Saccharin Stimulates Insulin Secretion Dependent on Sweet Taste Receptor-Induced Activation of PLC Signaling Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Animals
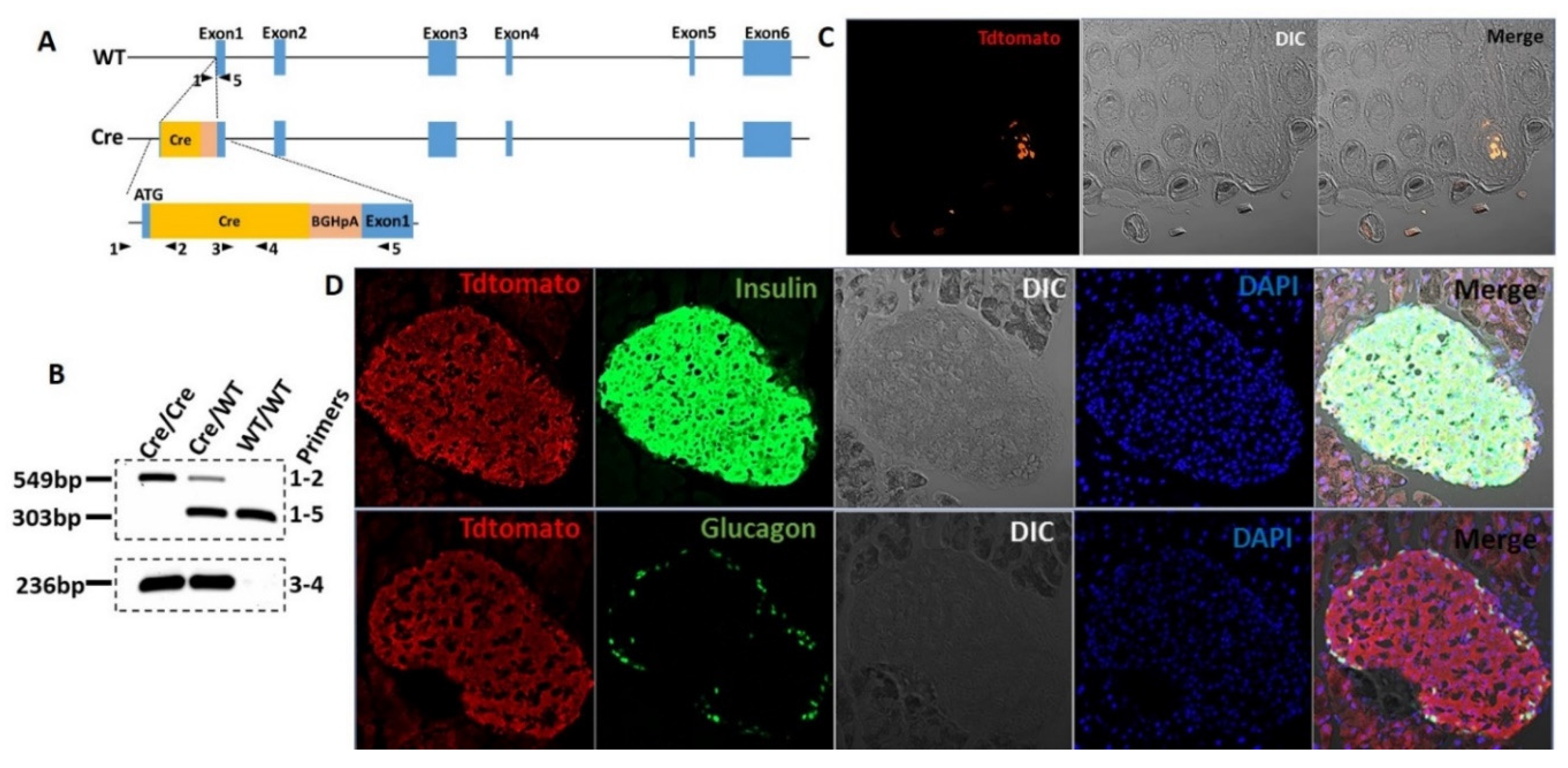
2.2. T1r2-Cre Knock-in (KI) Mouse
2.3. T1R2 Reporter Mice
2.4. Cell Culture
2.5. In Vivo Experiments
2.6. Static Insulin Secretion
2.7. Calcium Imaging
2.8. Total Internal Reflection (TIRF) Microscopy
2.9. Immunofluorescence Microscopy
2.10. Statistical Analysis
3. Results
3.1. The T1R2 Sweet Taste Receptor Is Expressed in Beta-Cells of Mouse Islets
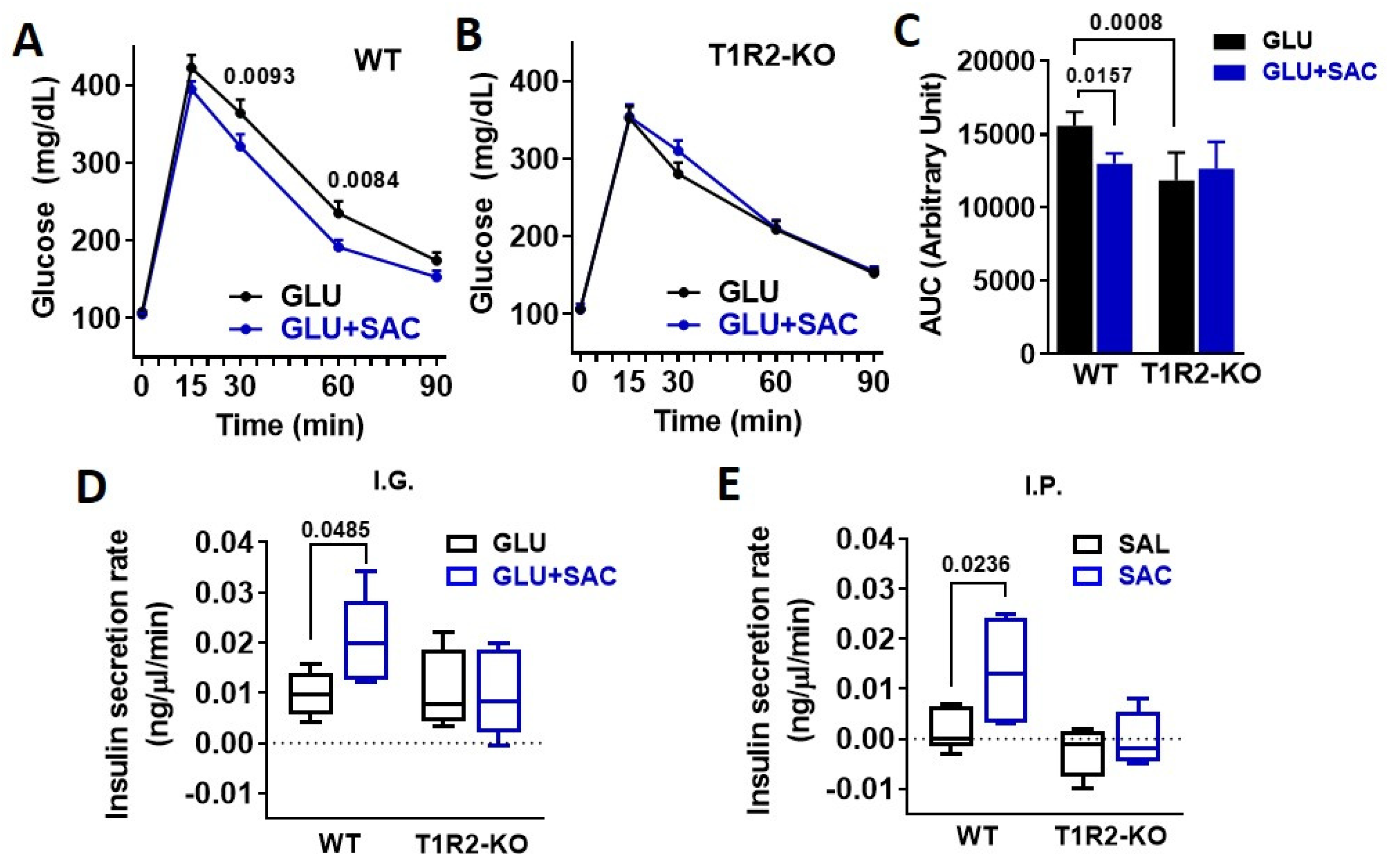
3.2. Saccharin Potentiates Insulin Secretion In Vivo Dependent on STR
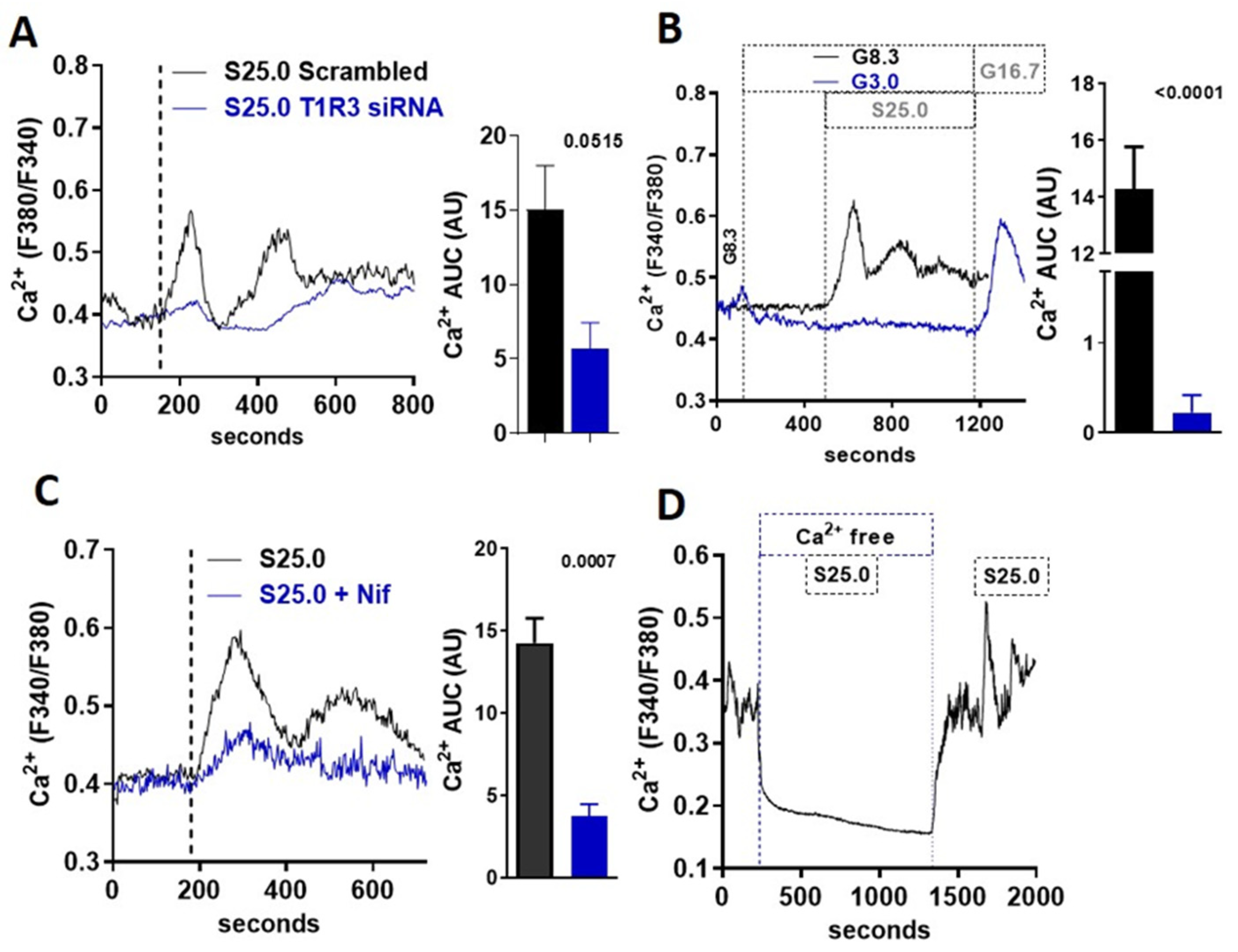
3.3. Saccharin-Induced Signaling Is Dependent on STR and Calcium Flux
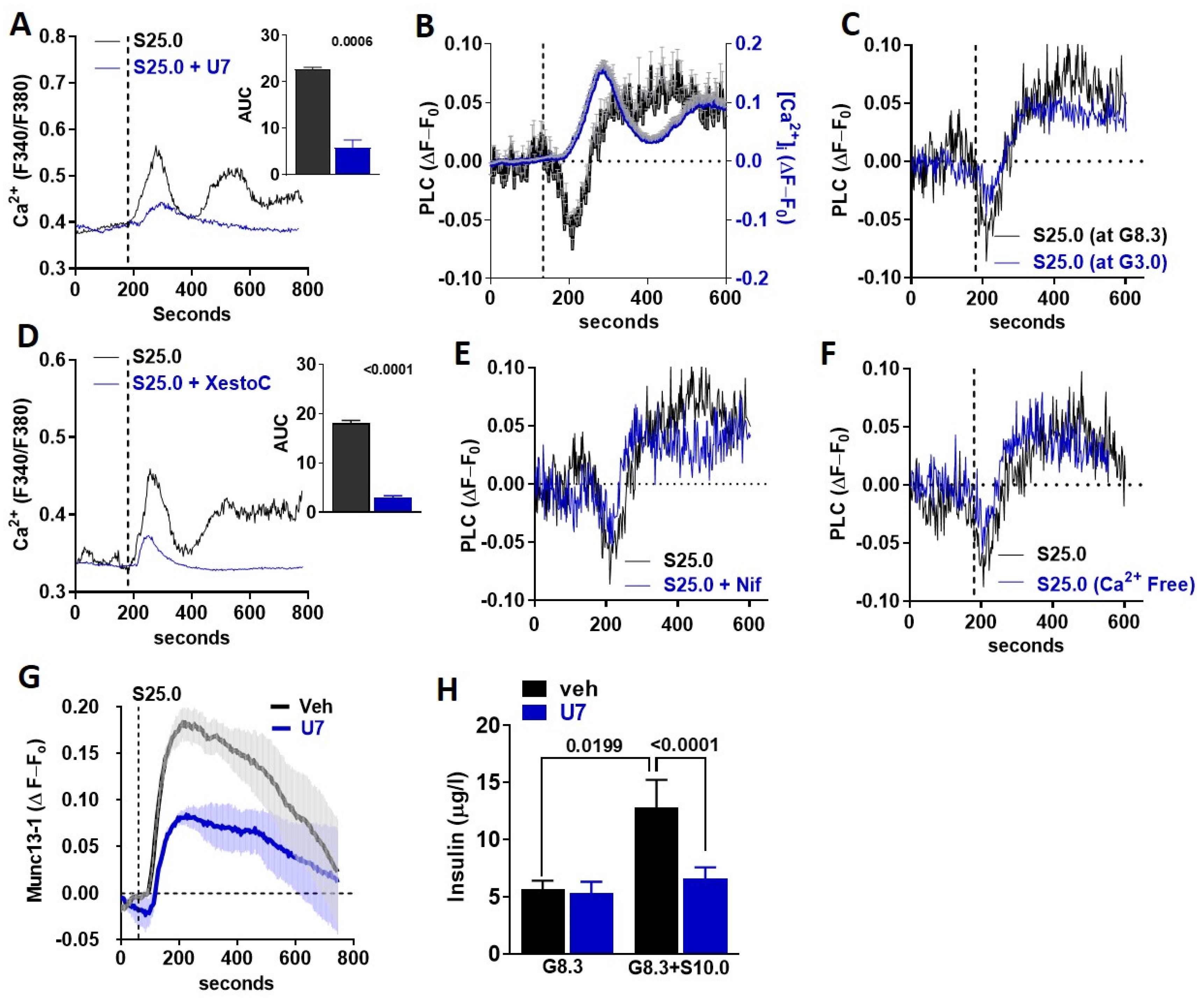
3.4. Saccharin Stimulates Insulin Secretion Dependent on PLC Activation
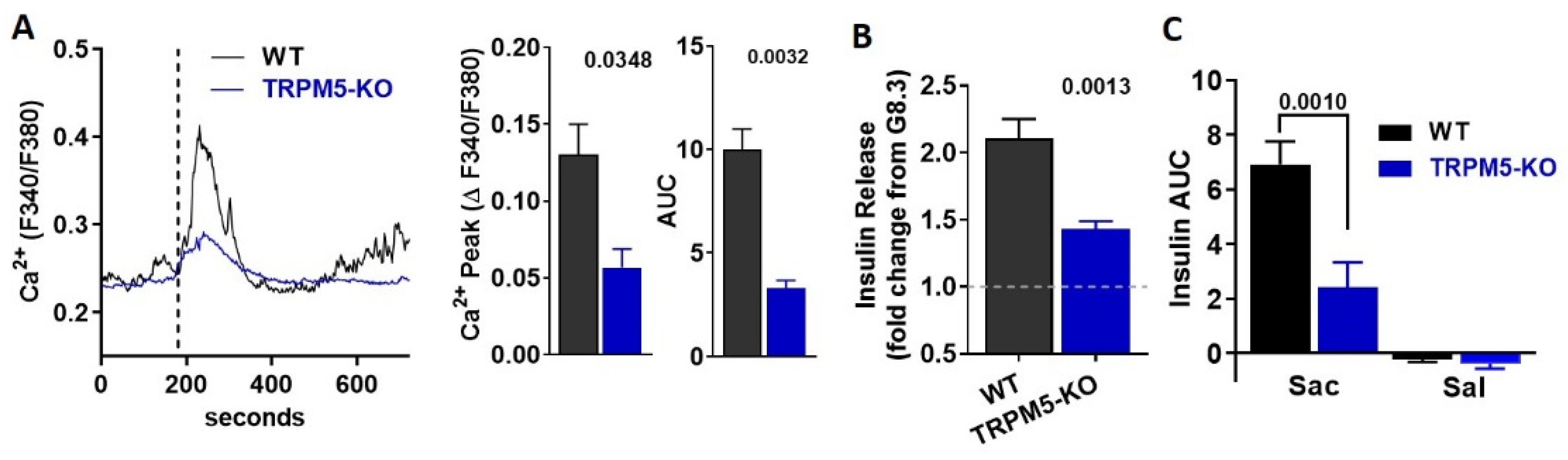
3.5. TRPM5 Mediates the Effects of Saccharin-Induced Activation of STR Signaling in Beta-Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sylvetsky, A.C.; Welsh, J.A.; Brown, R.J.; Vos, M.B. Low-calorie sweetener consumption is increasing in the United States. Am. J. Clin. Nutr. 2012, 96, 640–646. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Serrano, J.; Smith, K.R.; Crouch, A.L.; Sharma, V.; Yi, F.; Vargova, V.; LaMoia, T.E.; Dupont, L.M.; Serna, V.; Tang, F.; et al. High-dose saccharin supplementation does not induce gut microbiota changes or glucose intolerance in healthy humans and mice. Microbiome 2021, 9, 1–18. [Google Scholar]
