Quantitative Assessment of Periodontal Bacteria Using a Cell-Based Immunoassay with Functionalized Quartz Crystal Microbalance
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
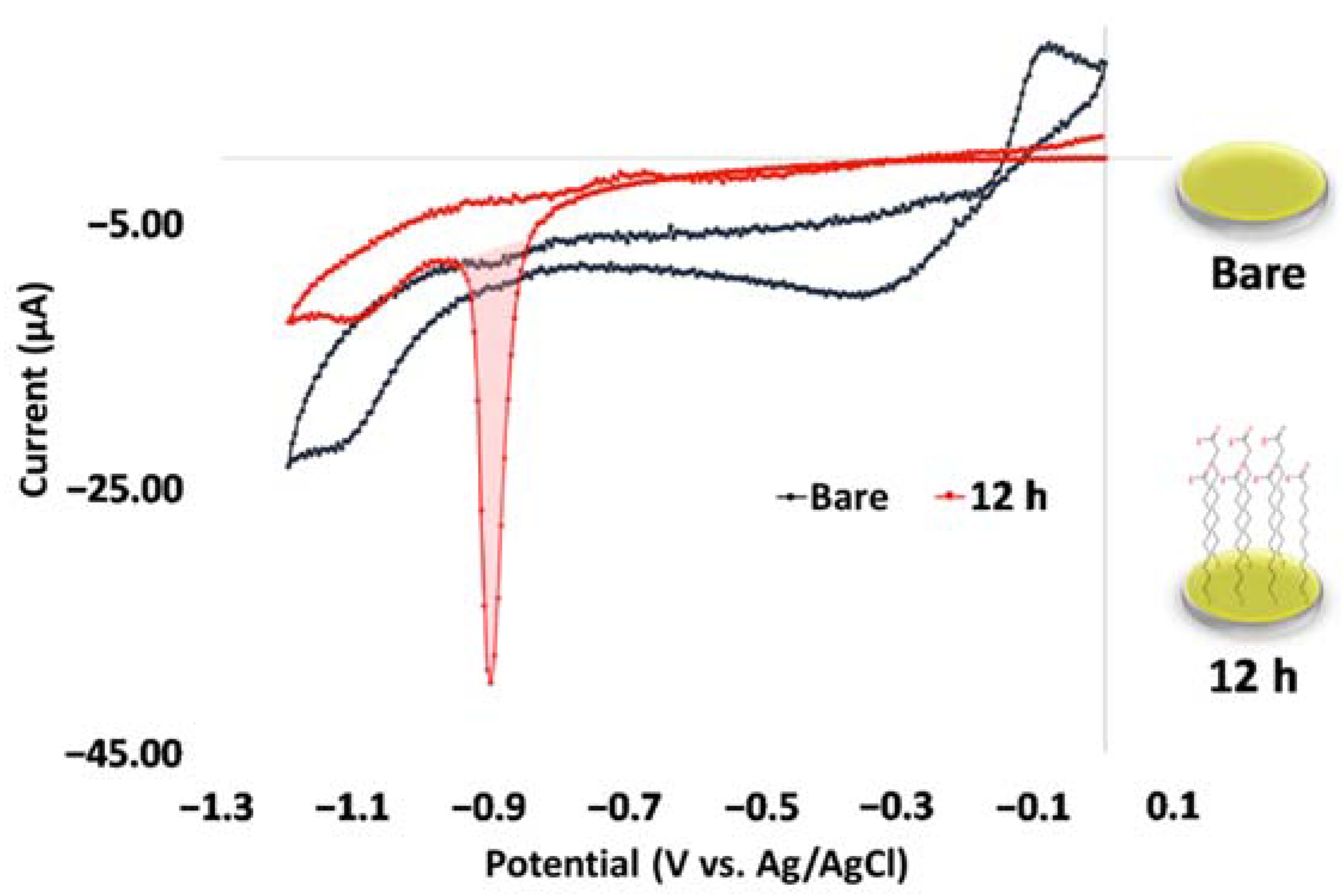
2.2. Self-Assembled Monolayer and Surface Characterization
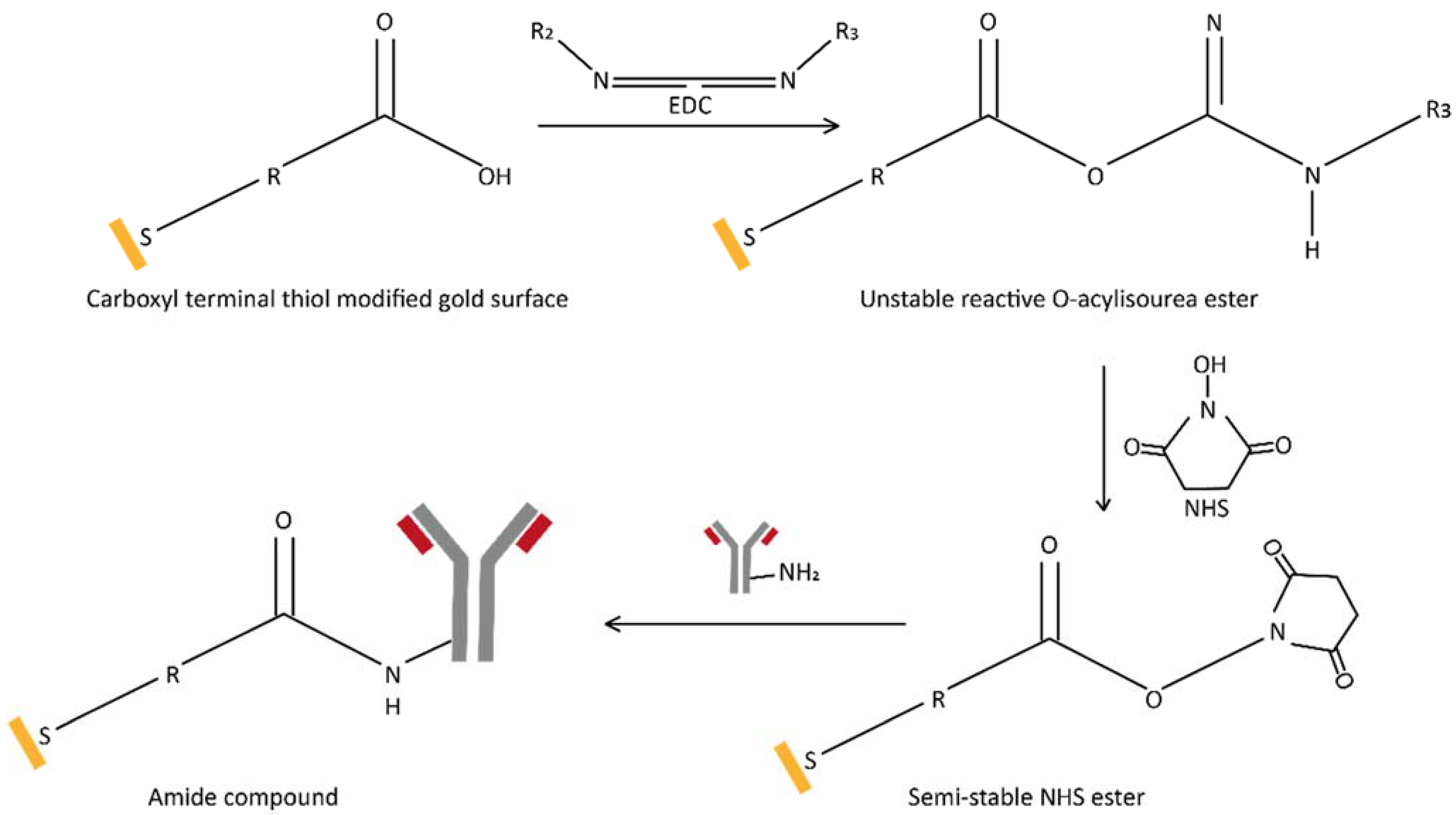
2.3. Antibody Immobilization on a Carboxy-Terminated Self-Assembled Monolayer Surface
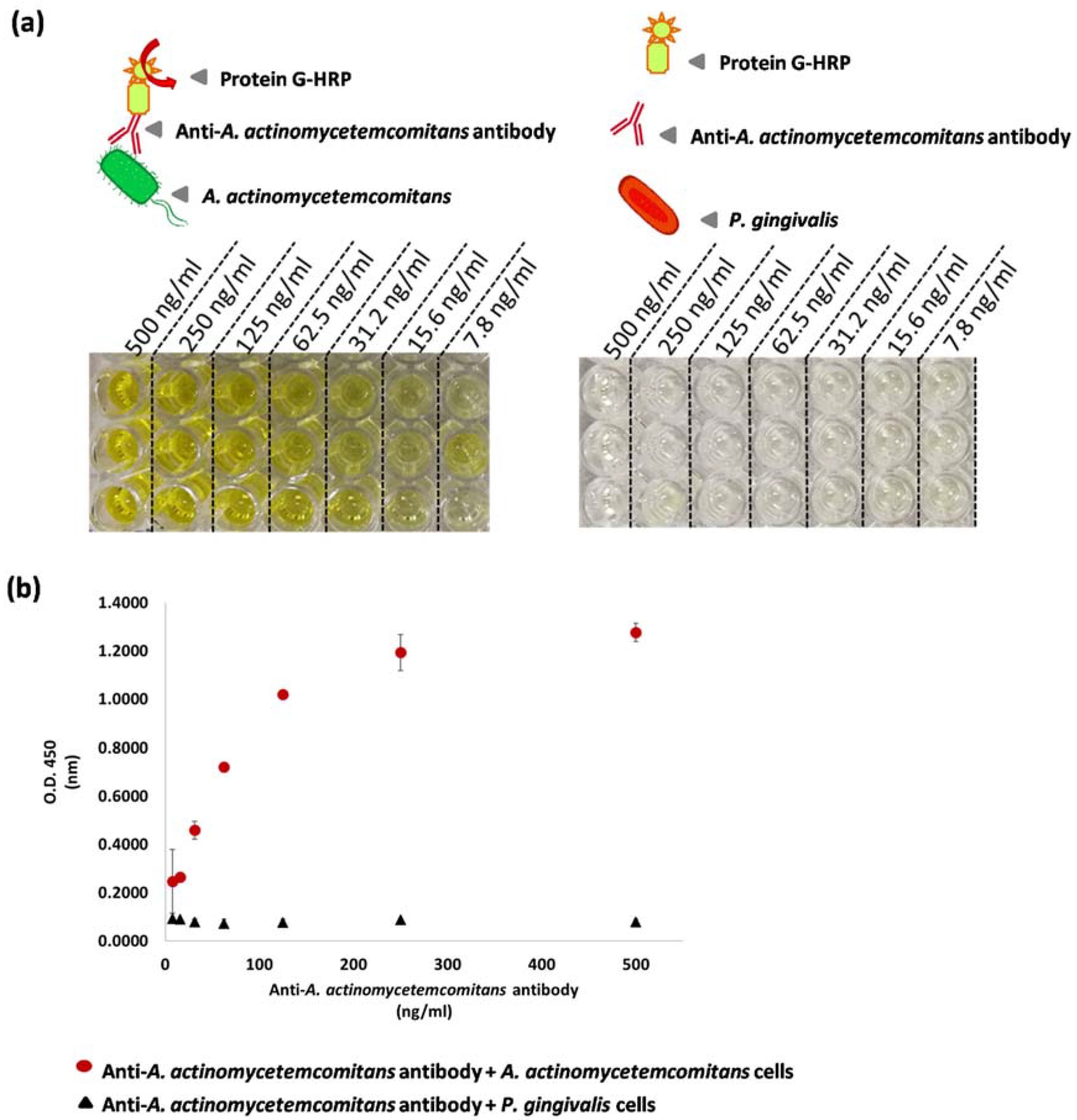
2.4. Enzyme-Linked Immunosorbent Assay
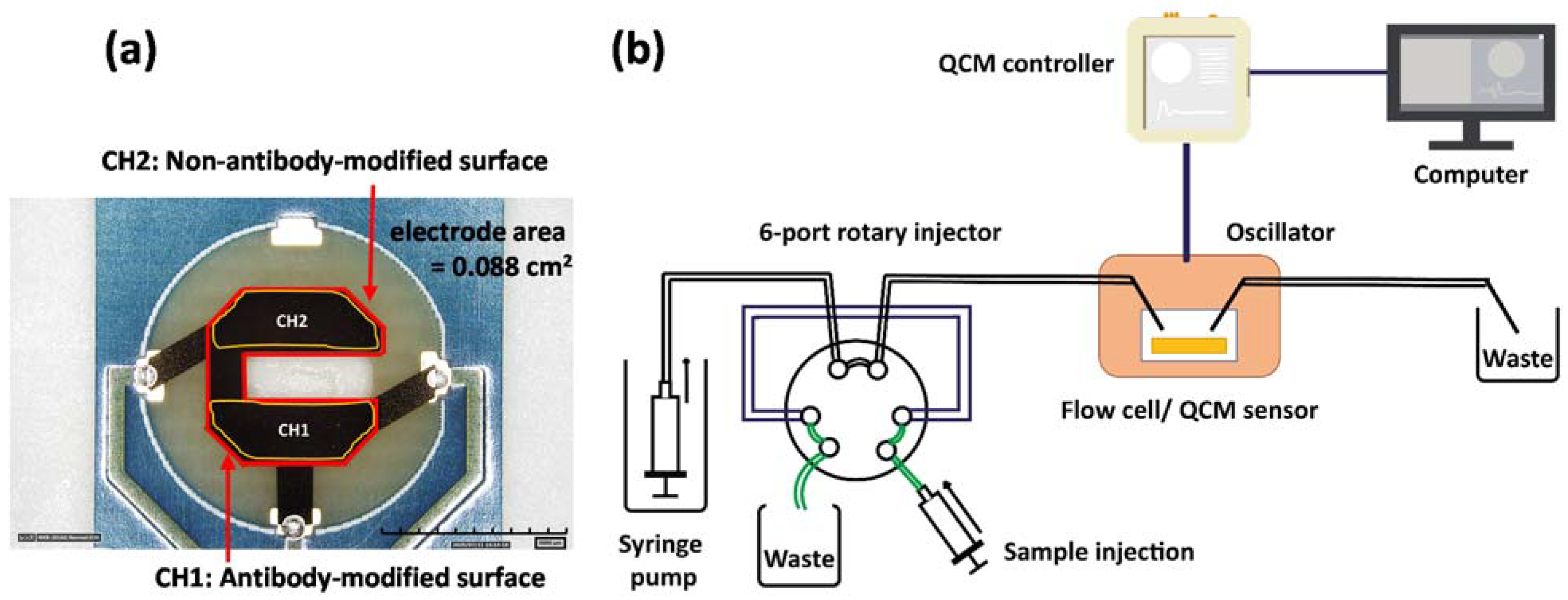
2.5. Fabrication of the QCM Immunosensor
2.6. Quartz Crystal Microbalance Measurement
3. Results and Discussion
3.1. Characterization of the Self-Assembled Monolayer of 11-MUA
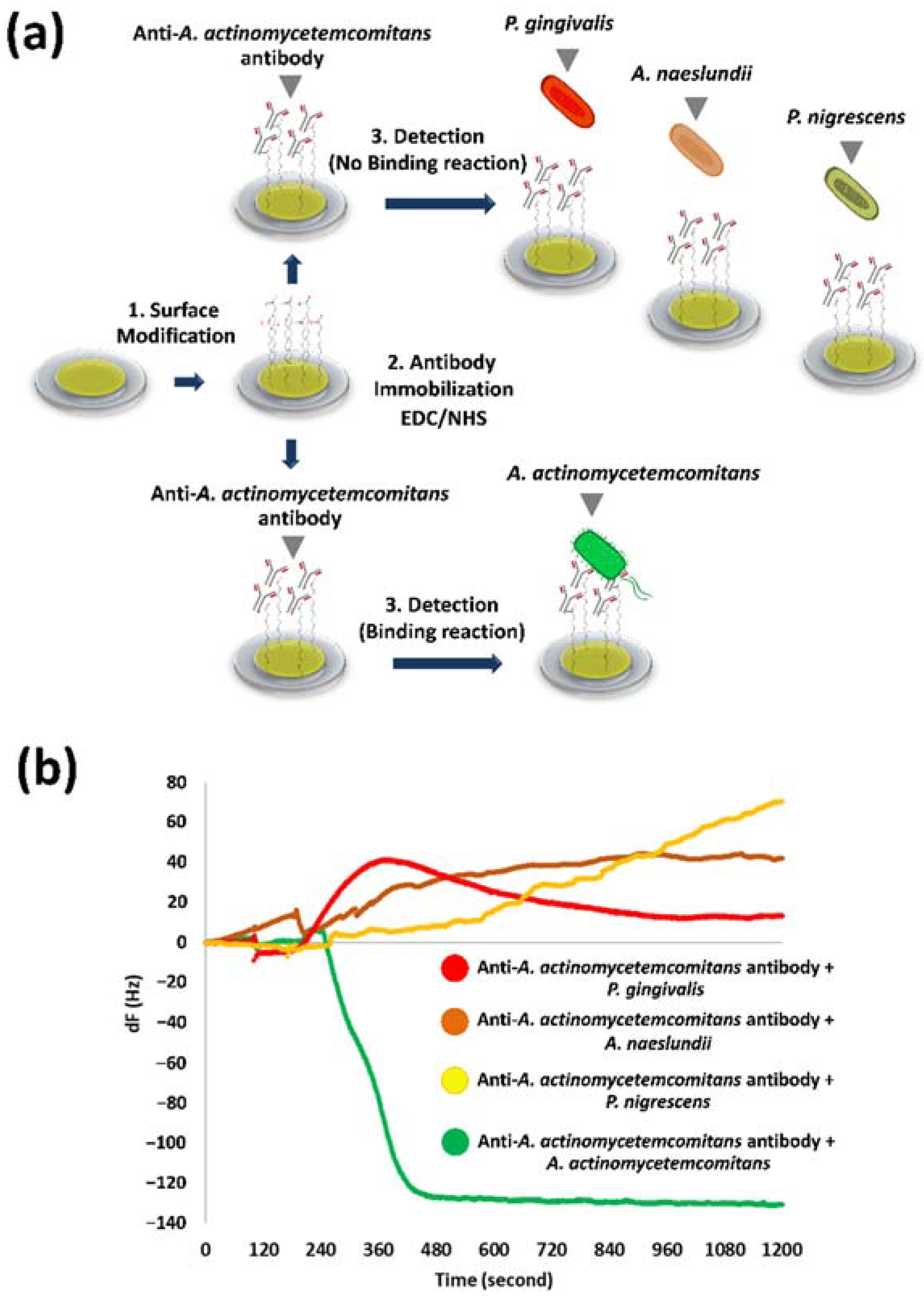
3.2. Specificity of Anti-A. actinomycetemcomitans Antibody
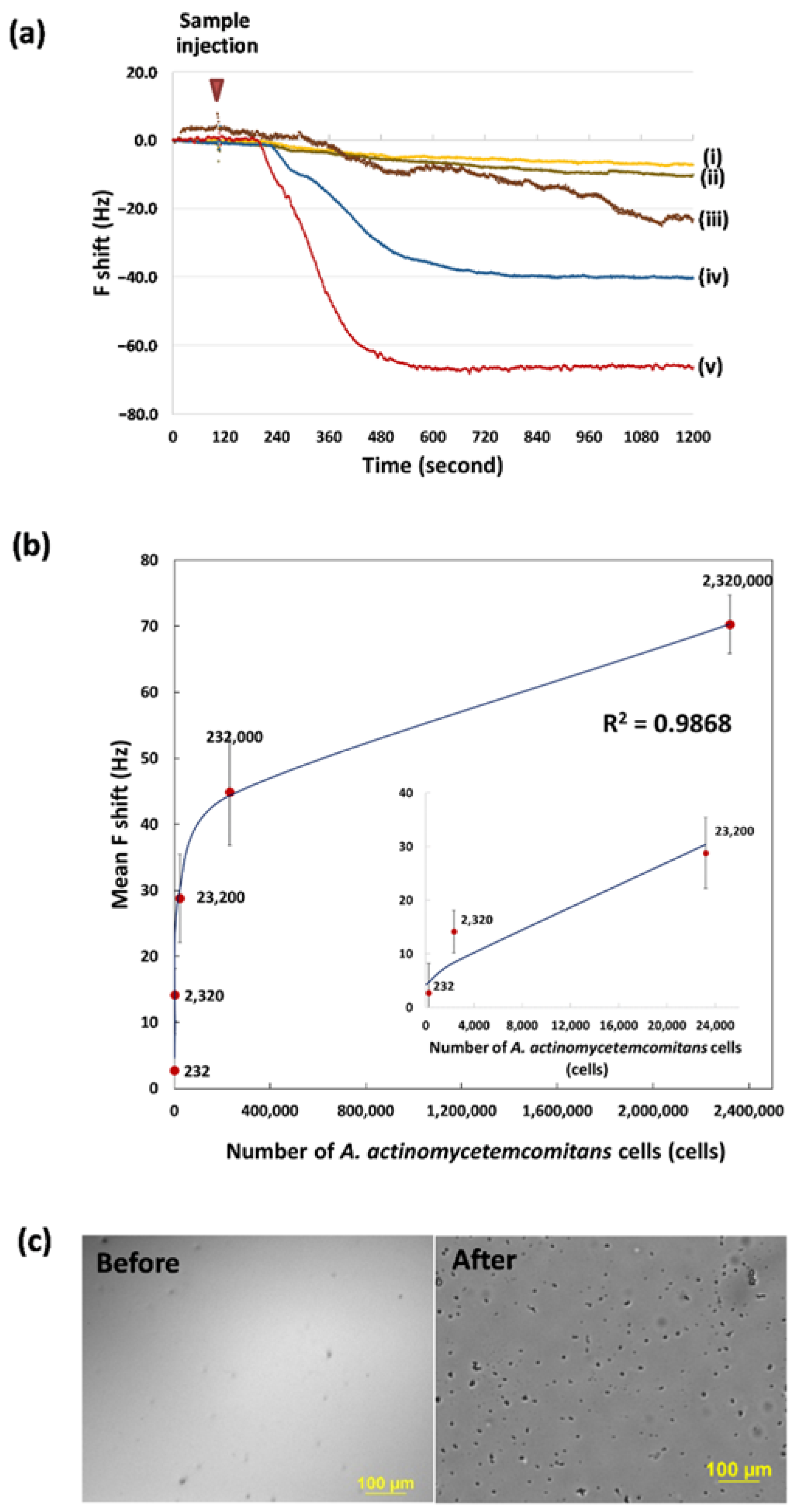
3.3. Determination of the Bacteria Concentration and Calibration Curves
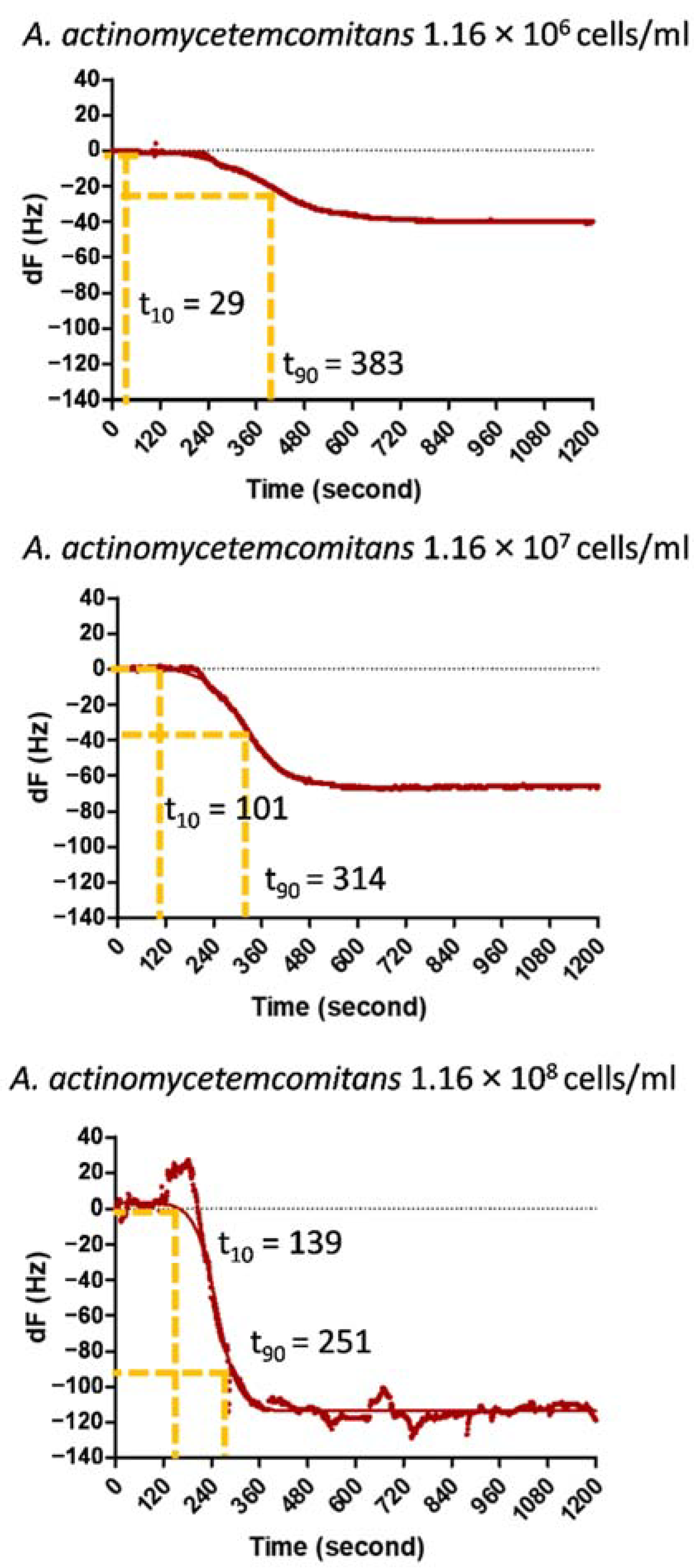
3.4. Response Time Analysis for the Frequency Shift
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chang, H.-J.; Lee, S.-J.; Yong, T.-H.; Shin, N.-Y.; Jang, B.-G.; Kim, J.-E.; Huh, K.-H.; Lee, S.-S.; Heo, M.-S.; Choi, S.-C.; et al. Deep Learning Hybrid Method to Automatically Diagnose Periodontal Bone Loss and Stage Periodontitis. Sci. Rep. 2020, 10, 7531. [Google Scholar] [CrossRef]
- He, W.; You, M.; Wan, W.; Xu, F.; Li, F.; Li, A. Point-of-Care Periodontitis Testing: Biomarkers, Current Technologies, and Perspectives. Trends Biotechnol. 2018, 36, 1127–1144. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002. [Google Scholar] [CrossRef] [Green Version]
