Glucose Biosensor Based on Dendritic Gold Nanostructures Electrodeposited on Graphite Electrode by Different Electrochemical Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment of the Working Electrode and Optimization of Electrochemical DGN Synthesis
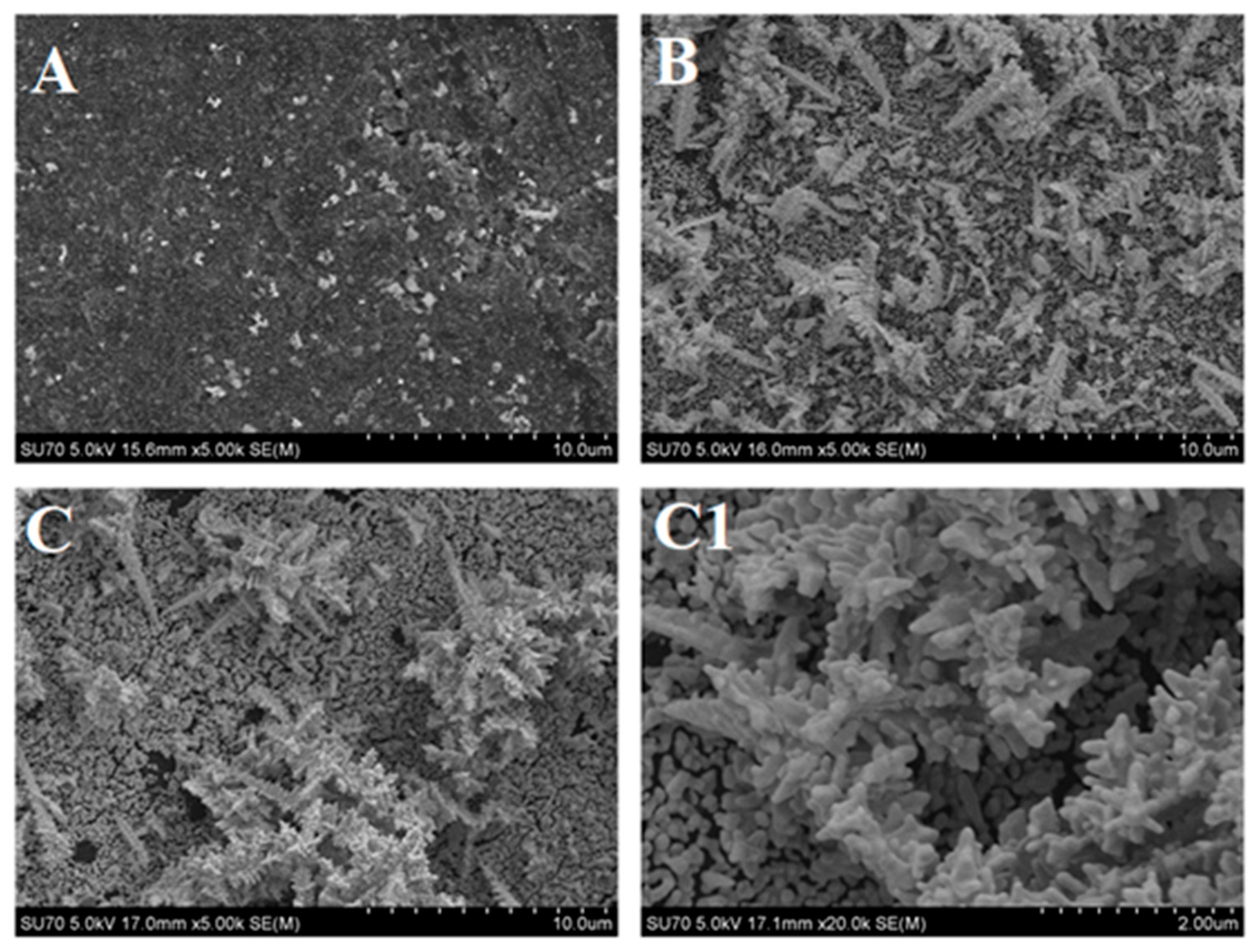
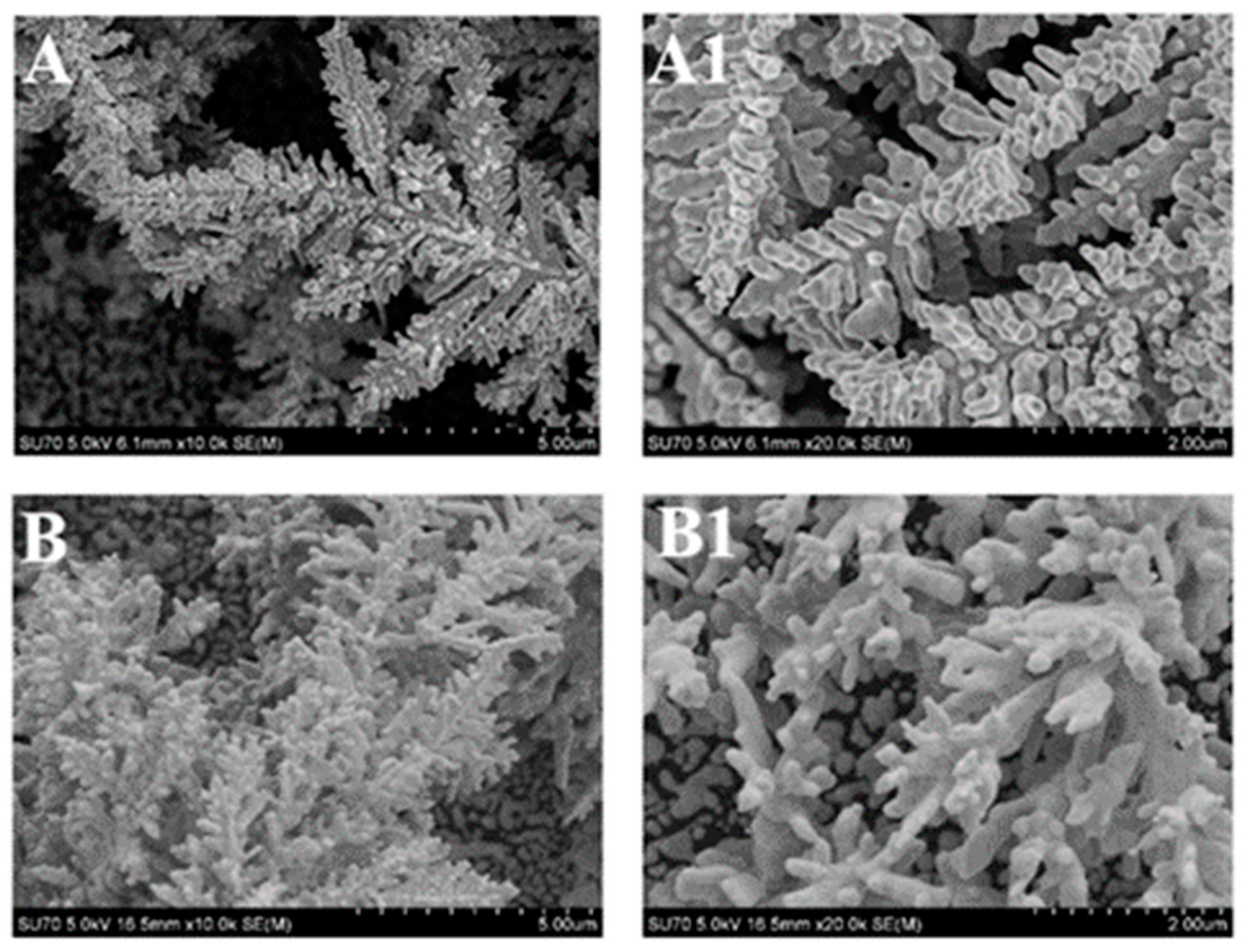
2.3. Imaging of DGN/GR Electrode by Field Emission Scanning Electron Microscopy
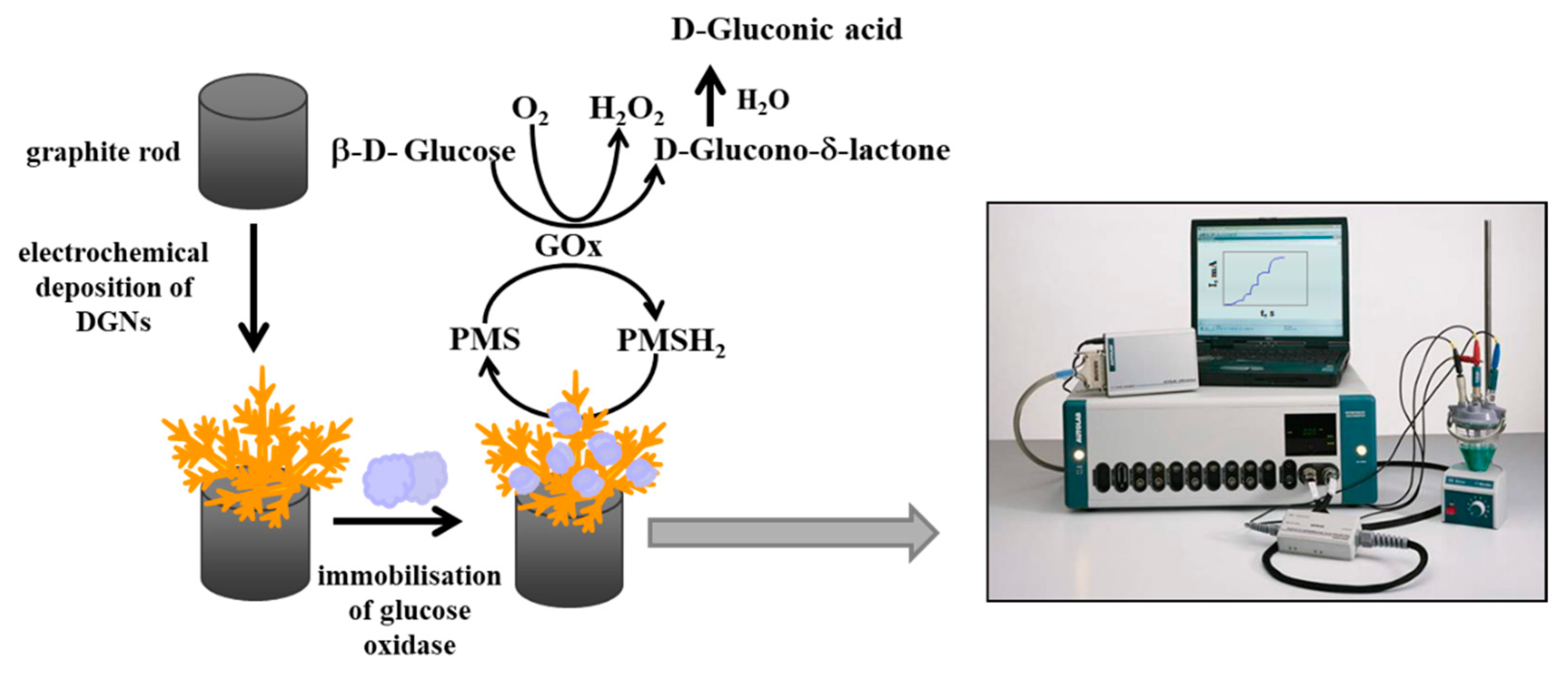
2.4. Immobilization of GOx on GR Electrode Premodified with Electrochemically Deposited DGNs
2.5. Electrochemical Measurements
2.6. Calculations
3. Results and Discussion
3.1. The Optimization of Electrochemical Deposition of DGNs
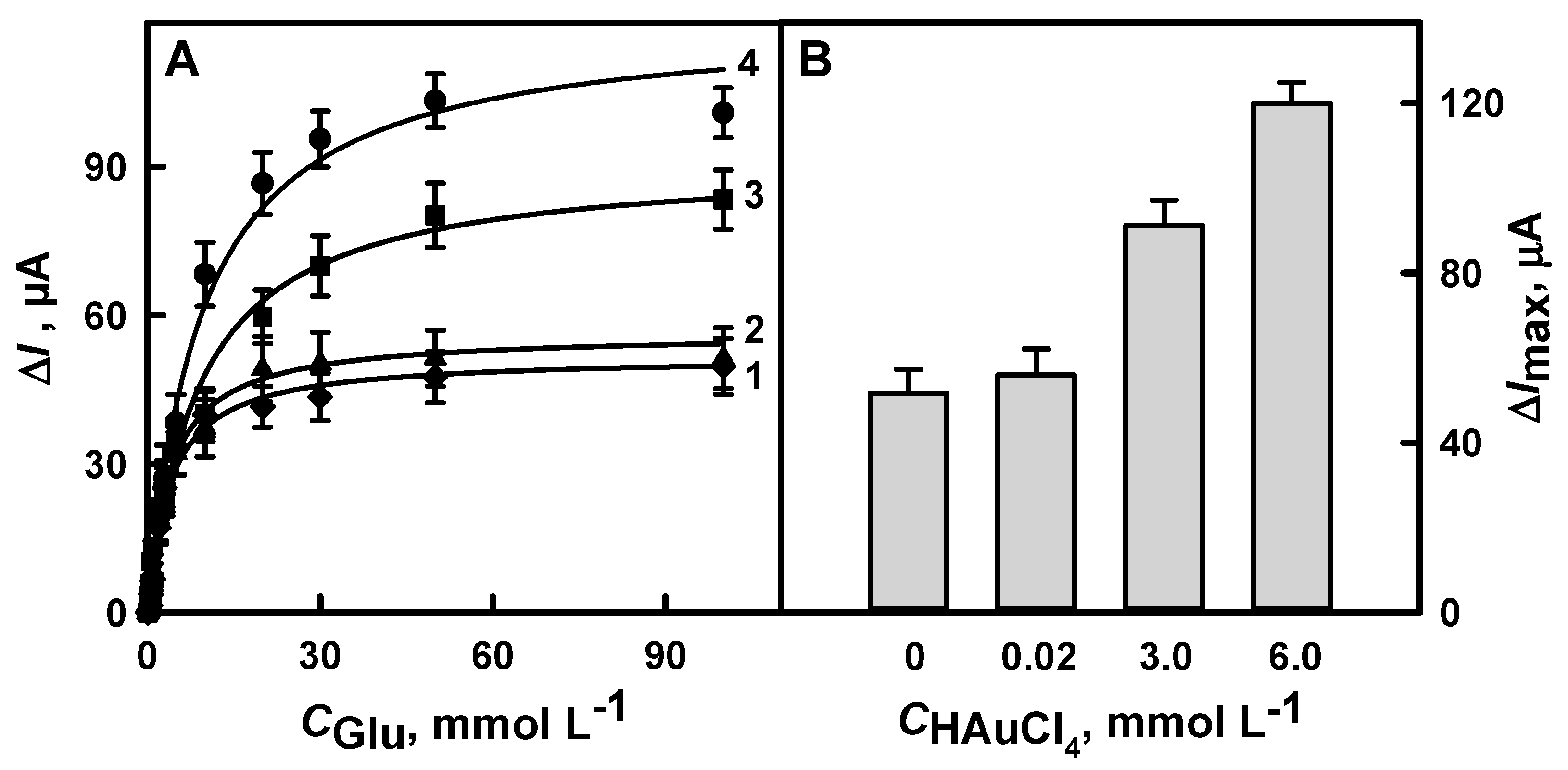
3.1.1. The Selection of Optimal HAuCl4 Solution Concentration
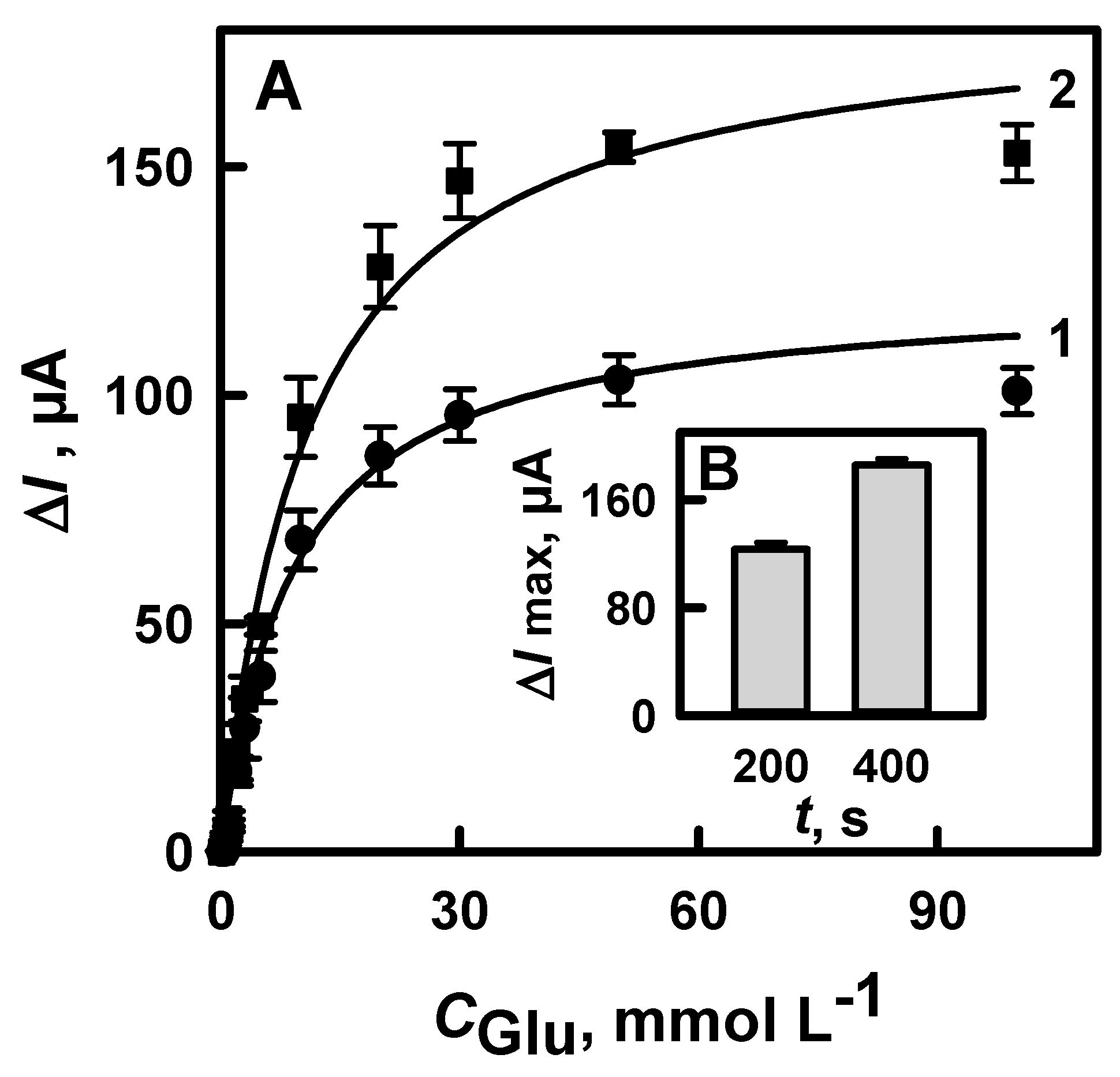
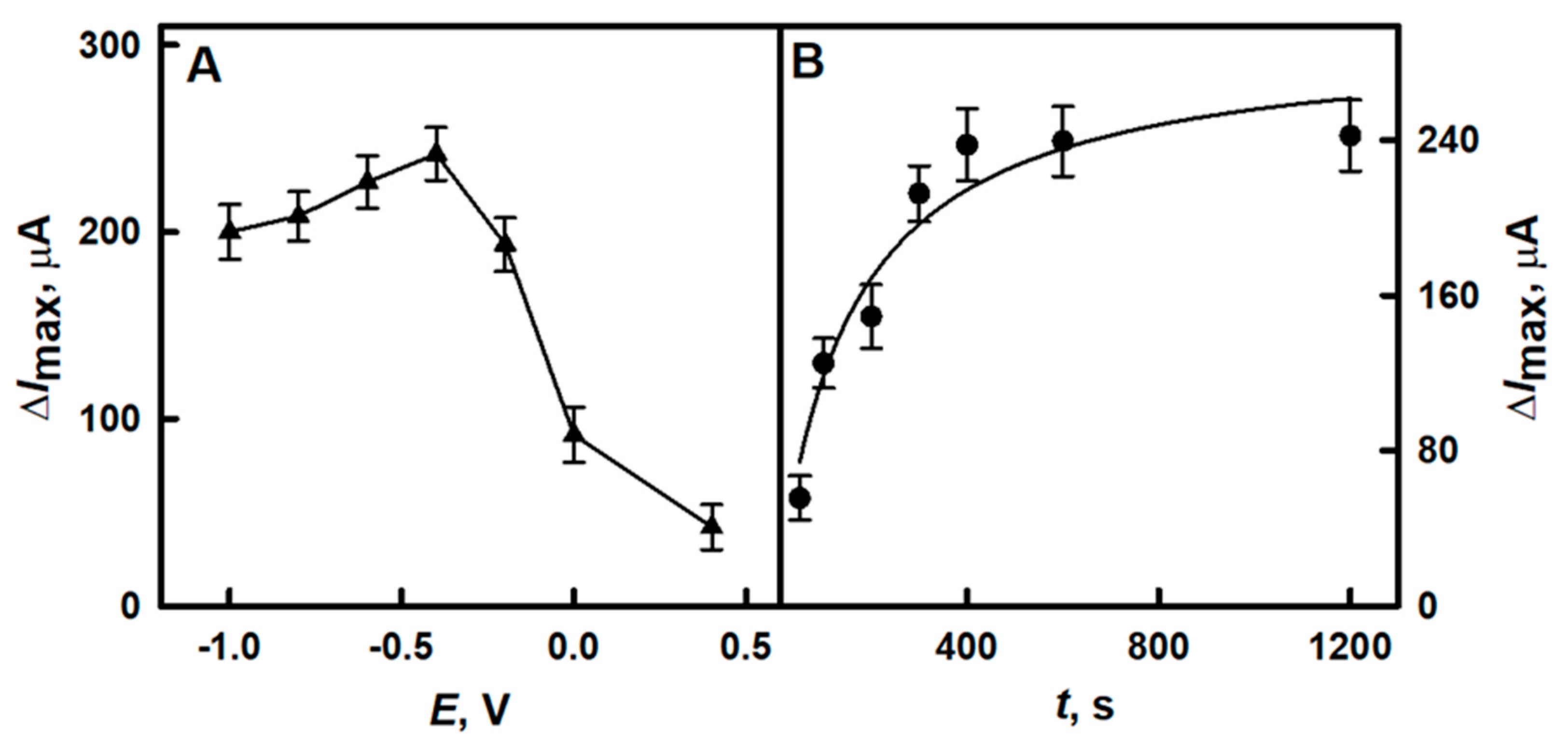
3.1.2. The Selection of the Optimal Time for the Electrochemical Deposition of DGNs
3.1.3. The Selection of the Optimal Potential for Electrochemical Deposition of DGNs
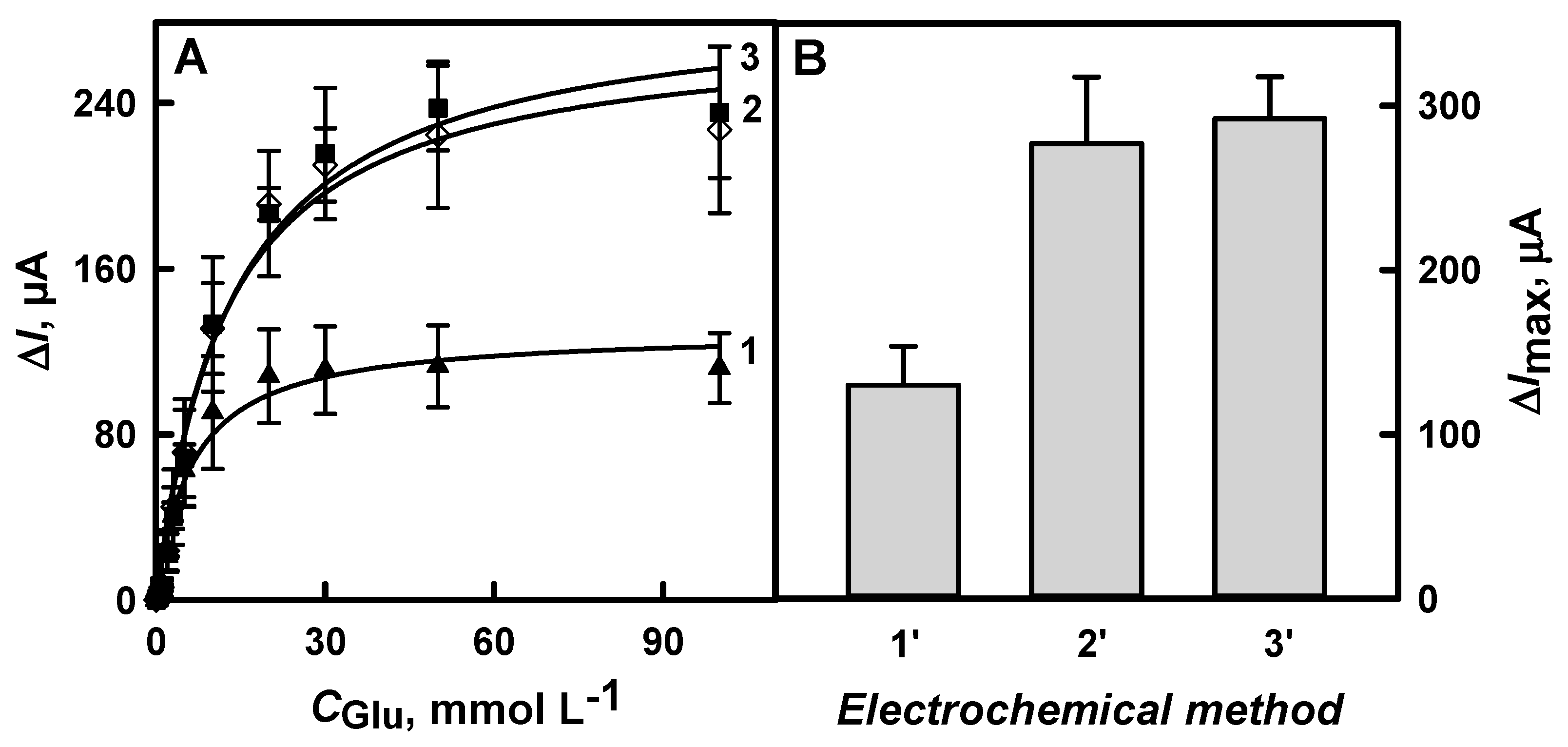
3.1.4. The Comparison of Glucose Biosensors Developed Using Electrodes Modified with DGNs Synthetized by Different Electrochemical Methods
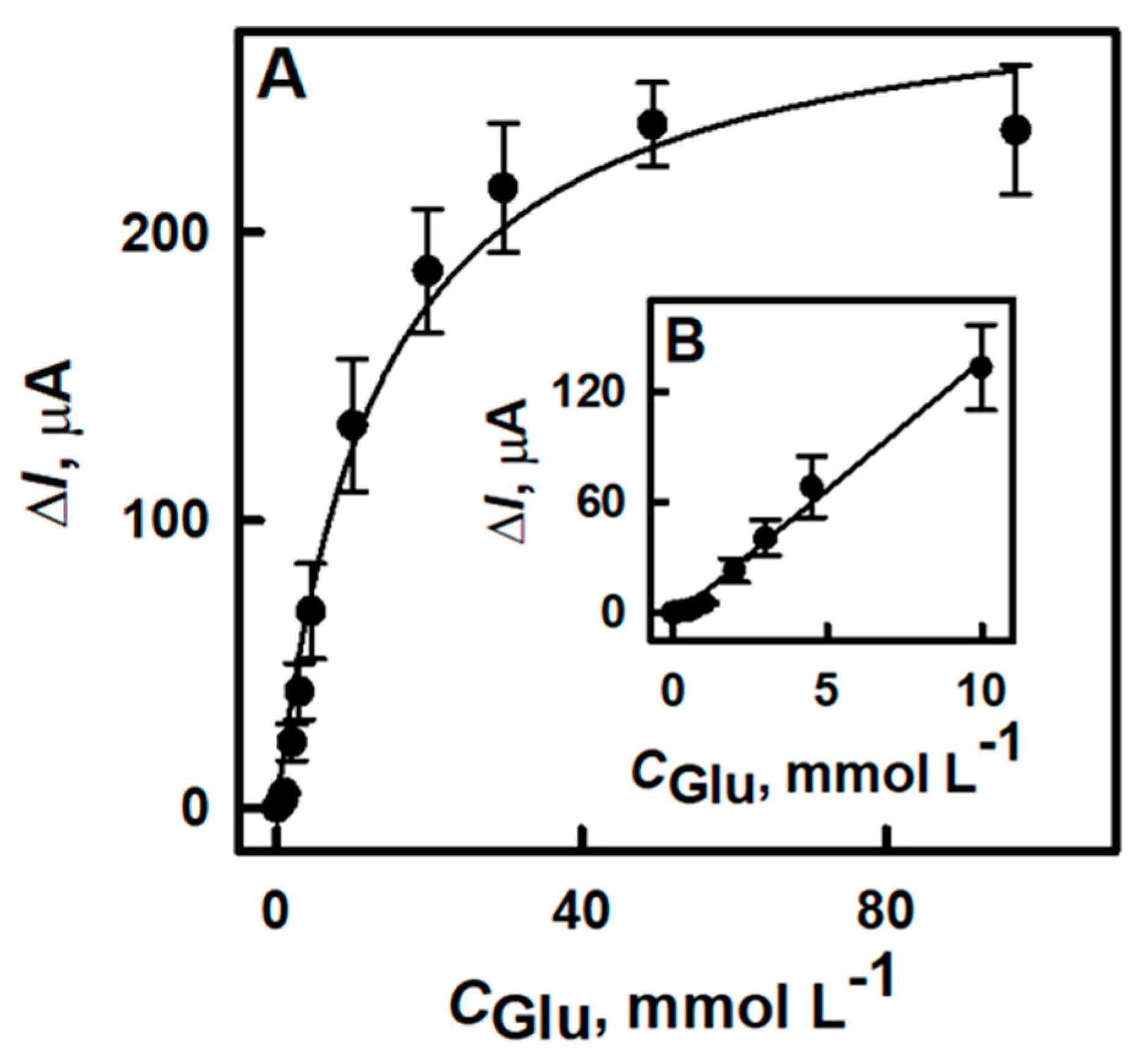
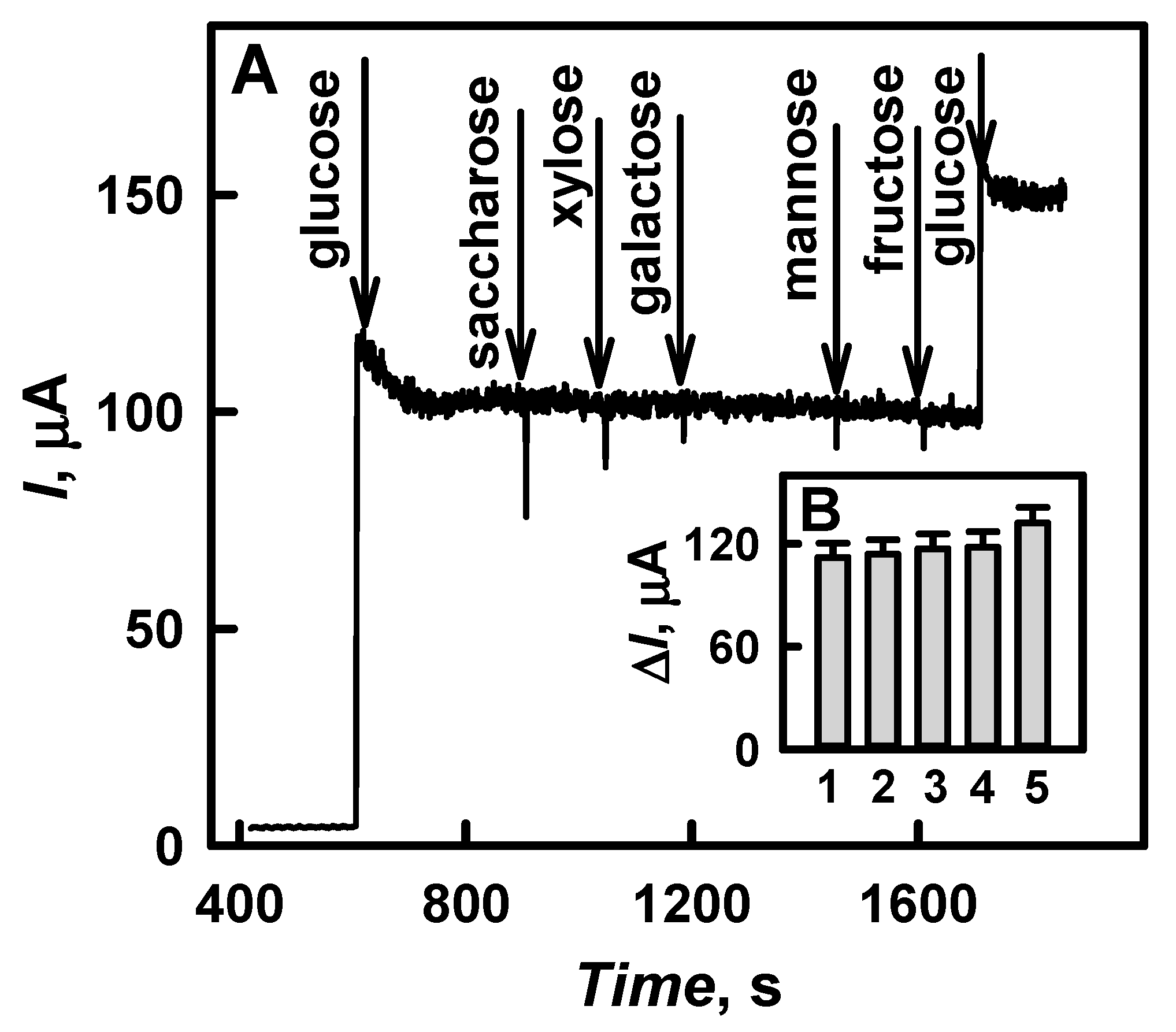
3.2. The Characterization of the Developed Biosensor Based on Electrochemically Deposited DGNs
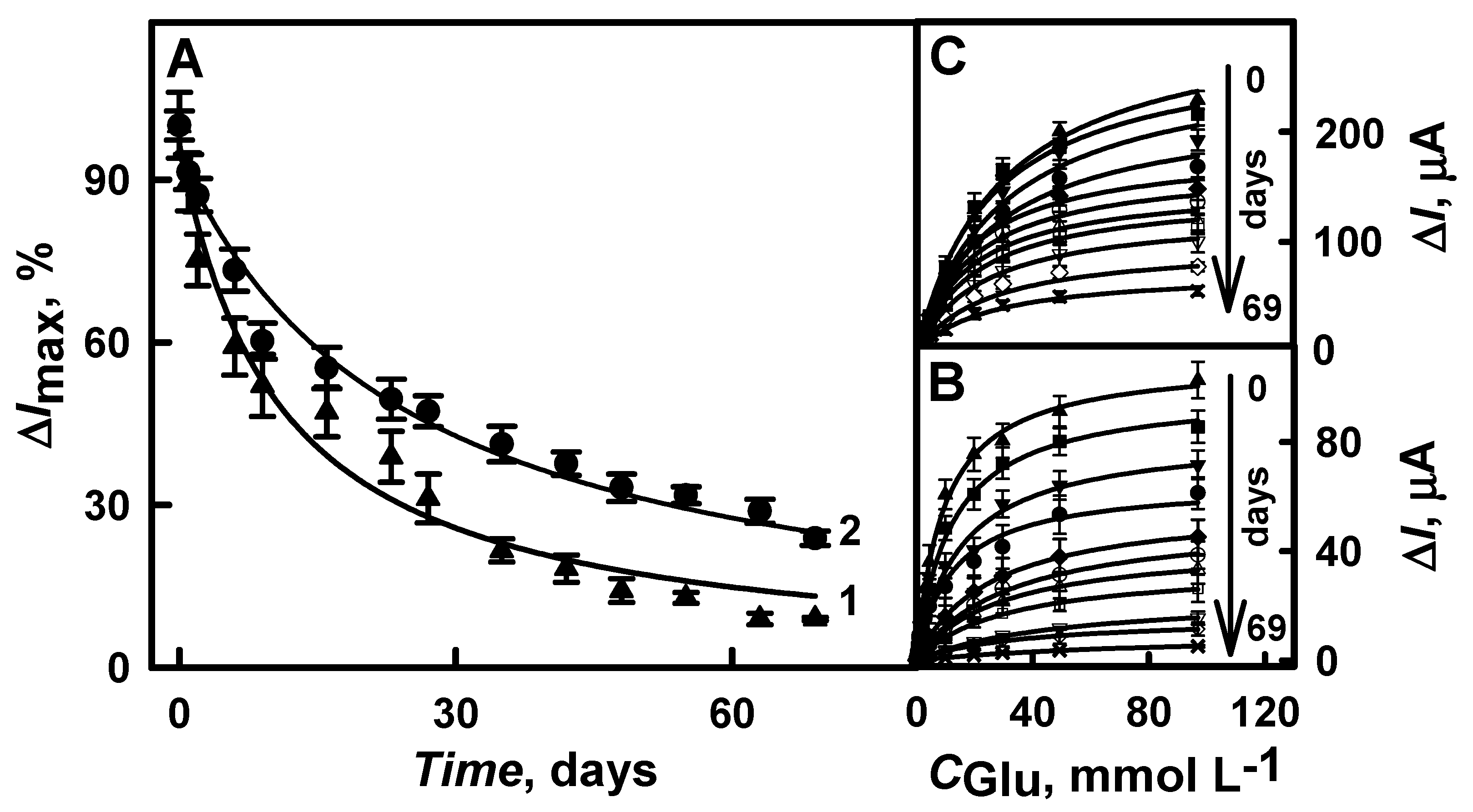
3.3. The Stability of the Developed Glucose Biosensor
3.4. Application of the Developed Biosensor for the Determination of Glucose in Human Serum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, N.; Zhao, P.; Astruc, D. Anisotropic gold nanoparticles: Synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef]