- Higgins, K.A.; Mattes, R.D. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am. J. Clin. Nutr. 2019, 109, 1288–1301. [Google Scholar] [CrossRef]
- Cooper, P.; Wahlqvist, M.; Simpson, R. Sucrose Versus Saccharin as an Added Sweetener in Non-Insulin-Dependent Diabetes: Short- And Medium-Term Metabolic Effects. Diabet. Med. 1988, 5, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Malaisse, W.J.; Vanonderbergen, A.; Louchami, K.; Jijakli, H.; Malaisse-Lagae, F. Effects of artificial sweeteners on insulin release and cationic fluxes in rat pancreatic islets. Cell. Signal. 1998, 10, 727–733. [Google Scholar] [CrossRef]
- Malaisse, W.J.; Malaisse-Lagae, F. Bitter taste of monosaccharide pentaacetate esters. Biochem. Mol. Boil. Int. 1997, 43, 1367–1371. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nagasawa, M.; Yamada, S.; Hara, A.; Mogami, H.; Nikolaev, V.O.; Lohse, M.J.; Shigemura, N.; Ninomiya, Y.; Kojima, I. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE 2009, 4, e5106. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, G.A.; Soundarapandian, M.M.; Tyrberg, B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc. Natl. Acad. Sci. USA 2012, 109, E524–E532. [Google Scholar] [CrossRef]
- Smith, N.J.; Grant, J.N.; Moon, J.I.; So, S.S.; Finch, A.M. Critically evaluating sweet taste receptor expression and signaling through a molecular pharmacology lens. FEBS J. 2021, 288, 2660–2672. [Google Scholar] [CrossRef]
- Smith, K.; Karimian Azari, E.; LaMoia, T.E.; Hussain, T.; Vargova, V.; Karolyi, K.; Veldhuis, P.P.; Arnoletti, J.P.; de la Fuente, S.G.; Pratley, R.E.; et al. T1R2 receptor-mediated glucose sensing in the upper intestine potentiates glucose absorption through activation of local regulatory pathways. Mol. Metab. 