Nitrogen-Doped Graphene-Based Sensor for Electrochemical Detection of Piroxicam, a NSAID Drug for COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Instruments
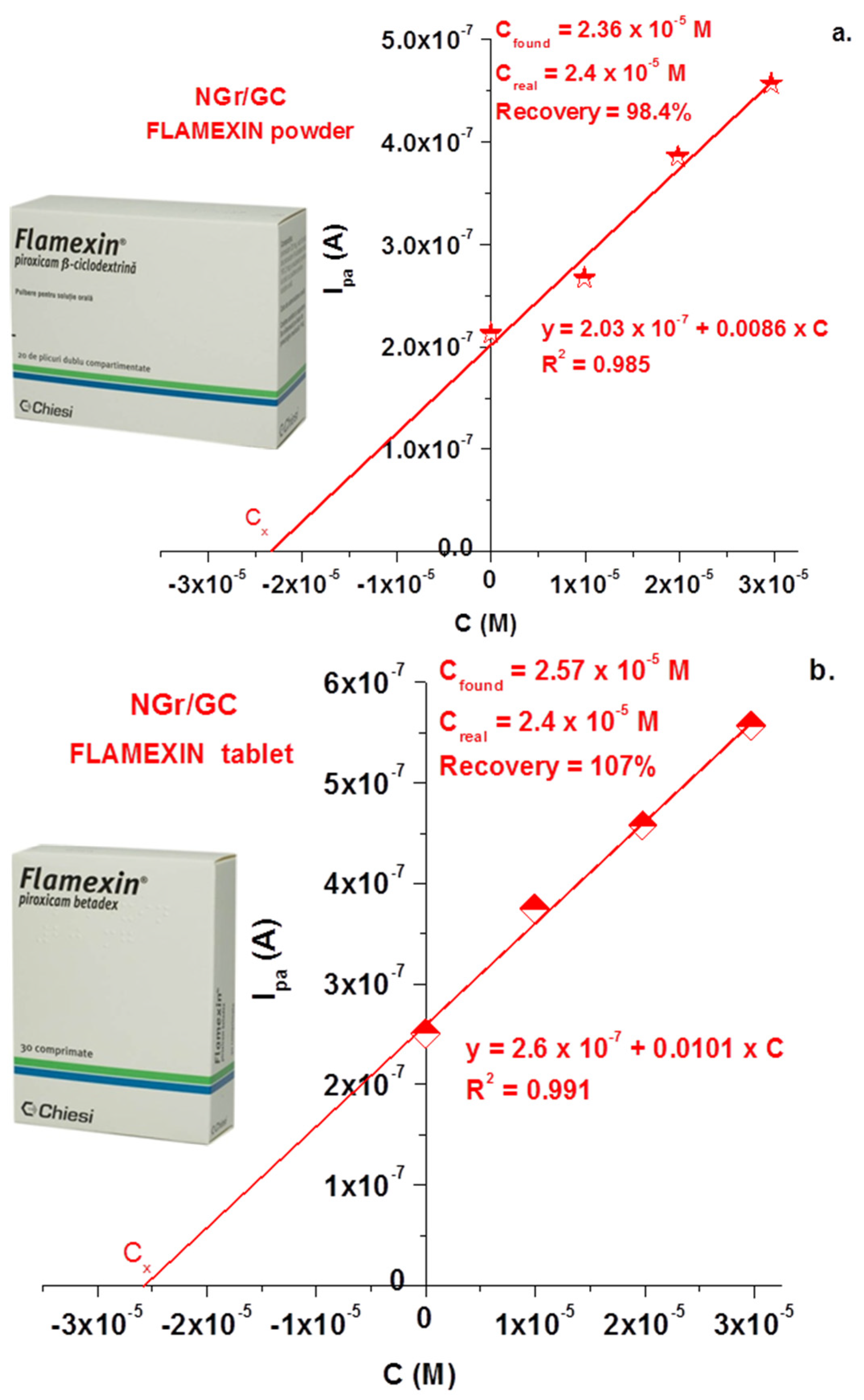
3. Results
3.1. Morphological and Structural Characterization of GO and NGr Samples
3.2. Electrochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mostafa, G.A.E.; Al-Dosseri, A.S.; Al-Badr, A.A. Piroxicam. Profiles Drug Subst. Excip. Relat. Methodol. 2020, 45, 199–474. [Google Scholar] [PubMed]
- Mostafa, A.; Kandeil, A.; Elshaier, Y.A.M.M.; Kutkat, O.; Moatasim, Y.; Rashad, A.A.; Shehata, M.; Gomaa, M.R.; Mahrous, N.; Mahmoud, S.H.; et al. Fda-approved drugs with potent in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2. Pharmaceuticals 2020, 13, 443. [Google Scholar] [CrossRef] [PubMed]
- Vineet, K.; Jain, V.; Deb, A.; Yadav, A. Role of Piroxicam in Controlling Fever in COVID 19—An Observational Case Series Study. Indian J. Appl. Res. 2021, 24–25. [Google Scholar] [CrossRef]
- Prakash, L.; Jain, A.D.; Dhar, S.; Jamal, S. Effect of Sublingual Piroxicam on Hypoxia in Cytokine Storm Induced COVID 19 Pneumonia-Descriptive Study of Clinicians’ Experiences in 2003 Consecutive Cases. Acta Sci. Orthop. 2021, 4, 2581–8635. [Google Scholar]
- Peter, A.E.; Sandeep, B.V.; Rao, B.G.; Kalpana, V.L. Calming the Storm: Natural Immunosuppressants as Adjuvants to Target the Cytokine Storm in COVID-19. Front. Pharmacol. 2021, 11, 1–22. [Google Scholar] [CrossRef]
- Macdougall, L.G.; Rothberg, A.D. Piroxicam poisoning in a 2-year-old child. S. Afr. Med. J. 1984, 66, 31–33. [Google Scholar]
- Liu, W.; Wang, W.D.; Waang, W.; Bai, S.; Dybowski, C. Influence of structure on the spectroscopic properties of the polymorphs of piroxicam. J. Phys. Chem. B 2010, 114, 16641–16649. [Google Scholar] [CrossRef]
- Mishnev, A.; Kiselovs, G. New crystalline forms of piroxicam. Z. Für Nat. B. 2013, 68, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Rele, R.V.; Sawant, S.A. A validated simple titrimetric method for the quantitative determination of piroxicam from pharmaceutical dosages. Anal. Chem. Indian J. 2013, 13, 69–76. [Google Scholar]
- Amin, A.S. Spectrophotometric determination of piroxicam and tenoxicam in pharmaceutical formulations using alizarin. J. Pharm. Biomed. Anal. 2002, 29, 729–736. [Google Scholar] [CrossRef]
- Mandrescu, M.; Spac, A.F. Dorneanu, Spectrophotometric determination of piroxicam using ferric ferricyanide as reagent. Rev. Med.-Chir. Soc. Med. Nat. 2009, 113, 2009. [Google Scholar]
- Gölcü, A.; Çeşme, M. A Simple and Validated Spectrophotometric Method for Determination of Piroxicam in Dosage Forms and Biological Fluids. J. Sci. Eng. Res. 2017, 4, 210–217. [Google Scholar]