- Hajishengallis, G.; Liang, S.; Payne, M.A.; Hashim, A.; Jotwani, R.; Eskan, M.A.; McIntosh, M.L.; Alsam, A.; Kirkwood, K.L.; Lambris, J.D.; et al. Low-Abundance Biofilm Species Orchestrates Inflammatory Periodontal Disease through the Commensal Microbiota and Complement. Cell Host Microbe 2011, 10, 497–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontology 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Nomura, Y.; Takeuchi, H.; Okamoto, M.; Sogabe, K.; Okada, A.; Hanada, N. Chair-side detection of Prevotella Intermedia in mature dental plaque by its fluorescence. Photodiagn. Photodyn. Ther. 2017, 18, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yoneda, M.; Hirofuji, T. Mixed Red-Complex Bacterial Infection in Periodontitis. Int. J. Dent. 2013, 2013, 587279. [Google Scholar] [CrossRef]
- Park, O.J.; Yi, H.; Jeon, J.H.; Kang, S.S.; Koo, K.T.; Kum, K.Y.; Chun, J.; Yun, C.H.; Han, S.H. Pyrosequencing Analysis of Subgingival Microbiota in Distinct Periodontal Conditions. J. Dent. Res. 2015, 94, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Scapoli, L.; Girardi, A.; Palmieri, A.; Martinelli, M.; Cura, F.; Lauritano, D.; Carinci, F. Quantitative Analysis of Periodontal Pathogens in Periodontitis and Gingivitis. J. Biol. Regul. Homeost. Agents 2015, 29, 101–110. [Google Scholar] [PubMed]
- Torrungruang, K.; Jitpakdeebordin, S.; Charatkulangkun, O.; Gleebbua, Y. Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Treponema denticola/Prevotella intermedia Co-Infection are Associated with Severe Periodontitis in a Thai Population. PLoS ONE 2015, 10, e0136646. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for Whole-Cell Bacterial Detection. Clin. Microbiol. Rev. 2014, 27, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malagi, S. Chairside Diagnostic Test Kits In Periodontics—A Review. Int. Arab J. Dent. 2015, 30, 99–102. [Google Scholar]