- Jeerapan, I.; Sonsaard, T.; Nacapricha, D. Applying nanomaterials to modern biomedical electrochemical detection of metabolites, electrolytes, and pathogens. Chemosensors 2020, 8, 71. [Google Scholar] [CrossRef]
- Piña, S.; Candia-Onfray, C.; Hassan, N.; Jara-Ulloa, P.; Contreras, D.; Salazar, R. Glassy carbon electrode modified with C/Au nanostructured materials for simultaneous determination of hydroquinone and catechol in water matrices. Chemosensors 2021, 9, 88. [Google Scholar] [CrossRef]
- Zou, H.; Ren, G.; Shang, M.; Wang, W. One-step, seedless, fabrication of three-dimensional gold meso-flowers (3D-AuMFs) with high activities in catalysis and surface enhanced Raman scattering. Mater. Chem. Phys. 2016, 176, 115–120. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, B.; Mitzscherling, S.; Masic, A.; Li, L.; Bargheer, M.; Möhwald, H. Preparation of gold nanostars and their study in selective catalytic reactions. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 20–25. [Google Scholar] [CrossRef]
- Feng, J.J.; Lv, Z.Y.; Qin, S.F.; Li, A.Q.; Fei, Y.; Wang, A.J. N-methylimidazole-assisted electrodeposition of Au porous textile-like sheet arrays and its application to electrocatalysis. Electrochim. Acta 2013, 102, 312–318. [Google Scholar] [CrossRef]
- Lin, T.H.; Lin, C.W.; Liu, H.H.; Sheu, J.T.; Hung, W.H. Potential-controlled electrodeposition of gold dendrites in the presence of cysteine. Chem. Commun. 2011, 47, 2044–2046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, F.; Yu, X.; Liu, H.; Fu, Y.; Wang, Z.; Jiang, L.; Li, X. Polyelectrolyte multilayer as matrix for electrochemical deposition of gold clusters: Toward super-hydrophobic surface. J. Am. Chem. Soc. 2004, 126, 3064–3065. [Google Scholar] [CrossRef]
