Ionogels Based on a Single Ionic Liquid for Electronic Nose Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Iron Oxide Particles
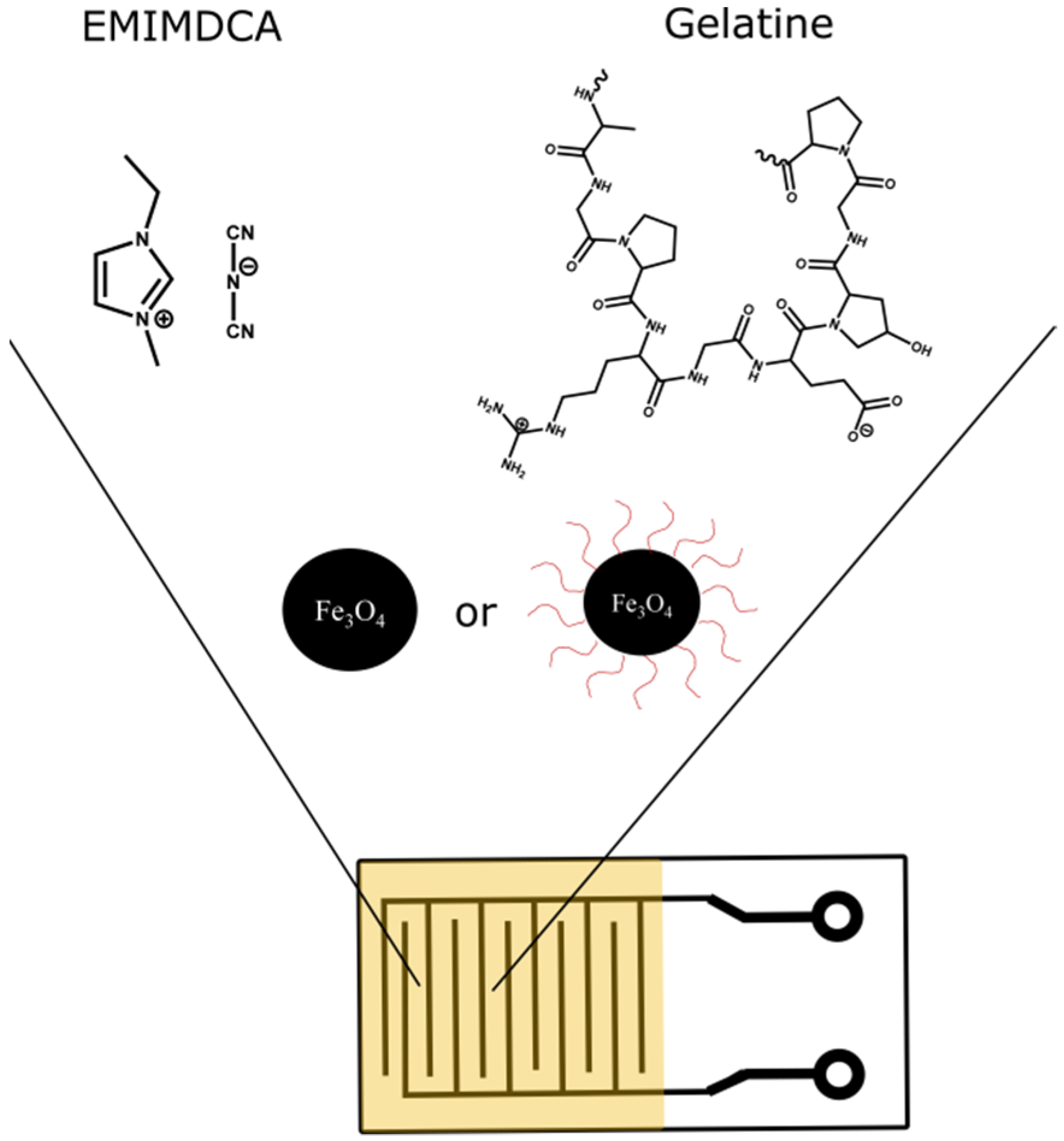
2.3. Ionogel Preparation
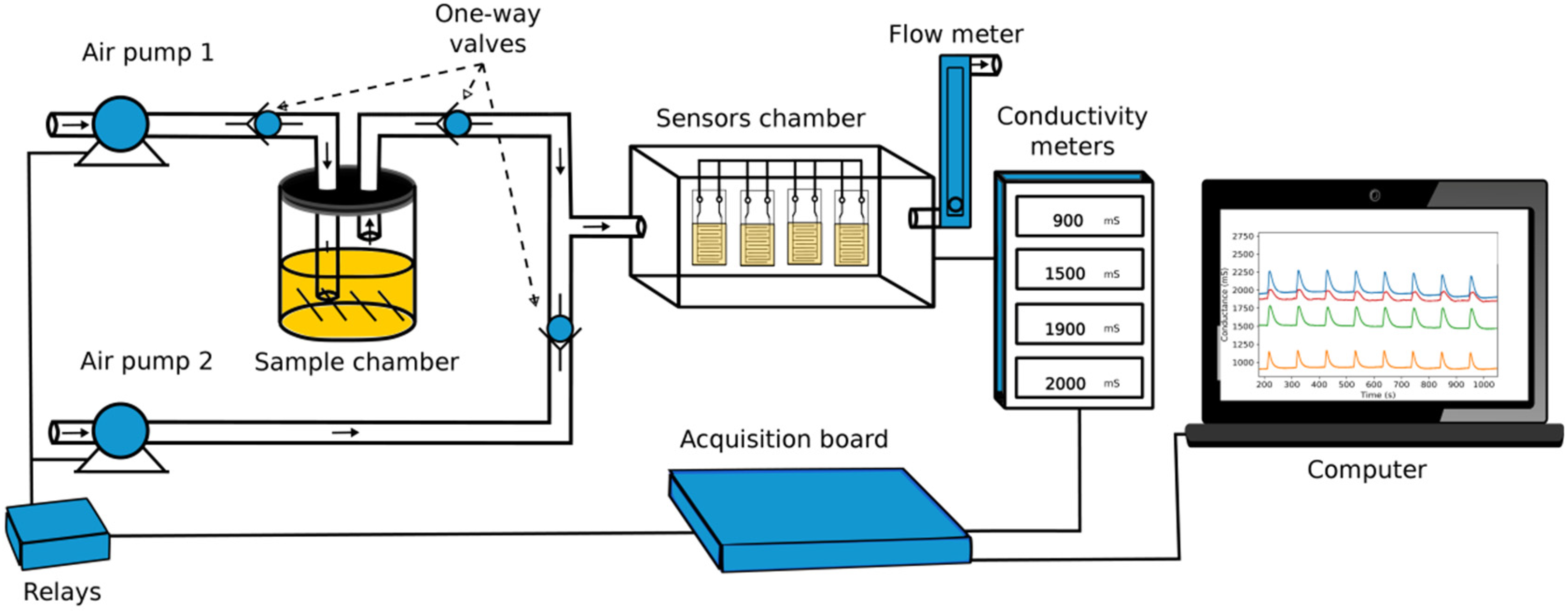
2.4. Sensor Preparation and Application in e-Nose
2.5. Data Treatment
3. Results and Discussion
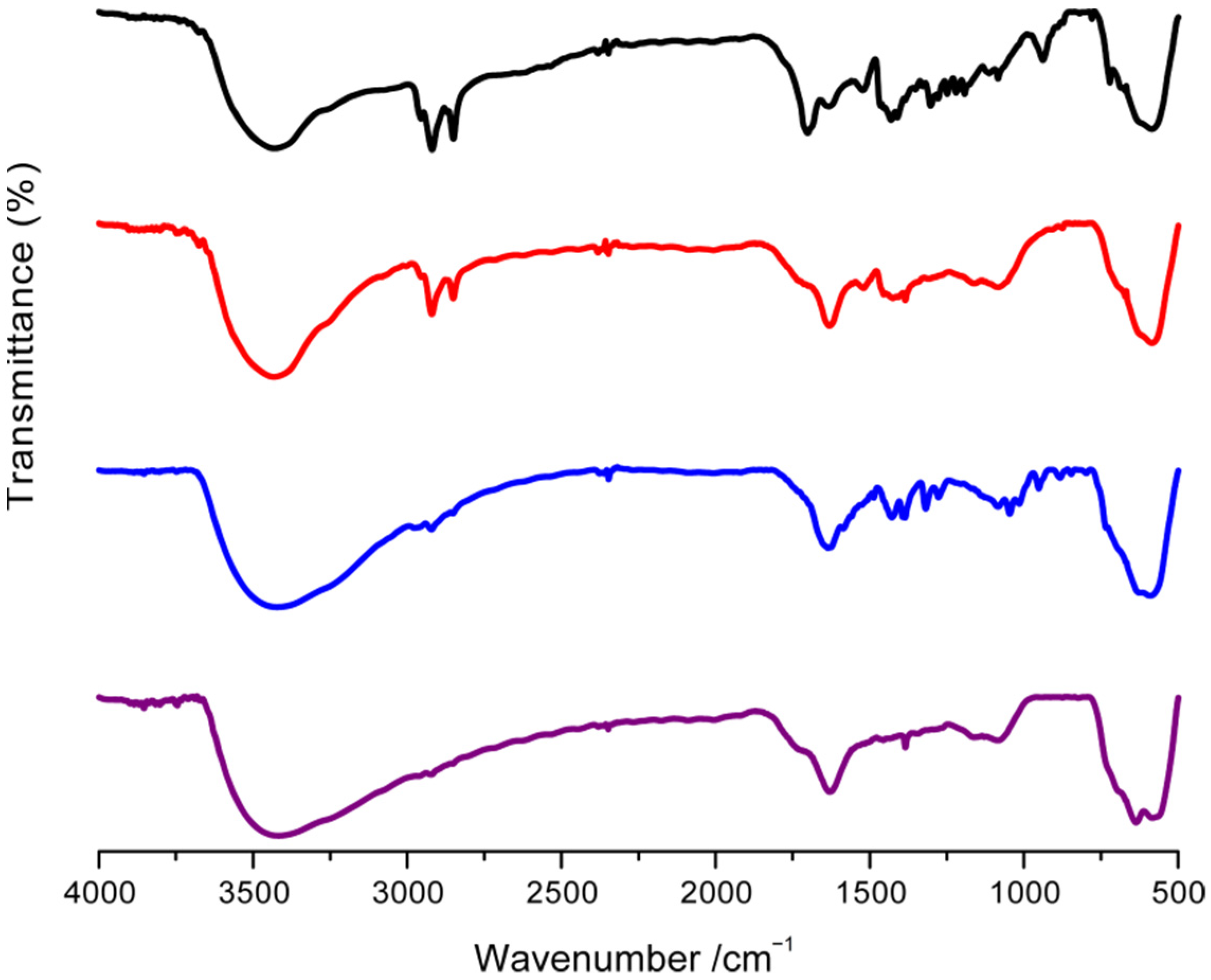
3.1. Characterization of Fe3O4
3.2. The Sensors
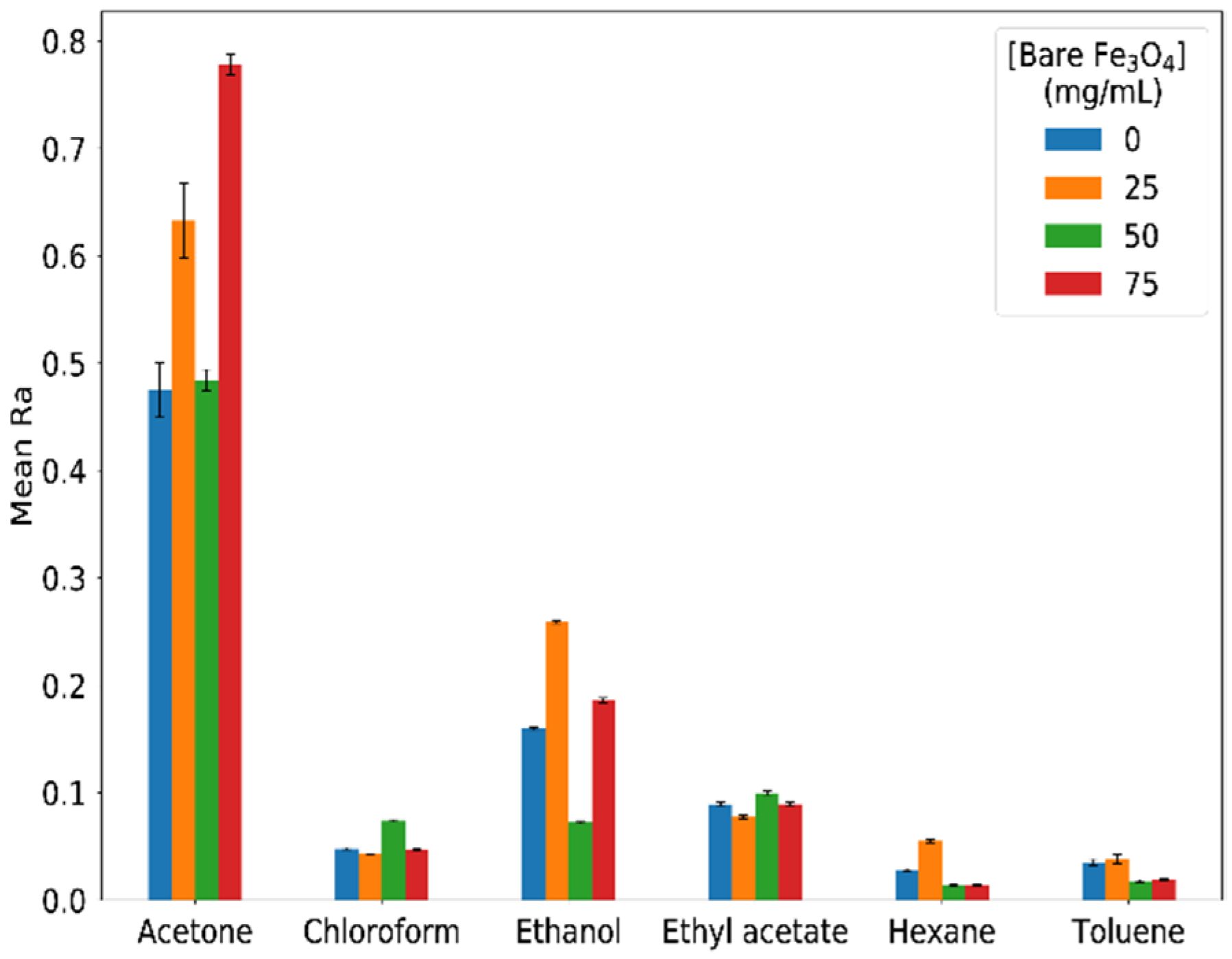
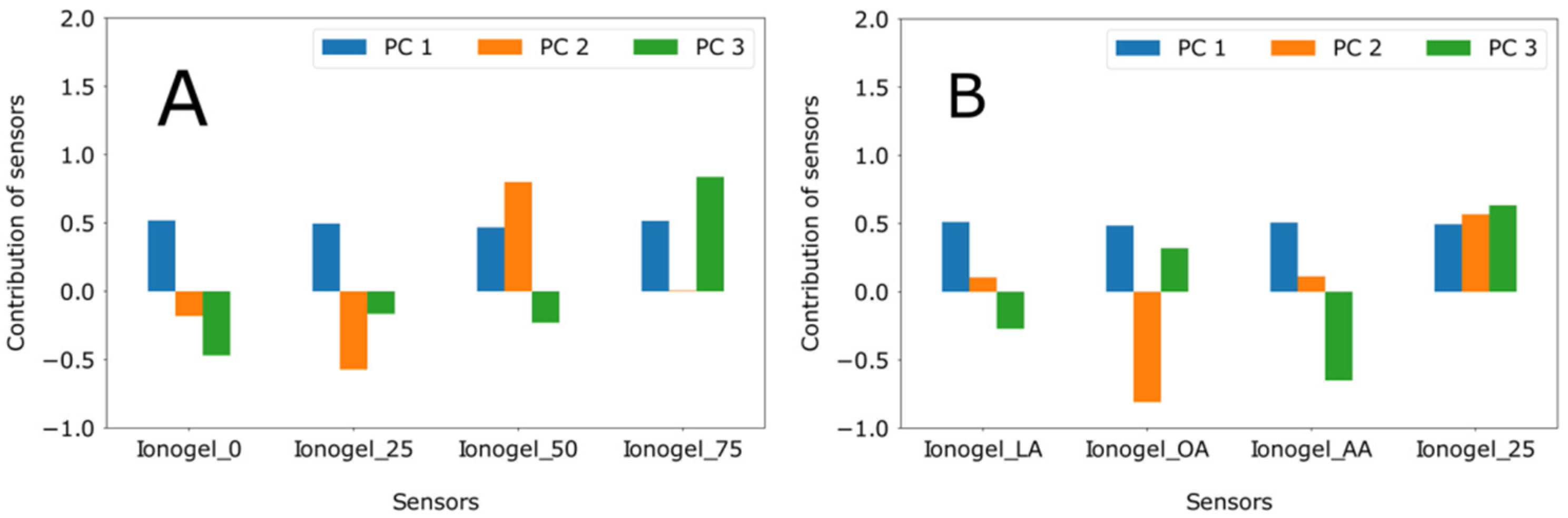
3.3. Effect of Particle Concentration in the Ionogels
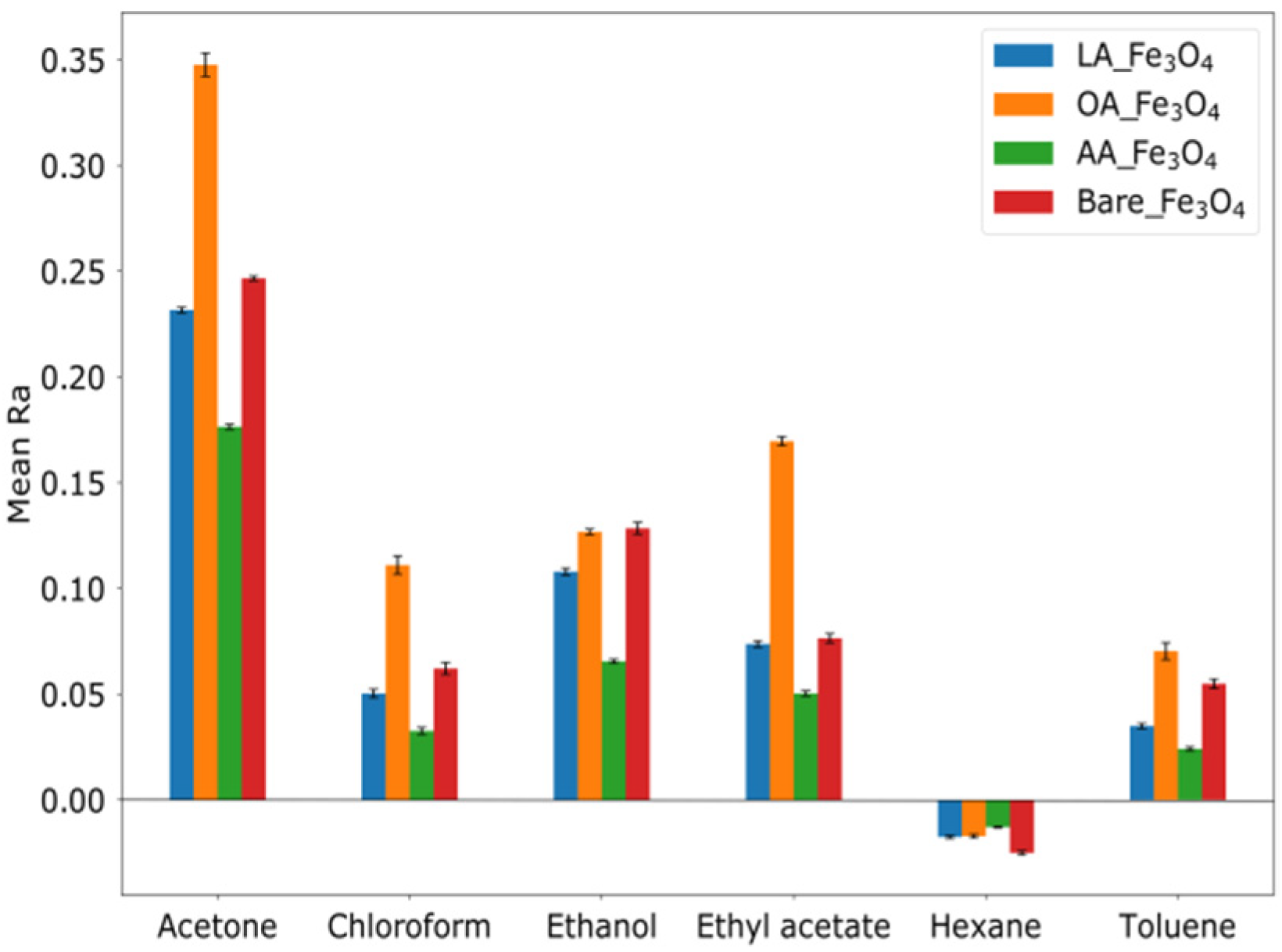
3.4. Effect of Particle Functionalization in Ionogels
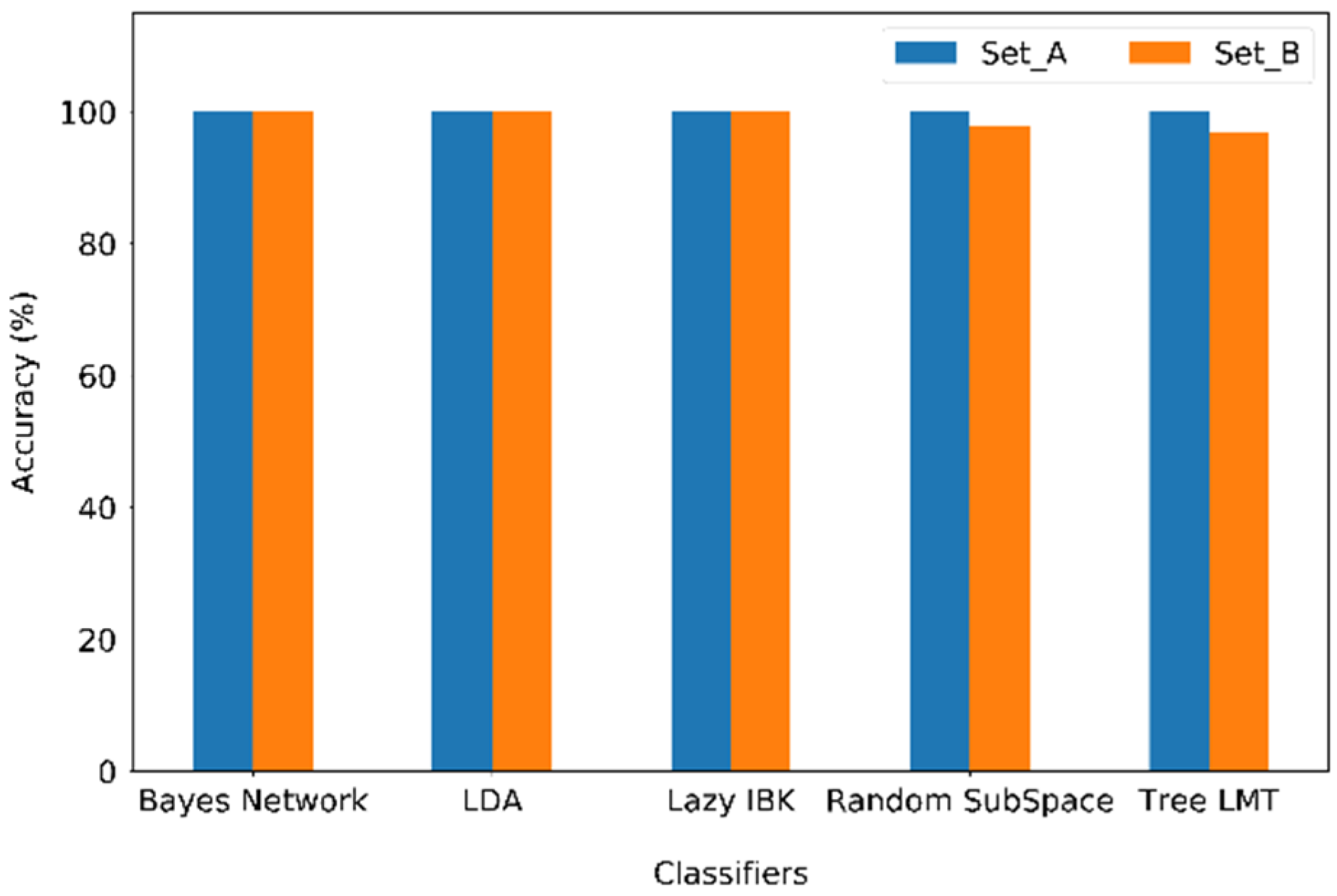
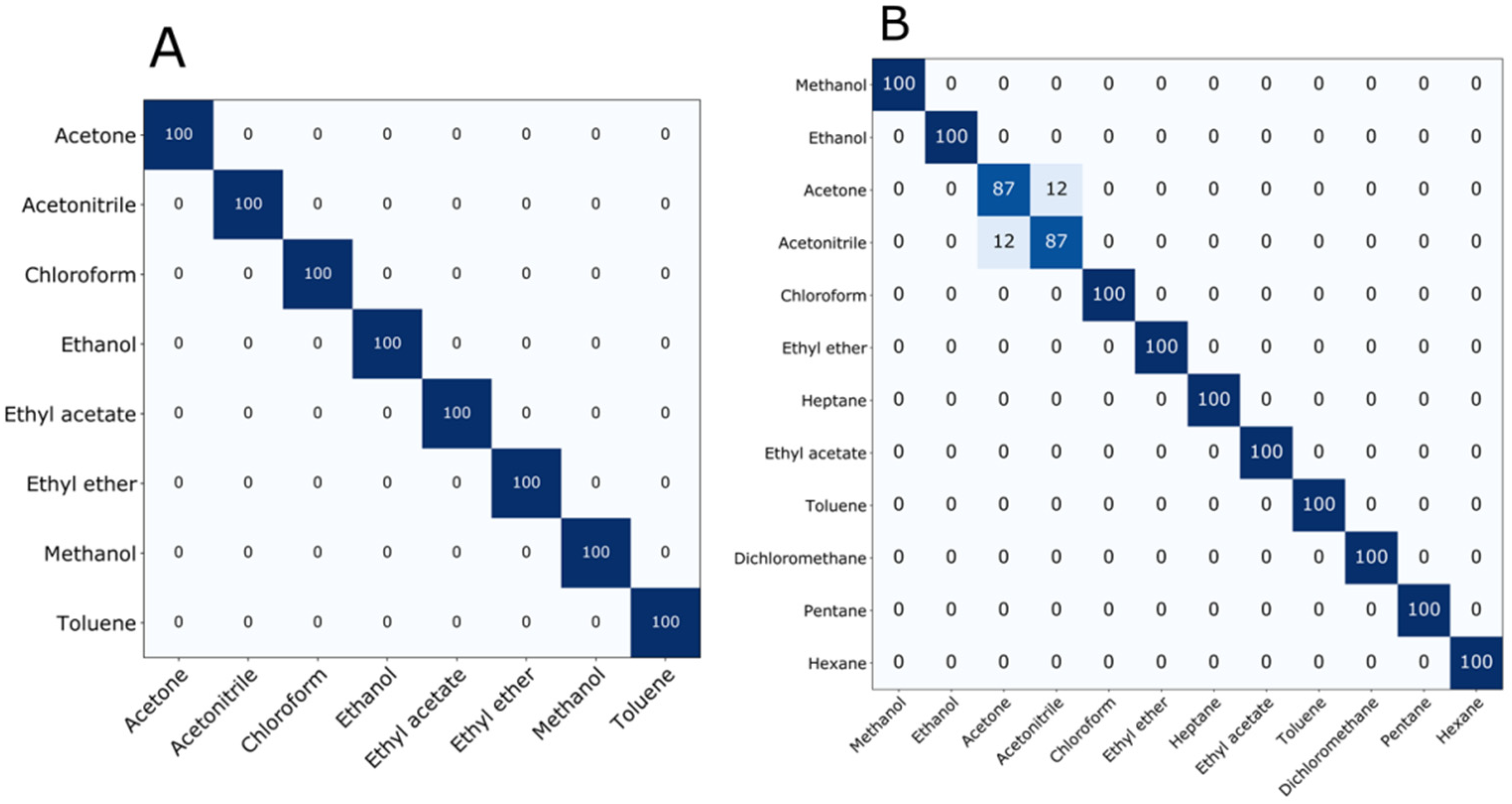
3.5. Ionogels in e-Nose to Classify Distinct VOCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Keaveney, S.T.; Haines, R.S.; Harper, J.B. Ionic liquid solvents: The importance of microscopic interactions in predicting organic reaction outcomes. Pure Appl. Chem. 2017, 89, 745–757. [Google Scholar] [CrossRef][Green Version]
- Zolfigol, M.A.; Khazaei, A.; Moosavi-Zare, A.R.; Zare, A. 3-Methyl-1-sulfonic acid imidazolium chloride as a new, Efficient and recyclable catalyst and solvent for the preparation of N-sulfonyl imines at room Temperature. J. Iran. Chem. Soc. 2010, 7, 646–651. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Sasikumar, B.; Arthanareeswaran, G.; Ismail, A.F. Recent progress in ionic liquid membranes for gas separation. J. Mol. Liq. 2018, 266, 330–341. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef]