- Ravishankar, P.; Mithra, D.; Chakraborty, P.; Kumar, A. Chairside diagnostics in periodontics. SRM J. Res. Dent. Sci. 2017, 8, 78–81. [Google Scholar] [CrossRef]
- Yamamoto, Y. PCR in diagnosis of infection: Detection of bacteria in cerebrospinal fluids. Clin. Diagn. Lab. Immunol. 2002, 9, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Brosel-Oliu, S.; Abramova, N.; Uria, N.; Bratov, A. Impedimetric transducers based on interdigitated electrode arrays for bacterial detection—A review. Anal. Chim. Acta 2019, 1088, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Penn, L.S.; Xi, J. Quartz crystal microbalance: Sensing cell-substrate adhesion and beyond. Biosens. Bioelectron. 2018, 99, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Uekubo, A.; Hiratsuka, K.; Aoki, A.; Takeuchi, Y.; Abiko, Y.; Izumi, Y. Effect of antimicrobial photodynamic therapy using rose bengal and blue light-emitting diode on Porphyromonas gingivalis in vitro: Influence of oxygen during treatment. Laser Ther. 2016, 25, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komazaki, R.; Katagiri, S.; Takahashi, H.; Maekawa, S.; Shiba, T.; Takeuchi, Y.; Kitajima, Y.; Ohtsu, A.; Udagawa, S.; Sasaki, N.; et al. Periodontal pathogenic bacteria, Aggregatibacter actinomycetemcomitans affect non-alcoholic fatty liver disease by altering gut microbiota and glucose metabolism. Sci. Rep. 2017, 7, 13950. [Google Scholar] [CrossRef]