- Zeeb, M.; Tayebi Jamil, P.; Berenjian, A.; Ganjali, M.R.; Talei Bavil Olyai, M.R. Quantitative analysis of piroxicam using temperature-controlled ionic liquid dispersive liquid phase microextraction followed by stopped-flow injection spectrofluorimetry. DARU J. Pharm. Sci. 2013, 21, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Zhang, Y.; Chen, F.; Chen, L. Chemiluminescence method for the determination of piroxicam by the enhancement of the tris-(4,7-diphenyl-1,10- Phenanthrolinedisulphonic acid) ruthenium(II) (RuBPS)-cerium(IV) system and its application. Luminescence 2009, 24, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Otarola, J.; Lista, A.G.; Fernández Band, B.; Garrido, M. Capillary electrophoresis to determine entrapment efficiency of a nanostructured lipid carrier loaded with piroxicam. J. Pharm. Anal. 2015, 5, 70–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starek, M.; Krzek, J.; Rotkegel, P. TLC determination of piroxicam, tenoxicam, celecoxib and rofecoxib in biological material. J. Anal. Chem. 2015, 70, 351–359. [Google Scholar] [CrossRef]
- Dragomiroiu, G.T.A.B.; Popa, D.E.; Velescu, B.Ș.; Andrieș, A.; Ordeanu, V.; Crenguța, A. Synthesis, characterization and microbiological activity evaluation of novel hard gelatine capsules with cefaclor and piroxicam. Farmacia 2016, 64, 887–895. [Google Scholar]
- Spac, A.F.; Miftode, A.M.; Asaftei, I.V.; Sandu, I. Quality by Design (QbD) approach to develop and validate a HPLC method for piroxicam from serum. Rev. Chim. 2018, 69, 2167–2171. [Google Scholar] [CrossRef]
- Calvo, A.M.; Santos, G.M.; Dionísio, T.J.; Marques, M.P.; Brozoski, D.T.; Lanchote, V.L.; Fernandes, M.H.R.; Faria, F.A.C.; Santos, C.F. Quantification of piroxicam and 5’-hydroxypiroxicam in human plasma and saliva using liquid chromatography-tandem mass spectrometry following oral administration. J. Pharm. Biomed. Anal. 2016, 120, 212–220. [Google Scholar] [CrossRef]
- Torriero, A.A.J.; Tonn, C.E.; Sereno, L.; Raba, J. Electrooxidation mechanism of non-steroidal anti-inflammatory drug piroxicam at glassy carbon electrode. J. Electroanal. Chem. 2006, 588, 218–225. [Google Scholar] [CrossRef]
- Abbaspour, A.; Mirzajani, R. Electrochemical monitoring of piroxicam in different pharmaceutical forms with multi-walled carbon nanotubes paste electrode. J. Pharm. Biomed. Anal. 2007, 44, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S.; Jokar, E.; Ghalkhani, M. Electrochemical determination of piroxicam on the surface of pyrolytic graphite electrode modified with a film of carbon nanoparticle-chitosan. Microchim. Acta 2010, 170, 141–146. