How Chemoresistive Sensors Can Learn from Heterogeneous Catalysis. Hints, Issues, and Perspectives
Abstract
1. Introduction
2. What Can Be Expected from Further Interaction with Metal Oxide Heterogeneous Catalysis
3. A Selection of Case Studies
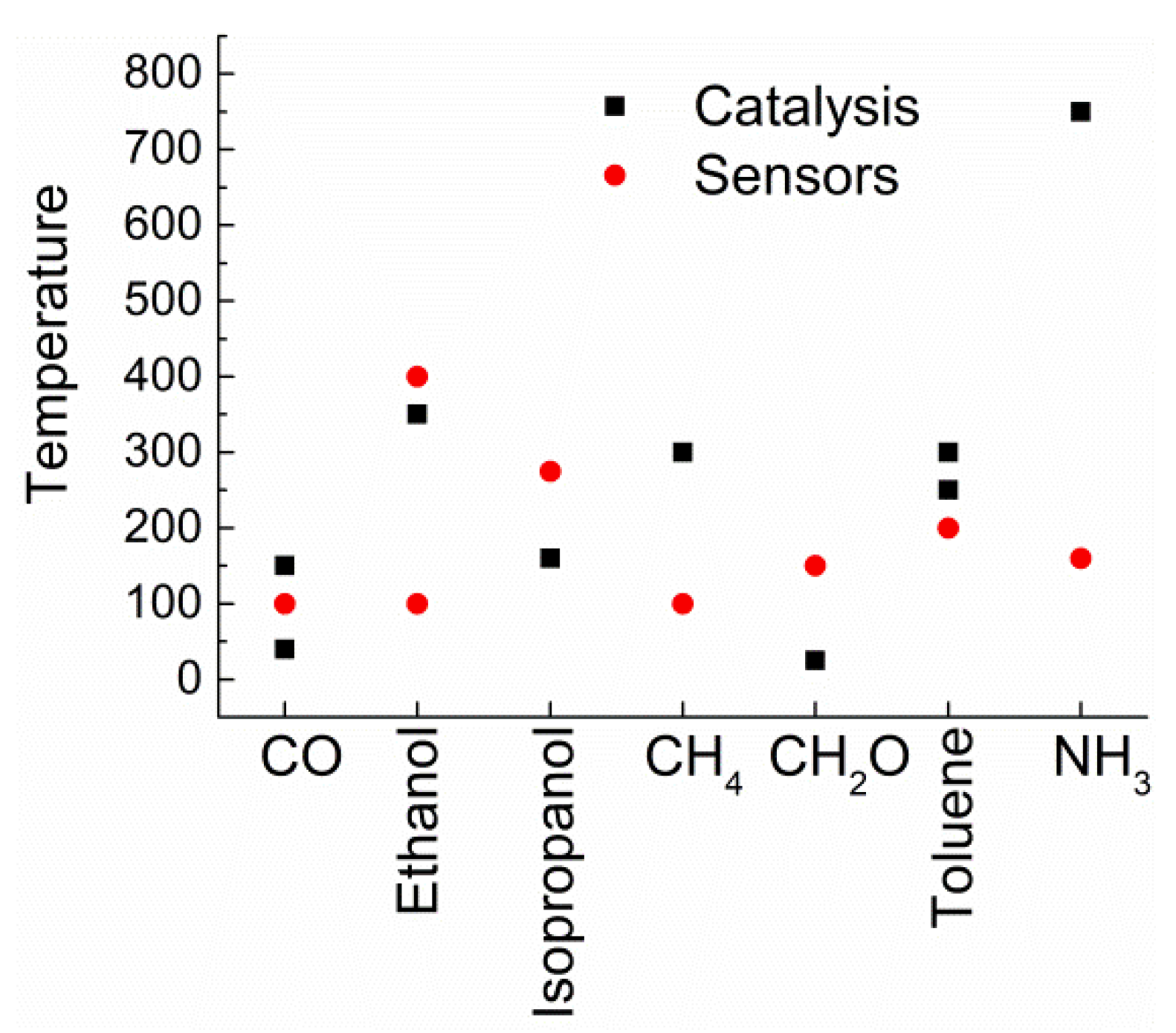
3.1. Co3O4
3.2. Manganese Oxides
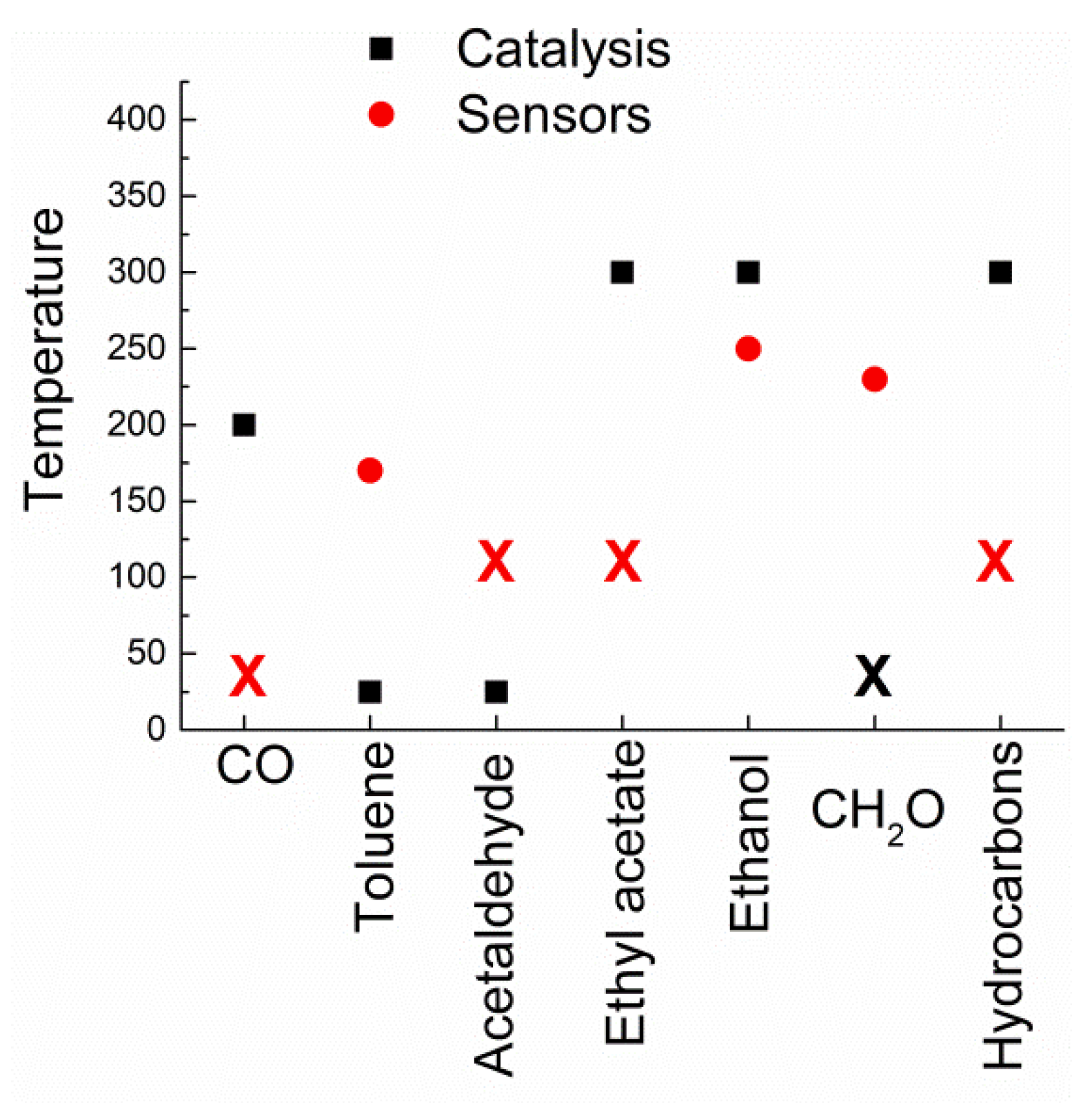
3.3. CuO and NiO
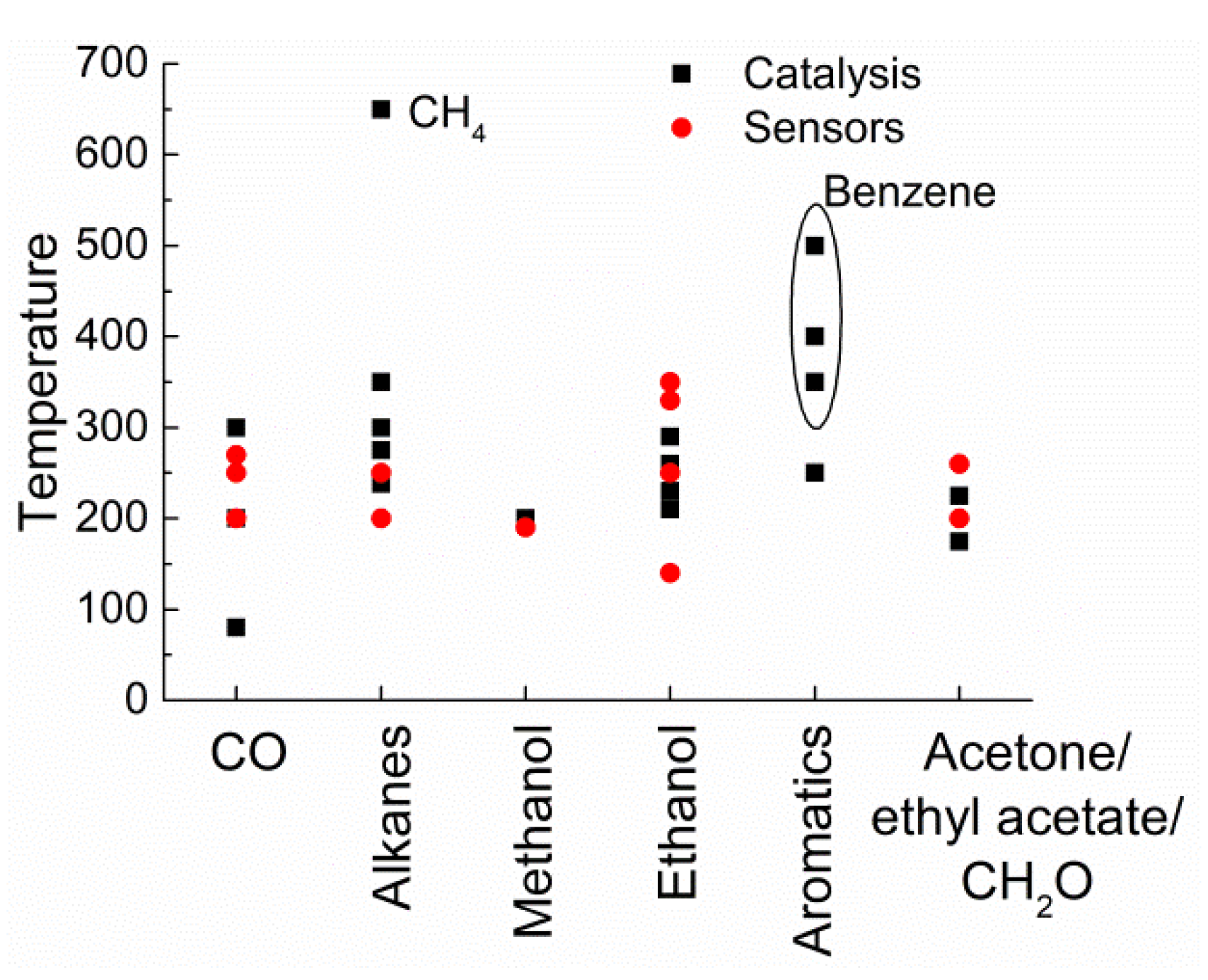
3.4. α-Cr2O3
3.5. Perovskites
3.6. α-Fe2O3
3.7. Semiconducting Oxide-Supported Catalysts
3.7.1. V2O5-TiO2 and SnO2-V2O5
3.7.2. Miscellanea of Other Semiconducting Oxide-Supported Catalysts
4. Reactions and Mechanisms
- (i)
- oxygen adsorption onto the sensor surface, implying charge extraction from the sensing material itself;
- (ii)
- reaction of the analyte with the sensor surface, implying consumption of surface oxygen, and restoring charge into the semiconducting sensing material.
1/2O2 + Co-☐-Co → Co-O-Co
OC-Co3+ + Co-O-Co → (CO2)-Co3+ + Co-☐-Co
(CO2)-Co3+ → CO2(g) + Co3+
5. Conclusions
- Metal oxide heterogeneous catalysts such as Co3O4, CuO and perovskites seem to have already been profitably exploited as chemoresistive sensors. However, several analytes such as alkanes and aromatics still deserve further attention.
- Other catalysts deserve more attention, such as α-Cr2O3 and manganese oxides.
- The field of supported (onto semiconducting oxides) catalysts seems very promising, and it deserves further investigation. It is only limited by the synthetic ability in preparing any novel material available by coupling single oxides: SnO2-MoO3, SnO2-WO3, TiO2-Fe2O3, etc.
- The design rule for perovskite catalysts is of potential interest for the design of chemosensors too.
- The presence and nature of oxygen vacancies deserve particular attention. This means that they must be carefully identified, characterized, and related to the ongoing reactions. This achievement only can provide further insight into the connections between heterogeneous catalysis and chemoresistive sensors.
- 6.
- The connection between catalysts and their applications as gas sensors is not straightforward. It may happen that a good catalyst for a given gaseous reaction does not provide an outstanding sensing response to the same gas, or vice versa. Recent findings suggest that, indeed, different species and/or mechanisms may be involved in the two application fields. This is just one stimulus more to deepen the knowledge of the sensing mechanisms, which can only beneficially draw from the well-established field of catalysis mechanisms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller, S.A.; Degler, D.; Feldmann, C.; Türk, M.; Moos, R.; Fink, K.; Studt, F.; Gerthsen, D.; Bârsan, N.; Grunwaldt, J.-D. Exploiting Synergies in Catalysis and Gas Sensing using Noble Metal-Loaded Oxide Composites. ChemCatChem 2018, 10, 864–880. [Google Scholar] [CrossRef]
- Xiong, M.; Gao, Z.; Qin, Y. Spillover in Heterogeneous Catalysis: New Insights and Opportunities. ACS Catal. 2021, 11, 3159–3172. [Google Scholar] [CrossRef]
- Conner, W.C.; Falconer, J.L. Spillover in Heterogeneous Catalysis. Chem. Rev. 1995, 95, 759–788. [Google Scholar] [CrossRef]
- Yamazoe, N.; Kurokawa, Y.; Seiyama, T. Effects of additives on semiconductor gas sensors. Sens. Actuators B 1983, 4, 283–289. [Google Scholar] [CrossRef]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Degler, D.; Müller, S.A.; Doronkin, D.E.; Wang, D.; Grunwaldt, J.-D.; Weimar, U.; Barsan, N. Platinum loaded tin dioxide: A model system for unravelling the interplay between heterogeneous catalysis and gas sensing. J. Mater. Chem. A 2018, 6, 2034–2046. [Google Scholar] [CrossRef]
- Degler, D.; Weimar, U.; Barsan, N. Current Understanding of the Fundamental Mechanisms of Doped and Loaded Semiconducting Metal-Oxide-Based Gas Sensing Materials. ACS Sens. 2019, 4, 2228–2249. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Henshaw, G.S.; Pratt, K.F.E.; Peat, R. Reaction–diffusion effects and systematic design of gas-sensitive resistors based on semiconducting oxides. J. Chem. Soc. Faraday Trans. 1995, 91, 4299–4307. [Google Scholar] [CrossRef]
- Védrine, J.C. Importance, features and uses of metal oxide catalysts in heterogeneous catalysis. Chin. J. Catal. 2019, 40, 1627–1636. [Google Scholar] [CrossRef]
- Védrine, J.C. Metal Oxides in Heterogeneous Oxidation Catalysis: State of the Art and Challenges for a More Sustainable World. ChemSusChem 2019, 12, 577–588. [Google Scholar] [CrossRef]
- Bion, N.; Can, F.; Courtois, X.; Duprez, D. Transition metal oxides for combustion and depollution processes. In Metal Oxides in Heterogeneous Catalysis; Védrine, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 287–353. [Google Scholar] [CrossRef]
- Xu, H.; Yan, N.; Qu, Z.; Liu, W.; Mei, J.; Huang, W.; Zhao, S. Gaseous Heterogeneous Catalytic Reactions over Mn-Based Oxides for Environmental Applications: A Critical Review. Environ. Sci. Technol. 2017, 51, 8879–8892. [Google Scholar] [CrossRef]
- Védrine, J.C. Heterogeneous Catalysis on Metal Oxides. Catalysts 2017, 7, 341. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional gas sensing nanomaterials: A panoramic view. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Kim, J.-S.; Lee, J.-H. Rational Design of Semiconductor-Based Chemiresistors and their Libraries for Next-Generation Artificial Olfaction. Adv. Mater. 2020, 32, 2002075. [Google Scholar] [CrossRef]
- Xu, J.M.; Cheng, J.P. The advances of Co3O4 as gas sensing materials: A review. J. Alloy. Compd. 2016, 686, 753–768. [Google Scholar] [CrossRef]
- Cunningham, D.A.H.; Kobayashi, T.; Kamijo, N.; Haruta, M. Influence of dry operating conditions: Observation of oscillations and low temperature CO oxidation over Co3O4 and Au/Co3O4 catalysts. Catal. Lett. 1994, 25, 257–264. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Liu, Z.-Q.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Wicker, S.; Großmann, K.; Bârsan, N.; Weimar, U. Co3O4—A systematic investigation of catalytic and gas sensing performance under variation of temperature, humidity, test gas and test gas concentration. Sens. Actuators B Chem. 2013, 185, 644–650. [Google Scholar] [CrossRef]
