Artificial-Intelligence-Assisted Discovery of Genetic Factors for Precision Medicine of Antiplatelet Therapy in Diabetic Peripheral Artery Disease
Abstract
1. Introduction
2. Materials and Methods
3. Results
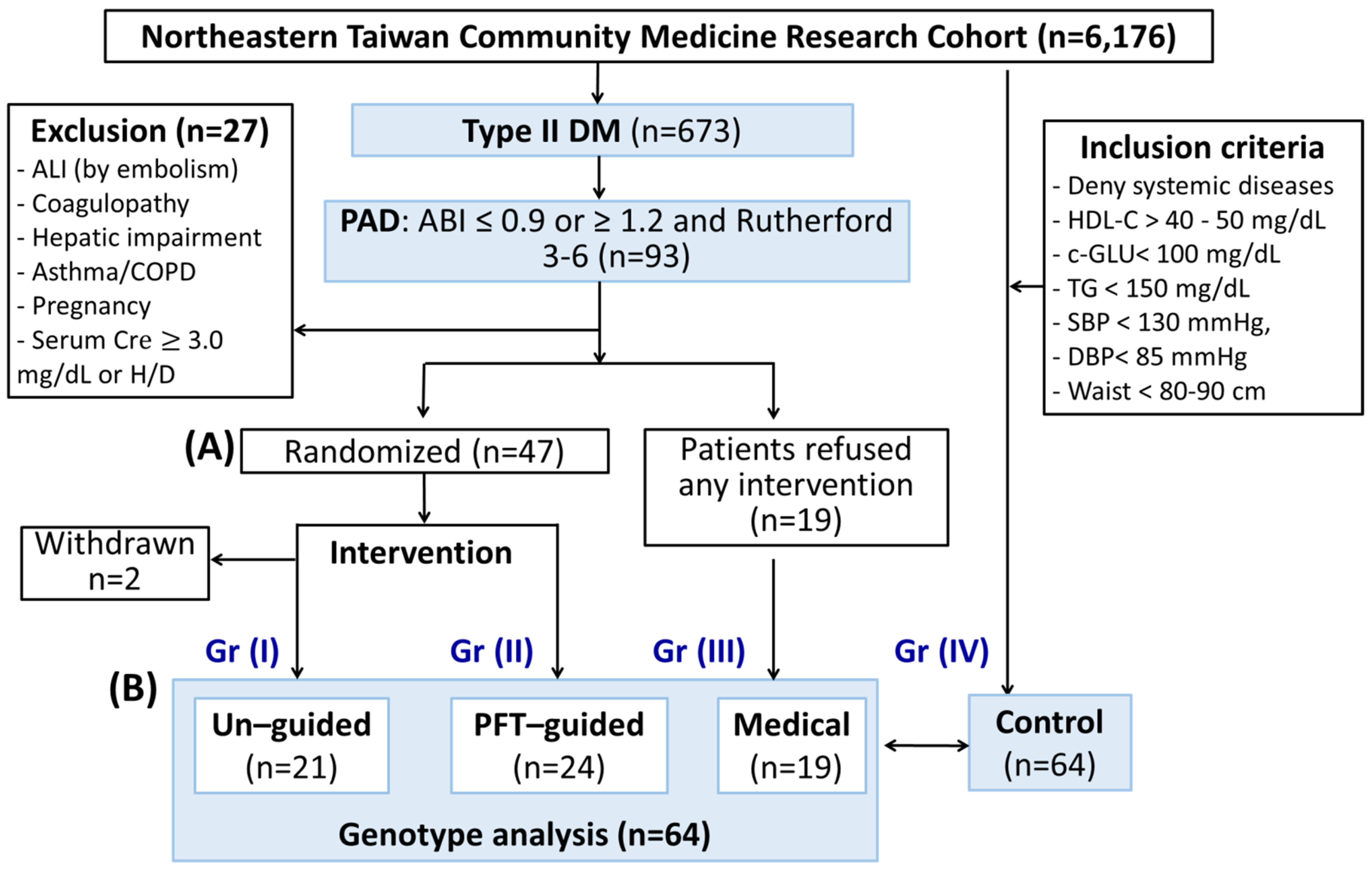
3.1. Subject Enrollment and Clinical Outcomes
3.1.1. Baseline Characteristics
3.1.2. Platelet Reactivity
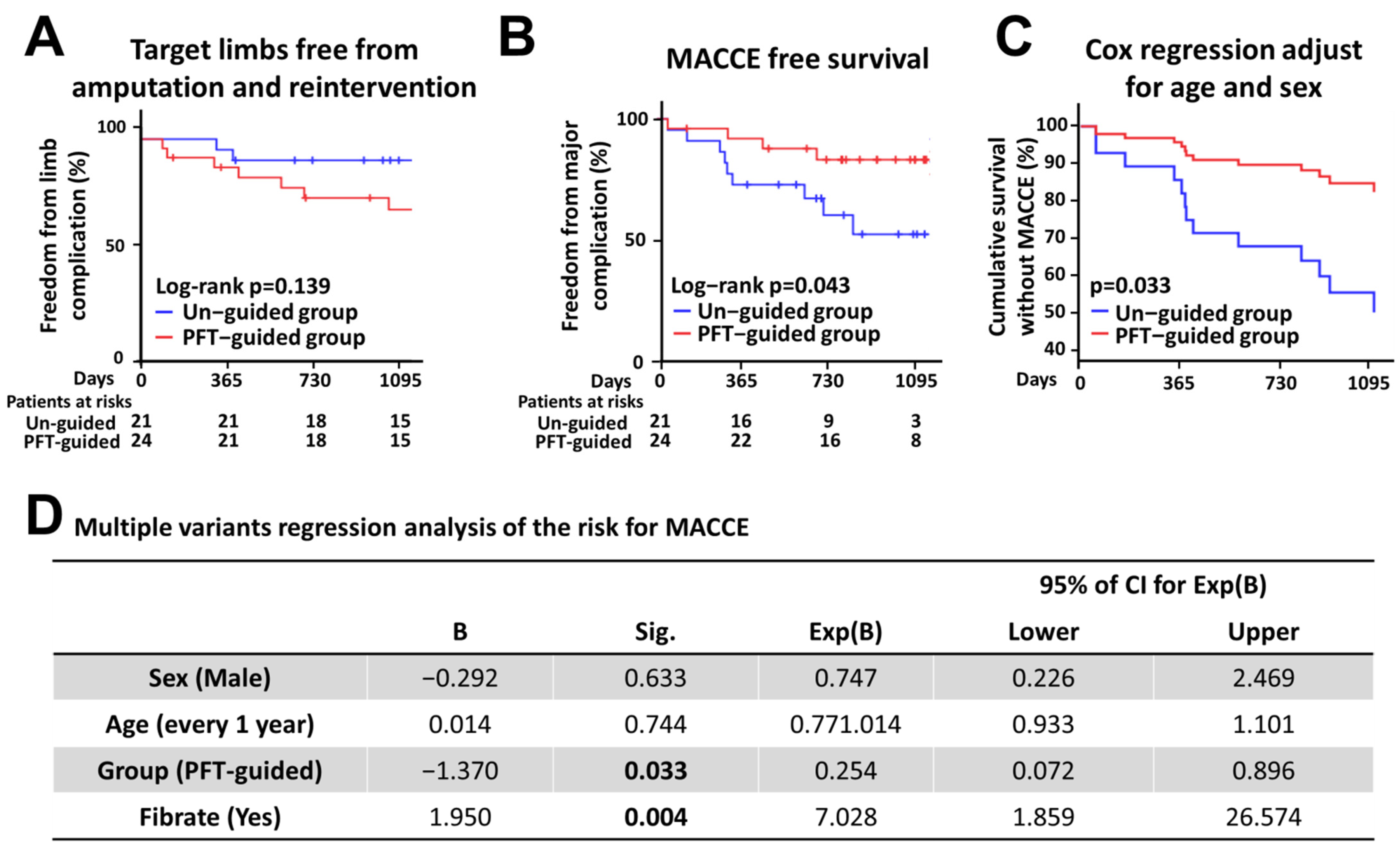
3.1.3. Clinical Outcomes
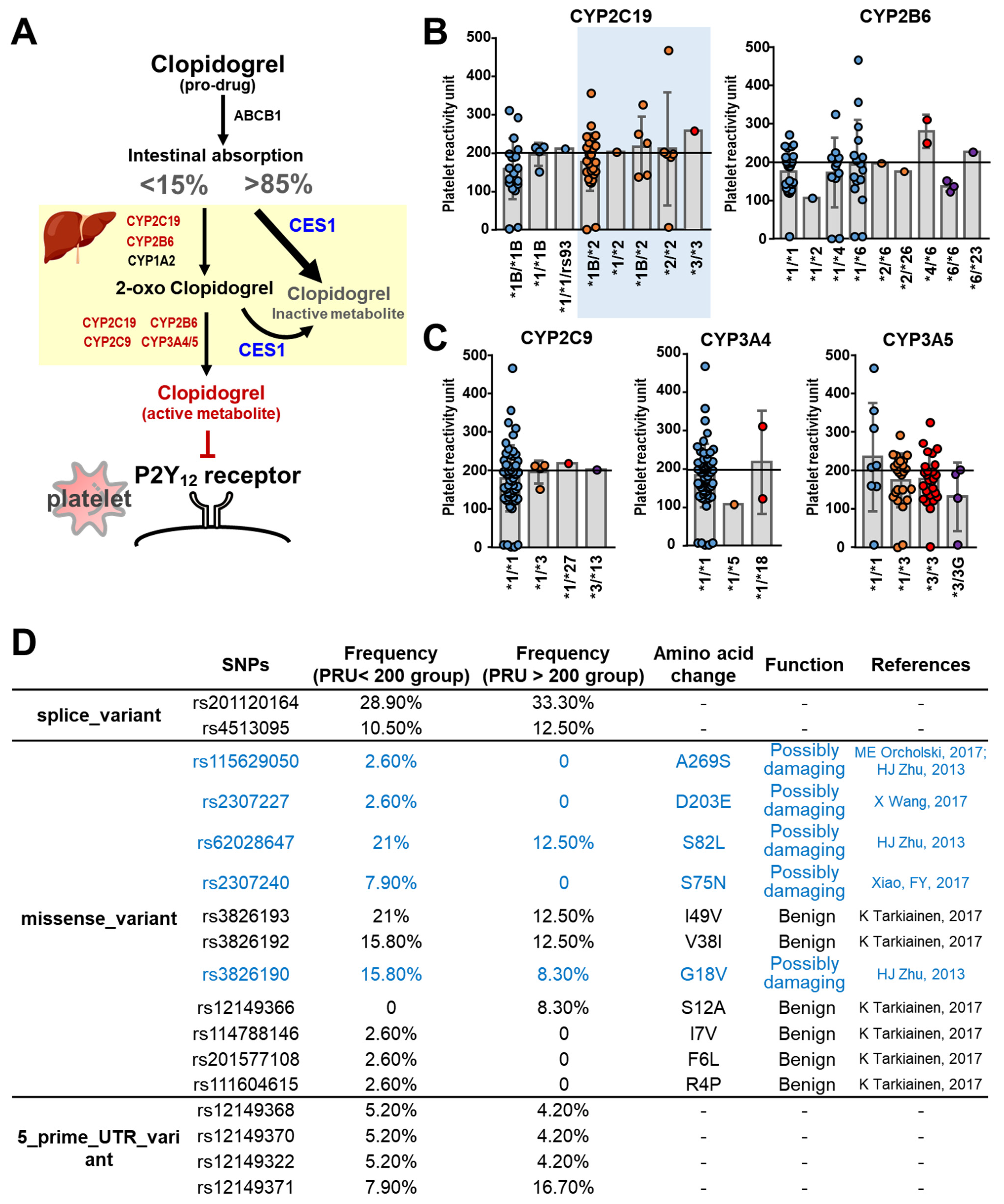
3.2. Pharmacogenetic Analyses of the Clopidogrel Metabolic Pathways
3.2.1. Cytochrome P450
3.2.2. CES1
3.2.3. P2Y12 Receptor
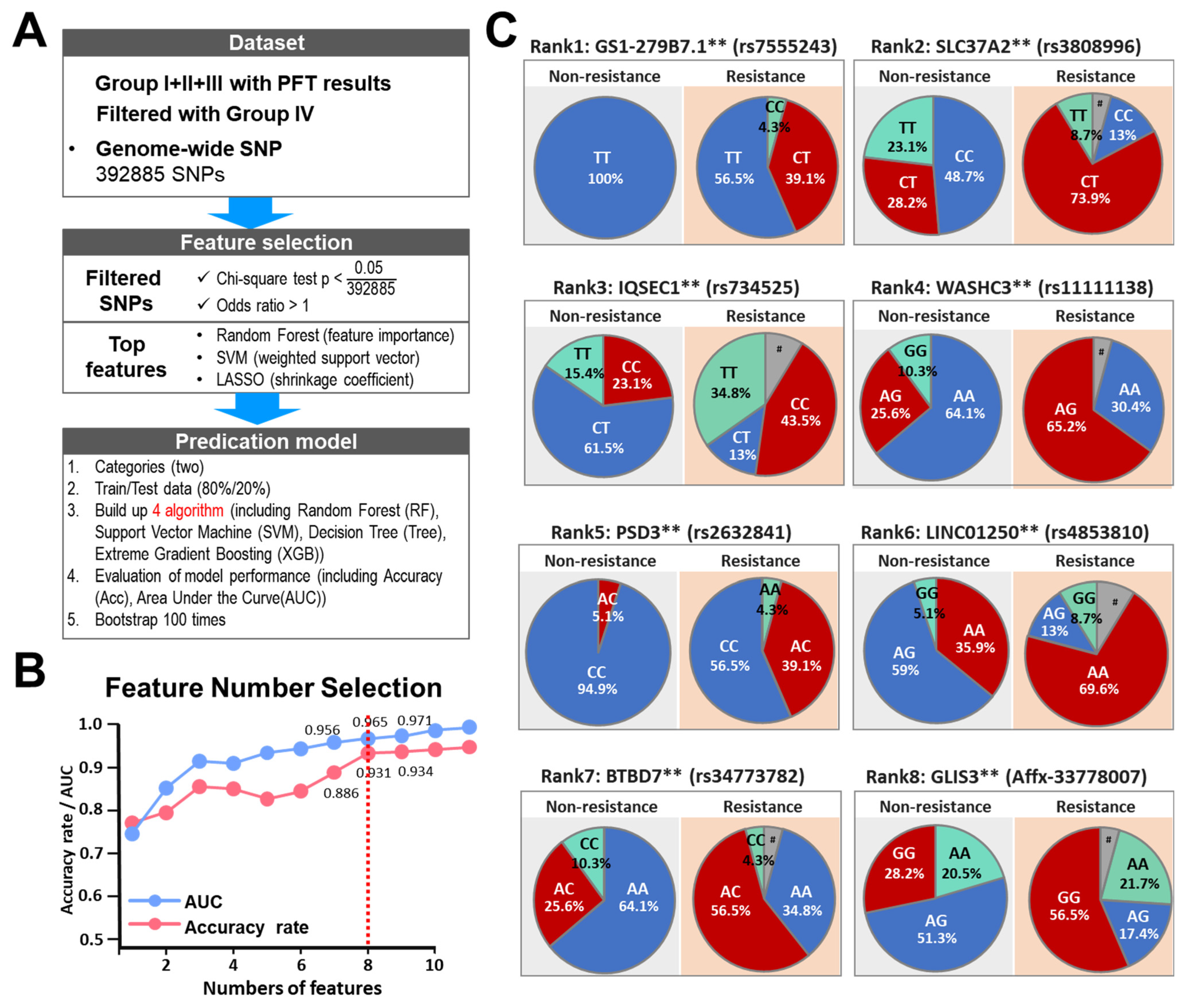
3.3. AI-Assisted Identification of SNP Signatures That Predispose towards Clopidogrel Resistance
4. Discussion
4.1. The Prognosis of Clopidogrel-Resistant PAD Patients
4.2. Ticagrelor Dosage
4.3. Cost-Effectiveness of Genotyping-Guided DAPT
4.4. Precision Medicine for DAPT: Resensitization of the P2Y12 Receptor
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABI | Ankle-brachial index |
| ACT | Activated clotting time |
| AI | Artificial intelligence |
| AUC | Area under curve |
| CES1 | Carboxylesterase 1 |
| DAPT | Dual anti-platelet therapy |
| EUCLID | Effects of Ticagrelor and Clopidogrel in Patients with Peripheral Artery Disease |
| IRB | Institutional Review Board |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| MACCEs | Major adverse cerebrovascular and cardiac events |
| NTCMRC | Northeastern Taiwan Community Medicine Research Cohort |
| PAD | Peripheral artery disease |
| PFT | Platelet function test |
| PRU | Platelet reactivity in units |
| RF | Random Forest |
| SNP | Single-nucleotide polymorphism |
| SVM | Support Vector Machine |
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Hao, G.; Chen, Z.; Zhang, L.; Shao, L.; Tian, Y.; Dong, Y.; Zheng, C.; Kang, Y.; et al. A national study of the prevalence and risk factors associated with peripheral arterial disease from China: The China Hypertension Survey, 2012. Int. J. Cardiol. 2019, 275, 165–170. [Google Scholar] [CrossRef]
- Johnston, L.E.; Stewart, B.T.; Yangni-Angate, H.; Veller, M.; Upchurch, G.R., Jr.; Gyedu, A.; Kushner, A.L. Peripheral Arterial Disease in Sub-Saharan Africa: A Review. JAMA Surg. 2016, 151, 564–572. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. Microvascular complications and foot care: Standards of medical care in diabetes. Diabetes Care 2021, 44, S151–S167. [Google Scholar] [CrossRef]
- Strobl, F.F.; Brechtel, K.; Schmehl, J.; Zeller, T.; Reiser, M.F.; Claussen, C.D.; Tepe, G. Twelve-Month Results of a Randomized Trial Comparing Mono With Dual Antiplatelet Therapy in Endovascularly Treated Patients With Peripheral Artery Disease. J. Endovasc. Ther. 2013, 20, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, E.; Gori, A.M.; Giusti, B.; Valenti, R.; Migliorini, A.; Basili, S.; Paniccia, R.; Elmahdy, M.F.; Pulli, R.; Pratesi, C.; et al. On-Treatment Platelet Reactivity is a Predictor of Adverse Events in Peripheral Artery Disease Patients Undergoing Percutaneous Angioplasty. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 545–552. [Google Scholar] [CrossRef]