2018, 17, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, J.; Hoon, M.A.; Ryba, N.J.; Zuker, C.S. The receptors and cells for mammalian taste. Nature 2006, 444, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.; Thompson, N.L.; Burghardt, T.P. Total internal inflection fluorescent microscopy. J. Microsc. 1983, 129, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Thore, S.; Wuttke, A.; Tengholm, A. Rapid turnover of phosphatidylinositol-4,5-bisphosphate in insulin-secreting cells mediated by Ca2+ and the ATP-to-ADP ratio. Diabetes 2007, 56, 818–826. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De, S.P.; Parys, J.B.; Callewaert, G.; Weidema, A.F.; Hill, E.; De, S.H.; Erneux, C.; Sorrentino, V.; Missiaen, L. Xestospongin C is an equally potent inhibitor of the inositol 1,4,5-trisphosphate receptor and the endoplasmic-reticulum Ca2+ pumps. Cell Calcium 1999, 26, 9–13. [Google Scholar]
- Kang, L.; He, Z.; Xu, P.; Fan, J.; Betz, A.; Brose, N.; Xu, T. Munc13-1 is required for the sustained release of insulin from pancreatic beta cells. Cell Metab. 2006, 3, 463–468. [Google Scholar] [CrossRef]
- Kwan, E.P.; Xie, L.; Sheu, L.; Nolan, C.J.; Prentki, M.; Betz, A.; Brose, N.; Gaisano, H.Y. Munc13-1 deficiency reduces insulin secretion and causes abnormal glucose tolerance. Diabetes 2006, 55, 1421–1429. [Google Scholar] [CrossRef]
- Kwan, E.P.; Xie, L.; Sheu, L.; Ohtsuka, T.; Gaisano, H.Y. Interaction between Munc13-1 and RIM is critical for glucagon-like peptide-1 mediated rescue of exocytotic defects in Munc13-1 deficient pancreatic beta-cells. Diabetes 2007, 56, 2579–2588. [Google Scholar] [CrossRef]