- Andrzejewska, E.; Marcinkowska, A.; Zgrzeba, A. Ionogels—Materials containing immobilized ionic liquids. Polimery 2017, 62, 344–352. [Google Scholar] [CrossRef]
- Viau, L.; Tourné-Péteilh, C.; Devoisselle, J.-M.; Vioux, A. Ionogels as drug delivery system: One-step sol–gel synthesis using imidazolium ibuprofenate ionic liquid. Chem. Commun. 2010, 46, 228–230. [Google Scholar] [CrossRef]
- Zhao, K.; Song, H.; Duan, X.; Wang, Z.; Liu, J.; Ba, X. Novel Chemical Cross-Linked Ionogel Based on Acrylate Terminated Hyperbranched Polymer with Superior Ionic Conductivity for High Performance Lithium-Ion Batteries. Polymers 2019, 11, 444. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, X.; Yang, S.; Zhang, Y.; Zhang, R.; Hu, S.; Bao, X.; Zhao, F.; Li, X.; Liu, Q. Design of sepiolite-supported ionogel-embedded composite membranes without proton carrier wastage for wide-temperature-range operation of proton exchange membrane fuel cells. J. Mater. Chem. A 2019, 7, 15288–15301. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Guo, J.; Yuan, C.; Yan, F. Ultrahigh Ionic Liquid Content Supramolecular Ionogels for Quasi-Solid-State Dye Sensitized Solar Cells. Electrochim. Acta 2015, 165, 98–104. [Google Scholar] [CrossRef]
- Joshi, N.; Rawat, K.; Solanki, P.R.; Bohidar, H.B. Biocompatible laponite ionogels based non-enzymatic oxalic acid sensor. Sens. Bio-Sens. Res. 2015, 5, 105–111. [Google Scholar] [CrossRef]