- Jia, H.; Chang, G.; Lei, M.; Hec, H.; Liu, X.; Shu, H.; Xia, T.; Su, J.; He, Y. Platinum nanoparticles decorated dendrite-like gold nanostructure on glassy carbon electrodes for enhancing electrocatalysis performance to glucose oxidation. Appl. Surf. Sci. 2016, 384, 58–64. [Google Scholar] [CrossRef]
- Yi, S.; Sun, L.; Lenaghan, S.C.; Wang, Y.; Chong, X.; Zhang, Z.; Zhang, M. One-step synthesis of dendritic gold nanoflowers with high surface-enhanced Raman scattering (SERS) properties. RSC Adv. 2013, 3, 10139–10144. [Google Scholar] [CrossRef]
- Huang, T.; Meng, F.; Qi, L. Controlled synthesis of dendritic gold nanostructures assisted by supramolecular complexes of surfactant with cyclodextrin. Langmuir 2010, 26, 7582–7589. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Pan, N.; Zhang, K.; Wang, Z.; Hu, H.; Wang, X. Fabrication of dendrite-like Au nanostructures and their enhanced photoluminescence emission. Phys. Stat. Sol. A 2007, 204, 3398–3404. [Google Scholar] [CrossRef]
- Shanmugam, M.; Kim, K. Electrodeposited gold dendrites at reduced graphene oxide as an electrocatalyst for nitrite and glucose oxidation. J. Electroanal. Chem. 2016, 776, 82–92. [Google Scholar] [CrossRef]
- Pang, S.; Kondo, T.; Kawai, T. Formation of dendrimer-like gold nanoparticle assemblies. Chem. Mater. 2005, 17, 3636–3641. [Google Scholar] [CrossRef]
- Bai, X.; Gao, Y.; Zheng, L. Galvanic replacement mediated growth of dendritic gold nanostructures with a three-fold symmetry and their applications to SERS. Cryst. Eng. Comm. 2011, 13, 3562–3568. [Google Scholar] [CrossRef]