- Navarese, E.P.; Wernly, B.; Lichtenauer, M.; Petrescu, A.M.; Kołodziejczak, M.; Lauten, A.; Frediani, L.; Veulemanns, V.; Wanha, W.; Wojakowski, W.; et al. Dual vs single antiplatelet therapy in patients with lower extremity peripheral artery disease—A meta-analysis. Int. J. Cardiol. 2018, 269, 292–297. [Google Scholar] [CrossRef]
- Tsigkou, V.; Siasos, G.; Rovos, K.; Tripyla, N.; Tousoulis, D. Peripheral artery disease and antiplatelet treatment. Curr. Opin. Pharmacol. 2018, 39, 43–52. [Google Scholar] [CrossRef]
- Wang, T.H.; Bhatt, D.L.; Fox, K.A.; Steinhubl, S.R.; Brennan, D.M.; Hacke, W.; Mak, K.-H.; Pearson, T.A.; Boden, W.E.; Steg, P.G.; et al. An analysis of mortality rates with dual-antiplatelet therapy in the primary prevention population of the CHARISMA trial. Eur. Heart J. 2007, 28, 2200–2207. [Google Scholar] [CrossRef]
- Ang, L.; Palakodeti, V.; Khalid, A.; Tsimikas, S.; Idrees, Z.; Tran, P.; Clopton, P.; Zafar, N.; Bromberg-Marin, G.; Keramati, S.; et al. Elevated Plasma Fibrinogen and Diabetes Mellitus Are Associated With Lower Inhibition of Platelet Reactivity With Clopidogrel. J. Am. Coll. Cardiol. 2008, 52, 1052–1059. [Google Scholar] [CrossRef]
- Hess, C.N.; Norgren, L.; Ansel, G.M.; Capell, W.H.; Fletcher, J.P.; Fowkes, F.G.R.; Gottsater, A.; Hitos, K.; Jaff, M.R.; Nordanstig, J.; et al. A Structured review of antithrombotic therapy in peripheral artery disease with a focus on revascularization: A TASC (InterSociety Consensus for the Management of Peripheral Artery Disease) Initiative. Circulation 2017, 135, 2534–2555. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.R.; Fowkes, F.G.R.; Heizer, G.; Berger, J.S.; Baumgartner, I.; Held, P.; Katona, B.G.; Mahaffey, K.W.; Norgren, L.; Jones, W.S.; et al. Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease. N. Engl. J. Med. 2017, 376, 32–40. [Google Scholar] [CrossRef]
- Lewis, J.P.; Backman, J.D.; Reny, J.-L.; Bergmeijer, T.O.; Mitchell, B.D.; Ritchie, M.D.; Déry, J.-P.; Pakyz, R.E.; Gong, L.; Ryan, K.; et al. Pharmacogenomic polygenic response score predicts ischaemic events and cardiovascular mortality in clopidogrel-treated patients. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 203–210. [Google Scholar] [CrossRef]
- Martis, S.; Peter, I.; Hulot, J.-S.; Kornreich, R.; Desnick, R.J.; Scott, S.A. Multi-ethnic distribution of clinically relevant CYP2C genotypes and haplotypes. Pharmacogenom. J. 2013, 13, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Guirgis, M.; Thompson, P.; Jansen, S. Review of aspirin and clopidogrel resistance in peripheral arterial disease. J. Vasc. Surg. 2017, 66, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.S.; Bergmeijer, T.; Gong, L.; Reny, J.; Lewis, J.P.; Mitchell, B.D.; Alexopoulos, D.; Aradi, D.; Altman, R.B.; Bliden, K.; et al. Genomewide Association Study of Platelet Reactivity and Cardiovascular Response in Patients Treated With Clopidogrel: A Study by the International Clopidogrel Pharmacogenomics Consortium. Clin. Pharmacol. Ther. 2020, 108, 1067–1077. [Google Scholar] [CrossRef]
- Bachtiar, M.; Jin, Y.; Wang, J.; Tan, T.W.; Chong, S.S.; Ban, K.H.K.; Lee, C.G.L. Architecture of population-differentiated polymorphisms in the human genome. PLoS ONE 2019, 14, e0224089. [Google Scholar] [CrossRef]