- Hai, W.; Goda, T.; Takeuchi, H.; Yamaoka, S.; Horiguchi, Y.; Matsumoto, A.; Miyahara, Y. Human influenza virus detection using sialyllactose-functionalized organic electrochemical transistors. Sens. Actuators B Chem. 2018, 260, 635–641. [Google Scholar] [CrossRef]
- Goda, T.; Miyahara, Y. Thermo-responsive molecular switches for ATP using hairpin DNA aptamers. Biosens. Bioelectron. 2011, 26, 3949–3952. [Google Scholar] [CrossRef]
- Poirier, G.E.; Tarlov, M.J.; Rushmeier, H.E. Two-Dimensional Liquid Phase and the px.sqroot.3 Phase of Alkanethiol Self-Assembled Monolayers on Au(111). Langmuir 1994, 10, 3383–3386. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Goda, T.; Matsumoto, A.; Takeuchi, H.; Yamaoka, S.; Miyahara, Y. Direct and label-free influenza virus detection based on multisite binding to sialic acid receptors. Biosens. Bioelectron. 2017, 92, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Reeves, B.D.; Young, M.; Grieco, P.A.; Suci, P. Aggregatibacter actinomycetemcomitans biofilm killing by a targeted ciprofloxacin prodrug. Biofouling 2013, 29, 1005–1014. [Google Scholar] [CrossRef] [Green Version]
- Gmur, R. Monoclonal antibodies for the rapid identification in clinical samples of Peptostreptococcus micros and Actinobacillus actinomycetemcomitans serotypes a, d, and e. Med. Microbiol. Lett. 1996, 5, 335–349. [Google Scholar]
- Papapanou, P.N.; Teanpaisan, R.; Obiechina, N.S.; Pithpornchaiyakul, W.; Pongpaisal, S.; Pisuithanakan, S.; Baelum, V.; Fejerskov, O.; Dahlén, G. Periodontal microbiota and clinical periodontal status in a rural sample in southern Thailand. Eur. J. Oral Sci. 2002, 110, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Laine, M.L.; Moustakis, V.; Koumakis, L.; Potamias, G.; Loos, B.G. Modeling Susceptibility to Periodontitis. J. Dent. Res. 2012, 92, 45–50. [Google Scholar] [CrossRef]