- Deng, J.; Wang, L.; Lou, Z.; Zhang, T. Fast response/recovery performance of comb-like Co3O4 nanostructure. RSC Adv. 2014, 4, 21115–21120. [Google Scholar] [CrossRef]
- Dissanayake, S.; Wasalathanthri, N.; Amin, A.S.; He, J.; Poges, S.; Rathnayake, D.; Suib, S.L. Mesoporous Co3O4 catalysts for VOC elimination: Oxidation of 2-propanol. Appl. Catal. A Gen. 2020, 590, 117366. [Google Scholar] [CrossRef]
- Fedorov, F.S.; Solomatin, M.A.; Uhlemann, M.; Oswald, S.; Kolosov, D.A.; Morozov, A.; Varezhnikov, A.S.; Ivanov, M.A.; Grebenko, A.K.; Sommer, M.; et al. Quasi-2D Co3O4 nanoflakes as an efficient gas sensor versus alcohol VOCs. J. Mater. Chem. A 2020, 8, 7214–7228. [Google Scholar] [CrossRef]
- Choi, K.-I.; Kim, H.-R.; Kim, K.-M.; Liu, D.; Cao, G.; Lee, J.-H. C2H5OH sensing characteristics of various Co3O4 nanostructures prepared by solvothermal reaction. Sens. Actuators B Chem. 2010, 146, 183–189. [Google Scholar] [CrossRef]
- Marcoccia, C.G.; Peluso, M.A.; Sambeth, J.E. Synthesis, characterization and catalytic properties of cobalt oxide recovered from spent lithium-ion batteries. Mol. Catal. 2020, 481, 110223. [Google Scholar] [CrossRef]
- Lü, Y.; Zhan, W.; He, Y.; Wang, Y.; Kong, X.; Kuang, Q.; Xie, Z.; Zheng, L. MOF-Templated Synthesis of Porous Co3O4 Concave Nanocubes with High Specific Surface Area and Their Gas Sensing Properties. ACS Appl. Mater. Interfaces 2014, 6, 4186–4195. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Xiao, W.; Wang, S.; Ren, Z.; Ding, J.; Gao, P.-X. Boosting catalytic propane oxidation over PGM-free Co3O4 nanocrystal aggregates through chemical leaching: A comparative study with Pt and Pd based catalysts. Appl. Catal. B Environ. 2018, 226, 585–595. [Google Scholar] [CrossRef]
- Hu, L.; Peng, Q.; Li, Y. Selective Synthesis of Co3O4 Nanocrystal with Different Shape and Crystal Plane Effect on Catalytic Property for Methane Combustion. J. Am. Chem. Soc. 2008, 130, 16136–16137. [Google Scholar] [CrossRef]
- Epifani, M.; Tang, P.-Y.; Genç, A.; Morante, J.R.; Arbiol, J.; Díaz, R.; Wicker, S. The Ethylhexanoate Route to Metal Oxide Nanocrystals: Synthesis of CoO Nanooctahedra from Co(II) 2-Ethylhexanoate. Eur. J. Inorg. Chem. 2016, 2016, 3963–3968. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zhang, L.; Jiang, D. Surface oxygen vacancies on Co3O4 mediated catalytic formaldehyde oxidation at room temperature. Catal. Sci. Technol. 2016, 6, 3845–3853. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, N.-J.; Park, H.J.; Kim, J.; Lee, D.-S.; Song, H. A Hollow Assembly and Its Three-Dimensional Network Formation of Single-Crystalline Co3O4 Nanoparticles for Ultrasensitive Formaldehyde Gas Sensors. J. Phys. Chem. C 2014, 118, 25994–26002. [Google Scholar] [CrossRef]
- Zhang, W.; Díez-Ramírez, J.; Anguita, P.; Descorme, C.; Valverde, J.L.; Giroir-Fendler, A. Nanocrystalline Co3O4 catalysts for toluene and propane oxidation: Effect of the precipitation agent. Appl. Catal. B Environ. 2020, 273, 118894. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, R.; Wang, L.; Lou, Z.; Zhang, T. Enhanced sensing performance of the Co3O4 hierarchical nanorods to NH3 gas. Sens. Actuators B Chem. 2015, 209, 449–455. [Google Scholar] [CrossRef]
- Petryk, J.; Kołakowska, E. Cobalt oxide catalysts for ammonia oxidation activated with cerium and lanthanum. Appl. Catal. B Environ. 2000, 24, 121–128. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef]
- Chabre, Y.; Pannetier, J. Structural and electrochemical properties of the proton/γ-MnO2 system. Prog. Solid State Chem. 1995, 23, 1–130. [Google Scholar] [CrossRef]
- Albering, J.H. Structural Chemistry of Manganese Dioxide and Related Compounds. In Handbook of Battery Materials; Daniel, C., Besenhard, J.O., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 87–123. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B Environ. 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Lamaita, L.; Peluso, M.A.; Sambeth, J.E.; Thomas, H.J. Synthesis and characterization of manganese oxides employed in VOCs abatement. Appl. Catal. B Environ. 2005, 61, 114–119. [Google Scholar] [CrossRef]
- Miao, L.; Wang, J.; Zhang, P. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde. Appl. Surf. Sci. 2019, 466, 441–453. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, J.; Nie, L. Progress of Catalytic Oxidation of Formaldehyde over Manganese Oxides. ChemistrySelect 2019, 4, 12085–12098. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Cheng, W.; Wu, F.; Lu, X.; Li, Z. Controlled synthesis of diverse manganese oxide-based catalysts for complete oxidation of toluene and carbon monoxide. Chem. Eng. J. 2014, 244, 59–67. [Google Scholar] [CrossRef]
- Bigiani, L.; Zappa, D.; Maccato, C.; Gasparotto, A.; Sada, C.; Comini, E.; Barreca, D. Hydrogen Gas Sensing Performances of p-Type Mn3O4 Nanosystems: The Role of Built-in Mn3O4/Ag and Mn3O4/SnO2 Junctions. Nanomaterials 2020, 10, 511. [Google Scholar] [CrossRef]
- Bigiani, L.; Maccato, C.; Carraro, G.; Gasparotto, A.; Sada, C.; Comini, E.; Barreca, D. Tailoring Vapor-Phase Fabrication of Mn3O4 Nanosystems: From Synthesis to Gas-Sensing Applications. ACS Appl. Nano Mater. 2018, 1, 2962–2970. [Google Scholar] [CrossRef]
- Na, C.W.; Park, S.-Y.; Chung, J.-H.; Lee, J.-H. Transformation of ZnO Nanobelts into Single-Crystalline Mn3O4 Nanowires. ACS Appl. Mater. Interfaces 2012, 4, 6565–6572. [Google Scholar] [CrossRef]
- Tian, X.; Yang, L.; Qing, X.; Yu, K.; Wang, X. Trace level detection of hydrogen gas using birnessite-type manganese oxide. Sens. Actuators B Chem. 2015, 207, 34–42. [Google Scholar] [CrossRef]