- Yuan, Q.X.; Teng, L.P.; Zhou, J.Y.; Liu, C.P.; Guo, J.; Liu, L.J.; De, W.; Liu, C. Characterization of Munc13-1 and insulin secretion during pancreatic development in rats. J. Endocrinol. Investig. 2008, 31, 630–635. [Google Scholar] [CrossRef]
- Zhao, S.; Mugabo, Y.; Iglesias, J.; Xie, L.; Delghingaro-Augusto, V.; Lussier, R.; Peyot, M.L.; Joly, E.; Taib, B.; Davis, M.A.; et al. α/β-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab. 2014, 19, 993–1007. [Google Scholar] [CrossRef]
- Sheu, L.; Pasyk, E.A.; Ji, J.; Huang, X.; Gao, X.; Varoqueaux, F.; Brose, N.; Gaisano, H.Y. Regulation of insulin exocytosis by Munc13-1. J. Biol. Chem. 2003, 278, 27556–27563. [Google Scholar] [CrossRef]
- Friedrich, R.; Gottfried, I.; Ashery, U. Munc13-1 Translocates to the Plasma Membrane in a Doc2B- and Calcium-Dependent Manner. Front. Endocrinol. 2013, 4, 119. [Google Scholar] [CrossRef]
- Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Mueller, K.L.; Cook, B.; Wu, D.; Zuker, C.S.; Ryba, N.J. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell 2003, 112, 293–301. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Margolskee, R.; Liman, E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J. Neurosci. 2007, 27, 5777–5786. [Google Scholar] [CrossRef]
- Colsoul, B.; Schraenen, A.; Lemaire, K.; Quintens, R.; Van Lommel, L.; Segal, A.; Owsianik, G.; Talavera, K.; Voets, T.; Margolskee, R.F.; et al. Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5-/- mice. Proc. Natl. Acad. Sci. USA 2010, 107, 5208–5213. [Google Scholar] [CrossRef]
