Recognition and Sensing of Chiral Organic Molecules by Chiral Porphyrinoids: A Review
Abstract
1. Introduction
2. Chiral Recognition of Amino Acids and Their Esters
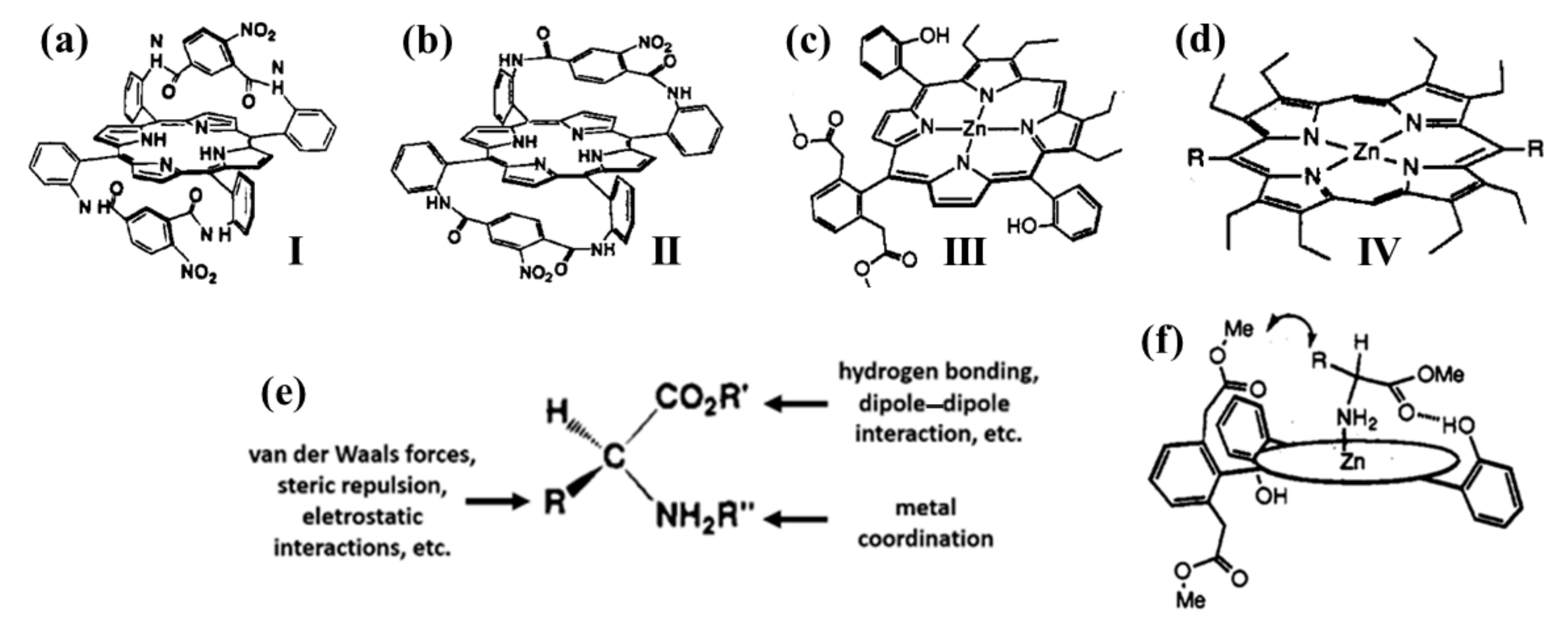
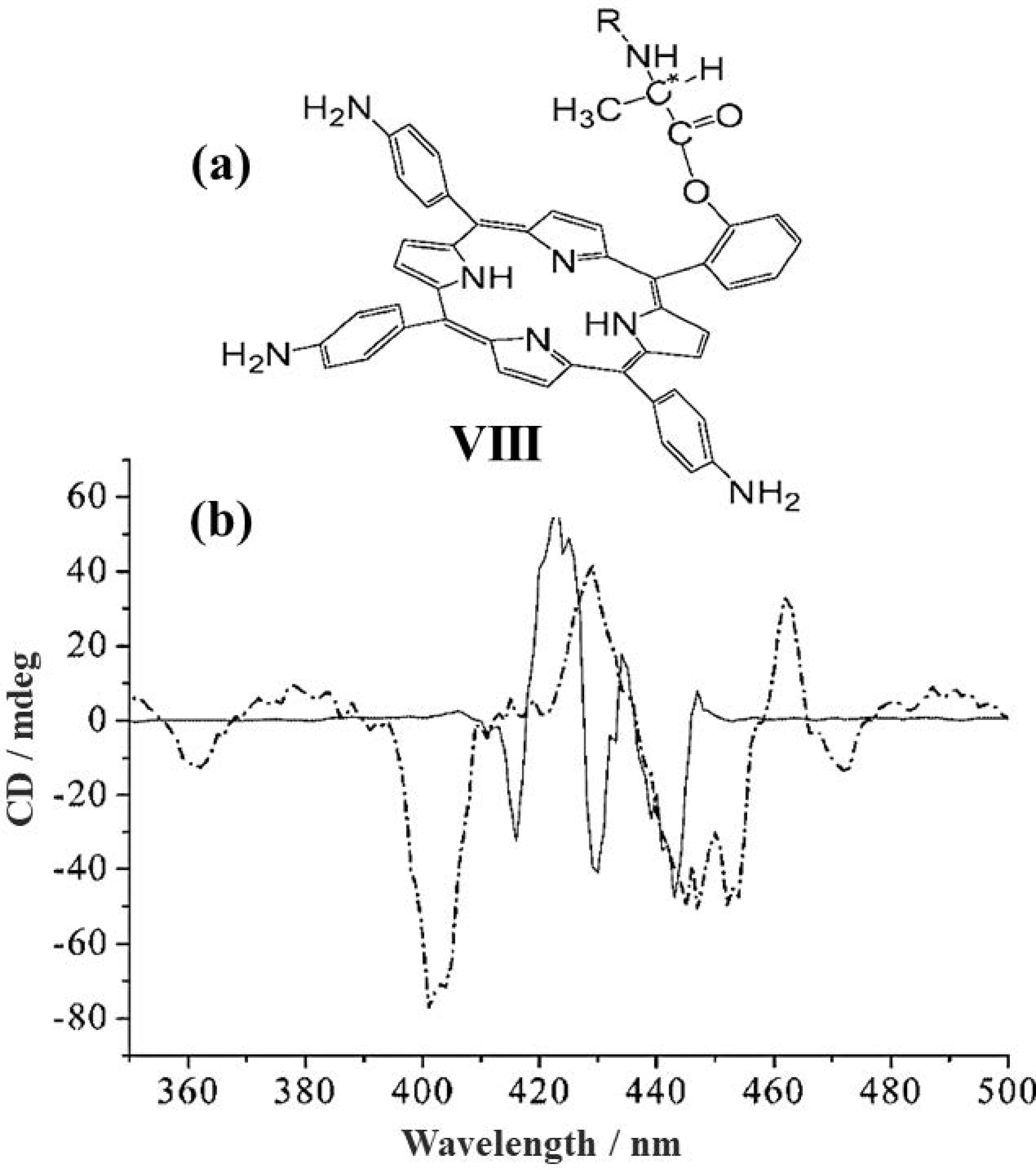
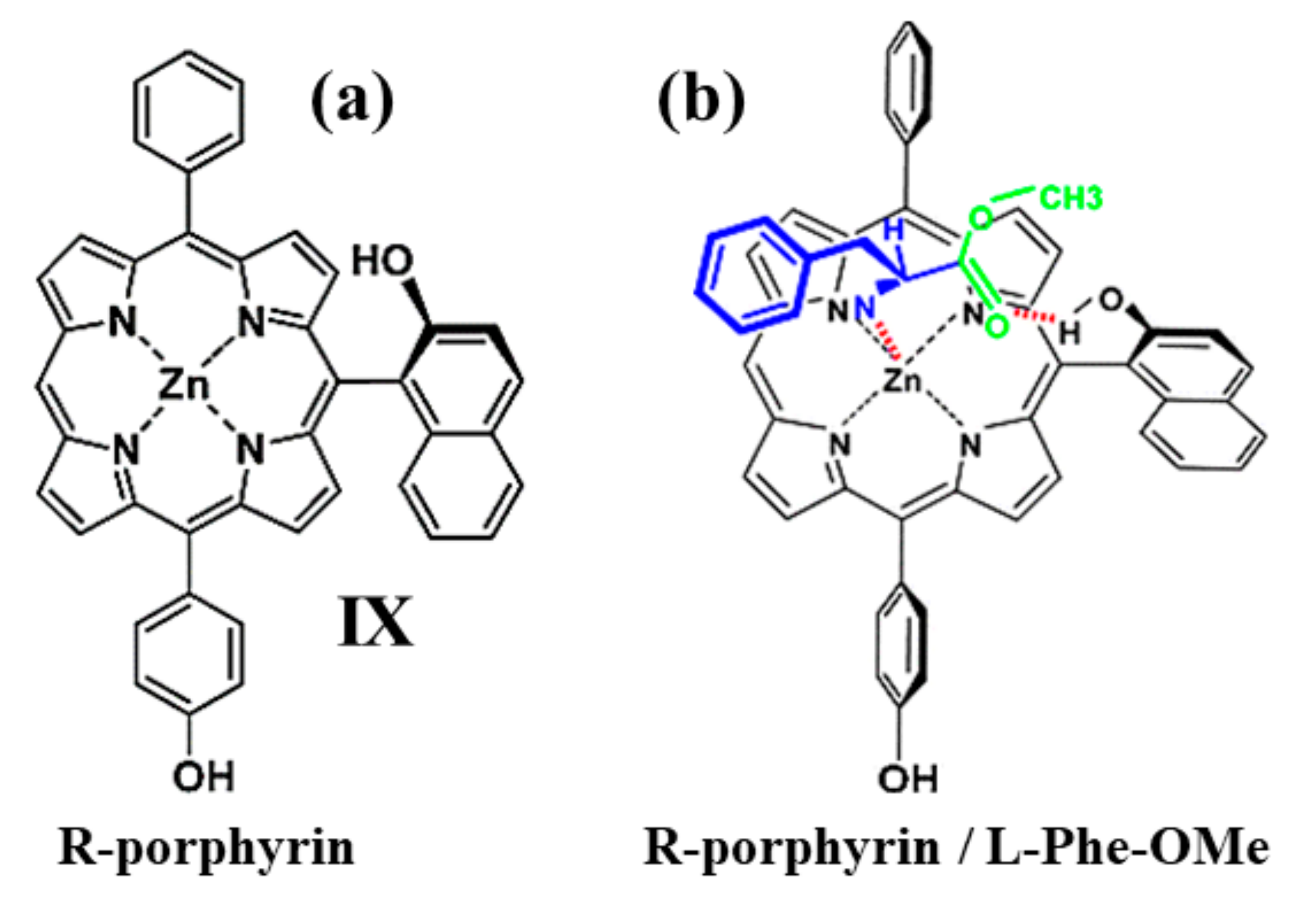
2.1. Monomeric Porphynoids
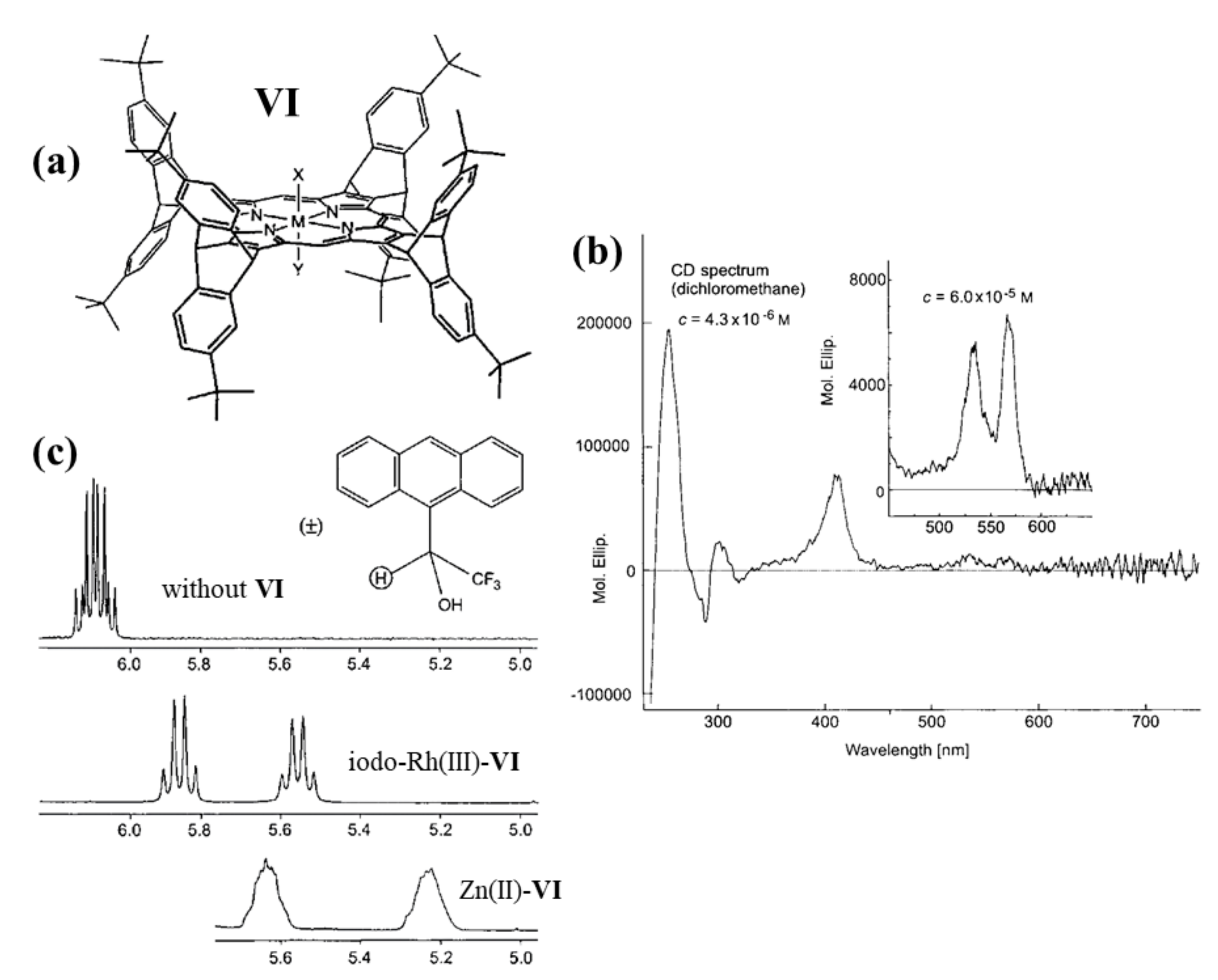
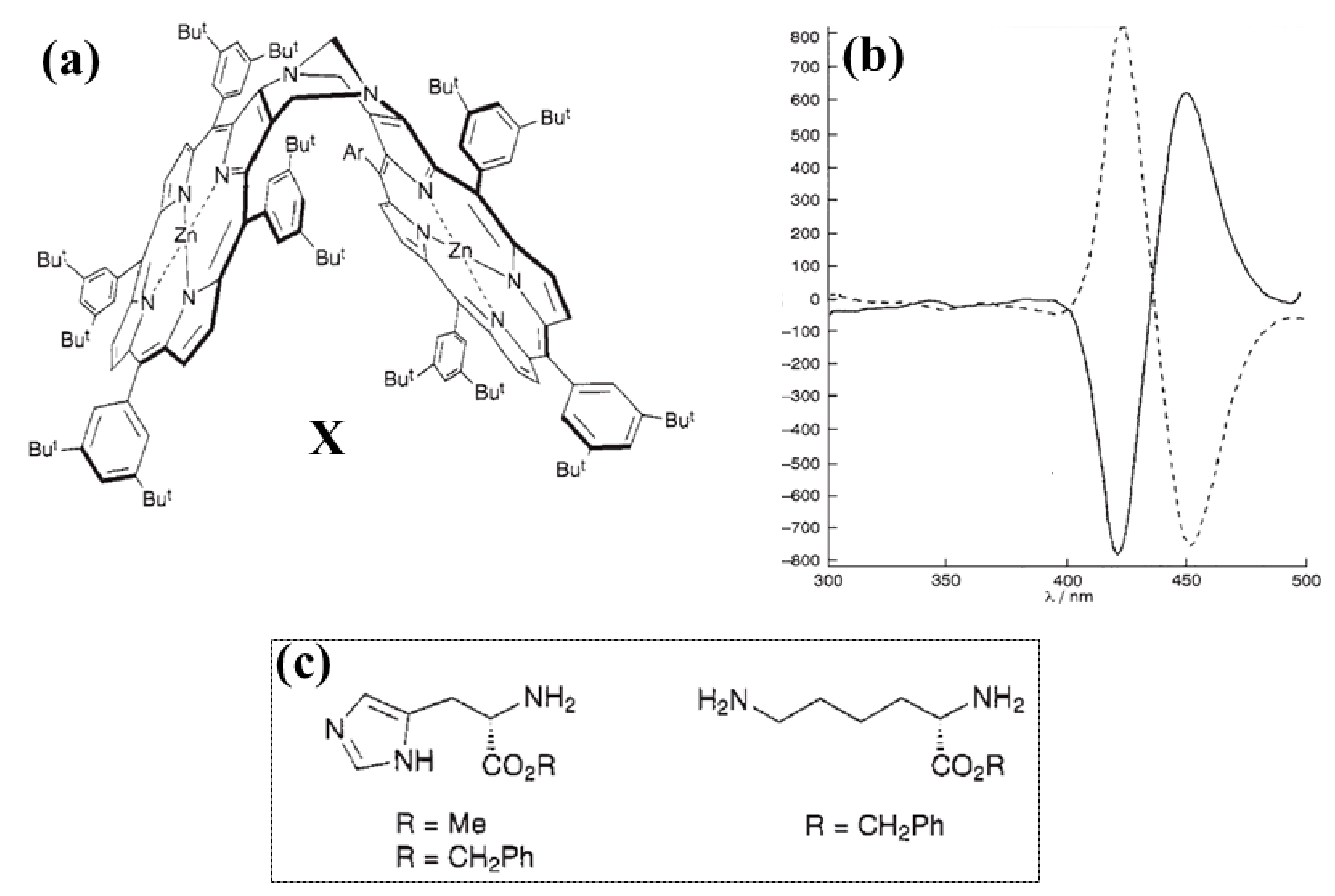
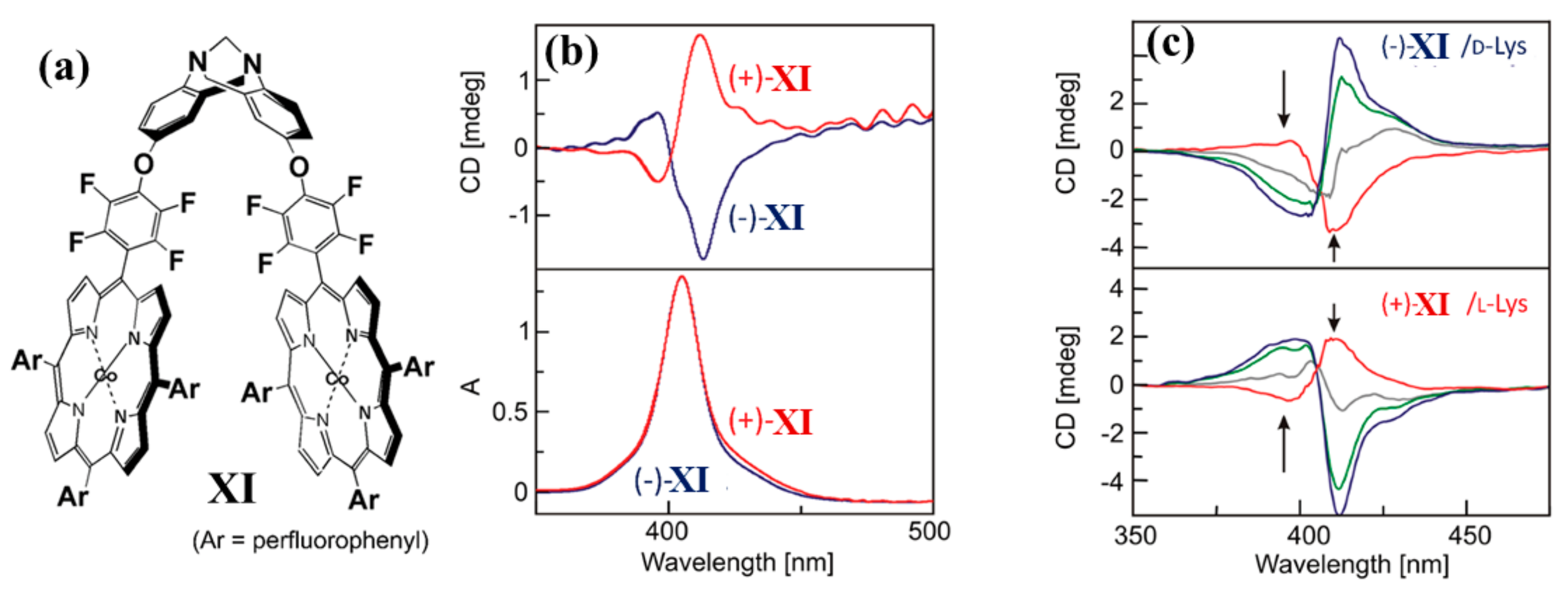
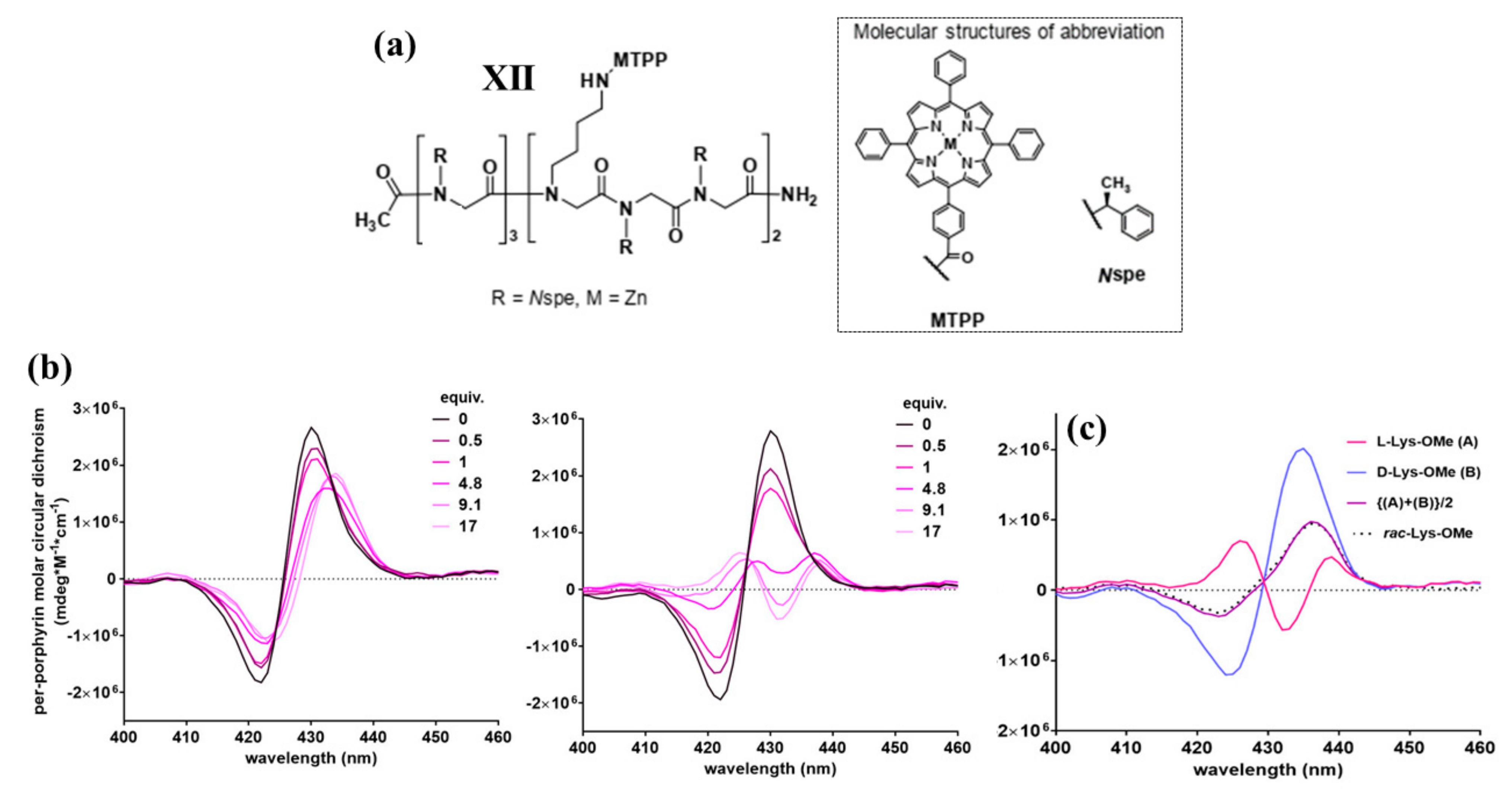
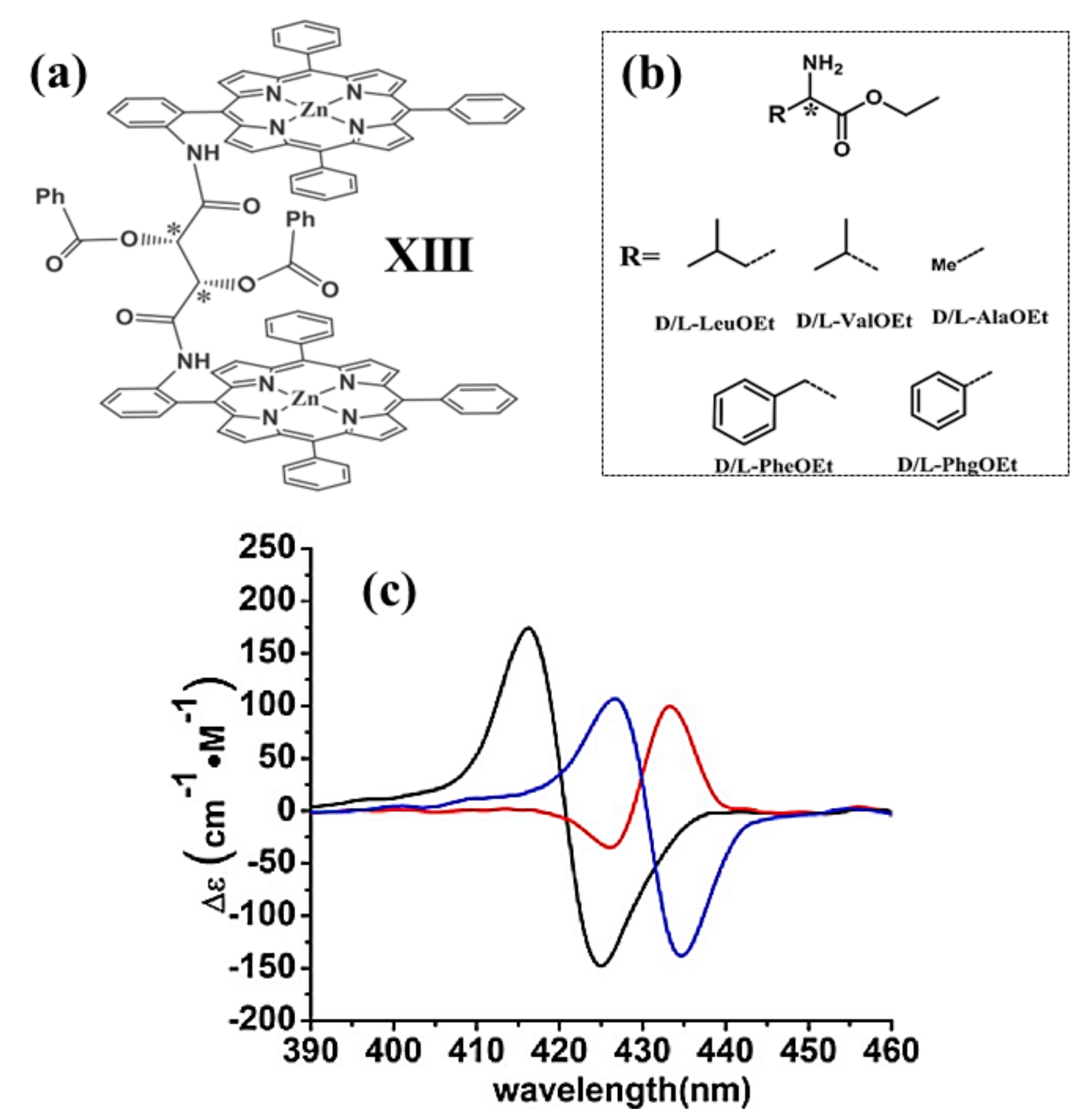
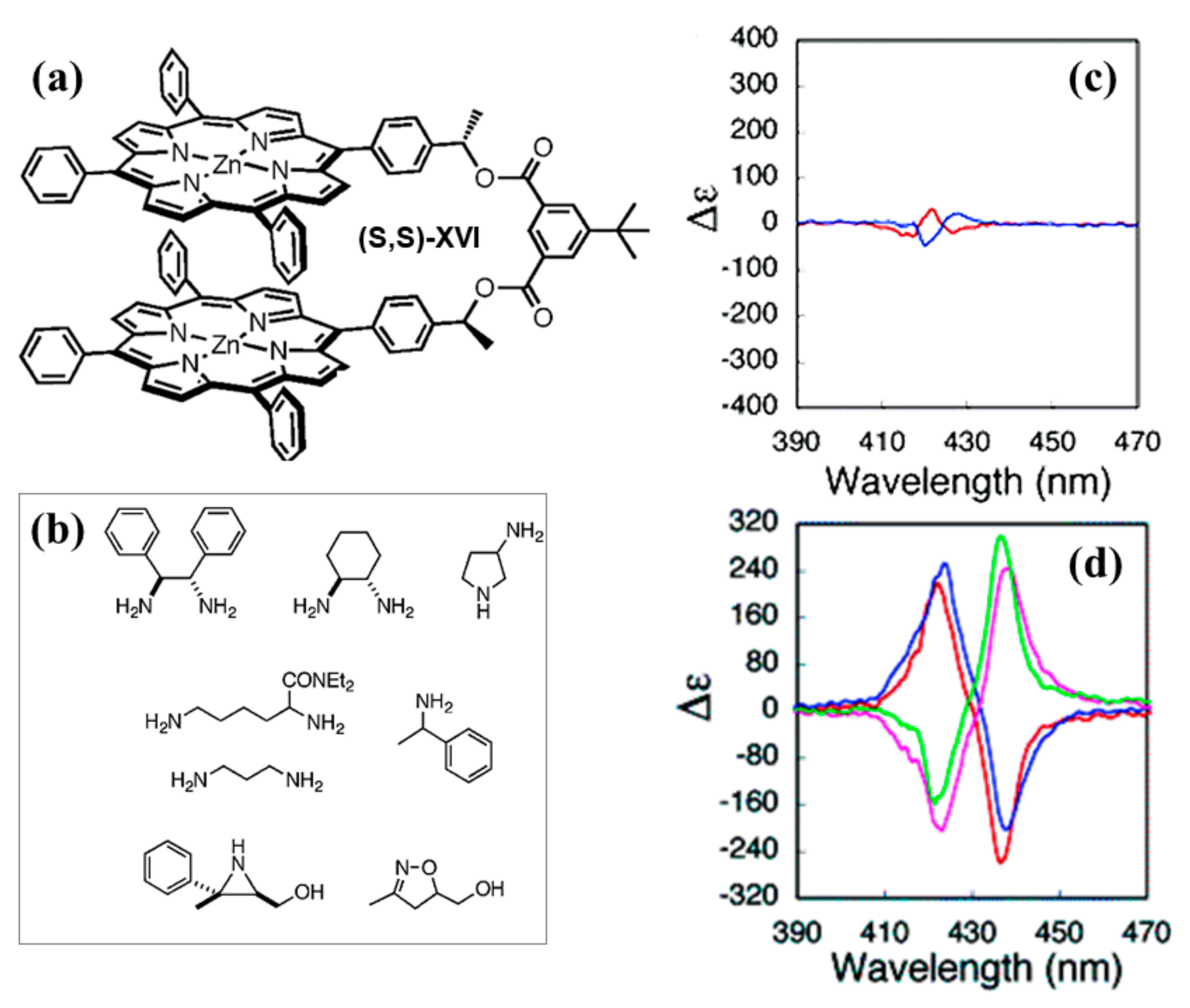
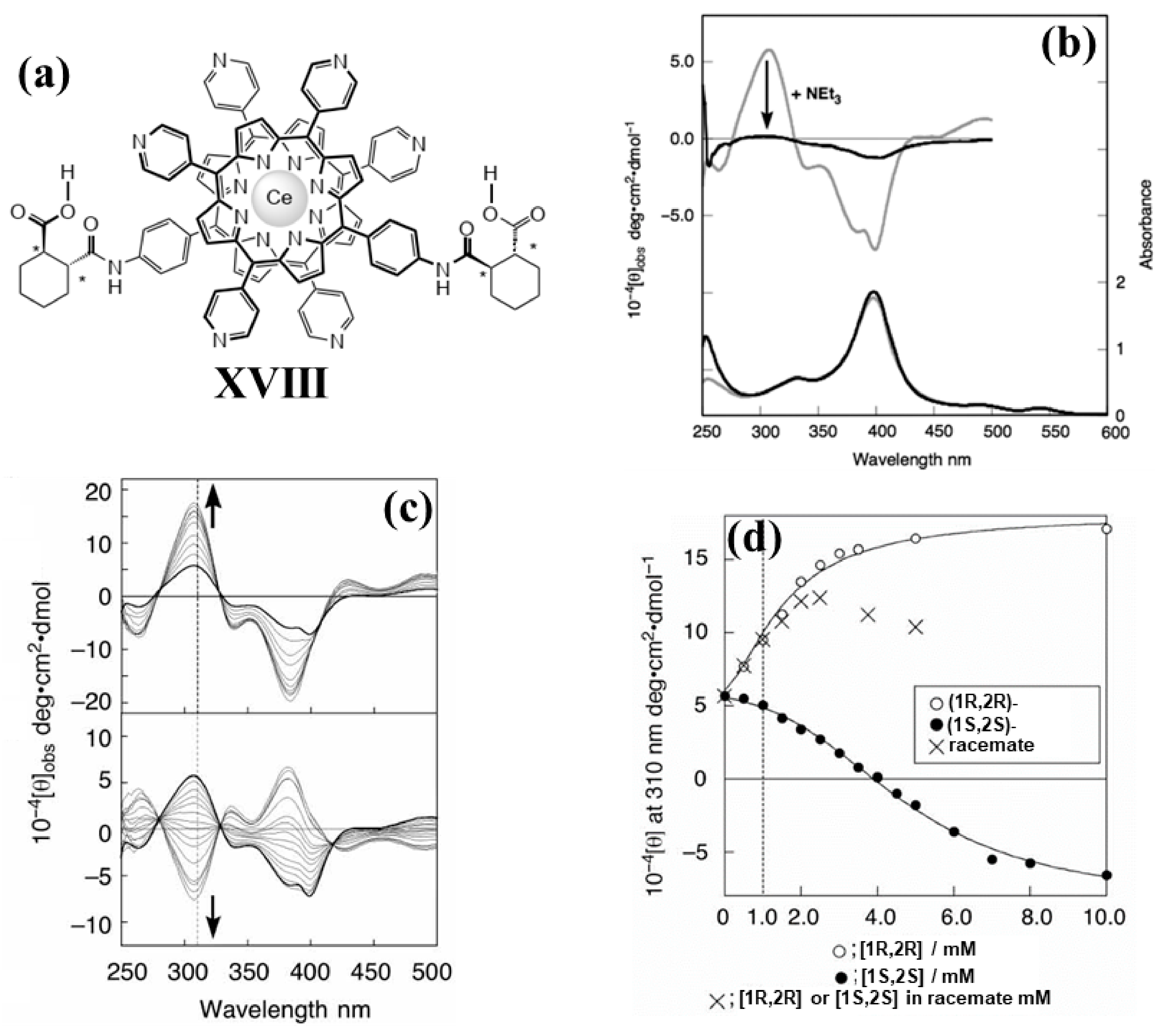
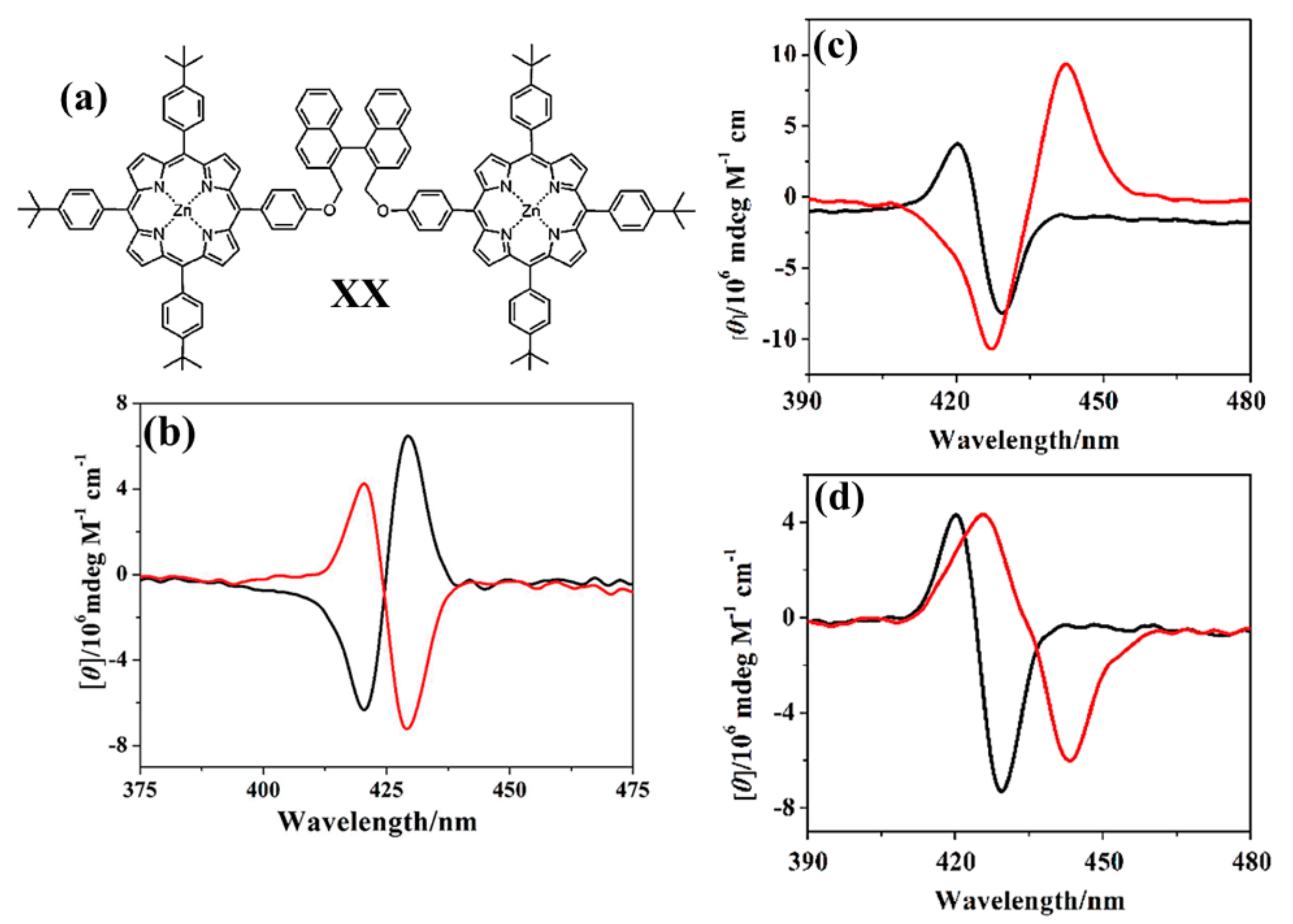
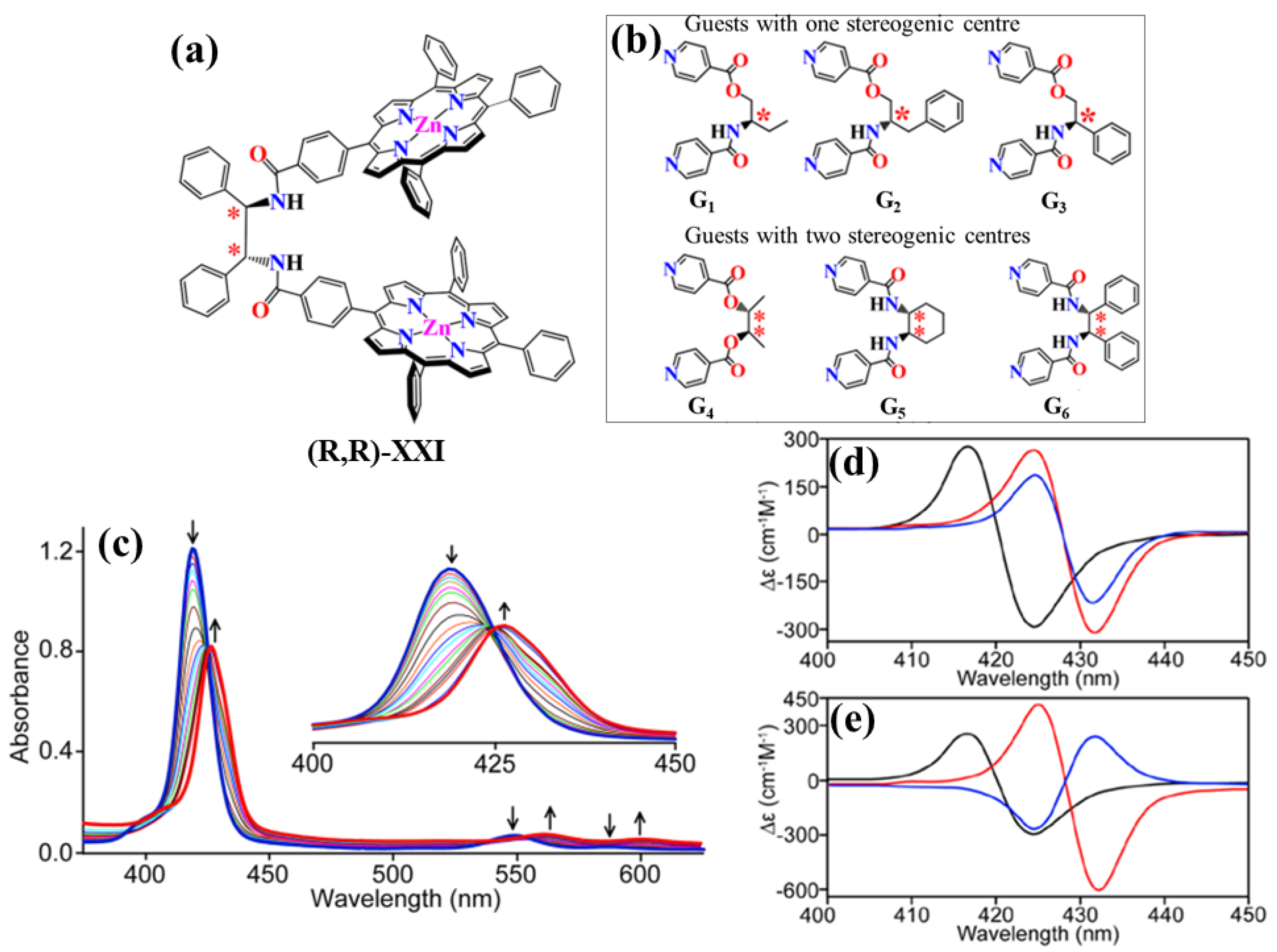
2.2. Bis-Porphyrin Systems and Porphyrin-Tweezers
3. Chiral Recognition of Anionic and Neutral Guests
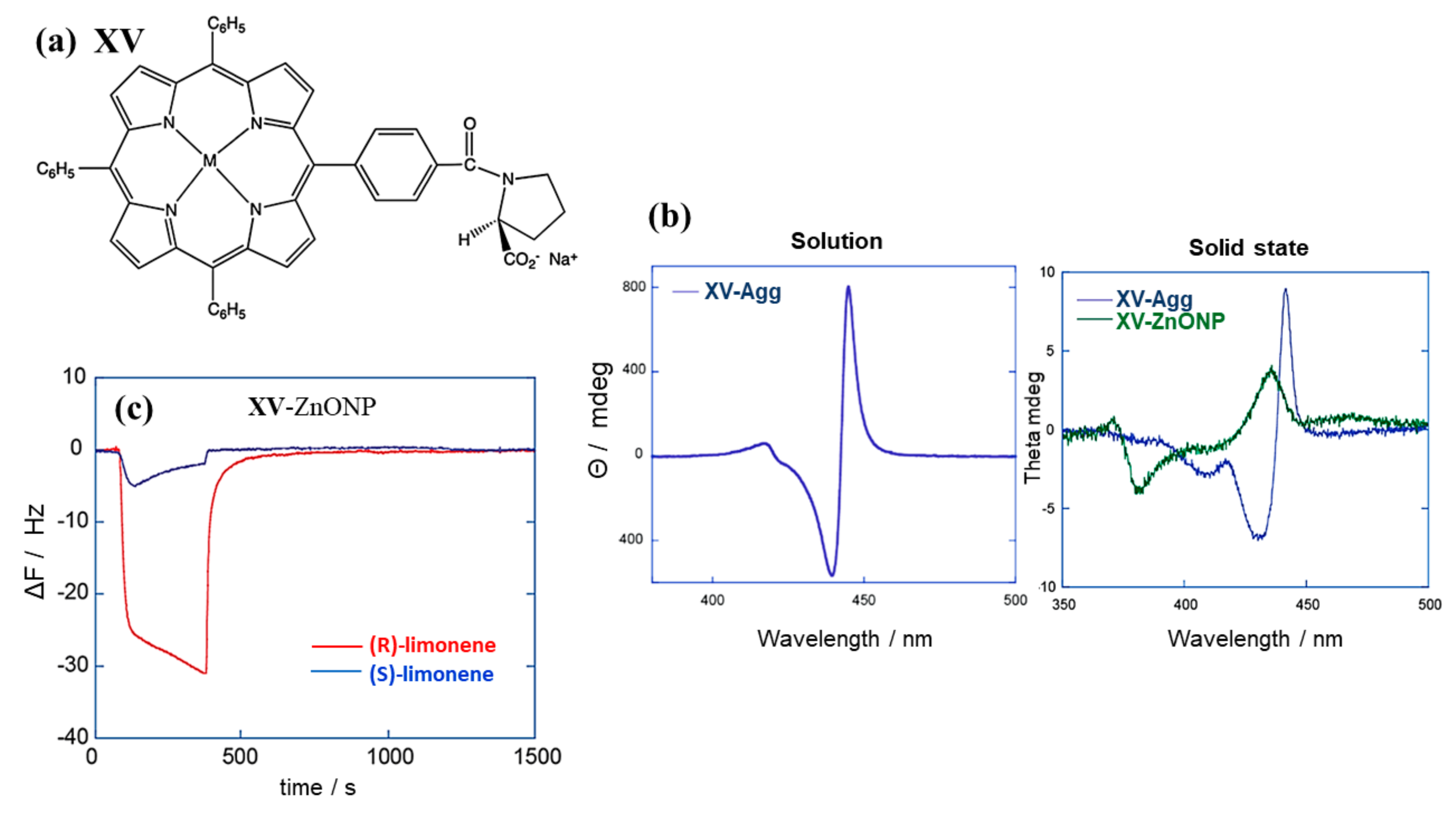
3.1. Monomeric Porphynoids
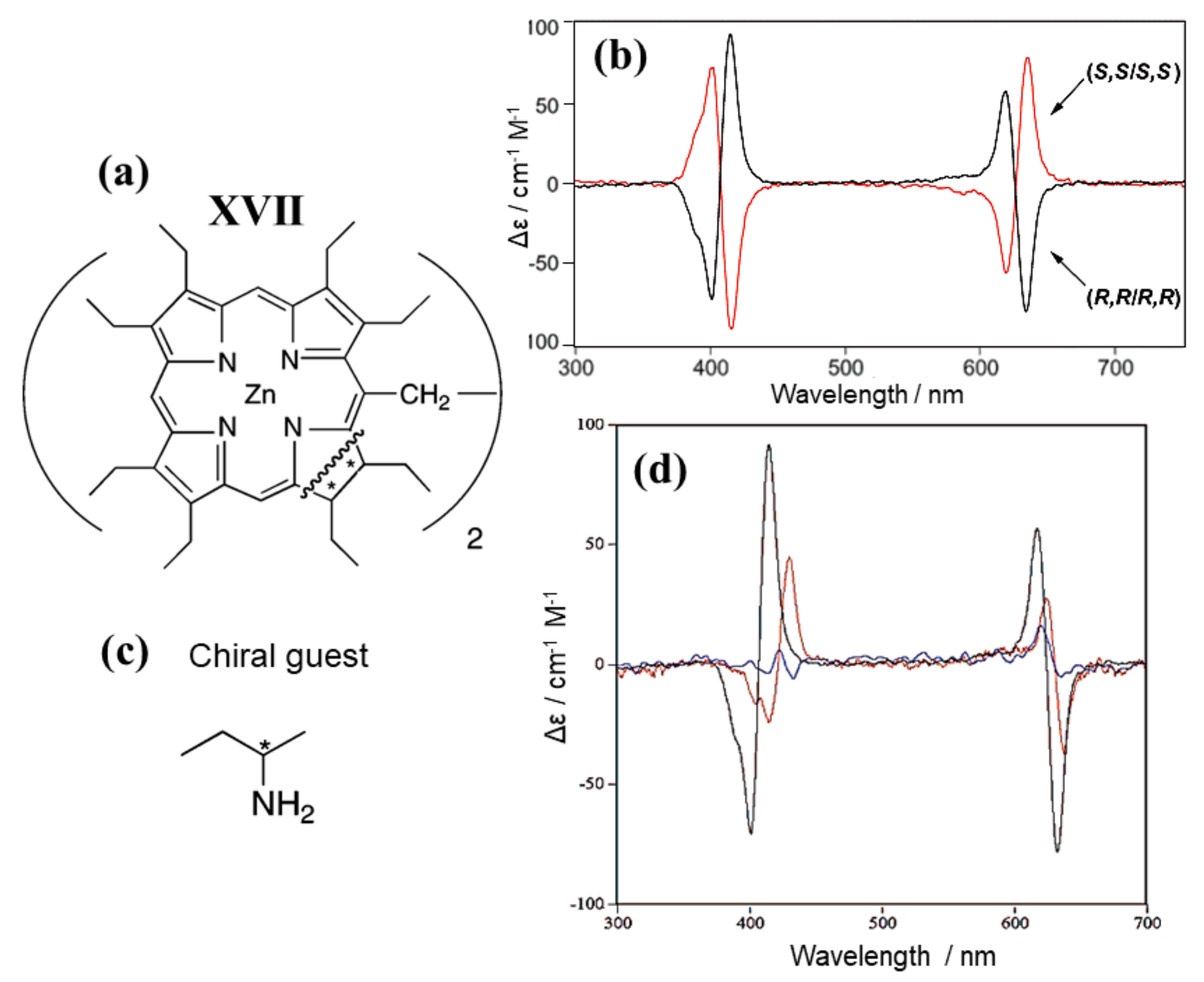
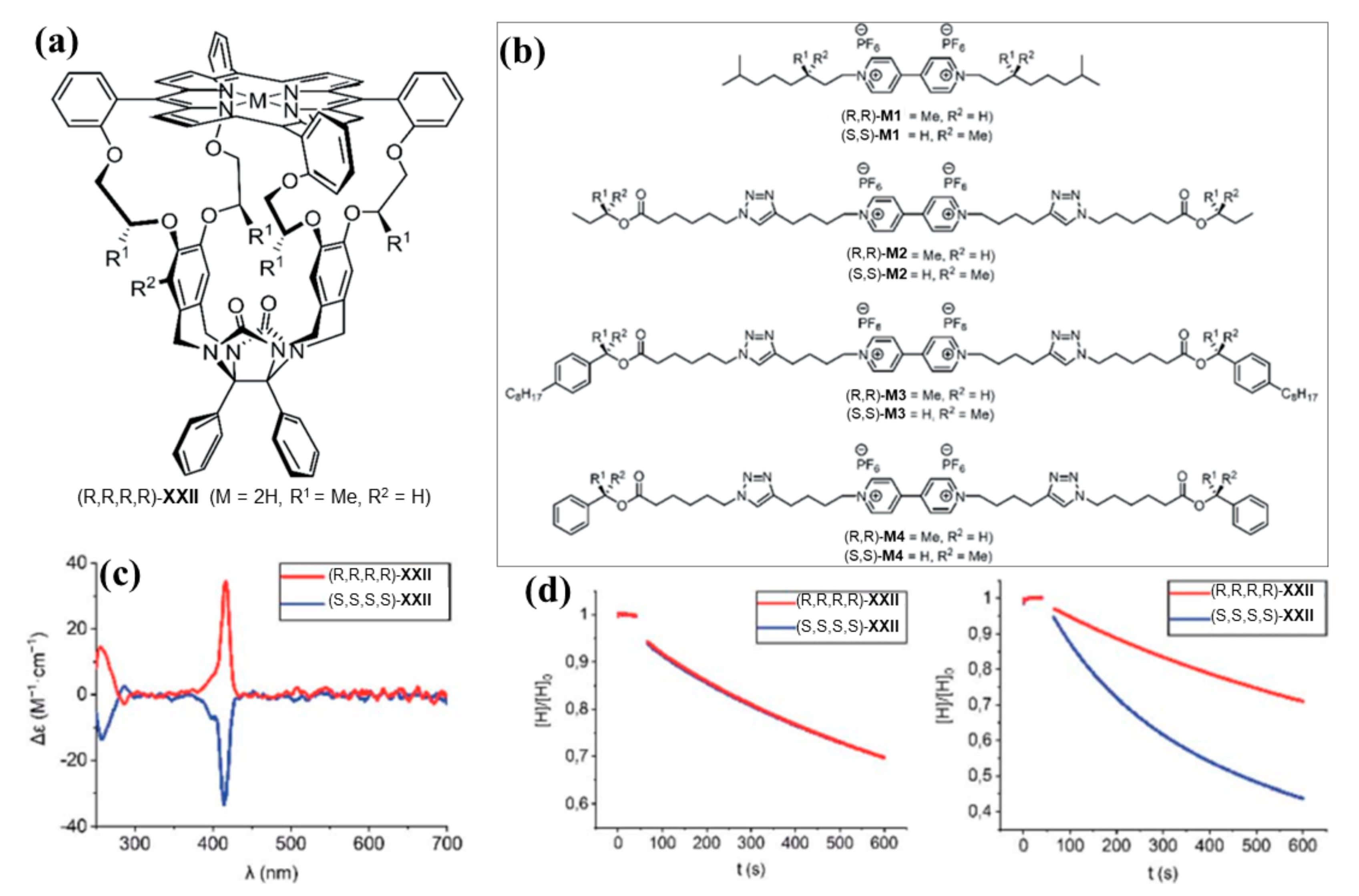
3.2. Bis-Porphyrin Systems and Porphyrin-Tweezers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baron, R.; McCammon, J.A. Molecular recognition and ligand association. Annu. Rev. Phys. Chem. 2013, 64, 151–175. [Google Scholar] [CrossRef]
- Persch, E.; Dumele, O.; Diederich, F. Molecular recognition in chemical and biological systems. Angew. Chem. Int. Ed. 2015, 54, 3290–3327. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Benincori, T.; Penoni, A.; Vaghi, L.; Cirilli, R.; Abbate, S.; Longhi, G.; Mazzeo, G.; Grecchi, S.; Panigati, M.; et al. Highly enantioselective “inherently chiral” electroactive materials based on a 2,2′-biindole atropisomeric scaffold. Chem. Sci. 2019, 10, 2708–2717. [Google Scholar] [CrossRef]
- Ousaka, N.; Yamamoto, S.; Iida, H.; Iwata, T.; Ito, S.; Hijikata, Y.; Irle, S.; Yashima, E. Water-mediated deracemization of a bisporphyrin helicate assisted by diastereoselective encapsulation of chiral guests. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Baars, M.W.P.L.; Meijer, E.W. Host-Guest Chemistry of Dendritic Molecules. In Dendrimers II. Topics in Current Chemistry; Vögtle, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; Volume 210, pp. 131–182. [Google Scholar]
- Gattuso, G.; Notti, A.; Pappalardo, S.; Parisi, M.F.; Pisagatti, I. Recognition in water of bioactive substrates by a sulphonato p-tert-butylcalix[5]arene. Supramol. Chem. 2014, 26, 597–600. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Yamamura, H.; Inoue, C.; Kawai, M.; Osaka, I.; Arakawa, R.; Shiba, K.; Sato, A.; Young, H.K.; Selvapalam, N.; et al. Chiral recognition in cucurbituril cavities. J. Am. Chem. Soc. 2006, 128, 14871–14880. [Google Scholar] [CrossRef]