- Stobbe, E.R.; de Boer, B.A.; Geus, J.W. The reduction and oxidation behaviour of manganese oxides. Catal. Today 1999, 47, 161–167. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Zedan, A.F.; Mohamed, A.T.; El-Shall, M.S.; AlQaradawi, S.Y.; AlJaber, A.S. Tailoring the reducibility and catalytic activity of CuO nanoparticles for low temperature CO oxidation. RSC Adv. 2018, 8, 19499–19511. [Google Scholar] [CrossRef]
- Steinhauer, S. Gas Sensors Based on Copper Oxide Nanomaterials: A Review. Chemosensors 2021, 9, 51. [Google Scholar] [CrossRef]
- Rydosz, A. The Use of Copper Oxide Thin Films in Gas-Sensing Applications. Coatings 2018, 8, 425. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.; Zhou, T.; Lou, Z.; Deng, J.; Zhang, T. Concave Cu2O octahedral nanoparticles as an advanced sensing material for benzene (C6H6) and nitrogen dioxide (NO2) detection. Sens. Actuators B Chem. 2016, 223, 311–317. [Google Scholar] [CrossRef]
- Park, H.J.; Choi, N.-J.; Kang, H.; Jung, M.Y.; Park, J.W.; Park, K.H.; Lee, D.-S. A ppb-level formaldehyde gas sensor based on CuO nanocubes prepared using a polyol process. Sens. Actuators B Chem. 2014, 203, 282–288. [Google Scholar] [CrossRef]
- Steinhauer, S.; Chapelle, A.; Menini, P.; Sowwan, M. Local CuO Nanowire Growth on Microhotplates: In Situ Electrical Measurements and Gas Sensing Application. ACS Sens. 2016, 1, 503–507. [Google Scholar] [CrossRef]
- Wang, F.; Li, H.; Yuan, Z.; Sun, Y.; Chang, F.; Deng, H.; Xie, L.; Li, H. A highly sensitive gas sensor based on CuO nanoparticles synthetized via a sol–gel method. RSC Adv. 2016, 6, 79343–79349. [Google Scholar] [CrossRef]
- Li, D.; Tang, Y.; Ao, D.; Xiang, X.; Wang, S.; Zu, X. Ultra-highly sensitive and selective H2S gas sensor based on CuO with sub-ppb detection limit. Int. J. Hydrogen Energy 2019, 44, 3985–3992. [Google Scholar] [CrossRef]
- Parravano, G. The Catalytic Oxidation of Carbon Monoxide on Nickel Oxide. I. Pure Nickel Oxide. J. Am. Chem. Soc. 1953, 75, 1448–1451. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Y.; Liu, X.; Liu, Y.; Liu, J.; Teng, B. Pinpointing the active sites and reaction mechanism of CO oxidation on NiO. Phys. Chem. Chem. Phys. 2019, 21, 17852–17858. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Jiang, Z.; Yang, R.; Jiang, Z.; Pan, Y.; Shangguan, W. New insight into the enhanced activity of ordered mesoporous nickel oxide in formaldehyde catalytic oxidation reactions. J. Catal. 2018, 361, 370–383. [Google Scholar] [CrossRef]
- Xia, Y.; Lin, M.; Ren, D.; Li, Y.; Hu, F.; Chen, W. Preparation of high surface area mesoporous nickel oxides and catalytic oxidation of toluene and formaldehyde. J. Porous Mater. 2017, 24, 621–629. [Google Scholar] [CrossRef]
- Bai, G.; Dai, H.; Deng, J.; Liu, Y.; Qiu, W.; Zhao, Z.; Li, X.; Yang, H. The microemulsion preparation and high catalytic performance of mesoporous NiO nanorods and nanocubes for toluene combustion. Chem. Eng. J. 2013, 219, 200–208. [Google Scholar] [CrossRef]
- Ye, Y.; Zhao, Y.; Ni, L.; Jiang, K.; Tong, G.; Zhao, Y.; Teng, B. Facile synthesis of unique NiO nanostructures for efficiently catalytic conversion of CH4 at low temperature. Appl. Surf. Sci. 2016, 362, 20–27. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Swart, H.C.; Motaung, D.E. A review on recent progress of p-type nickel oxide based gas sensors: Future perspectives. J. Alloy. Compd. 2019, 805, 267–294. [Google Scholar] [CrossRef]
- Lin, L.; Liu, T.; Miao, B.; Zeng, W. Hydrothermal fabrication of uniform hexagonal NiO nanosheets: Structure, growth and response. Mater. Lett. 2013, 102–103, 43–46. [Google Scholar] [CrossRef]
- Miao, R.; Zeng, W.; Gao, Q. Hydrothermal synthesis of novel NiO nanoflowers assisted with CTAB and SDS respectively and their gas-sensing properties. Mater. Lett. 2017, 186, 175–177. [Google Scholar] [CrossRef]
- Liu, B.; Yang, H.; Zhao, H.; An, L.; Zhang, L.; Shi, R.; Wang, L.; Bao, L.; Chen, Y. Synthesis and enhanced gas-sensing properties of ultralong NiO nanowires assembled with NiO nanocrystals. Sens. Actuators B Chem. 2011, 156, 251–262. [Google Scholar] [CrossRef]
- Urso, M.; Leonardi, S.G.; Neri, G.; Petralia, S.; Conoci, S.; Priolo, F.; Mirabella, S. Acetone sensing and modelling by low-cost NiO nanowalls. Mater. Lett. 2020, 262, 127043. [Google Scholar] [CrossRef]
- Castro-Hurtado, I.; Malagù, C.; Morandi, S.; Pérez, N.; Mandayo, G.G.; Castaño, E. Properties of NiO sputtered thin films and modeling of their sensing mechanism under formaldehyde atmospheres. Acta Mater. 2013, 61, 1146–1153. [Google Scholar] [CrossRef]
- Cho, N.G.; Hwang, I.-S.; Kim, H.-G.; Lee, J.-H.; Kim, I.-D. Gas sensing properties of p-type hollow NiO hemispheres prepared by polymeric colloidal templating method. Sens. Actuators B Chem. 2011, 155, 366–371. [Google Scholar] [CrossRef]
- Yao, Y.-F.Y. The oxidation of hydrocarbons and CO over metal oxides: II. α-Cr2O3. J. Catal. 1973, 28, 139–149. [Google Scholar] [CrossRef]
- Sinha, A.K.; Suzuki, K. Three-Dimensional Mesoporous Chromium Oxide: A Highly Efficient Material for the Elimination of Volatile Organic Compounds. Angew. Chem. Int. Ed. 2005, 44, 271–273. [Google Scholar] [CrossRef]
- Rotter, H.; Landau, M.V.; Carrera, M.; Goldfarb, D.; Herskowitz, M. High surface area chromia aerogel efficient catalyst and catalyst support for ethylacetate combustion. Appl. Catal. B Environ. 2004, 47, 111–126. [Google Scholar] [CrossRef]
- Xia, Y.; Dai, H.; Jiang, H.; Deng, J.; He, H.; Au, C.T. Mesoporous Chromia with Ordered Three-Dimensional Structures for the Complete Oxidation of Toluene and Ethyl Acetate. Environ. Sci. Technol. 2009, 43, 8355–8360. [Google Scholar] [CrossRef]
- Bumajdad, A.; Al-Ghareeb, S.; Madkour, M.; Al Sagheer, F. Non-noble, efficient catalyst of unsupported α-Cr2O3 nanoparticles for low temperature CO Oxidation. Sci. Rep. 2017, 7, 14788. [Google Scholar] [CrossRef]