- Ersoez, B.; Bauersfeld, M.-L.; Wöllenstein, J. Ionogel—Based Composite Material for CO2 Sensing Deposited on a Chemiresistive Transducer. Proceedings 2016, 1, 314. [Google Scholar] [CrossRef]
- Chrobok, A.; Baj, S.; Pudło, W.; Jarzębski, A. Supported ionic liquid phase catalysis for aerobic oxidation of primary alcohols. Appl. Catal. A 2010, 389, 179–185. [Google Scholar] [CrossRef]
- Vittoz, P.-F.; El Siblani, H.; Bruma, A.; Rigaud, B.; Sauvage, X.; Fernandez, C.; Vicente, A.; Barrier, N.; Malo, S.; Levillain, J.; et al. Insight in the Alginate Pd-Ionogels—Application to the Tsuji–Trost Reaction. ACS Sustain. Chem. Eng. 2018, 6, 5192–5197. [Google Scholar] [CrossRef]
- Netto, M.M.O.; Gonçalves, W.B.; Li, R.W.C.; Gruber, J. Biopolymer based ionogels as active layers in low-cost gas sensors for electronic noses. Sens. Actuators B Chem. 2020, 315, 128025. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, M.; Adhikari, B. Advances of electronic nose and its application in fresh foods: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2700–2710. [Google Scholar] [CrossRef]
- Vos, H.G.J.; Stevan, S.L., Jr.; Ayub, R.A. Peach growth cycle monitoring using an electronic nose. Comput. Electron. Agric. 2019, 163, 104858. [Google Scholar] [CrossRef]
- Gamboa, J.C.R.; Albarracin, E.S.; da Silva, A.J.; Ferreira, T.A.E. Electronic nose dataset for detection of wine spoilage thresholds. Data Br. 2019, 25, 104202. [Google Scholar] [CrossRef]