- Lee, J.H.; Kamada, K.; Enomoto, N.; Hojo, J. Seeding method for three-dimensional dendritic growth of gold nanoparticles stabilized by hexatrimethylammonium bromide. Chem. Lett. 2007, 36, 728–729. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, L.; Zhao, D.; Fei, Z.; Lu, Q.; Dyson, P.J. Fabrication of dendritic gold nanoparticles by use of an ionic polymer template. Langmuir 2008, 24, 2699–2704. [Google Scholar] [CrossRef]
- Tang, X.L.; Jiang, P.; Ge, G.L.; Tsuji, M.; Xie, S.S.; Guo, Y.J. Poly(N-vinyl-2-pyrrolidone) (PVP)-capped dendritic gold nanoparticles by a one-step hydrothermal route and their high SERS effect. Langmuir 2008, 24, 1763–1768. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, K.; Su, B.; Jiang, L. Superhydrophobicity-mediated electrochemical reaction along the solid-liquid -gas triphase interface: Edge-growth of gold architectures. Adv. Mater. 2014, 26, 1124–1128. [Google Scholar] [CrossRef]
- Heli, H.; Amirizadeh, O. Non-enzymatic glucose biosensor based on hyperbranched pine-like gold nanostructures. Mater. Sci. Eng. C 2016, 63, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Shu, H.; Cao, L.; Chang, G.; He, H.; Zhang, Y.; He, Y. Direct electrodeposition of gold nanostructures onto glassy carbon electrodes for non-enzymatic detection of glucose. Electrochim. Acta 2014, 132, 524–532. [Google Scholar] [CrossRef]
- Das, A.K.; Samdani, J.; Kim, H.Y.; Lee, J.H. Nicotinamide adenine dinucleotide assisted direct electrodeposition of gold nanodendrites and its electrochemical application. Electrochim. Acta 2015, 158, 129–137. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Z.; Miao, Z.; Ma, M.; Zhang, Y.; Zhang, C.; Wang, W.; Han, B.; Chen, Q. One step electrodeposotion of dendritic gold nanostructures on β-lactoglobulin-functionalized reduced graphene oxide for glucose sensing. Talanta 2015, 144, 823–829. [Google Scholar] [CrossRef]