- Mahara, A.; Yamaoka, T. Continuous separation of cells of high osteoblastic differentiation potential from mesenchymal stem cells on an antibody-immobilized column. Biomaterials 2010, 31, 4231–4237. [Google Scholar] [CrossRef]
- Myung, J.H.; Launiere, C.A.; Eddington, D.T.; Hong, S. Enhanced Tumor Cell Isolation by a Biomimetic Combination of E-selectin and anti-EpCAM: Implications for the Effective Separation of Circulating Tumor Cells (CTCs). Langmuir 2010, 26, 8589–8596. [Google Scholar] [CrossRef] [Green Version]
- Myung, J.H.; Gajjar, K.A.; Chen, J.; Molokie, R.E.; Hong, S. Differential detection of tumor cells using a combination of cell rolling, multivalent binding, and multiple antibodies. Anal. Chem. 2014, 86, 6088–6094. [Google Scholar] [CrossRef] [Green Version]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Ren, C.; Zhu, T.; Wang, K.; Jiang, Z.; Ma, Y. Comparison of the different responses of surface plasmon resonance and quartz crystal microbalance techniques at solid–liquid interfaces under various experimental conditions. Analyst 2015, 140, 1323–1336. [Google Scholar] [CrossRef]
- Zhu, T.; Pei, Z.; Huang, J.; Xiong, C.; Shi, S.; Fang, J. Detection of bacterial cells by impedance spectra via fluidic electrodes in a microfluidic device. Lab Chip 2010, 10, 1557–1560. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, D.; Hou, B. Selective and specific detection of sulfate-reducing bacteria using potentiometric stripping analysis. Talanta 2010, 82, 1608–1611. [Google Scholar] [CrossRef]