- Ezhilan, M.; Nesakumar, N.; Babu, K.J.; Srinandan, C.S.; Rayappan, J.B.B. Freshness Assessment of Broccoli using Electronic Nose. Measurement 2019, 145, 735–743. [Google Scholar] [CrossRef]
- John, A.T.; Murugappan, K.; Nisbet, D.R.; Tricoli, A. An Outlook of Recent Advances in Chemiresistive Sensor-Based Electronic Nose Systems for Food Quality and Environmental Monitoring. Sensors 2021, 21, 2271. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.R.; Li, R.W.C.; Takahashi, E.S.; Rehder, G.P.; Ceccantini, G.; Gruber, J. Wood identification by a portable low-cost polymer-based electronic nose. RSC Adv. 2016, 6, 109945. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, H.; Raj, V.B.; Nimal, A.T.; Gupta, V.; Singh, V.K. Trace Detection of Nerve Agent Simulant in the Fuel Vapour Environment using Metal Oxide/Surface Acoustic Wave E-Nose. Def. Sci. J. 2020, 70, 520–528. [Google Scholar] [CrossRef]
- Deshmukh, S.; Bandyopadhyay, R.; Bhattacharyya, N.; Pandey, R.A.; Jana, A. Application of electronic nose for industrial odors and gaseous emissions measurement and monitoring—An overview. Talanta 2015, 144, 329–340. [Google Scholar] [CrossRef]
- Sun, H.; Tian, F.; Liang, Z.; Sun, T.; Yu, B.; Yang, S.X.; He, Q.; Zhang, L.; Liu, X. Sensor Array Optimization of Electronic Nose for Detection of Bacteria in Wound Infection. IEEE Trans. Ind. Electron. 2017, 64, 7350–7358. [Google Scholar] [CrossRef]
- Lim, S.H.; Martino, R.; Anikst, V.; Xu, Z.; Mix, S.; Benjamin, R.; Schub, H.; Eiden, M.; Rhodes, P.A.; Banaei, N. Rapid Diagnosis of Tuberculosis from Analysis of Urine Volatile Organic Compounds. ACS Sens. 2016, 1, 852–856. [Google Scholar] [CrossRef]