- Corradini, R.; Sforza, S.; Tedeschi, T.; Marchelli, R. Chirality as a tool in nucleic acid recognition: Principles and relevance in biotechnology and in medicinal chemistry. Chirality 2007, 19, 269–294. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Q.; Liu, M. Enantioselective Recognition by Chiral Supramolecular Gels. Chem. Asian J. 2016, 11, 2642–2649. [Google Scholar] [CrossRef]
- Farinone, M.; Urbańska, K.; Pawlicki, M. BODIPY- and Porphyrin-Based Sensors for Recognition of Amino Acids and Their Derivatives. Molecules 2020, 25, 4523. [Google Scholar] [CrossRef]
- Gaeta, M.; Rodolico, E.; Fragalà, M.E.; Pappalardo, A.; Pisagatti, I.; Gattuso, G.; Notti, A.; Parisi, M.F.; Purrello, R.; D’Urso, A. Self-assembly of discrete porphyrin/calix[4]tube complexes promoted by potassium ion encapsulation. Molecules 2021, 26, 704. [Google Scholar] [CrossRef] [PubMed]
- Ceccacci, F.; Mancini, G.; Sferrazza, A.; Villani, C. pH variation as the switch for chiral recognition in a biomembrane model. J. Am. Chem. Soc. 2005, 127, 13762–13763. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Nishimura, T. Detection and Amplification of Chirality by Helical Polymers. Chem. Eur. J. 2004, 10, 42–51. [Google Scholar] [CrossRef]
- Guo, D.S.; Wang, K.; Liu, Y. Selective binding behaviors of p-sulfonatocalixarenes in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 1–21. [Google Scholar] [CrossRef]
- Randazzo, R.; Gaeta, M.; Gangemi, C.M.A.; Fragalà, M.E.; Purrello, R.; D’Urso, A. Chiral recognition of L- and D-amino acid by porphyrin supramolecular aggregates. Molecules 2019, 24, 84. [Google Scholar] [CrossRef]
- Lintuluoto, J.M.; Borovkov, V.V.; Inoue, Y. Direct determination of absolute configuration of monoalcohols by bis (magnesium porphyrin). J. Am. Chem. Soc. 2002, 124, 13676–13677. [Google Scholar] [CrossRef]
- De Luca, G.; Romeo, A.; Scolaro, L.M.; Pasternack, R.F. Conformations of a model protein revealed by an aggregating CuII porphyrin: Sensing the difference. Chem. Commun. 2010, 46, 389–391. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, Y.; Zhu, M.; Zhao, L.; Wei, L.; Bian, Y. Stereochemistry and solid-state structure of an intrinsically chiral meso-patterned porphyrin: Case study by NMR and single-crystal X-ray diffraction analysis. J. Org. Chem. 2013, 78, 9949–9955. [Google Scholar] [CrossRef]
- Gaeta, M.; Randazzo, R.; Cristaldi, D.A.; D’Urso, A.; Purrello, R.; Fragalà, M.E. ZnTPPS demetalation: Role of polyelectrolytes on aggregation after protonation in acid. J. Porphyr. Phthalocyanines 2017, 21, 426–430. [Google Scholar] [CrossRef]