- Bachtiar, M.; Ooi, B.N.S.; Wang, J.; Jin, Y.; Tan, T.W.; Chong, S.S.; Lee, C.G.L. Towards precision medicine: Interrogating the human genome to identify drug pathways associated with potentially functional, population-differentiated polymorphisms. Pharm. J. 2019, 19, 516–527. [Google Scholar] [CrossRef]
- Ducci, K.; Liistro, F.; Porto, I.; Ventoruzzo, G.; Angioli, P.; Falsini, G.; Vergallo, R.; Bolognese, L. Ticagrelor versus clopidogrel in patients undergoing implantation of paclitaxel-eluting stent in the femoropopliteal district: A randomized pilot study using frequency-domain optical coherence tomography. Int. J. Cardiol. 2020, 304, 192–197. [Google Scholar] [CrossRef]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Aradi, D.; Jacobshagen, C.; Gross, L.; Trenk, D.; Geisler, T.; Orban, M.; Hadamitzky, M.; Merkely, B.; Kiss, R.G.; et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): A randomised, open-label, multicentre trial. Lancet 2017, 390, 1747–1757. [Google Scholar] [CrossRef]
- Notarangelo, F.M.; Maglietta, G.; Bevilacqua, P.; Cereda, M.; Merlini, P.A.; Villani, G.Q.; Moruzzi, P.; Patrizi, G.; Malagoli Tagliazucchi, G.; Crocamo, A.; et al. Pharmacogenomic approach to selecting antiplatelet therapy in patients with acute coronary syndromes: The PHARMCLO Trial. J. Am. Coll. Cardiol. 2018, 71, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- So, D.Y.F.; Wells, G.A.; McPherson, R.; Labinaz, M.; Le May, M.R.; Glover, C.; Dick, A.J.; Froeschl, M.; Marquis, J.-F.; On behalf of the CAPITAL investigators; et al. A prospective randomized evaluation of a pharmacogenomic approach to antiplatelet therapy among patients with ST-elevation myocardial infarction: The RAPID STEMI study. Pharm. J. 2016, 16, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Galea, S.; Abdalla, S.M. Precision Medicine Approaches and the Health of Populations: Study Design Concerns and Considerations. Perspect. Biol. Med. 2018, 61, 527–536. [Google Scholar] [CrossRef]
- Gim, J.A.; Kwon, Y.; Lee, H.A.; Lee, K.R.; Kim, S.; Choi, Y.; Kim, Y.K.; Lee, H. A Machine Learning-based identification of genes affecting the pharmacokinetics of tacrolimus using the DMET(TM) plus platform. Int. J. Mol. Sci. 2020, 21, 2517. [Google Scholar] [CrossRef]
- Podda, G.; Grossi, E.; Palmerini, T.; Buscema, P.M.; Femia, E.A.; Della Riva, D.; de Servi, S.; Calabrò, P.; Piscione, F.; Maffeo, D.; et al. Prediction of high on-treatment platelet reactivity in clopidogrel-treated patients with acute coronary syndromes. Int. J. Cardiol. 2017, 240, 60–65. [Google Scholar] [CrossRef]
- Hardman, R.L.; Jazaeri, O.; Yi, J.; Smith, M.; Gupta, R. Overview of Classification Systems in Peripheral Artery Disease. Semin. Interv. Radiol. 2014, 31, 378–388. [Google Scholar] [CrossRef]
- Garabedian, T.; Alam, S. High residual platelet reactivity on clopidogrel: Its significance and therapeutic challenges overcoming clopidogrel resistance. Cardiovasc. Diagn. Ther. 2013, 3, 23–37. [Google Scholar] [CrossRef]