- Santini, G.; Mores, N.; Penas, A.; Capuano, R.; Mondino, C.; Trove, A.; Macagno, F.; Zini, G.; Cattani, P.; Martinelli, E.; et al. Electronic Nose and Exhaled Breath NMR-based Metabolomics Applications in Airways Disease. Curr. Top. Med. Chem. 2016, 16, 1610–1630. [Google Scholar] [CrossRef]
- Xia, Q.S.; Zhang, Z.C.; Liu, Y.J.; Leng, J.S. Buckypaper and its composites for aeronautic applications. Compos. Part B Eng. 2020, 199, 108231. [Google Scholar] [CrossRef]
- Ravishankar, B.; Nayak, S.K.; Kader, M.A. Hybrid composites for automotive applications—A review. J. Reinf. Plast Compos. 2019, 38, 835–845. [Google Scholar] [CrossRef]
- Park, S.J.; Park, C.S.; Yoon, H. Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids. Polymers 2017, 9, 155. [Google Scholar] [CrossRef]
- Zheng, Y.G.; Li, H.Y.; Shen, W.F.; Jian, J.W. Wearable electronic nose for human skin odor identification: A preliminary study. Sens. Actuators A Phys. 2019, 285, 395–405. [Google Scholar] [CrossRef]
- Swe, M.M.; Eamsa-Ard, T.; Srikhirin, T.; Kerdcharoen, T. Monitoring the Freshness Level of Beef Using Nanocomposite Gas Sensors in Electronic nose. In Proceedings of the 2019 IEEE International Conference on Consumer Electronics—Asia (ICCE-Asia), Bangkok, Thailand, 12–14 June 2019; pp. 100–103. [Google Scholar] [CrossRef]
- Carvalho, T.; Vidinha, P.; Vieira, B.R.; Li, R.W.C.; Gruber, J. Ion Jelly: A novel sensing material for gas sensors and electronic noses. J. Mater. Chem. C 2014, 2, 696–700. [Google Scholar] [CrossRef]