- Romeo, A.; Castriciano, M.A.; Zagami, R.; Pollicino, G.; Monsù Scolaro, L.; Pasternack, R.F. Effect of zinc cations on the kinetics of supramolecular assembly and the chirality of porphyrin J-aggregates. Chem. Sci. 2017, 8, 961–967. [Google Scholar] [CrossRef]
- Monti, D.; Nardis, S.; Stefanelli, M.; Paolesse, R.; Di Natale, C.; D’Amico, A. Porphyrin-based nanostructures for sensing applications. J. Sens. 2009, 2009. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef]
- Panda, M.K.; Ladomenou, K.; Coutsolelos, A.G. Porphyrins in bio-inspired transformations: Light-harvesting to solar cell. Coord. Chem. Rev. 2012, 256, 2601–2627. [Google Scholar] [CrossRef]
- Jurow, M.; Schuckman, A.E.; Batteas, J.D.; Drain, C.M. Porphyrins as molecular electronic components of functional devices. Coord. Chem. Rev. 2010, 254, 2297–2310. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Kano, K. Porphyrin-cyclodextrin supramolecular complexes as myoglobin model in water. Colloid Polym. Sci. 2008, 286, 79–84. [Google Scholar] [CrossRef]
- Silva, E.F.F.; Serpa, C.; Dąbrowski, J.M.; Monteiro, C.J.P.; Formosinho, S.J.; Stochel, G.; Urbanska, K.; Simões, S.; Pereira, M.M.; Arnaut, L.G. Mechanisms of singlet-oxygen and superoxide-ion generation by porphyrins and bacteriochlorins and their implications in photodynamic therapy. Chem. Eur. J. 2010, 16, 9273–9286. [Google Scholar] [CrossRef]
- Mateos-Timoneda, M.A.; Crego-Calama, M.; Reinhoudt, D.N. Supramolecular chirality of self-assembled systems in solution. Chem. Soc. Rev. 2004, 33, 363–372. [Google Scholar] [CrossRef]
- Huang, X.; Fujioka, N.; Pescitelli, G.; Koehn, F.E.; Williamson, R.T.; Nakanishi, K.; Berova, N. Absolute configurational assignments of secondary amines by CD-sensitive dimeric zinc porphyrin host. J. Am. Chem. Soc. 2002, 124, 10320–10335. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Ikbal, S.A.; Petrovic, A.G.; Berova, N.; Rath, S.P. Complexation of Chiral Zinc-Porphyrin Tweezer with Achiral Diamines: Induction and Two-Step Inversion of Interporphyrin Helicity Monitored by ECD. Inorg. Chem. 2017, 56, 3849–3860. [Google Scholar] [CrossRef]
- Kurtán, T.; Nesnas, N.; Li, Y.Q.; Huang, X.; Nakanishi, K.; Berova, N. Chiral recognition by CD-sensitive dimeric zinc porphyrin host. 1. Chiroptical protocol for absolute configurational assignments of monoalcohols and primary monoamines. J. Am. Chem. Soc. 2001, 123, 5962–5973. [Google Scholar] [CrossRef]