- Price, M.J.; Endemann, S.; Gollapudi, R.R.; Valencia, R.; Stinis, C.T.; Levisay, J.P.; Ernst, A.; Sawhney, N.S.; Schatz, R.A.; Teirstein, P.S. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur. Heart J. 2008, 29, 992–1000. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, N.; Tai, H.; Juang, J.J.; Wu, C.; Lin, L.; Hwang, J.; Lin, J.; Chiang, F.; Tsai, C. CYP2C19 Polymorphism is Associated With Amputation Rates in Patients Taking Clopidogrel After Endovascular Intervention for Critical Limb Ischaemia. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.; Ghouri, M.A.; Kovacs, F. Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database Syst. Rev. 2012, 2012, CD002071. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulos, S.; Pastromas, G.; Katsanos, K.; Kitrou, P.; Karnabatidis, D.; Siablis, D. Platelet responsiveness to clopidogrel treatment after peripheral endovascular procedures—The preclop study: Clinical impact and optimal cut-off value of high on treatment platelet reactivity. J. Vasc. Interv. Radiol. 2014, 25, S92. [Google Scholar] [CrossRef]
- Thott, O.; Granath, F.; Malmstedt, J.; Wahlgren, C.M. Editor’s Choice—Dual antiplatelet therapy improves outcome in diabetic patients undergoing endovascular femoropopliteal stenting for critical limb ischaemia. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 403–410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, S.; Agafonov, O.; Azab, A.; Stokowy, T.; Hovig, E. Accuracy and efficiency of germline variant calling pipelines for human genome data. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Numanagić, I.; Malikić, S.; Ford, M.; Qin, X.; Toji, L.; Radovich, M.; Skaar, T.C.; Pratt, V.M.; Berger, B.; Scherer, S.; et al. Allelic decomposition and exact genotyping of highly polymorphic and structurally variant genes. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Lin, J.-C.; Chen, L.-K.; Hsiao, W.W.-W.; Fan, C.-T.; Ko, M.L. Next Chapter of the Taiwan Biobank: Sustainability and Perspectives. Biopreservation Biobanking 2019, 17, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.J.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J.; Wang, X.; Gawronski, B.E.; Brinda, B.J.; Angiolillo, D.J.; Markowitz, J.S. Carboxylesterase 1 as a determinant of clopidogrel metabolism and activation. J. Pharmacol. Exp. Ther. 2013, 344, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, F.; Sundararajan, S. Use of Dual Antiplatelet Therapy Following Ischemic Stroke. Stroke 2020, 51, e78–e80. [Google Scholar] [CrossRef] [PubMed]
- Markus, H.S.; Droste, D.W.; Kaps, M.; Larrue, V.; Lees, K.R.; Siebler, M.; Ringelstein, E.B. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: The Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation 2005, 111, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Pogue, J.; Hart, R.G.; Hohnloser, S.H.; Pfeffer, M.A.; Chrolavicius, S.; Yusuf, S.; Fox, K.A. Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 360, 2066–2078. [Google Scholar] [CrossRef]
- Alberts, M.J.; Bhatt, D.L.; Mas, J.-L.; Ohman, E.M.; Hirsch, A.T.; Röther, J.; Salette, G.; Goto, S.; Smith, S.C.; Liau, C.-S.; et al. Three-year follow-up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur. Heart J. 2009, 30, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Ynsaurriaga, F.A.; Barrios, V.; Amaro, M.B.; Martí-Almor, J.; Martínez, J.G.; Duque, J.A.; Ruiz-Ortiz, M.; Vázquez-García, R.; Muñoz, A.V. Chronic Coronary Syndrome: Overcoming Clinical Practice Guidelines. The role of the COMPASS Strategy. Curr. Cardiol. Rev. 2021, 17, 294–305. [Google Scholar] [CrossRef]