- Hussain, A.; Semeano, A.T.S.; Palma, S.I.C.J.; Pina, A.S.; Almeida, J.; Medrado, B.F.; Pádua, A.C.C.S.; Carvalho, A.L.; Dionísio, M.; Li, R.W.C.; et al. Tunable Gas Sensing Gels by Cooperative Assembly. Adv. Funct. Mater. 2017, 27, 1700803. [Google Scholar] [CrossRef]
- Semeano, A.T.S.; Maffei, D.F.; Palma, S.; Li, R.W.C.; Franco, B.D.G.M.; Roque, A.C.A.; Gruber, J. Tilapia Fish microbial spoilage monitored by a single optical gas sensor. Food Control 2018, 89, 72–76. [Google Scholar] [CrossRef]
- Gil-González, N.; Benito-Lopez, F.; Castaño, E.; Morant-Miñana, M.C. Imidazole-based ionogel as room temperature benzene and formaldehyde sensor. Microchim. Acta 2020, 187, 638. [Google Scholar] [CrossRef]
- Palma, S.I.C.J.; Marciello, M.; Carvalho, A.; Veintemillas-Verdaguer, S.; del Puerto Morales, M.; Roque, A.C.A. Effects of phase transfer ligands on monodisperse iron oxide magnetic nanoparticles. J. Colloid Interface Sci. 2015, 437, 147–155. [Google Scholar] [CrossRef]
- Da Rocha, R.T.; Gutz, I.G.R.; do Lago, C.L. A Low-Cost and High-Performance Conductivity Meter. J. Chem. Educ. 1997, 74, 572. [Google Scholar] [CrossRef]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Moré, R.; Scholz, M.; Busse, G.; Busse, L.; Paulmann, C.; Tolkiehn, M.; Techert, S. Hydrogen bond dynamics in crystalline β-9-anthracene carboxylic acid—a combined crystallographic and spectroscopic study. Phys. Chem. Chem. Phys. 2012, 14, 10187–10195. [Google Scholar] [CrossRef]