- Torres, N.; Noriega, L.; Tovar, A.R. Nutrient modulation of insulin secretion. Vitam. Horm. 2009, 80, 217–244. [Google Scholar]
- Bailey, C.J.; Day, C.; Knapper, J.M.; Turner, S.L.; Flatt, P.R. Antihyperglycaemic effect of saccharin in diabetic ob/ob mice. Br. J. Pharmacol. 1997, 120, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B.; Gregersen, S.; Poulsen, C.R.; Hermansen, K. Stevioside acts directly on pancreatic beta cells to secrete insulin: Actions independent of cyclic adenosine monophosphate and adenosine triphosphate-sensitive K+-channel activity. Metabolism 2000, 49, 208–214. [Google Scholar] [CrossRef]
- Liang, Y.; Maier, V.; Steinbach, G.; Lalic, L.; Pfeiffer, E.F. The effect of artificial sweetener on insulin secretion. II. Stimulation of insulin release from isolated rat islets by Acesulfame K (in vitro experiments). Horm. Metab. Res. 1987, 19, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Steinbach, G.; Maier, V.; Pfeiffer, E.F. The effect of artificial sweetener on insulin secretion. 1. The effect of acesulfame K on insulin secretion in the rat (studies in vivo). Horm. Metab. Res. 1987, 19, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.; Zuker, C.S. Mammalian sweet taste receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Erlenbach, I.; Ryba, N.J.; Zuker, C.S. The receptors for mammalian sweet and umami taste. Cell 2003, 115, 255–266. [Google Scholar] [CrossRef]
- Renwick, A.G. The disposition of saccharin in animals and man—A review. Food Chem. Toxicol. 1985, 23, 429–435. [Google Scholar] [CrossRef]
- Teff, K.L.; Devine, J.; Engelman, K. Sweet taste: Effect on cephalic phase insulin release in men. Physiol. Behav. 1995, 57, 1089–1095. [Google Scholar] [CrossRef]