- Orme, R.C.; Parker, W.A.E.; Thomas, M.R.; Judge, H.M.; Baster, K.; Sumaya, W.; Morgan, K.P.; McMellon, H.C.; Richardson, J.D.; Grech, E.D.; et al. Study of two dose regimens of ticagrelor compared with clopidogrel in patients undergoing percutaneous coronary intervention for stable coronary artery disease (STEEL-PCI). Circulation 2018, 138, 1290–1300. [Google Scholar] [CrossRef]
- Choi, K.-N.; Jin, H.-Y.; Shin, H.-C.; Park, Y.-A.; Seo, J.-S.; Jang, J.-S.; Yang, T.-H.; Kim, D.-K.; Kim, D.-S. Comparison of the Antiplatelet Effects of Once and Twice Daily Low-Dose Ticagrelor and Clopidogrel After Percutaneous Coronary Intervention. Am. J. Cardiol. 2017, 120, 201–206. [Google Scholar] [CrossRef]
- Wang, H.; Qi, J.; Li, Y.; Tang, Y.; Li, C.; Li, J.; Han, Y. Pharmacodynamics and pharmacokinetics of ticagrelor vs. clopidogrel in patients with acute coronary syndromes and chronic kidney disease. Br. J. Clin. Pharmacol. 2017, 84, 88–96. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Storey, R.; Steg, P.G.; Cohen, M.; Kuder, J.; Goodrich, E.; Nicolau, J.; Parkhomenko, A.; López-Sendón, J.; et al. Ticagrelor for Prevention of Ischemic Events After Myocardial Infarction in Patients With Peripheral Artery Disease. J. Am. Coll. Cardiol. 2016, 67, 2719–2728. [Google Scholar] [CrossRef]
- Guo, B.; Tan, Q.; Guo, D.; Shi, Z.; Zhang, C.; Guo, W. Patients carrying CYP2C19 loss of function alleles have a reduced response to clopidogrel therapy and a greater risk of in-stent restenosis after endovascular treatment of lower extremity peripheral arterial disease. J. Vasc. Surg. 2014, 60, 993–1001. [Google Scholar] [CrossRef]
- Pastromas, G.; Spiliopoulos, S.; Katsanos, K.; Diamantopoulos, A.; Kitrou, P.; Karnabatidis, D.; Siablis, D. Clopidogrel Responsiveness in Patients Undergoing Peripheral Angioplasty. Cardiovasc. Interv. Radiol. 2013, 36, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Becker, R.C.; Wojdyla, D.M.; Emanuelsson, H.; Hiatt, W.R.; Horrow, J.; Husted, S.; Mahaffey, K.W.; Steg, P.G.; Storey, R.; et al. Cardiovascular events in acute coronary syndrome patients with peripheral arterial disease treated with ticagrelor compared with clopidogrel: Data from the PLATO Trial. Eur. J. Prev. Cardiol. 2015, 22, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Leesar, M.A.; Ahmed, H.; Lendel, V.; Rodriguez, G.; Mutlu, D.; Cawich, I.; Prasad, A.; Oglesby, M.; Marmagkiolis, K.; et al. Impact of ticagrelor and aspirin versus clopidogrel and aspirin in symptomatic patients with peripheral arterial disease: Thrombus burden assessed by optical coherence tomography. Cardiovasc. Revascularization Med. 2018, 19, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.W.; Chew, D.P.; Kader, M.A.S.A.; Ako, J.; Bahl, V.K.; Chan, M.; Park, K.W.; Chandra, P.; Hsieh, I.-C.; Huan, D.Q.; et al. 2020 Asian Pacific Society of Cardiology Consensus Recommendations on the Use of P2Y12 Receptor Antagonists in the Asia-Pacific Region. Eur. Cardiol. Rev. 2021, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Motovska, Z.; Bhatt, D.L. 12 months of DAPT after acute coronary syndrome still beats 6 months. Lancet 2018, 391, 1240–1242. [Google Scholar] [CrossRef]