- Moré, R.; Busse, G.; Hallmann, J.; Paulmann, C.; Scholz, M.; Techert, S. Photodimerization of Crystalline 9-Anthracenecarboxylic Acid: A Nontopotactic Autocatalytic Transformation. J. Phys. Chem. C 2010, 114, 4142–4148. [Google Scholar] [CrossRef]
- Vidinha, P.; Lourenço, N.M.T.; Pinheiro, C.; Brás, A.R.; Carvalho, T.; Santos-Silva, T.; Mukhopadhyay, A.; Romão, M.J.; Parola, J.; Dionisio, M.; et al. Ion jelly: A tailor-made conducting material for smart electrochemical devices. Chem. Commun. 2008, 5842–5844. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, H.; Wu, J. Chemiresistive ionogel sensor array for the detection and discrimination of volatile organic vapor. Sens. Actuators B Chem. 2014, 202, 105–113. [Google Scholar] [CrossRef]
- Bonhôte, P.; Dias, A.-P.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, Highly Conductive Ambient-Temperature Molten Salts. Inorg. Chem. 1996, 35, 1168–1178. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A.; Torres, M.-J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Hall, M.; Frank, E.; Holmes, G.; Pfahringer, B.; Reutemann, P.; Witten, I.H. The WEKA data mining software. ACM SIGKDD Explor. Newsl. 2009, 11, 10–18. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kumar, V.; Bonyani, M.; Majhi, S.M.; Bang, J.H.; Kim, J.-Y.; Kim, H.W.; Kim, S.S.; Kim, K.-H. Conducting Polymer Nanofibers based Sensors for Organic and Inorganic Gaseous Compounds. Asian J. Atmos. Environ. 2020, 14, 85–104. [Google Scholar] [CrossRef]