- Just, T.; Pau, H.W.; Engel, U.; Hummel, T. Cephalic phase insulin release in healthy humans after taste stimulation? Appetite 2008, 51, 622–627. [Google Scholar] [CrossRef]
- Kokrashvili, Z.; Mosinger, B.; Margolskee, R.F. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann. N. Y. Acad. Sci. 2009, 1170, 91–94. [Google Scholar] [CrossRef]
- Karimian Azari, E.; Smith, K.R.; Yi, F.; Osborne, T.F.; Bizzotto, R.; Mari, A.; Pratley, R.E.; Kyriazis, G.A. Inhibition of sweet chemosensory receptors alters insulin responses during glucose ingestion in healthy adults: A randomized crossover interventional study. Am. J. Clin. Nutr. 2017, 105, 1001–1009. [Google Scholar] [CrossRef]
- Thompson, M.M.; Mayer, J. Hypoglycemic effects of saccharin in experimental animals. Am. J. Clin. Nutr. 1959, 7, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Pacini, G.; Ahren, B.; D’Argenio, D.Z. Glucagon increases insulin levels by stimulating insulin secretion without effect on insulin clearance in mice. Peptides 2017, 88, 74–79. [Google Scholar] [CrossRef]
- Thorens, B. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes. Metab. 2011, 13 (Suppl. 1), 82–88. [Google Scholar] [CrossRef]
- Serrano, J.; Seflova, J.; Park, J.; Pribadi, M.; Sanematsu, K.; Shigemura, N.; Serna, V.; Yi, F.; Mari, A.; Procko, E.; et al. The Ile191Val is a partial loss-of-function variant of the TAS1R2 sweet-taste receptor and is associated with reduced glucose excursions in humans. Mol. Metab. 2021, 54, 101339. [Google Scholar] [CrossRef] [PubMed]
