dysfunction in Sedentary, Obese Humans Is Mediated by NADPH Oxidase. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2412–2420. [Google Scholar] [CrossRef]
- Dann, S.G.; Selvaraj, A.; Thomas, G. mTOR Complex1-S6K1 signaling: At the crossroads of obesity, diabetes and cancer. Trends Mol. Med. 2007, 13, 252–259. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Martinez-Lemus, L.A.; Sowers, J.R. Overnutrition, mTOR signaling, and cardiovascular diseases. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1198–R1206. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ming, X.F. mTOR signalling: The molecular interface connecting metabolic stress, aging and cardiovascular diseases. Obes. Rev. 2012, 13 (Suppl. 2), 58–68. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Liu, H.; Gao, F.; Luo, H.; Lin, H.; Meng, L.; Jiang, C.; Guo, Y.; Chi, J.; Guo, H. Folic acid delays development of atherosclerosis in low-density lipoprotein receptor-deficient mice. J. Cell. Mol. Med. 2018, 22, 3183–3191. [Google Scholar] [CrossRef]
- Zhang, L.; Ussher, J.R.; Oka, T.; Cadete, V.J.J.; Wagg, C.; Lopaschuk, G.D. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc. Res. 2011, 89, 148–156. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, Y.; Turdi, S.; Ren, J. Adiponectin knockout accentuates high fat diet-induced obesity and cardiac dysfunction: Role of autophagy. Biochim. Biophys. Acta 2013, 1832, 1136–1148. [Google Scholar] [CrossRef]
- Lin, H.; Wang, T.; Ruan, Y.; Liu, K.; Li, H.; Wang, S.; Li, M.; Liu, J. Rapamycin Supplementation May Ameliorate Erectile Function in Rats with Streptozotocin-Induced Type 1 Diabetes by Inducing Autophagy and Inhibiting Apoptosis, Endothelial Dysfunction, and Corporal Fibrosis. J. Sex. Med. 2018, 15, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Aboseif, S.R.; Lue, T.F. Fundamentals and hemodynamics of penile erection. Cardiovasc. Interv. Radiol. 1988, 11, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; Peiró, C.; Cuevas, P.; Gabancho, S.; Fernández, A.; González-Corrochano, R.; La Fuente, J.M.; Baron, A.D.; Chen, K.S.; de Tejada, I.S. The novel antioxidant, AC3056 (2,6-di-t-butyl-4-((Dimethyl-4-Methoxyphenylsilyl)Methyloxy)Phenol), reverses erectile dysfunction in diabetic rats and improves NO-mediated responses in penile tissue from diabetic men. J. Sex. Med. 2009, 6, 373–387. [Google Scholar] [CrossRef]
- Bivalacqua, T.J.; Armstrong, J.S.; Biggerstaff, J.; Abdel-Mageed, A.B.; Kadowitz, P.J.; Hellstrom, W.J.G.; Champion, H.C. Gene transfer of extracellular SOD to the penis reduces O2-· and improves erectile function in aged rats. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1408–H1421. [Google Scholar] [CrossRef]
- Wingard, C.; Fulton, D.; Husain, S.; Traish, A. Altered penile vascular reactivity and erection in the Zucker obese-diabetic rat. J. Sex. Med. 2007, 4, 348–363. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Cong, R.; Tian, Y.; Chen, C.; Wang, Y.; Zhang, Q.; Zhou, X.; Ji, C.; Meng, X.; et al. Restoration of erectile function by suppression of corporal apoptosis and oxidative stress with losartan in aged rats with erectile dysfunction. Andrology 2020, 8, 769–779. [Google Scholar] [CrossRef]
- Yafi, F.A.; Jenkins, L.; Albersen, M.; Corona, G.; Isidori, A.M.; Goldfarb, S.; Maggi, M.; Nelson, C.J.; Parish, S.; Salonia, A.; et al. Erectile dysfunction. Nat. Rev. Dis. Primers 2016, 2, 16300. [Google Scholar] [CrossRef]