- Rajesh, H.; Ozkan, U.S. Complete oxidation of ethanol, acetaldehyde and ethanol/methanol mixtures over copper oxide and copper-chromium oxide catalysts. Ind. Eng. Chem. Res. 1993, 32, 1622–1630. [Google Scholar] [CrossRef]
- Miremadi, B.K.; Singh, R.C.; Chen, Z.; Morrison, S.R.; Colbow, K. Chromium oxide gas sensors for the detection of hydrogen, oxygen and nitrogen oxide. Sens. Actuators B Chem. 1994, 21, 1–4. [Google Scholar] [CrossRef]
- An, G.; Zhang, Y.; Liu, Z.; Miao, Z.; Han, B.; Miao, S.; Li, J. Preparation of porous chromium oxide nanotubes using carbon nanotubes as templates and their application as an ethanol sensor. Nanotechnology 2007, 19, 035504. [Google Scholar] [CrossRef]
- Ma, H.; Xu, Y.; Rong, Z.; Cheng, X.; Gao, S.; Zhang, X.; Zhao, H.; Huo, L. Highly toluene sensing performance based on monodispersed Cr2O3 porous microspheres. Sens. Actuators B Chem. 2012, 174, 325–331. [Google Scholar] [CrossRef]
- Ding, C.; Ma, Y.; Lai, X.; Yang, Q.; Xue, P.; Hu, F.; Geng, W. Ordered Large-Pore Mesoporous Cr2O3 with Ultrathin Framework for Formaldehyde Sensing. ACS Appl. Mater. Interfaces 2017, 9, 18170–18177. [Google Scholar] [CrossRef]
- Pokhrel, S.; Simion, C.E.; Quemener, V.; Bârsan, N.; Weimar, U. Investigations of conduction mechanism in Cr2O3 gas sensing thick films by ac impedance spectroscopy and work function changes measurements. Sens. Actuators B Chem. 2008, 133, 78–83. [Google Scholar] [CrossRef]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceramics 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Raymond, M.V.; Smyth, D.M. Defects and charge transport in perovskite ferroelectrics. J. Phys. Chem. Solids 1996, 57, 1507–1511. [Google Scholar] [CrossRef]
- Ji, Q.; Bi, L.; Zhang, J.; Cao, H.; Zhao, X.S. The role of oxygen vacancies of ABO3 perovskite oxides in the oxygen reduction reaction. Energy Environ. Sci. 2020, 13, 1408–1428. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, T.; Dou, B.-S.; Wang, C.-X.; Xie, X.-F.; Yu, Z.-L.; Fan, S.-R.; Fan, Z.-R.; Wang, L.-C. A comparative study on perovskite-type mixed oxide catalysts A′xA1− xBO3−λ (A′ = Ca, Sr, A = La, B = Mn, Fe, Co) for NH3 oxidation. J. Catal. 1989, 120, 88–107. [Google Scholar] [CrossRef]
- Wells, A.F. Structural Inorganic Chemistry, 3rd ed.; Clarendon Press: Oxford, UK, 1962; pp. 494–499. [Google Scholar]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Bhalla, A.S.; Guo, R.; Roy, R. The perovskite structure—A review of its role in ceramic science and technology. Mater. Res. Innov. 2000, 4, 3–26. [Google Scholar] [CrossRef]
- Evans, H.A.; Wu, Y.; Seshadri, R.; Cheetham, A.K. Perovskite-related ReO3-type structures. Nat. Rev. Mater. 2020, 5, 196–213. [Google Scholar] [CrossRef]
- Sebastian, M.T. ABO3 Type Perovskites. In Dielectric Materials for Wireless Communication; Sebastian, M.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 161–203. [Google Scholar] [CrossRef]
- Bashan, V.; Ust, Y. Perovskite catalysts for methane combustion: Applications, design, effects for reactivity and partial oxidation. Int. J. Energy Res. 2019, 43, 7755–7789. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751. [Google Scholar] [CrossRef] [PubMed]
- Labhasetwar, N.; Saravanan, G.; Megarajan, S.K.; Manwar, N.; Khobragade, R.; Doggali, P.; Grasset, F. Perovskite-type catalytic materials for environmental applications. Sci. Technol. Adv. Mater. 2015, 16, 036002. [Google Scholar] [CrossRef]
- Fierro, J.L.G. Structure and composition of perovskite surface in relation to adsorption and catalytic properties. Catal. Today 1990, 8, 153–174. [Google Scholar] [CrossRef]
- Tejuca, L.G.; Fierro, J.L.G.; Tascón, J.M.D. Structure and Reactivity of Perovskite-Type Oxides. In Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: Cambridge, MA, USA, 1989; Volume 36, pp. 237–328. [Google Scholar]
- Misono, M. Chapter 3—Catalysis of Perovskite and Related Mixed Oxides. In Studies in Surface Science and Catalysis; Misono, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 176, pp. 67–95. [Google Scholar]
- Parravano, G. Catalytic Activity of Lanthanum and Strontium Manganite. J. Am. Chem. Soc. 1953, 75, 1497–1498. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H. Perovskite-Related Oxides as Oxidation—Reduction Catalysts. In Advanced Materials in Catalysis; Burton, J.J., Garten, R.L., Eds.; Academic Press: Cambridge, MA, USA, 1977; pp. 129–180. [Google Scholar] [CrossRef]
- Dowden, D.A. Crystal and Ligand Field Models of Solid Catalysts. Catal. Rev. 1972, 5, 1–32. [Google Scholar] [CrossRef]
- Karki, S.B.; Hona, R.K.; Ramezanipour, F. Effect of Structure on Sensor Properties of Oxygen-Deficient Perovskites, A2BB′O5 (A = Ca, Sr; B = Fe; B′ = Fe, Mn) for Oxygen, Carbon Dioxide and Carbon Monoxide Sensing. J. Electron. Mater. 2020, 49, 1557–1567. [Google Scholar] [CrossRef]
- Nakamura, T.; Misono, M.; Yoneda, Y. Catalytic Properties of Perovskite-type Mixed Oxides, La1−xSrxCoO3. Bull. Chem. Soc. Jpn. 1982, 55, 394–399. [Google Scholar] [CrossRef]
- Taguchi, H.; Yamasaki, S.; Itadani, A.; Yosinaga, M.; Hirota, K. CO oxidation on perovskite-type LaCoO3 synthesized using ethylene glycol and citric acid. Catal. Commun. 2008, 9, 1913–1915. [Google Scholar] [CrossRef]
- Panich, N.M.; Pirogova, G.N.; Korosteleva, R.I.; Voronin, Y.V. Oxidation of CO and hydrocarbons over perovskite-type complex oxides. Russ. Chem. Bull. 1999, 48, 694–697. [Google Scholar] [CrossRef]
- Viswanathan, B. CO Oxidation and NO Reduction on Perovskite Oxides. Catal. Rev. 1992, 34, 337–354. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- Fergus, J.W. Perovskite oxides for semiconductor-based gas sensors. Sens. Actuators B Chem. 2007, 123, 1169–1179. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Song, P.; Qin, H.; An, K.; Wang, X.; Jiang, M. CO-sensing properties of perovskite La0.68Pb0.32FeO3 nano-materials. Sens. Actuators B Chem. 2006, 119, 315–318. [Google Scholar] [CrossRef]