- Damak, S.; Rong, M.; Yasumatsu, K.; Kokrashvili, Z.; Varadarajan, V.; Zou, S.; Jiang, P.; Ninomiya, Y.; Margolskee, R.F. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 2003, 301, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Damak, S.; Rong, M.; Yasumatsu, K.; Kokrashvili, Z.; Perez, C.A.; Shigemura, N.; Yoshida, R.; Mosinger, B., Jr.; Glendinning, J.I.; Ninomiya, Y.; et al. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem. Senses 2006, 31, 253–264. [Google Scholar] [CrossRef]
- Luttrell, L.M.; Maudsley, S.; Bohn, L.M. Fulfilling the Promise of “Biased” G Protein-Coupled Receptor Agonism. Mol. Pharmacol. 2015, 88, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.L.; Kalwat, M.A.; McGlynn, K.; Cobb, M.H. Sucralose activates an ERK1/2-ribosomal protein S6 signaling axis. FEBS Open Bio 2017, 7, 174–186. [Google Scholar] [CrossRef]
- Bauer, C.S.; Woolley, R.J.; Teschemacher, A.G.; Seward, E.P. Potentiation of exocytosis by phospholipase C-coupled G-protein-coupled receptors requires the priming protein Munc13-1. J. Neurosci. 2007, 27, 212–219. [Google Scholar] [CrossRef]
- Martin, R.; Durroux, T.; Ciruela, F.; Torres, M.; Pin, J.P.; Sanchez-Prieto, J. The metabotropic glutamate receptor mGlu7 activates phospholipase C, translocates munc-13-1 protein, and potentiates glutamate release at cerebrocortical nerve terminals. J. Biol. Chem. 2010, 285, 17907–17917. [Google Scholar] [CrossRef]
- Enklaar, T.; Brixel, L.R.; Zabel, B.U.; Prawitt, D. Adding efficiency: The role of the CAN ion channels TRPM4 and TRPM5 in pancreatic islets. Islets 2010, 2, 337–338. [Google Scholar] [CrossRef]