- Gremmel, T.; Xhelili, E.; Steiner, S.; Koppensteiner, R.; Kopp, C.W.; Panzer, S. Response to antiplatelet therapy and platelet reactivity to thrombin receptor activating peptide-6 in cardiovascular interventions: Differences between peripheral and coronary angioplasty. Atherosclerosis 2014, 232, 119–124. [Google Scholar] [CrossRef]
- Pereira, N.L.; Rihal, C.; Lennon, R.; Marcus, G.; Shrivastava, S.; Bell, M.R.; So, D.; Geller, N.; Goodman, S.G.; Hasan, A.; et al. Effect of CYP2C19 Genotype on ischemic outcomes during oral P2Y12 inhibitor therapy: A Meta-Analysis. JACC Cardiovasc. Interv. 2021, 14, 739–750. [Google Scholar] [CrossRef]
- Wallentin, L.; James, S.; Storey, R.; Armstrong, M.; Barratt, B.; Horrow, J.; Husted, S.; Katus, H.; Steg, P.G.; Shah, S.H.; et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: A genetic substudy of the PLATO trial. Lancet 2010, 376, 1320–1328. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Wang, Z.; Wang, S.; Zhang, H.; Wang, Y.; Lu, C.; Xuan, H.; Wang, C.; Li, D.; et al. Efficacy and safety of low dose ticagrelor in patients with acute coronary syndrome: A systematic review and meta-analysis. Postgrad. Med. J. 2020, 96, 693–702. [Google Scholar] [CrossRef]
- Theidel, U.; Asseburg, C.; Giannitsis, E.; Katus, H. Cost-effectiveness of ticagrelor versus clopidogrel for the prevention of atherothrombotic events in adult patients with acute coronary syndrome in Germany. Clin. Res. Cardiol. 2013, 102, 447–458. [Google Scholar] [CrossRef][Green Version]
- Barbieri, M.; Drummond, M.; Willke, R.; Chancellor, J.; Jolain, B.; Towse, A. Variability of Cost-Effectiveness Estimates for Pharmaceuticals in Western Europe: Lessons for Inferring Generalizability. Value Health 2005, 8, 10–23. [Google Scholar] [CrossRef]
- Johnson, S.G.; Gruntowicz, D.; Chua, T.; Morlock, R.J. Financial Analysis of CYP2C19 Genotyping in Patients Receiving Dual Antiplatelet Therapy Following Acute Coronary Syndrome and Percutaneous Coronary Intervention. J. Manag. Care Spéc. Pharm. 2015, 21, 552–557. [Google Scholar] [CrossRef]
- Frelinger, A.L., 3rd; Bhatt, D.L.; Lee, R.D.; Mulford, D.J.; Wu, J.; Nudurupati, S.; Nigam, A.; Lampa, M.; Brooks, J.K.; Barnard, M.R.; et al. Clopidogrel pharmacokinetics and pharmacodynamics vary widely despite exclusion or control of polymorphisms (CYP2C19, ABCB1, PON1), noncompliance, diet, smoking, co-medications (including proton pump inhibitors), and pre-existent variability in platelet function. J. Am. Coll. Cardiol. 2013, 61, 872–879. [Google Scholar]
- Nasyuhana Sani, Y.; Sheau Chin, L.; Luen Hui, L.; Mohd Redhuan Shah Edwin, N.E.; Teck Hwa, G.; Serebruany, V.L.; Kah Hay, Y. The CYP2C19(∗)1/(∗)2 Genotype does not adequately predict clopidogrel response in healthy Malaysian volunteers. Cardiol. Res. Pract. 2013, 2013, 128795. [Google Scholar] [CrossRef]
- Kanamarlapudi, V.; Owens, S.E.; Saha, K.; Pope, R.J.; Mundell, S.J. ARF6-Dependent Regulation of P2Y Receptor Traffic and Function in Human Platelets. PLoS ONE 2012, 7, e43532. [Google Scholar] [CrossRef]
- Cunningham, M.R.; Aungraheeta, R.; Mundell, S.J. Pathophysiological consequences of receptor mistraffic: Tales from the platelet P2Y 12 receptor. Mol. Cell. Endocrinol. 2017, 449, 74–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, W.; Karim, Z.A.; Whiteheart, S.W. Arf6 plays an early role in platelet activation by collagen and convulxin. Blood 2006, 107, 3145–3152. [Google Scholar] [CrossRef]