- Li, Y.; Fang, L.; Cheng, P.; Deng, J.; Jiang, L.; Huang, H.; Zheng, J. An electrochemical immunosensor for sensitive detection of Escherichia coli O157:H7 using C60 based biocompatible platform and enzyme functionalized Pt nanochains tracing tag. Biosens. Bioelectron. 2013, 49, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gu, C.; Qian, F.; Li, Y.; Zhang, J.Z. Highly Sensitive Detection of Proteins and Bacteria in Aqueous Solution Using Surface-Enhanced Raman Scattering and Optical Fibers. Anal. Chem. 2011, 83, 5888–5894. [Google Scholar] [CrossRef]
- Gasparyan, V.K.; Bazukyan, I.L. Lectin sensitized anisotropic silver nanoparticles for detection of some bacteria. Anal. Chim. Acta 2013, 766, 83–87. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, R.; Wang, Y.; Su, X.; Ying, Y.; Wang, J.; Li, Y. Evaluation of different micro/nanobeads used as amplifiers in QCM immunosensor for more sensitive detection of E. coli O157:H7. Biosens. Bioelectron. 2011, 29, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Farias, B.C.; Souza, P.R.E.; Ferreira, B.; Melo, R.S.A.; Machado, F.B.; Gusmão, E.S.; Cimões, R. Occurrence of periodontal pathogens among patients with chronic periodontitis. Braz. J. Microbiol. 2012, 43, 909–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nastych, O.; Goncharuk-Khomyn, M.; Foros, A.; Cavalcanti, A.; Yavuz, I.; Tsaryk, V. Comparison of Bacterial Load Parameters in Subgingival Plaque during Peri-implantitis and Periodontitis Using the RT-PCR Method. Acta Stomatol. Croat. 2020, 54, 32–43. [Google Scholar] [CrossRef] [PubMed]