- Cheng, H.; Beck, A.; Launay, P.; Gross, S.A.; Stokes, A.J.; Kinet, J.P.; Fleig, A.; Penner, R. TRPM4 controls insulin secretion in pancreatic beta-cells. Cell Calcium. 2007, 41, 51–61. [Google Scholar] [CrossRef]
- Marigo, V.; Courville, K.; Hsu, W.H.; Feng, J.M.; Cheng, H. TRPM4 impacts on Ca2+ signals during agonist-induced insulin secretion in pancreatic beta-cells. Mol. Cell. Endocrinol. 2009, 299, 194–203. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, J.; Meshram, N.N.; Soundarapandian, M.M.; Smith, K.R.; Mason, C.; Brown, I.S.; Tyrberg, B.; Kyriazis, G.A. Saccharin Stimulates Insulin Secretion Dependent on Sweet Taste Receptor-Induced Activation of PLC Signaling Axis. Biomedicines 2022, 10, 120. https://doi.org/10.3390/biomedicines10010120
Serrano J, Meshram NN, Soundarapandian MM, Smith KR, Mason C, Brown IS, Tyrberg B, Kyriazis GA. Saccharin Stimulates Insulin Secretion Dependent on Sweet Taste Receptor-Induced Activation of PLC Signaling Axis. Biomedicines. 2022; 10(1):120. https://doi.org/10.3390/biomedicines10010120
Chicago/Turabian StyleSerrano, Joan, Nishita N. Meshram, Mangala M. Soundarapandian, Kathleen R. Smith, Carter Mason, Ian S. Brown, Björn Tyrberg, and George A. Kyriazis. 2022. "Saccharin Stimulates Insulin Secretion Dependent on Sweet Taste Receptor-Induced Activation of PLC Signaling Axis" Biomedicines 10, no. 1: 120. https://doi.org/10.3390/biomedicines10010120
APA StyleSerrano, J., Meshram, N. N., Soundarapandian, M. M., Smith, K. R., Mason, C., Brown, I. S., Tyrberg, B., & Kyriazis, G. A. (2022). Saccharin Stimulates Insulin Secretion Dependent on Sweet Taste Receptor-Induced Activation of PLC Signaling Axis. Biomedicines, 10(1), 120. https://doi.org/10.3390/biomedicines10010120