- Lesniewski, L.A.; Seals, D.R.; Walker, A.E.; Henson, G.D.; Blimline, M.W.; Trott, D.W.; Bosshardt, G.C.; LaRocca, T.J.; Lawson, B.R.; Zigler, M.C.; et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell 2017, 16, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, A.G.; Yepuri, G.; Carvas, J.M.; Stein, S.; Matter, C.M.; Scerri, I.; Ruffieux, J.; Montani, J.P.; Ming, X.F.; Yang, Z. Hyperactive S6K1 mediates oxidative stress and endothelial dysfunction in aging: Inhibition by resveratrol. PLoS ONE 2011, 6, e19237. [Google Scholar] [CrossRef] [PubMed]
- Rezabakhsh, A.; Ahmadi, M.; Khaksar, M.; Montaseri, A.; Malekinejad, H.; Rahbarghazi, R.; Garjani, A. Rapamycin inhibits oxidative/nitrosative stress and enhances angiogenesis in high glucose-treated human umbilical vein endothelial cells: Role of autophagy. Biomed. Pharmacother. 2017, 93, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.L.; Lowenstein, C.J.; Bredt, D.S.; Chang, T.S.K.; Snyder, S.H. Nitric oxide: A physiologic mediator of penile erection. Science 1992, 257, 401–403. [Google Scholar] [CrossRef]
- Reho, J.J.; Guo, D.F.; Rahmouni, K. Mechanistic Target of Rapamycin Complex 1 Signaling Modulates Vascular Endothelial Function Through Reactive Oxygen Species. J. Am. Heart Assoc. 2019, 8, e010662. [Google Scholar] [CrossRef]
- Musicki, B.; Liu, T.; Lagoda, G.A.; Strong, T.D.; Sezen, S.F.; Johnson, J.M.; Burnett, A.L. Hypercholesterolemia-induced erectile dysfunction: Endothelial nitric oxide synthase (eNOS) uncoupling in the mouse penis by NAD(P)H oxidase. J. Sex. Med. 2010, 7, 3023–3032. [Google Scholar] [CrossRef]
- Fraga-Silva, R.A.; Costa-Fraga, F.P.; Montecucco, F.; Sturny, M.; Faye, Y.; Mach, F.; Pelli, G.; Shenoy, V.; da Silva, R.F.; Raizada, M.K.; et al. Diminazene protects corpus cavernosum against hypercholesterolemia-induced injury. J. Sex. Med. 2015, 12, 289–302. [Google Scholar] [CrossRef]
- Nunes, K.P.; Teixeira, C.E.; Priviero, F.B.M.; Toque, H.A.; Webb, R.C. Beneficial effect of the soluble guanylyl cyclase stimulator BAY 41-2272 on impaired penile erection in db/db−/− type II diabetic and obese mice. J. Pharmacol. Exp. Ther. 2015, 353, 330–339. [Google Scholar] [CrossRef]
- Li, M.; Zhuan, L.; Wang, T.; Rao, K.; Yang, J.; Yang, J.; Quan, W.; Liu, J.; Ye, Z. Apocynin improves erectile function in diabetic rats through regulation of NADPH oxidase expression. J. Sex. Med. 2012, 9, 3041–3050. [Google Scholar] [CrossRef]
- Jin, L.; Lagoda, G.; Leite, R.; Webb, R.C.; Burnett, A.L. NADPH oxidase activation: A mechanism of hypertension-associated erectile dysfunction. J. Sex. Med. 2008, 5, 544–551. [Google Scholar] [CrossRef]
- Silva, F.H.; Mónica, F.Z.; Báu, F.R.; Brugnerotto, A.F.; Priviero, F.B.M.; Toque, H.A.; Antunes, E. Superoxide anion production by NADPH oxidase plays a major role in erectile dysfunction in middle-aged rats: Prevention by antioxidant therapy. J. Sex. Med. 2013, 10, 960–971. [Google Scholar] [CrossRef]
- Stolk, J.; Hiltermann, T.J.; Dijkman, J.H.; Verhoeven, A.J. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol. 1994, 11, 95–102. [Google Scholar] [CrossRef]
- Sánchez, A.; Contreras, C.; Martínez, M.P.; Climent, B.; Benedito, S.; García-Sacristán, A.; Hernández, M.; Prieto, D. Role of neural NO synthase (nNOS) uncoupling in the dysfunctional nitrergic vasorelaxation of penile arteries from insulin-resistant obese Zucker rats. PLoS ONE 2012, 7, e36027. [Google Scholar] [CrossRef]