- Song, P.; Wang, Q.; Yang, Z. The effects of annealing temperature on the CO-sensing property of perovskite La0.8Pb0.2Fe0.8Cu0.2O3 nanoparticles. Sens. Actuators B Chem. 2009, 141, 109–115. [Google Scholar] [CrossRef]
- Giang, H.T.; Duy, H.T.; Ngan, P.Q.; Thai, G.H.; Toan, N.N. Hydrocarbon gas sensing of nano-crystalline perovskite oxides LnFeO3 (Ln=La, Nd and Sm). Sens. Actuators B Chem. 2011, 158, 246–251. [Google Scholar] [CrossRef]
- Lee, Y.N.; Lago, R.M.; Fierro, J.L.G.; Cortés, V.; Sapiña, F.; Martínez, E. Surface properties and catalytic performance for ethane combustion of La1−xKxMnO3+δ perovskites. Appl. Catal. A Gen. 2001, 207, 17–24. [Google Scholar] [CrossRef]
- Alifanti, M.; Bueno, G.; Parvulescu, V.; Parvulescu, V.I.; Corberán, V.C. Oxidation of ethane on high specific surface SmCoO3 and PrCoO3 perovskites. Catal. Today 2009, 143, 309–314. [Google Scholar] [CrossRef]
- Nitadori, T.; Kurihara, S.; Misono, M. Catalytic properties of La1−xA′xMnO3 (A′ = Sr, Ce, Hf). J. Catal. 1986, 98, 221–228. [Google Scholar] [CrossRef]
- Merino, N.A.; Barbero, B.P.; Grange, P.; Cadús, L.E. La1−xCaxCoO3 perovskite-type oxides: Preparation, characterisation, stability, and catalytic potentiality for the total oxidation of propane. J. Catal. 2005, 231, 232–244. [Google Scholar] [CrossRef]
- Asada, T.; Kayama, T.; Kusaba, H.; Einaga, H.; Teraoka, Y. Preparation of alumina-supported LaFeO3 catalysts and their catalytic activity for propane combustion. Catal. Today 2008, 139, 37–42. [Google Scholar] [CrossRef]
- Sui, Z.-J.; Vradman, L.; Reizner, I.; Landau, M.V.; Herskowitz, M. Effect of preparation method and particle size on LaMnO3 performance in butane oxidation. Catal. Commun. 2011, 12, 1437–1441. [Google Scholar] [CrossRef]
- Szabo, V.; Bassir, M.; Gallot, J.E.; Van Neste, A.; Kaliaguine, S. Perovskite-type oxides synthesised by reactive grinding: Part III. Kinetics of n-hexane oxidation over LaCo(1−x)FexO3. Appl. Catal. B Environ. 2003, 42, 265–277. [Google Scholar] [CrossRef]
- Levasseur, B.; Kaliaguine, S. Methanol oxidation on LaBO3 (B = Co, Mn, Fe) perovskite-type catalysts prepared by reactive grinding. Appl. Catal. A Gen. 2008, 343, 29–38. [Google Scholar] [CrossRef]
- Pecchi, G.; Reyes, P.; Zamora, R.; Cadús, L.E.; Fierro, J.L.G. Surface properties and performance for VOCs combustion of LaFe1−yNiyO3 perovskite oxides. J. Solid State Chem. 2008, 181, 905–912. [Google Scholar] [CrossRef]
- Najjar, H.; Batis, H. La–Mn perovskite-type oxide prepared by combustion method: Catalytic activity in ethanol oxidation. Appl. Catal. A Gen. 2010, 383, 192–201. [Google Scholar] [CrossRef]
- Białobok, B.; Trawczyński, J.; Miśta, W.; Zawadzki, M. Ethanol combustion over strontium- and cerium-doped LaCoO3 catalysts. Appl. Catal. B Environ. 2007, 72, 395–403. [Google Scholar] [CrossRef]
- Stege, W.P.; Cadús, L.E.; Barbero, B.P. La1−xCaxMnO3 perovskites as catalysts for total oxidation of volatile organic compounds. Catal. Today 2011, 172, 53–57. [Google Scholar] [CrossRef]
- Spinicci, R.; Faticanti, M.; Marini, P.; De Rossi, S.; Porta, P. Catalytic activity of LaMnO3 and LaCoO3 perovskites towards VOCs combustion. J. Mol. Catal. A Chem. 2003, 197, 147–155. [Google Scholar] [CrossRef]
- Qin, J.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Liang, Y. Three-dimensionally ordered macroporous La1−xMgxFeO3 as high performance gas sensor to methanol. J. Alloy. Compd. 2015, 635, 194–202. [Google Scholar] [CrossRef]
- Obayashi, H.; Sakurai, Y.; Gejo, T. Perovskite-type oxides as ethanol sensors. J. Solid State Chem. 1976, 17, 299–303. [Google Scholar] [CrossRef]
- Ge, X.; Liu, Y.; Liu, X. Preparation and gas-sensitive properties of LaFe1−yCoyO3 semiconducting materials. Sens. Actuators B Chem. 2001, 79, 171–174. [Google Scholar] [CrossRef]
- Song, P.; Qin, H.; Zhang, L.; An, K.; Lin, Z.; Hu, J.; Jiang, M. The structure, electrical and ethanol-sensing properties of La1−xPbxFeO3 perovskite ceramics with x ≤ 0.3. Sens. Actuators B Chem. 2005, 104, 312–316. [Google Scholar] [CrossRef]
- Cao, K.; Cao, E.; Zhang, Y.; Hao, W.; Sun, L.; Peng, H. The influence of nonstoichiometry on electrical transport and ethanol sensing characteristics for nanocrystalline LaFexO3−δ sensors. Sens. Actuators B Chem. 2016, 230, 592–599. [Google Scholar] [CrossRef]
- Xiang, J.; Chen, X.; Zhang, X.; Gong, L.; Zhang, Y.; Zhang, K. Preparation and characterization of Ba-doped LaFeO3 nanofibers by electrospinning and their ethanol sensing properties. Mater. Chem. Phys. 2018, 213, 122–129. [Google Scholar] [CrossRef]
- Hao, P.; Qu, G.-M.; Song, P.; Yang, Z.-X.; Wang, Q. Synthesis of Ba-doped porous LaFeO3 microspheres with perovskite structure for rapid detection of ethanol gas. Rare Met. 2021, 40, 1651–1661. [Google Scholar] [CrossRef]
- Einaga, H.; Hyodo, S.; Teraoka, Y. Complete Oxidation of Benzene Over Perovskite-Type Oxide Catalysts. Top. Catal. 2010, 53, 629–634. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, L.; Dai, H.; He, H.; Au, C.T. Hydrothermally fabricated single-crystalline strontium-substituted lanthanum manganite microcubes for the catalytic combustion of toluene. J. Mol. Catal. A Chem. 2009, 299, 60–67. [Google Scholar] [CrossRef]
- Blasin-Aubé, V.; Belkouch, J.; Monceaux, L. General study of catalytic oxidation of various VOCs over La0.8Sr0.2MnO3+x perovskite catalyst—influence of mixture. Appl. Catal. B Environ. 2003, 43, 175–186. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, L.; Dai, H.; He, H.; Au, C.T. Strontium-Doped Lanthanum Cobaltite and Manganite: Highly Active Catalysts for Toluene Complete Oxidation. Ind. Eng. Chem. Res. 2008, 47, 8175–8183. [Google Scholar] [CrossRef]
- Niu, J.; Deng, J.; Liu, W.; Zhang, L.; Wang, G.; Dai, H.; He, H.; Zi, X. Nanosized perovskite-type oxides La1−xSrxMO3−δ (M=Co, Mn; x=0, 0.4) for the catalytic removal of ethylacetate. Catal. Today 2007, 126, 420–429. [Google Scholar] [CrossRef]