- Proni, G.; Pescitelli, G.; Huang, X.; Quraishi, N.Q.; Nakanishi, K.; Berova, N. Configurational assignment of α-chiral carboxylic acids by complexation to dimeric Zn-porphyrin: Host-guest structure, chiral recognition and circular dichroism. Chem. Commun. 2002, 2, 1590–1591. [Google Scholar] [CrossRef]
- Ishii, H.; Chen, Y.; Miller, R.A.; Karady, S.; Nakanishi, K.; Berova, N. Chiral recognition of cyclic α-hydroxyketones by CD-sensitive zinc tetraphenylporphyrin tweezer. Chirality 2005, 17, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Angelini, N.; Micali, N.; Mineo, P.; Scamporrino, E.; Villari, V.; Vitalini, D. Uncharged water-soluble Co(II)—Porphyrin: A receptor for aromatic α-amino acids. J. Phys. Chem. B 2005, 109, 18645–18651. [Google Scholar] [CrossRef]
- Borovkov, V.V.; Lintuluoto, J.M.; Inoue, Y. Supramolecular chirogenesis in zinc porphyrins: Mechanism, role of guest structure, and application for the absolute configuration determination. J. Am. Chem. Soc. 2001, 123, 2979–2989. [Google Scholar] [CrossRef]
- Gholami, H.; Anyika, M.; Zhang, J.; Vasileiou, C.; Borhan, B. Host–Guest Assembly of a Molecular Reporter with Chiral Cyanohydrins for Assignment of Absolute Stereochemistry. Chem. Eur. J. 2016, 22, 9235–9239. [Google Scholar] [CrossRef]
- Borovkov, V. Supramolecular chirality in porphyrin chemistry. Symmetry 2014, 6, 256–294. [Google Scholar] [CrossRef]
- Ferrand, Y.; Le Maux, P.; Simonneaux, G. Highly enantioselective synthesis of cyclopropylphosphonates catalyzed by chiral ruthenium porphyrins. Org. Lett. 2004, 6, 3211–3214. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, H.; Mizutani, T. Multifunctional and Chiral Porphyrins: Model Receptors for Chiral Recognition. Acc. Chem. Res. 1998, 31, 81–89. [Google Scholar] [CrossRef]
- Konishi, K.; Takahata, Y.; Aida, T.; Inoue, S.; Kuroda, R. Chiral N-Substituted Porphyrins Related to Heme Inactivation Products. First Crystallographic Determination of Absolute Stereochemistry and Correlation with Circular Dichroism. J. Am. Chem. Soc. 1993, 115, 1169–1170. [Google Scholar] [CrossRef]
- Lee, H.; Hong, K.-I.; Jang, W.-D. Design and applications of molecular probes containing porphyrin derivatives. Coord. Chem. Rev. 2018, 354, 46–73. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, C.; Wang, Y.; Lang, J.-P. Crystallographic and DFT studies on host-guest complexes consisting of zinc bisporphyrinates and 1-phenylethylamine. J. Coord. Chem. 2019, 72, 1156–1170. [Google Scholar] [CrossRef]