| Incubation Time (h) | AUC (QSAM, C) | Packing Density (Molecule·nm−2) | Weight11-MUA on Au Surface (ng) |
|---|---|---|---|
| 12 | 1.28 × 10−5 | 4.54 | 144.5 |
| (n = 4) | |||
| 24 | 1.26 × 10−5 | 4.46 | 142.3 |
| (n = 2) |
| A. actinomycetemcomitans Cells Density (Cells/mL) | t10 | t90 | Response Time (τ =t90 − t10) | |
|---|---|---|---|---|
| 1.16 × 106 | N1 | 124 | 302 | 177 |
| N2 | 29 | 383 | 354 | |
| N3 | 185 | 384 | 198 | |
| mean | 113 | 356 | 243 | |
| SD | 64.2 | 38.5 | 78.6 | |
| 1.16 × 107 | N1 | 230 | 274 | 44 |
| N2 | 101 | 314 | 212 | |
| N3 | 24 | 409 | 385 | |
| mean | 119 | 332 | 214 | |
| SD | 84.7 | 56.7 | 138.9 | |
| 1.16 × 108 | N1 | 139 | 251 | 112 |
| N2 | 137 | 289 | 152 | |
| N3 | 201 | 339 | 138 | |
| mean | 159 | 293 | 134 | |
| SD | 29.6 | 36.0 | 16.5 | |
| Target Pathogen | Transducer | Sensing Strategy | Limit of Detection | Ref. |
|---|---|---|---|---|
| P. gingivalis | Microfluidic cell-hydrodynamic focusing | No immobilization, impedance reading during sample flow | 103 cells/mL | [32] |
| E. coli | ||||
| Sulfate-reducing bacteria | Glassy carbon electrode | Potentiometric analysis, bacterial processing is required | 2.3 × 10–2.3 × 107 CFU/mL | [33] |
| Heat-killed E. coli | Saturated calomel electrode | Amperometric detection of secondary antibody using glucose oxidase | 3 × 101−3.2 × 106 CFU/mL | [34] |
| S. oneidensis | SERS | Silver nanoparticles sandwiched by analyte binding on optical fiber tip | 106 cells/mL | [35] |
| E. coli | SPR | Lectin-functionalized anisotropic silver nanoparticles | 1.5 × 104 CFU/mL | [36] |
| S. aureus | ||||
| B. subtilis | ||||