- Xiao, H.; Xue, C.; Song, P.; Li, J.; Wang, Q. Preparation of porous LaFeO3 microspheres and their gas-sensing property. Appl. Surf. Sci. 2015, 337, 65–71. [Google Scholar] [CrossRef]
- Yao, P.-J.; Wang, J.; Chu, W.-L.; Hao, Y.-W. Preparation and characterization of La1−xSrxFeO3 materials and their formaldehyde gas-sensing properties. J. Mater. Sci. 2013, 48, 441–450. [Google Scholar] [CrossRef]
- Menesklou, W.; Schreiner, H.-J.; Härdtl, K.H.; Ivers-Tiffée, E. High temperature oxygen sensors based on doped SrTiO3. Sens. Actuators B Chem. 1999, 59, 184–189. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on Sensing Applications of Perovskite Nanomaterials. Chemosensors 2020, 8, 55. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Kim, I.-D. Recent advances in ABO3 perovskites: Their gas-sensing performance as resistive-type gas sensors. J. Korean Ceram. Soc. 2020, 57, 24–39. [Google Scholar] [CrossRef]
- Queraltó, A.; Graf, D.; Frohnhoven, R.; Fischer, T.; Vanrompay, H.; Bals, S.; Bartasyte, A.; Mathur, S. LaFeO3 Nanofibers for High Detection of Sulfur-Containing Gases. ACS Sustain. Chem. Eng. 2019, 7, 6023–6032. [Google Scholar] [CrossRef]
- Ma, X.-H.; Li, H.-Y.; Kweon, S.-H.; Jeong, S.-Y.; Lee, J.-H.; Nahm, S. Highly Sensitive and Selective PbTiO3 Gas Sensors with Negligible Humidity Interference in Ambient Atmosphere. ACS Appl. Mater. Interfaces 2019, 11, 5240–5246. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.A.; Sackmann, A.; Weimar, U.; Bârsan, N. Acetylene- and Ethylene-Sensing Mechanism for LaFeO3-Based Gas Sensors: Operando Insights. J. Phys. Chem. C 2020, 124, 7317–7326. [Google Scholar] [CrossRef]
- Mirzaei, A.; Hashemi, B.; Janghorban, K. α-Fe2O3 based nanomaterials as gas sensors. J. Mater. Sci. Mater. Electron. 2016, 27, 3109–3144. [Google Scholar] [CrossRef]
- Cuong, N.D.; Khieu, D.Q.; Hoa, T.T.; Quang, D.T.; Viet, P.H.; Dai Lam, T.; Hoa, N.D.; Van Hieu, N. Facile synthesis of α-Fe2O3 nanoparticles for high-performance CO gas sensor. Mater. Res. Bull. 2015, 68, 302–307. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Y. A review of the α-Fe2O3 (hematite) nanotube structure: Recent advances in synthesis, characterization, and applications. Nanoscale 2020, 12, 10912–10932. [Google Scholar] [CrossRef]
- Wang, P.; Sui, L.; Yu, H.; Zhang, X.; Cheng, X.; Gao, S.; Zhao, H.; Huo, L.; Xu, Y.; Wu, H. Monodispersed hollow α-Fe2O3 ellipsoids via [C12mim][PF6]-assistant synthesis and their excellent n-butanol gas-sensing properties. Sens. Actuators B Chem. 2021, 326, 128796. [Google Scholar] [CrossRef]
- Ma, T.; Zheng, L.; Zhao, Y.; Xu, Y.; Zhang, J.; Liu, X. Highly Porous Double-Shelled Hollow Hematite Nanoparticles for Gas Sensing. ACS Appl. Nano Mater. 2019, 2, 2347–2357. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Z.; Li, H.; Sun, M.; Han, S.; Cai, C.; Shen, W.; Fu, Y. Ultrafast Response/Recovery and High Selectivity of the H2S Gas Sensor Based on α-Fe2O3 Nano-Ellipsoids from One-Step Hydrothermal Synthesis. ACS Appl. Mater. Interfaces 2019, 11, 12761–12769. [Google Scholar] [CrossRef]
- Tian, K.; Wang, X.-X.; Yu, Z.-Y.; Li, H.-Y.; Guo, X. Hierarchical and Hollow Fe2O3 Nanoboxes Derived from Metal–Organic Frameworks with Excellent Sensitivity to H2S. ACS Appl. Mater. Interfaces 2017, 9, 29669–29676. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, Z.; Wang, N.; Huang, Y.; Wang, J.; Liu, W.; Fu, Y.; Wang, Z. Facile synthesis of α-Fe2O3 micro-ellipsoids by surfactant-free hydrothermal method for sub-ppm level H2S detection. Mater. Des. 2016, 110, 532–539. [Google Scholar] [CrossRef]
- Li, P.; Miser, D.E.; Rabiei, S.; Yadav, R.T.; Hajaligol, M.R. The removal of carbon monoxide by iron oxide nanoparticles. Appl. Catal. B Environ. 2003, 43, 151–162. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, Z.; Li, X.; Hu, B.; Xie, Y. Thermally Stable Hematite Hollow Nanowires. Inorg. Chem. 2004, 43, 6540–6542. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, Y.; Wang, Y.; Bao, F.; Zhou, L.; Wei, X.; Zhang, Y.; Zheng, Q. Quasicubic α-Fe2O3 Nanoparticles with Excellent Catalytic Performance. J. Phys. Chem. B 2006, 110, 3093–3097. [Google Scholar] [CrossRef]
- Barbosa, A.L.; Herguido, J.; Santamaria, J. Methane combustion over unsupported iron oxide catalysts. Catal. Today 2001, 64, 43–50. [Google Scholar] [CrossRef]
- Dobosz, J.; Zawadzki, M. Total oxidation of lean propane over α-Fe2O3 using microwaves as an energy source. React. Kinet. Mech. Catal. 2015, 114, 157–172. [Google Scholar] [CrossRef]
- Solsona, B.; García, T.; Sanchis, R.; Soriano, M.D.; Moreno, M.; Rodríguez-Castellón, E.; Agouram, S.; Dejoz, A.; Nieto, J.L. Total oxidation of VOCs on mesoporous iron oxide catalysts: Soft chemistry route versus hard template method. Chem. Eng. J. 2016, 290, 273–281. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron oxide surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef]
- Guerrero-Pérez, M.O. Supported, bulk and bulk-supported vanadium oxide catalysts: A short review with an historical perspective. Catal. Today 2017, 285, 226–233. [Google Scholar] [CrossRef]
- Wachs, I.E. Catalysis science of supported vanadium oxide catalysts. Dalton Trans. 2013, 42, 11762–11769. [Google Scholar] [CrossRef] [PubMed]
- Gannoun, C.; Delaigle, R.; Ghorbel, A.; Gaigneaux, E.M. V2O5/TiO2 and V2O5/TiO2–SO42− catalysts for the total oxidation of chlorobenzene: One-step sol–gel preparation vs. two-step impregnation. Catal. Sci. Technol. 2019, 9, 2344–2350. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Liu, X.; Zhu, T.; Guo, Y.; Qi, H. Catalytic oxidation of chlorinated benzenes over V2O5/TiO2 catalysts: The effects of chlorine substituents. Catal. Today 2015, 241, 92–99. [Google Scholar] [CrossRef]