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Malekzadeh, G.; Derakhshan, A.A. Boehmite nano particle modified carbon paste electrode for determination of piroxicam. Sens. Actuators B: Chem. 2014, 201, 378–386. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Sheikhshoaie, I.; Samadzadeh, A. Simultaneous electrochemical determination of levodopa and piroxicam using a glassy carbon electrode modified with a ZnO-Pd/CNT nanocomposite. RSC Adv. 2018, 8, 26707–26712. [Google Scholar] [CrossRef] [Green Version]
- Dehdashti, A.; Babaei, A. Highly Sensitive Electrochemical Sensor Based on Pt Doped NiO Nanoparticles/MWCNTs Nanocomposite Modified Electrode for Simultaneous Sensing of Piroxicam and Amlodipine. Electroanalysis 2020, 32, 1017–1024. [Google Scholar] [CrossRef]
- Feizollahi, A.; Rafati, A.A.; Assari, P.; Joghani, R.A. Simple and Fast Determination of Piroxicam in Pharmaceutical and Real Samples Using Glassy Carbon Electrode Modified with Copper Nano-particles. J. Electrochem. Soc. 2020, 167, 067521. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Fatibello-filho, O.S.C. Determination of piroxicam and nimesulide using an electrochemical sensor based on reduced graphene oxide and PEDOT: PSS. J. Electroanal. Chem. 2017, 799, 547–555. [Google Scholar] [CrossRef]
- Bagher, M.; Karimian, N. Development of piroxicam sensor based on molecular imprinted polymer-modified carbon paste electrode. Mater. Sci. Eng. C 2011, 31, 1844–1851. [Google Scholar]
- Dhanalakshmi, N.; Priya, T.; Thennarasu, S.; Sivanesan, S.; Thinakaran, N. Synthesis and electrochemical properties of environmental free L-glutathione grafted graphene oxide/ZnO nanocomposite for highly selective piroxicam sensing. J. Pharm. Anal. 2021, 11, 48–56. [Google Scholar] [CrossRef]
- Santos, A.M.; Wong, A.; Vicentini, F.C.; Fatibello-Filho, O. Simultaneous voltammetric sensing of levodopa, piroxicam, ofloxacin and methocarbamol using a carbon paste electrode modified with graphite oxide and β-cyclodextrin. Microchim. Acta 2019, 186, 174. [Google Scholar] [CrossRef]
- Kaushal, S. Heteroatom-doped graphene as sensing materials. RSC Adv. 2020, 10, 28608–28629. [Google Scholar] [CrossRef]
- Talukder, N.; Wang, Y.; Babu, B.; Soo, E. Nitrogen-doped graphene nanomaterials for electrochemical catalysis/reactions: A review on chemical structures and stability. Carbon 2021, 185, 198–214. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Matson, D.W.; Li, J.; Lin, Y. Nitrogen-Doped Graphene and Its Application in Electrochemical Biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Pogacean, F.; Socaci, C.; Pruneanu, S.; Biris, A.R.; Coros, M.; Magerusan, L.; Katona, G.; Turcu, R.; Borodi, G. Graphene based nanomaterials as chemical sensors for hydrogen peroxide—A comparison study of their intrinsic peroxidase catalytic behavior. Sens. Actuators B Chem. 2015, 213, 474–483. [Google Scholar] [CrossRef]