- Moravec, R.; Conger, K.K.; D’Souza, R.; Allison, A.B.; Casanova, J.E. BRAG2/GEP100/IQSec1 interacts with clathrin and regulates alpha5beta1 integrin endocytosis through activation of ADP ribosylation factor 5 (Arf5). J. Biol. Chem. 2012, 287, 31138–31147. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Yamauchi, Y.; Hongu, T.; Funakoshi, Y.; Ohbayashi, N.; Hasegawa, H.; Kanaho, Y. Activation of the Small G Protein Arf6 by Dynamin2 through Guanine Nucleotide Exchange Factors in Endocytosis. Sci. Rep. 2015, 5, 14919. [Google Scholar] [CrossRef]
- Onodera, T.; Sakai, T.; Hsu, J.C.-F.; Matsumoto, K.; Chiorini, J.A.; Yamada, K.M. Btbd7 Regulates Epithelial Cell Dynamics and Branching Morphogenesis. Science 2010, 329, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fedoseienko, A.; Chen, B.; Burstein, E.; Jia, D.; Billadeau, D.D. Endosomal receptor trafficking: Retromer and beyond. Traffic 2018, 19, 578–590. [Google Scholar] [CrossRef] [PubMed]

| Group | I | II | I vs. II | III | IV |
|---|---|---|---|---|---|
| Un-guided (n = 21) | PFT-guided (n = 24) | p | Medical (n = 19) | Control (n = 64) | |
| Sex, male (n, %) | 11(52.4%) | 16 (66.7%) | 0.15 | 15 (78.9%) | 42 (65.6%) |
| Age (years) | 63.0 ± 7.4 | 63.9 ± 6.3 | 0.44 | 63.7 ± 11.0 | 63.8 ± 13.3 |
| Body weight (Kg) | 68.9 ± 10.2 | 68.4 ± 15.4 | 0.60 | 63.2 ± 10.6 | 57.9 ± 6.7 |
| Ejection fraction (%) | 65.4 ± 8.5 | 69.0 ± 6.8 | 0.23 | N/A | N/A |
| Smoking (%) | 0.336 | ||||
| Current | 12 (57.1%) | 13 (54.2%) | 7 (36.8%) | 28 (43.8%) | |
| None | 7 (33.3%) | 5 (20.8%) | 12 (63.2%) | 36 (56.2%) | |
| Quit | 2 (9.5%) | 6 (25.0%) | |||
| Co-morbidity | |||||
| DM | 21 (100%) | 24 (100%) | 1.00 | 19 (100%) | 0 |
| Hypertension | 15 (71.4%) | 15 (62.5%) | 0.32 | 12 (63.2%) | 0 |
| CAD | 6 (28.6%) | 7 (29.2%) | 0.54 | 10 (52.6%) | 0 |
| Old CVA | 3 (14.3%) | 2 (8.3%) | 0.27 | 2 (10.5%) | 0 |
| CKD stage 2–3 | 3 (14.3%) | 4 (16.7%) | 0.50 | 2 (10.5%) | 0 |
| Medications | |||||
| Aspirin | 16 (76.2%) | 10 (41.7%) | 0.034 | 13 (68.4%) | 0 |
| Cilostazol | 6 (28.6%) | 8 (33.3%) | 0.759 | 0 (0%) | 0 |
| Pentoxifylline | 3 (14.3%) | 6 (25.0%) | 0.469 | 0 (0%) | 0 |
| Acrabose | 3 (14.3%) | 4 (16.7%) | 1.0 | 3 (15.8%) | 0 |
| Thiazolidinedione | 0 (0%) | 2 (8.3%) | 0.491 | 1 (5.3%) | 0 |
| DPP4 | 15 (71.4%) | 9 (37.5%) | 0.036 | 3 (15.8%) | 0 |
| Sulfonylurea | 11(52.4%) | 16 (66.7%) | 0.374 | 4 (21.1%) | 0 |
| Metformin | 17 (81.0%) | 14 (58.3%) | 0.121 | 5 (26.3%) | 0 |
| SGLT2 inhibitor | 2 (9.5%) | 2 (8.3%) | 1.0 | 6 (31.6%) | 0 |
| Diuretics | 6 (28.6%) | 8 (33.3%) | 0.759 | 3 (15.8 %) | 0 |
| CCA | 12 (57.1%) | 14 (58.3%) | 1.0 | 6 (31.6%) | 0 |
| Beta-blocker | 11 (52.4%) | 15 (62.5%) | 0.555 | 10 (52.6%) | 0 |
| ACEI | 2 (9.5%) | 3 (12.5%) | 1.0 | 2 (10.5%) | 0 |
| ARB | 16 (76.2%) | 15 (62.5%) | 0.356 | 14 (73.7%) | 0 |
| Nitrate | 3 (14.3%) | 1 (4.2%) | 0.326 | 6 (31.6%) | 0 |
| Statin | 15 (71.4%) | 21 (87.5%) | 0.267 | 11 (57.9%) | 0 |
| Fibrate | 3 (14.3%) | 3 (12.5%) | 1.0 | 1 (5.3%) | 0 |
| Ezetimibe | 5 (23.8%) | 5 (20.8%) | 1.0 | 2 (10.5%) | 0 |
| Biochemistry data | |||||
| Total cholesterol (mg/dL) | 154.4 ± 28.8 | 160.5 ± 44.7 | 0.94 | 150.6 ± 26.4 | 199.7 ± 35.3 |