- Zhuo, C.-C.; Li, L.; Hu, C.-J.; Lang, J.-P. Host-guest assembly for highly sensitive probing of a chiral mono-alcohol with a zinc trisporphyrinate. Sci. Rep. 2017, 7, 3829. [Google Scholar] [CrossRef]
- Hu, Q.; Zhuo, C.; Wang, Y.; Hu, C.; Lang, J. Chirality Transfer from Chiral Monoamines to an m-Phthalic Diamide-Linked Zinc Bisporphyrinate with a Benzylamide Substituent. Inorg. Chem. 2017, 56, 10204–10214. [Google Scholar] [CrossRef]
- Hu, T.; Hu, C.; Wang, Y.; Young, D.J.; Lang, J.-P. Stoichiometrically controlled chirality inversion in zinc bisporphyrinate–monoamine complexes. Dalt. Trans. 2018, 47, 5503–5512. [Google Scholar] [CrossRef]
- Hu, T.; Liu, T.; Zhang, Z.; Wang, Y.; Yang, Y.; Young, D.J.; Hu, C.; Lang, J.-P. Precise control of chirality transfer by adjusting the alkyl substituents of guests. Dye. Pigment. 2019, 160, 692–699. [Google Scholar] [CrossRef]
- Hembury, G.A.; Borovkov, V.V.; Inoue, Y. Chirality-sensing supramolecular systems. Chem. Rev. 2008, 108, 1–73. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, B.; Levon, K. Potentiometric sensing of chiral amino acids. Chem. Mater. 2003, 15, 2774–2779. [Google Scholar] [CrossRef]
- Cavazzini, A.; Nadalini, G.; Dondi, F.; Gasparrini, F.; Ciogli, A.; Villani, C. Study of mechanisms of chiral discrimination of amino acids and their derivatives on a teicoplanin-based chiral stationary phase. J. Chromatogr. A 2004, 1031, 143–158. [Google Scholar] [CrossRef]
- Fu, Y.; Han, Q.; Chen, Q.; Wang, Y.; Zhou, J.; Zhang, Q. A new strategy for chiral recognition of amino acids. Chem. Commun. 2012, 48, 2322–2324. [Google Scholar] [CrossRef] [PubMed]
- Carmo dos Santos, N.A.; Badetti, E.; Licini, G.; Abbate, S.; Longhi, G.; Zonta, C. A stereodynamic fluorescent probe for amino acids. Circular dichroism and circularly polarized luminescence analysis. Chirality 2018, 30, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Cuq, J.L. Chemistry, analysis, nutritional value, and toxicology of tryptophan in food. A review. J. Agric. Food Chem. 1988, 36, 1079–1093. [Google Scholar] [CrossRef]
- Cifuentes, A. Recent advances in the application of capillary electromigration methods for food analysis. Electrophoresis 2006, 27, 283–303. [Google Scholar] [CrossRef]
- Herrero, M.; Ibáñez, E.; Martín-Álvarez, P.J.; Cifuentes, A. Analysis of chiral amino acids in conventional and transgenic maize. Anal. Chem. 2007, 79, 5071–5077. [Google Scholar] [CrossRef][Green Version]
- Kuroda, Y.; Kato, Y.; Higashioji, T.; Ogoshi, H. New Chiral Porphyrins—Syntheses and Molecular Recognition of Amino Acid Esters. Angew. Chem. Int. Ed. Engl. 1993, 32, 723–724. [Google Scholar] [CrossRef]