- Moon, S.W.; Lee, G.-D.; Park, S.S.; Hong, S.-S. Catalytic combustion of chlorobenzene over V2O5/TiO2 catalysts prepared by the precipitation-deposition method. React. Kinet. Catal. Lett. 2004, 82, 303–310. [Google Scholar] [CrossRef]
- Debecker, D.P.; Bouchmella, K.; Delaigle, R.; Eloy, P.; Poleunis, C.; Bertrand, P.; Gaigneaux, E.M.; Mutin, P.H. One-step non-hydrolytic sol-gel preparation of efficient V2O5-TiO2 catalysts for VOC total oxidation. Appl. Catal. B Environ. 2010, 94, 38–45. [Google Scholar] [CrossRef]
- Guerrero-Pérez, M.O. V-Containing Mixed Oxide Catalysts for Reduction–Oxidation-Based Reactions with Environmental Applications: A Short Review. Catalysts 2018, 8, 564. [Google Scholar] [CrossRef]
- Haber, J. Fifty years of my romance with vanadium oxide catalysts. Catal. Today 2009, 142, 100–113. [Google Scholar] [CrossRef]
- Grzybowska-Swierkosz, B. Vanadia-titania catalysts for oxidation of o-xylene and other hydrocarbons. Appl. Catal. A Gen. 1997, 157, 263–310. [Google Scholar] [CrossRef]
- Bond, G.C.; Tahir, S.F. Vanadium oxide monolayer catalysts Preparation, characterization and catalytic activity. Appl. Catal. 1991, 71, 1–31. [Google Scholar] [CrossRef]
- Deo, G.; Wachs, I.E. Reactivity of Supported Vanadium Oxide Catalysts: The Partial Oxidation of Methanol. J. Catal. 1994, 146, 323–334. [Google Scholar] [CrossRef]
- Carrero, C.A.; Schloegl, R.; Wachs, I.E.; Schomaecker, R. Critical Literature Review of the Kinetics for the Oxidative Dehydrogenation of Propane over Well-Defined Supported Vanadium Oxide Catalysts. ACS Catal. 2014, 4, 3357–3380. [Google Scholar] [CrossRef]
- Pan, J.-B.; Huang, J.-M.; Li, J.-X.; Jiang, Z.-Y.; Lan, J.-L.; Qian, C.; Chen, X.-Z. Ammoxidation of 2-picoline catalyzed by modified V2O5/TiO2. Monatsh. Chem. 2014, 145, 1365–1369. [Google Scholar] [CrossRef]
- Cavalli, P.; Cavani, F.; Manenti, I.; Trifiro, F. Ammoxidation of alkylaromatics over V2O5/TiO2 catalysts. Catal. Today 1987, 1, 245–255. [Google Scholar] [CrossRef]
- Epifani, M.; Díaz, R.; Force, C.; Comini, E.; Andreu, T.; Zamani, R.R.; Arbiol, J.; Siciliano, P.; Faglia, G.; Morante, J.R. Colloidal Counterpart of the TiO2-Supported V2O5 System: A Case Study of Oxide-on-Oxide Deposition by Wet Chemical Techniques. Synthesis, Vanadium Speciation, and Gas-Sensing Enhancement. J. Phys. Chem. C 2013, 117, 20697–20705. [Google Scholar] [CrossRef]
- Epifani, M.; Comini, E.; Siciliano, P.; Faglia, G.; Morante, J.R. Evidence of catalytic activation of anatase nanocrystals by vanadium oxide surface layer: Acetone and ethanol sensing properties. Sens. Actuators B Chem. 2015, 217, 193–197. [Google Scholar] [CrossRef]
- Ai, M. The oxidation activity and acid-base properties of SnO2-based binary catalysts: I. The SnO2 V2O5 system. J. Catal. 1975, 40, 318–326. [Google Scholar] [CrossRef]
- Ono, T.; Nakagawa, Y.; Kubokawa, Y. Catalytic Activity of V–Sn Oxides for Oxidation Reactions. Bull. Chem. Soc. Jpn. 1981, 54, 343–347. [Google Scholar] [CrossRef]
- Bordoni, S.; Castellani, F.; Cavani, F.; Trifiro, F.; Gazzano, M. Nature of Vanadium Species in SnO2-V2O5-Based Catalysts-Chemistry of Preparation, Characterization, Thermal-Stability and Reactivity in Ethane Oxidative Dehydrogenation over V-Sn Mixed Oxides. J. Chem. Soc. Faraday Trans. 1994, 90, 2981–3000. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Bartolini, A.; Ghisletti, D.; Nalli, M.; Santucci, A. SnO2-V2O5-based catalysts - Nature of surface species and their activity in o-xylene oxidation. J. Chem. Soc. Faraday Trans. 1996, 92, 4321–4330. [Google Scholar] [CrossRef]
- Glinski, M.; Kijenski, J.; Jelen, T. Monolayer oxide catalysts from alkoxide precursors. 4. Vanadia, titania and stibia on SiO2, SnO2, and TiO2 in 2-propanol oxidation. React. Kinet. Catal. Lett. 1997, 60, 33–39. [Google Scholar] [CrossRef]
- Pillai, S.K.; Gheevarghese, O.; Sugunan, S. Catalytic properties of V2O5/SnO2 towards vapour-phase Beckmann rearrangement of cyclohexanone oxime. Appl. Catal. A Gen. 2009, 353, 130–136. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Dong, J.; Qin, N.; Xu, J. Selective BTEX sensor based on a SnO2/V2O5 composite. Sens. Actuators B Chem. 2013, 186, 126–131. [Google Scholar] [CrossRef]
- Kim, H.S.; Jin, C.H.; Park, S.H.; Lee, C.M. Structural, luminescent, and NO2 sensing properties of SnO2-core/V2O5-shell nanorods. J. Electroceramics 2013, 30, 6–12. [Google Scholar] [CrossRef]
- Wang, C.T.; Chen, M.T.; Lai, D.L. Vanadium-Tin Oxide Nanoparticles with Gas-Sensing and Catalytic Activity. J. Am. Ceram. Soc. 2011, 94, 4471–4477. [Google Scholar] [CrossRef]
- Patrono, P.; Laginestra, A.; Ramis, G.; Busca, G. Conversion of 1-Butene over WO3-TiO2 Catalysts. Appl. Catal. A Gen. 1994, 107, 249–266. [Google Scholar] [CrossRef]
- Lu, G.; Li, X.; Qu, Z.; Zhao, Q.; Li, H.; Shen, Y.; Chen, G. Correlations of WO3 species and structure with the catalytic performance of the selective oxidation of cyclopentene to glutaraldehyde on WO3/TiO2 catalysts. Chem. Eng. J. 2010, 159, 242–246. [Google Scholar] [CrossRef]
- Ulgen, A.; Hoelderich, W.F. Conversion of glycerol to acrolein in the presence of WO3/TiO2 catalysts. Appl. Catal. A Gen. 2011, 400, 34–38. [Google Scholar] [CrossRef]
- Onfroy, T.; Lebarbier, V.; Clet, G.; Houalla, M. Quantitative relationship between the nature of surface species and the catalytic activity of tungsten oxides supported on crystallized titania. J. Mol. Catal. A Chem. 2010, 318, 1–7. [Google Scholar] [CrossRef]
- Epifani, M.; Díaz, R.; Force, C.; Comini, E.; Manzanares, M.; Andreu, T.; Genç, A.; Arbiol, J.; Siciliano, P.; Faglia, G.; et al. Surface Modification of TiO2 Nanocrystals by WOx Coating or Wrapping: Solvothermal Synthesis and Enhanced Surface Chemistry. ACS Appl. Mater. Interfaces 2015, 7, 6898–6908. [Google Scholar] [CrossRef]
- Epifani, M.; Comini, E.; Díaz, R.; Genç, A.; Andreu, T.; Siciliano, P.; Morante, J.R. Acetone sensors based on TiO2 nanocrystals modified with tungsten oxide species. J. Alloy. Compd. 2016, 665, 345–351. [Google Scholar] [CrossRef]