| E. coli O157:H7 | QCM | Antibody for capture, antibody-functionalized nanoparticles for signal enhancement | 106 cells/mL | [37] |
| A. actinomycetemcomitans | QCM | Antibody immobilization by using 11-MUA-EDC/NHS | 1.16 × 104 cells/mL | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodphukdeekul, S.; Tabata, M.; Ratanaporncharoen, C.; Takeuchi, Y.; Somboon, P.; Boonlue, W.; Miyahara, Y.; Sriyudthsak, M. Quantitative Assessment of Periodontal Bacteria Using a Cell-Based Immunoassay with Functionalized Quartz Crystal Microbalance. Chemosensors 2021, 9, 159. https://doi.org/10.3390/chemosensors9070159
Rodphukdeekul S, Tabata M, Ratanaporncharoen C, Takeuchi Y, Somboon P, Boonlue W, Miyahara Y, Sriyudthsak M. Quantitative Assessment of Periodontal Bacteria Using a Cell-Based Immunoassay with Functionalized Quartz Crystal Microbalance. Chemosensors. 2021; 9(7):159. https://doi.org/10.3390/chemosensors9070159
Chicago/Turabian StyleRodphukdeekul, Satit, Miyuki Tabata, Chindanai Ratanaporncharoen, Yasuo Takeuchi, Pakpum Somboon, Watcharee Boonlue, Yuji Miyahara, and Mana Sriyudthsak. 2021. "Quantitative Assessment of Periodontal Bacteria Using a Cell-Based Immunoassay with Functionalized Quartz Crystal Microbalance" Chemosensors 9, no. 7: 159. https://doi.org/10.3390/chemosensors9070159
APA StyleRodphukdeekul, S., Tabata, M., Ratanaporncharoen, C., Takeuchi, Y., Somboon, P., Boonlue, W., Miyahara, Y., & Sriyudthsak, M. (2021). Quantitative Assessment of Periodontal Bacteria Using a Cell-Based Immunoassay with Functionalized Quartz Crystal Microbalance. Chemosensors, 9(7), 159. https://doi.org/10.3390/chemosensors9070159