- Yasuhisa Kuroda, R.; Kato, Y.; Higashioji, T.; Hasegawa, J.; Kawanami, S.; Takahashi, M.; Shiraishi, N.; Tanabe, K.; Ogoshi, H. Chiral Amino Acid Recognition by a Porphyrin-Based Artificial. J. Am. Chem. Soc 1995, 117, 10950–10958. [Google Scholar] [CrossRef]
- Mizutani, E.T.; Ema, T.; Tomita, T.; Kuroda, Y.; Ogoshi, H. Design and Synthesis of a Trifunctional Chiral Porphyrin with C2 Symmetry as a Chiral Recognition Host for Amino Acid. J. Am. Chem. Soc 1994, 116, 4240–4250. [Google Scholar] [CrossRef]
- Mizutani, T.; Ema, T.; Yoshida, T.; Kuroda, Y.; Ogoshi, H. Recognition of α-Amino Acid Esters by Zinc Porphyrin Derivatives via Coordination and Hydrogen Bonding Interactions. Evidence for Two-Point Fixation from Thermodynamic and Induced Circular Dichroism Spectroscopic Studies. Inorg. Chem. 1993, 32, 2072–2077. [Google Scholar] [CrossRef]
- Wang, C.Z.; Zhu, Z.A.; Li, Y.; Chen, Y.T.; Wen, X.; Miao, F.M.; Chan, W.L.; Chan, A.S. Chiral recognition of amino acid esters by zinc porphyrin derivatives. New J. Chem. 2001, 25, 801–806. [Google Scholar] [CrossRef]
- Schwenninger, R.; Ramondenc, Y.; Wurst, K.; Schlögl, J.; Kräutler, B. A Highly Enantiopure Biconcave Porphyrin with Effective D4-Structure. Chem. Eur. J. 2000, 6, 1214–1223. [Google Scholar] [CrossRef]
- Schwenninger, R.; Schlögl, J.; Maynollo, J.; Gruber, K.; Ochsenbein, P.; Bürgi, H.-B.; Konrat, R.; Kräutler, B. Metal Complexes of a Biconcave Porphyrin with D4-Structure—Versatile Chiral Shift Agents. Chem. Eur. J. 2001, 7, 2676–2686. [Google Scholar] [CrossRef]
- Imai, H.; Munakata, H.; Uemori, Y.; Sakura, N. Chiral Recognition of Amino Acids and Dipeptides by a Water-Soluble Zinc Porphyrin. Inorg. Chem. 2004, 43, 1211–1213. [Google Scholar] [CrossRef]
- Fa, H.B.; Zhao, L.; Wang, X.Q.; Yu, J.H.; Huang, Y.B.; Yang, M.; Wang, D.J. Chiral recognition of mesoporous SBA-15 with an incorporated chiral porphyrin. Eur. J. Inorg. Chem. 2006, 2006, 4355–4361. [Google Scholar] [CrossRef]
- Crossley, M.J.; Hambley, T.W.; MacKay, L.G.; Try, A.C.; Walton, R. Porphyrin analogues of Tröger’s base: Large chiral cavities with a bimetallic binding site. J. Chem. Soc. Chem. Commun. 1995, 1077–1079. [Google Scholar] [CrossRef]
- Crossley, M.J.; MacKay, L.G.; Try, A.C. Enantioselective recognition of histidine and lysine esters by porphyrin chiral clefts and detection of amino acid conformations in the bound state. J. Chem. Soc. Chem. Commun. 1995, 1925–1927. [Google Scholar] [CrossRef]