| Entry | Sensor | Fe3O4 conc. in Water (mg/L) | Fe3O4_coating conc. in Organic Solvent (mg/L) |
|---|---|---|---|
| 1 | Ionogel_0 | - | - |
| 2 | Ionogel_25 | 25 | - |
| 3 | Ionogel_50 | 50 | - |
| 4 | Ionogel_75 | 75 | - |
| 5 | Ionogel_AO | 0 | 25 |
| 6 | Ionogel_AL | 0 | 25 |
| 7 | Ionogel_AA | 0 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, W.B.; Cervantes, E.P.; Pádua, A.C.C.S.; Santos, G.; Palma, S.I.C.J.; Li, R.W.C.; Roque, A.C.A.; Gruber, J. Ionogels Based on a Single Ionic Liquid for Electronic Nose Application. Chemosensors 2021, 9, 201. https://doi.org/10.3390/chemosensors9080201
Gonçalves WB, Cervantes EP, Pádua ACCS, Santos G, Palma SICJ, Li RWC, Roque ACA, Gruber J. Ionogels Based on a Single Ionic Liquid for Electronic Nose Application. Chemosensors. 2021; 9(8):201. https://doi.org/10.3390/chemosensors9080201
Chicago/Turabian StyleGonçalves, Wellington B., Evelyn P. Cervantes, Ana C. C. S. Pádua, Gonçalo Santos, Susana I. C. J. Palma, Rosamaria W. C. Li, Ana C. A. Roque, and Jonas Gruber. 2021. "Ionogels Based on a Single Ionic Liquid for Electronic Nose Application" Chemosensors 9, no. 8: 201. https://doi.org/10.3390/chemosensors9080201
APA StyleGonçalves, W. B., Cervantes, E. P., Pádua, A. C. C. S., Santos, G., Palma, S. I. C. J., Li, R. W. C., Roque, A. C. A., & Gruber, J. (2021). Ionogels Based on a Single Ionic Liquid for Electronic Nose Application. Chemosensors, 9(8), 201. https://doi.org/10.3390/chemosensors9080201