- Hau, N.Y.; Yang, P.; Liu, C.; Wang, J.; Lee, P.H.; Feng, S.P. Aminosilane-assisted electrodeposition of gold nanodendrites and their catalytic properties. Sci. Rep. 2017, 7, 39839. [Google Scholar] [CrossRef]
- Purohit, B.; Kumar, A.; Mahato, K.; Chandra, P. Novel sensing assembly comprising engineered gold dendrites and MWCNT-AuNPs nanohybrid for acetaminophen detection in human urine. Electroanalysis 2020, 32, 561–570. [Google Scholar] [CrossRef]
- Rafatmah, E.; Hemmateenejad, B. Dendrite gold nanostructures electrodeposited on paper fibers: Application to electrochemical non-enzymatic determination of glucose. Sens. Actuators B Chem. 2020, 304, 127335. [Google Scholar] [CrossRef]
- Zaki, M.H.M.; Mohd, Y.; Chin, L.Y. Surface properties of nanostructured gold coatings electrodeposited at different potentials. Int. J. Electrochem. Sci. 2020, 15, 11401–11415. [Google Scholar] [CrossRef]
- Feng, J.J.; Li, A.Q.; Lei, Z.; Wang, A.J. Low-potential synthesis of “clean” Au nanodendrites and their high performance toward ethanol oxidation. ACS Appl. Mater. Inerfaces 2012, 4, 2570–2576. [Google Scholar] [CrossRef]
- Lv, Z.Y.; Li, A.Q.; Fei, Y.; Li, Z.; Chen, J.R.; Wang, A.J.; Feng, J.J. Facile and controlled electrochemical route to three-dimensional hierarchical dendritic gold nanostructures. Electrochim. Acta 2013, 109, 136–144. [Google Scholar] [CrossRef]