- Staerz, A.; Weimar, U.; Barsan, N. Understanding the Potential of WO₃ Based Sensors for Breath Analysis. Sensors 2016, 16, 1815. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.M.; Narsimha, K.; Rao, P.K. Effect of Method of Preparation on the Reactivity of Molybdena Supported on Titania. Langmuir 1991, 7, 1551–1553. [Google Scholar] [CrossRef]
- Segawa, K.; Soeya, T.; Kim, D.S. Supported Molybdenum Oxide Catalyst. Structure and Chemistry of Oxidized, Reduced, and Sulfided Surfaces. J. Jpn. Pet. Inst. 1990, 33, 347–359. [Google Scholar] [CrossRef][Green Version]
- Hu, H.; Wachs, I.E. Catalytic Properties of Supported Molybdenum Oxide Catalysts—In-Situ Raman and Methanol Oxidation Studies. J. Phys. Chem. 1995, 99, 10911–10922. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Niwa, M.; Murakami, Y. Morphology of molybdena supported on various oxides and its activity for methanol oxidation. J. Phys. Chem. 1990, 94, 1477–1482. [Google Scholar] [CrossRef]
- Brückman, K.; Grzybowska, B.; Che, M.; Tatibouët, J.M. Methanol oxidation on MoO3/TiO2 catalysts. Appl. Catal. A Gen. 1993, 96, 279–288. [Google Scholar] [CrossRef]
- Zhang, W.; Desikan, A.; Oyama, S.T. Effect of Support in Ethanol Oxidation on Molybdenum Oxide. J. Phys. Chem. 1995, 99, 14468–14476. [Google Scholar] [CrossRef]
- Shimada, H.; Sato, T.; Yoshimura, Y.; Hiraishi, J.; Nishijima, A. Support effect on the catalytic activity and properties of sulfided molybdenum catalysts. J. Catal. 1988, 110, 275–284. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Bhaskar, T.; Seela, K.K.; Lakshmi, K.S.; Reddy, K.R. Characterization and reactivity of molybdenum oxide catalysts supported on anatase and rutile polymorphs of titania. Appl. Catal. A Gen. 2001, 208, 291–305. [Google Scholar] [CrossRef]
- Epifani, M.; Kaciulis, S.; Mezzi, A.; Altamura, D.; Giannini, C.; Tang, P.; Morante, J.R.; Arbiol, J.; Siciliano, P.; Comini, E.; et al. Solvothermal Synthesis, Gas-Sensing Properties, and Solar Cell-Aided Investigation of TiO2–MoOx Nanocrystals. ChemNanoMat 2017, 3, 798–807. [Google Scholar] [CrossRef]
- Pârvulescu, V.I.; Grange, P.; Delmon, B. Catalytic removal of NO. Catal. Today 1998, 46, 233–316. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Mars, P.; van Krevelen, D.W. Oxidations carried out by means of vanadium oxide catalysts. Chem. Eng. Sci. 1954, 3, 41–59. [Google Scholar] [CrossRef]
- Doornkamp, C.; Ponec, V. The universal character of the Mars and Van Krevelen mechanism. J. Mol. Catal. A Chem. 2000, 162, 19–32. [Google Scholar] [CrossRef]
- Degler, D.; Wicker, S.; Weimar, U.; Barsan, N. Identifying the Active Oxygen Species in SnO2 Based Gas Sensing Materials: An Operando IR Spectrsocopy Study. J. Phys. Chem. C 2015, 119, 11792–11799. [Google Scholar] [CrossRef]


| Catalyzed Reactions | Composition | Sensing Application |
|---|---|---|
| CO oxidation, total oxidation of 2-propanol, ethanol, formaldehyde, toluene, methane, and ammonia | Co3O4 | CO, ethanol, ammonia, isopropanol toluene |
| Apart for CO and ethanol, the other applications deserve further developments. | ||
| Combustion of toluene, benzene, ethanol, and methane; formaldehyde and CO oxidation | MnO *x | H2, ethanol, acetone |
| Methane (possibly at high temperatures) | ||
| CO oxidation | CuO | Ethanol, CO, acetone, formaldehyde, benzene, H2S |
| CO oxidation, total oxidation of toluene, and formaldehyde and methane combustion | NiO | ethanol |
| CO, toluene, acetone, formaldehyde | ||
| CO oxidation, total oxidation of toluene, ethyl acetate, acetaldehyde, and various hydrocarbons | Cr2O3 | H2, ethanol, toluene, formaldehyde |
| CO, hydrocarbons, ethyl acetate, | ||
| CO oxidation, total oxidation of methane, ethanol, ethyl acetate, benzene, toluene, acetone, isopropanol, etc. | Perovskites (ABO3 general composition) | Methanol, ethanol, ammonia, alkanes, H2S, SO2, acetylene |
| Benzene, toluene, acetone, formaldehyde | ||
| CO oxidation, total oxidation of methane, propane, toluene | Fe2O3 | Acetone, ethanol, butanol, H2S |
| CO, Methane, propane, toluene | ||
| Total oxidation of chlorobenzenes and benzene; partial oxidation of o-xylene and of methanol; oxidative dehydrogenation of propane | TiO2-V2O5 | Ethanol, acetone |
| Benzene, toluene, chlorobenzenes, alkanes | ||
| Oxidation of CO, ethylene, propene, propane; oxidation of o-xylene and 2-propanol | SnO2-V2O5 | |
| Benzene and derivatives, alkanes and other hydrocarbons, alcohols | ||
| Isopropanol dehydration, cyclopentene oxidation | TiO2-WO3 | Acetone, ethanol |
| Alcohols, hydrocarbons | ||
| Partial oxidation of methanol and ethanol; dehydrosulfurization and ammoxidation reactions | TiO2-MoO3 | CO, acetone |
| Alcohols, H2S, other sulfur compounds, benzene, and derivatives |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monter-Guzmán, J.Y.M.; Chu, X.; Comini, E.; Epifani, M.; Zanella, R. How Chemoresistive Sensors Can Learn from Heterogeneous Catalysis. Hints, Issues, and Perspectives. Chemosensors 2021, 9, 193. https://doi.org/10.3390/chemosensors9080193
Monter-Guzmán JYM, Chu X, Comini E, Epifani M, Zanella R. How Chemoresistive Sensors Can Learn from Heterogeneous Catalysis. Hints, Issues, and Perspectives. Chemosensors. 2021; 9(8):193. https://doi.org/10.3390/chemosensors9080193
Chicago/Turabian StyleMonter-Guzmán, Jessica Yazmín Monter, Xiangfeng Chu, Elisabetta Comini, Mauro Epifani, and Rodolfo Zanella. 2021. "How Chemoresistive Sensors Can Learn from Heterogeneous Catalysis. Hints, Issues, and Perspectives" Chemosensors 9, no. 8: 193. https://doi.org/10.3390/chemosensors9080193
APA StyleMonter-Guzmán, J. Y. M., Chu, X., Comini, E., Epifani, M., & Zanella, R. (2021). How Chemoresistive Sensors Can Learn from Heterogeneous Catalysis. Hints, Issues, and Perspectives. Chemosensors, 9(8), 193. https://doi.org/10.3390/chemosensors9080193