- Tatar, A.; Valík, M.; Novotná, J.; Havlík, M.; Dolenský, B.; Král, V.; Urbanová, M. Preparation and enantioselectivity binding studies of a new chiral cobalt(II)porphyrin-Tröger’s base conjugate. Chirality 2014, 26, 361–367. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, B.; Seo, J. Metalloporphyrin dimers bridged by a peptoid helix: Host-guest interaction and chiral recognition. Molecules 2018, 23, 2741. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Hu, C.; Wang, Y. Enantioselectivity of a tartaric acid amide linked zinc bisporphyrinate towards amino acid esters. Dye. Pigment. 2020, 176, 108223. [Google Scholar] [CrossRef]
- Wu, X.; Starnes, S.D. L-nipecotic acid-porphyrin derivative: A chiral host with introverted functionality for chiral recognition. Org. Lett. 2012, 14, 3652–3655. [Google Scholar] [CrossRef]
- Stefanelli, M.; Magna, G.; Zurlo, F.; Caso, F.M.; Di Bartolomeo, E.; Antonaroli, S.; Venanzi, M.; Paolesse, R.; Di Natale, C.; Monti, D. Chiral Selectivity of Porphyrin-ZnO Nanoparticle Conjugates. ACS Appl. Mater. Interfaces 2019, 11, 12077–12087. [Google Scholar] [CrossRef]
- Ballantine, D.S., Jr.; White, R.M.; Martin, S.J.; Ricco, A.J.; Zellers, E.T.; Frye, G.C.; Wohltjen, H. Acoustic Wave Sensors: Theory, Design and Physico-Chemical Applications, 1st ed.; Academic Press: San Diego, CA, USA; Elsevier: San Diego, CA, USA, 1996; ISBN 9780080523330. [Google Scholar]
- Ema, T.; Ouchi, N.; Doi, T.; Korenaga, T.; Sakai, T. Highly sensitive chiral shift reagent bearing two zinc porphyrins. Org. Lett. 2005, 7, 3985–3988. [Google Scholar] [CrossRef]
- Borovkov, V.V.; Hembury, G.A.; Inoue, Y. Supramolecular chirogenesis with bis-chlorin versus bis-porphyrin hosts: Peculiarities of chirality induction and modulation of optical activity. J. Org. Chem. 2005, 70, 8743–8754. [Google Scholar] [CrossRef] [PubMed]
- Borovkov, V.V.; Muranaka, A.; Hembury, G.A.; Origane, Y.; Ponomarev, G.V.; Kobayashi, N.; Inoue, Y. Chiral bis-chlorin: Enantiomer resolution and absolute configuration determination. Org. Lett. 2005, 7, 1015–1018. [Google Scholar] [CrossRef]
- Ikeda, T.; Sada, K.; Shinkai, S.; Takeuchi, M. Enantioselective recognition of dicarboxylic acid guests based on an allosteric effect of a chiral double-decker porphyrin which changes the stoichiometry upon the guest binding. Supramol. Chem. 2011, 23, 59–64. [Google Scholar] [CrossRef]