- Chen, W.Y.; Mei, L.P.; Feng, J.J.; Yuan, T.; Wang, A.J.; Yu, H. Electrochemical determination of bisphenol A with a glassy carbon electrode modified with gold nanodendrites. Microchim. Acta 2015, 182, 703–709. [Google Scholar] [CrossRef]
- Huan, T.N.; Ganesh, T.; Kim, K.S.; Kim, S.; Han, S.H.; Chung, H. A three-dimentional gold nanodendrites network porous structure and its application for an electrochemical sensing. Biosens. Bioelectron. 2011, 27, 183–186. [Google Scholar] [CrossRef]
- Ye, W.; Yan, J.; Ye, Q.; Zhou, F. Template-free and direct electrochemical deposition of hierarchical dendritic gold microstructures: Growth and their multiple applications. J. Phys. Chem. C 2010, 114, 15617–15624. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, J.; Huang, W.; Li, Z. Electrochemical fabrication of clean dendritic Au supported Pt clusters for electrocatalytic oxidation of formic acid. Electrochim. Acta 2012, 70, 304–312. [Google Scholar] [CrossRef]
- Valera, A.E.; Nesbitt, N.T.; Archibald, M.M.; Naughton, M.J.; Chiles, T.C. On-chip electrochemical detection of cholera using a polypyrrole-functionalized dendritic gold sensor. ACS Sens. 2019, 4, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Vassilyev, Y.B.; Khazova, O.A.; Nikolaeva, N.N. Kinetics and mechanism of glucose electrooxidation on different electrode-catalysts part II. Effect of the nature of the electrode and the electrooxidation mechanism. J. Electroanal. Chem. 1985, 196, 127–144. [Google Scholar] [CrossRef]
- Lee, W.C.; Kim, K.B.; Gurudatt, N.G.; Hussain, K.K.; Choi, C.S.; Park, D.S.; Shim, Y.B. Comparison of enzymatic and non-enzymatic glucose sensors based on hierarchical Au-Ni alloy with conductive polymer. Biosens. Bioelectron. 2019, 130, 48–54. [Google Scholar] [CrossRef]