| HDL (mg/dL) | 39.1 ± 11.6 | 42.2 ± 13.7 | 0.35 | 41.3 ± 9.4 | 62.8 ± 14.3 |
| LDL (mg/dL) | 80.9 ± 23.9 | 92.3 ± 42.8 | 0.53 | 92.3 ± 24.5 | 126.7 ± 30.7 |
| Triglyceride (mg/dL) | 204.8 ± 126.5 | 149 ± 63.5 | 0.112 | 158.6 ± 100.4 | 96.9 ± 29.9 |
| Glycohemoglobin (%) | 7.99 ± 1.43 | 8.10 ± 1.82 | 0.84 | 7.24 ± 1.51 | 5.49 ± 0.31 |
| Fasting sugar (mg/dL) | 143.8 ± 39.3 | 140.7 ± 49.6 | 0.957 | 141.1 ± 60.9 | 90.0 ± 5.0 |
| Creatinine (mg/dL) | 1.22 ± 0.54 | 1.66 ± 0.77 | 0.063 | 0.96 ± 0.29 | 0.87 ± 0.15 |
| ALT (U/L) | 22.5 ± 12.1 | 23.5 ± 16.1 | 0.92 | 22.4 ± 9.6 | 22.1 ± 5.9 |
| Group | Un-Guided (n = 21) | PFT-Guided (n = 24) | p |
|---|---|---|---|
| PFT at baseline (PRU) | 231.5 ± 83.4 | 251.3 ± 86.2 | 0.937 |
| Clopidogrel resistance PRU > 234 (n, %) | 12 (57.1%) | 14 (58.3%) | 0.53 |
| PFT at 36 months (PRU) | 180.8 ± 66.2 | 89.9 ± 77.5 | 0.005 |
| Revascularization procedures | |||
| Bilateral (n, %) | 10 (27.6%) | 17 (70.8%) | 0.09 |
| Right only (n, %) | 6 (28.6%) | 5 (20.8%) | |
| Left only (n, %) | 5 (23.8%%) | 2 (8.3%) | |
| ABI at baseline | 0.84 ± 0.17 | 0.76 ± 0.15 | 0.05 |
| ABI at 36 months | 0.92 ± 0.15* | 0.89 ± 0.20 | 0.61 |
| Paired T test of ABI (pre-op vs. post-op) | p = 0.03 | p = 0.0006 | |
| Difference of ABI (%) | 14% ± 30% | 21% ± 29% | 0.28 |
| Primary Endpoint | |||
| Re-intervention | 2 (9.5%) | 3 (12.5%) | 0.47 |
| Amputation | 1 (4.8%) | 3 (12.5%) | 0.52 |
| Hemorrhagic episode | 0 (0%) | 0 (0%) | ns |
| Secondary Endpoint | |||
| MACCE (n, %) | 10 (47.6%) | 6 (25.0%) | 0.02 |
| Death (n, %) | 2 (9.5%) | 2 (8.3%) | 0.24 |
| Ischemic CVA (n, %) | 4 (19.0%) | 2 (8.3%) | 0.08 |
| AMI (n, %) | 5 (23.8%) | 2 (8.3%) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-H.; Chou, Y.-J.; Tsai, T.-H.; Hsu, P.W.-C.; Li, C.-H.; Chan, Y.-H.; Tsai, S.-F.; Ng, S.-C.; Chou, K.-M.; Lin, Y.-C.; et al. Artificial-Intelligence-Assisted Discovery of Genetic Factors for Precision Medicine of Antiplatelet Therapy in Diabetic Peripheral Artery Disease. Biomedicines 2022, 10, 116. https://doi.org/10.3390/biomedicines10010116
Yeh C-H, Chou Y-J, Tsai T-H, Hsu PW-C, Li C-H, Chan Y-H, Tsai S-F, Ng S-C, Chou K-M, Lin Y-C, et al. Artificial-Intelligence-Assisted Discovery of Genetic Factors for Precision Medicine of Antiplatelet Therapy in Diabetic Peripheral Artery Disease. Biomedicines. 2022; 10(1):116. https://doi.org/10.3390/biomedicines10010116
Chicago/Turabian StyleYeh, Chi-Hsiao, Yi-Ju Chou, Tsung-Hsien Tsai, Paul Wei-Che Hsu, Chun-Hsien Li, Yun-Hsuan Chan, Shih-Feng Tsai, Soh-Ching Ng, Kuei-Mei Chou, Yu-Ching Lin, and et al. 2022. "Artificial-Intelligence-Assisted Discovery of Genetic Factors for Precision Medicine of Antiplatelet Therapy in Diabetic Peripheral Artery Disease" Biomedicines 10, no. 1: 116. https://doi.org/10.3390/biomedicines10010116
APA StyleYeh, C.-H., Chou, Y.-J., Tsai, T.-H., Hsu, P. W.-C., Li, C.-H., Chan, Y.-H., Tsai, S.-F., Ng, S.-C., Chou, K.-M., Lin, Y.-C., Juan, Y.-H., Fu, T.-C., Lai, C.-C., Sytwu, H.-K., & Tsai, T.-F. (2022). Artificial-Intelligence-Assisted Discovery of Genetic Factors for Precision Medicine of Antiplatelet Therapy in Diabetic Peripheral Artery Disease. Biomedicines, 10(1), 116. https://doi.org/10.3390/biomedicines10010116