- Ema, T.; Ura, N.; Eguchi, K.; Ise, Y.; Sakai, T. Chiral porphyrin dimer with a macrocyclic cavity for intercalation of aromatic guests. Chem. Commun. 2011, 47, 6090–6092. [Google Scholar] [CrossRef] [PubMed]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The Exciton Model In Molecular Spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Matile, S.; Berova, N.; Nakanishi, K.; Novkova, S.; Philipova, I.; Blagoev, B. Porphyrins: Powerful Chromophores for Structural Studies by Exciton-Coupled Circular Dichroism. J. Am. Chem. Soc. 1995, 117, 7021–7022. [Google Scholar] [CrossRef]
- Berova, N.; Di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yang, H.; Li, X.; Wang, C.; Zhan, X.; Qi, D.; Bian, Y.; Jiang, J. Chiral Discrimination of Diamines by a Binaphthalene-Bridged Porphyrin Dimer. Inorg. Chem. 2017, 56, 8223–8231. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Ikbal, S.A.; Rath, S.P. Complexation of Chiral Zinc(II)Porphyrin Tweezer with Chiral Guests: Control, Discrimination and Rationalization of Supramolecular Chirality. Inorg. Chem. 2020, 59, 7795–7809. [Google Scholar] [CrossRef] [PubMed]
- Gilissen, P.J.; Slootbeek, A.D.; Ouyang, J.; Vanthuyne, N.; Bakker, R.; Elemans, J.A.A.W.; Nolte, R.J.M. Enantioselective synthesis of chiral porphyrin macrocyclic hosts and kinetic enantiorecognition of viologen guests. Chem. Sci. 2021, 12, 1661–1667. [Google Scholar] [CrossRef]
- Easson, L.H.; Stedman, E. Studies on the relationship between chemical constitution and physiological action. Biochem. J. 1933, 27, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Ogston, A.G. Interpretation of experiments on metabolic processes, using isotopic tracer elements [9]. Nature 1949, 163, 963. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A. Chiral recognition mechanisms. Anal. Chem. 2006, 78, 2093–2099. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Travagliante, G.; Gaeta, M.; Purrello, R.; D’Urso, A. Recognition and Sensing of Chiral Organic Molecules by Chiral Porphyrinoids: A Review. Chemosensors 2021, 9, 204. https://doi.org/10.3390/chemosensors9080204
Travagliante G, Gaeta M, Purrello R, D’Urso A. Recognition and Sensing of Chiral Organic Molecules by Chiral Porphyrinoids: A Review. Chemosensors. 2021; 9(8):204. https://doi.org/10.3390/chemosensors9080204
Chicago/Turabian StyleTravagliante, Gabriele, Massimiliano Gaeta, Roberto Purrello, and Alessandro D’Urso. 2021. "Recognition and Sensing of Chiral Organic Molecules by Chiral Porphyrinoids: A Review" Chemosensors 9, no. 8: 204. https://doi.org/10.3390/chemosensors9080204
APA StyleTravagliante, G., Gaeta, M., Purrello, R., & D’Urso, A. (2021). Recognition and Sensing of Chiral Organic Molecules by Chiral Porphyrinoids: A Review. Chemosensors, 9(8), 204. https://doi.org/10.3390/chemosensors9080204