- German, N.; Kausaite-Minkstimiene, A.; Ramanavicius, A.; Semashko, T.; Mikhailova, R.; Ramanaviciene, A. The use of different glucose oxidases for the development of an amperometric reagentless glucose biosensor based on gold nanoparticles covered by polypyrrole. Electrochim. Acta 2015, 169, 326–333. [Google Scholar] [CrossRef]
- Ramanavicius, A.; German, N.; Ramanaviciene, A. Evaluation of electron transfer in electrochemical system based on immobilized gold nanoparticles and glucose oxidase. J. Electrochem. Soc. 2017, 164, G45–G49. [Google Scholar] [CrossRef]
- German, N.; Ramanavicius, A.; Ramanaviciene, A. Electrochemical deposition of gold nanoparticles on graphite rod for glucose biosensing. Sens. Actuat. B Chem. 2014, 203, 25–34. [Google Scholar] [CrossRef]
- Xiao, X.; Li, H.; Wang, M.; Zhang, K.; Si, P. Examining the effects of self-assembled monolayers on nanoporous gold based amperometric glucose biosensors. Analyst 2014, 139, 488–494. [Google Scholar] [CrossRef]
- Scharifker, B.; Hills, G. Theoretical and experimental studies of multiple nucleation. Electrochim. Acta 1983, 28, 879–889. [Google Scholar] [CrossRef]
- German, N.; Ramanaviciene, A.; Voronovic, J.; Ramanavicius, A. Glucose biosensor based on graphite electrodes modified with glucose oxidase and colloidal gold nanoparticles. Microchim. Acta 2010, 168, 221–229. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Zhao, S.; Sun, Y.; Sun, C. Multilayered construction of glucose oxidase and gold nanoparticles on Au electrodes based on layer-by-layer covalent attachment. Electrochem. Commun. 2006, 8, 665–672. [Google Scholar] [CrossRef]