- Coros, M.; Varodi, C.; Pogacean, F.; Gal, E.; Pruneanu, S.M. Nitrogen-doped graphene: The influence of doping level on the charge-transfer resistance and apparent heterogeneous electron transfer rate. Sensors 2020, 20, 1815. [Google Scholar] [CrossRef] [Green Version]
- Coros, M.; Pogacean, F.; Turza, A.; Dan, M.; Berghian-Grosan, C.; Pana, I.O.; Pruneanu, S. Green synthesis, characterization and potential application of reduced graphene oxide. Physica E 2020, 119, 113971. [Google Scholar] [CrossRef]
- Nxumalo, E.N.; Mamba, B.B.; Kuvarega, A.T.; Mhlanga, S.D.; Yokwana, K.; Khenfouch, M.; Ray, S.C. Facile Synthesis of Nitrogen Doped Graphene Oxide from Graphite Flakes and Powders: A Comparison of Their Surface Chemistry. J. Nanosci. Nanotechnol. 2018, 18, 5470–5484. [Google Scholar]
- Razeghi, M.; Pircheraghi, G. TPU/graphene nanocomposites: Effect of graphene functionality on the morphology of separated hard domains in thermoplastic polyurethane. Polymer 2018, 148, 169–180. [Google Scholar] [CrossRef]
- Udayabhaskar, R.; Mangalaraja, R.V.; Pandiyarajan, T.; Karthikeyan, B.; Mansilla, H.D.; Contreras, D. Spectroscopic investigation on graphene-copper nanocomposites with strong UV emission and high catalytic activity. Carbon 2017, 124, 256–262. [Google Scholar] [CrossRef]
- Balzar, D. X-ray diffraction line broadening: Modeling and applications to high-Tc superconductors. J. Res. Natl. Inst. Stand. Technol. 1993, 98, 321. [Google Scholar] [CrossRef]
- Cushing, S.K.; Li, M.; Huang, F.; Wu, N. Origin of strong excitation wavelength dependent fluorescence of graphene oxide. ACS Nano 2014, 8, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Li, P.; Ouyang, J. Nitrogen-doped reduced graphene oxide prepared by simultaneous thermal reduction and nitrogen doping of graphene oxide in air and its application as an electrocatalyst. ACS Appl. Mater. Interfaces 2015, 7, 26952–26958. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A. Solid-state properties of pharmaceutical materials. Crystallogr. Rev. 2019, 26, 115–116. [Google Scholar] [CrossRef]
- Demiralay, E.Ç.; Yılmaz, H. Potentiometric pKa determination of piroxicam and tenoxicam in acetonitrile-water binary mixtures. SDU J. Sci. (E-J.) 2012, 7, 34–44. [Google Scholar]