- Channon, K.M. Tetrahydrobiopterin: Regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc. Med. 2004, 14, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Huyghe, E.; Zairi, A.; Nohra, J.; Kamar, N.; Plante, P.; Rostaing, L. Gonadal impact of target of rapamycin inhibitors (sirolimus and everolimus) in male patients: An overview. Transpl. Int. 2007, 20, 305–311. [Google Scholar] [CrossRef]
- Terlizzese, G.; Stubinski, R.; Casini, A.; Clerici, G.; Sangiorgi, G. A case report of pudendal arteries angioplasty with sirolimus drug-coated balloon and drug-eluting stent associated with intracavernous autologous peripheral blood mononuclear cells injection for untreatable vasculogenic erectile dysfunction. Eur. Heart J. Case Reports 2021, 5, ytab244. [Google Scholar] [CrossRef]
- Schönhofen, J.; Räber, L.; Knöchel, J.; Keo, H.H.; Regli, C.; Kostal, F.; Schumacher, M.C.; Sammarchi, L.; Bechir, M.; Diehm, N. Endovascular Therapy for Arteriogenic Erectile Dysfunction with a Novel Sirolimus-Eluting Stent. J. Sex. Med. 2021, 18, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Vlavcheski, F.; Giacca, A.; Tsiani, E. Attenuation of Free Fatty Acid (FFA)-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol is Linked to Activation of AMPK and Inhibition of mTOR and p70 S6K. Int. J. Mol. Sci. 2020, 21, 4900. [Google Scholar] [CrossRef]
| CD-Veh | CD-Rap | WD-Veh | WD-Rap | |
|---|---|---|---|---|
| Body Weight (g) | 27.6 ± 0.82 | 27.8 ± 0.55 | 31.6 ± 2.99 *† | 31.5 ± 2.92 *† |
| Lean Mass (g) | 22.3 ± 0.78 | 23.8 ± 0.58 | 22.6 ± 0.74 | 21.2 ± 0.39 |
| Fat Mass (g) | 3.42 ± 0.16 | 2.18 ± 0.13 | 6.56 ± 0.54 *† | 7.58 ± 0.51 *† |
| Body Fat % | 11.8 ± 0.62 | 10.6 ± 0.46 | 20.0 ± 1.48 *† | 23.6 ± 1.38 *† |
| Fasting Glucose (mg/dL) | 84.7 ± 7.52 | 93.8 ± 8.04 | 116 ± 8.30 | 118 ± 9.42 * |
| Nonfasting Glucose (mg/dL) | 132 ± 12.8 | 94.4 ± 3.48 | 141 ± 12.1 | 119 ± 5.39 † |
| Total Cholesterol (mg/dL) | 134 ± 4.2 | 196 ± 9.2 * | 169 ± 16.4 | 248 ± 7.2 *†‡ |
| Triglycerides (mg/dL) | 105 ± 16 § | 95.9 ± 9.6 | 75.2 ± 5.6 | 57.3 ± 3.0 |
| LDL-Cholesterol (mg/dL) | 57.8 ± 2.8 | 101 ± 6.1 * | 104 ± 15.8 * | 146 ± 4.4 *†‡ |
| HDL-Cholesterol (mg/dL) | 55.4 ± 2.0 | 75.7 ± 4.7 * | 73.1 ± 3.3 * | 90.6 ± 4.1 *‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Favor, J.D.; Pierre, C.J.; Bivalacqua, T.J.; Burnett, A.L. Rapamycin Suppresses Penile NADPH Oxidase Activity to Preserve Erectile Function in Mice Fed a Western Diet. Biomedicines 2022, 10, 68. https://doi.org/10.3390/biomedicines10010068
La Favor JD, Pierre CJ, Bivalacqua TJ, Burnett AL. Rapamycin Suppresses Penile NADPH Oxidase Activity to Preserve Erectile Function in Mice Fed a Western Diet. Biomedicines. 2022; 10(1):68. https://doi.org/10.3390/biomedicines10010068
Chicago/Turabian StyleLa Favor, Justin D., Clifford J. Pierre, Trinity J. Bivalacqua, and Arthur L. Burnett. 2022. "Rapamycin Suppresses Penile NADPH Oxidase Activity to Preserve Erectile Function in Mice Fed a Western Diet" Biomedicines 10, no. 1: 68. https://doi.org/10.3390/biomedicines10010068
APA StyleLa Favor, J. D., Pierre, C. J., Bivalacqua, T. J., & Burnett, A. L. (2022). Rapamycin Suppresses Penile NADPH Oxidase Activity to Preserve Erectile Function in Mice Fed a Western Diet. Biomedicines, 10(1), 68. https://doi.org/10.3390/biomedicines10010068