- Yuan, C.J.; Hsu, C.L.; Wang, S.C.; Chang, K.S. Eliminating the interference of ascorbic acid and uric acid to the amperometric glucose biosensor by cation exchangers membrane and size exclusion membrane. Electroanalysis 2005, 17, 2239–2245. [Google Scholar] [CrossRef]
| Added Concentration, mmol L−1 | Detected Concentration (n = 3), mmol L−1 | Relative Standard Deviation, % | Recovery Ratio, % |
|---|---|---|---|
| 0.500 | 0.470 | 5.63 | 94.0 |
| 1.00 | 0.960 | 4.91 | 96.0 |
| 2.00 | 1.95 | 4.81 | 97.5 |
| 3.00 | 2.94 | 4.39 | 98.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramanaviciene, A.; German, N.; Kausaite-Minkstimiene, A.; Ramanavicius, A. Glucose Biosensor Based on Dendritic Gold Nanostructures Electrodeposited on Graphite Electrode by Different Electrochemical Methods. Chemosensors 2021, 9, 188. https://doi.org/10.3390/chemosensors9080188
Ramanaviciene A, German N, Kausaite-Minkstimiene A, Ramanavicius A. Glucose Biosensor Based on Dendritic Gold Nanostructures Electrodeposited on Graphite Electrode by Different Electrochemical Methods. Chemosensors. 2021; 9(8):188. https://doi.org/10.3390/chemosensors9080188
Chicago/Turabian StyleRamanaviciene, Almira, Natalija German, Asta Kausaite-Minkstimiene, and Arunas Ramanavicius. 2021. "Glucose Biosensor Based on Dendritic Gold Nanostructures Electrodeposited on Graphite Electrode by Different Electrochemical Methods" Chemosensors 9, no. 8: 188. https://doi.org/10.3390/chemosensors9080188
APA StyleRamanaviciene, A., German, N., Kausaite-Minkstimiene, A., & Ramanavicius, A. (2021). Glucose Biosensor Based on Dendritic Gold Nanostructures Electrodeposited on Graphite Electrode by Different Electrochemical Methods. Chemosensors, 9(8), 188. https://doi.org/10.3390/chemosensors9080188