- Shaikh, T.; Uddin, S.; Talpur, F.N.; Khaskeli, A.R.; Agheem, M.H.; Shah, M.R.; Sherazi, T.H.; Siddiqui, S. Ultrasensitive Determination of Piroxicam at Diflunisal-Derived Gold Nanoparticle-Modified Glassy Carbon Electrode. J. Electron. Mater. 2017, 46, 10. [Google Scholar] [CrossRef]
- Asadpour-Zeynali, K.; Majidi, M.R.; Zarifi, M. Carbon ceramic electrode incorporated with zeolite ZSM-5 for determination of Piroxicam. Cent. Eur. J. Chem. 2010, 8, 155–162. [Google Scholar] [CrossRef] [Green Version]










| Sample | wt% | |||
|---|---|---|---|---|
| C | N | H | O | |
| GO | 52.76 | - | 1.76 | 45.48 |
| NGr | 79.75 | 5.86 | 1.54 | 12.85 |
| Electrode | Investigated Samples | Linear Range in Laboratory Solution/M | LOD/M | Ref. |
|---|---|---|---|---|
| CNTPE | Pharmaceutical samples | 4.5 × 10−7–1.5 × 10−5 | 3 × 10−7 | [21] |
| CNP-CS-PGE | Human blood plasma, capsule | 5 × 10−8–5 × 10−5 | 2.5 × 10−8 | [22] |
| BNP–CP | Blood serum, capsule, gel | 5 × 10−10–1 × 10−7 | 1.1 × 10−8 | [23] |
| GCE/ZnO–Pd/CNTs | Blood serum, water, tablets | 1 × 10−7–9 × 10−5 | 4 × 10−8 | [24] |
| Pt- NiO/MWCNTs/GCE | Human urine and serum | 6 × 10−7–3.2 × 10−4 | 6.1 × 10−8 | [25] |
| rGO-PEDOT/GCE | River water and pharmaceutical samples | 8.7 × 10−7–2.6 × 10−5 | 1.0 × 10−7 | [27] |
| glutathione-GO/ZnO/GCE | Blood serum, water, tablets | 1 × 10−7–5 × 10−4 | 1.8 × 10−9 | [29] |
| CD-GrO/CPE | Synthetic urine, river water | 1 × 10−6–1.5 × 10−5 | 1.05 × 10−7 | [30] |
| CCZME | Pharmaceutical samples | 2 × 10−7–5.01 × 10−5 | 2.9 × 10−7 | [46] |
| NGr/GC | Pharmaceutical samples | 1 × 10−6–5 × 10−5 | 3.03 × 10−7 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varodi, C.; Coros, M.; Pogăcean, F.; Ciorîţă, A.; Turza, A.; Pruneanu, S. Nitrogen-Doped Graphene-Based Sensor for Electrochemical Detection of Piroxicam, a NSAID Drug for COVID-19 Patients. Chemosensors 2022, 10, 47. https://doi.org/10.3390/chemosensors10020047
Varodi C, Coros M, Pogăcean F, Ciorîţă A, Turza A, Pruneanu S. Nitrogen-Doped Graphene-Based Sensor for Electrochemical Detection of Piroxicam, a NSAID Drug for COVID-19 Patients. Chemosensors. 2022; 10(2):47. https://doi.org/10.3390/chemosensors10020047
Chicago/Turabian StyleVarodi, Codruţa, Maria Coros, Florina Pogăcean, Alexandra Ciorîţă, Alexandru Turza, and Stela Pruneanu. 2022. "Nitrogen-Doped Graphene-Based Sensor for Electrochemical Detection of Piroxicam, a NSAID Drug for COVID-19 Patients" Chemosensors 10, no. 2: 47. https://doi.org/10.3390/chemosensors10020047
APA StyleVarodi, C., Coros, M., Pogăcean, F., Ciorîţă, A., Turza, A., & Pruneanu, S. (2022). Nitrogen-Doped Graphene-Based Sensor for Electrochemical Detection of Piroxicam, a NSAID Drug for COVID-19 Patients. Chemosensors, 10(2), 47. https://doi.org/10.3390/chemosensors10020047







