Antioxidant and Antiradical Properties of Some Examples of Flavonoids and Coumarins—Potentiometric Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Objects of Analysis
2.2. Cyclic Voltammetry
2.3. Sensor System for Determining Antioxidant Capacity (AOC)
2.4. Sensor System for Determining Antiradical Capacity (ARC)
3. Results
3.1. The Investigation of Electrochemical Activity by Cyclic Voltammetry
- The presence of an oxidation peak in the potential range of 0.21 to 0.28 V (Figure 1). Such compounds include luteolin, nordalbergin, 4-methylesculetin, 4-methyldaphnetin, and 7,8-dihydroxy-4-methyl-chroman-3-toluene-2-one. Compounds of this group contain in their structure a catechol fragment, which is characterized by reversible oxidation-reduction and an uncomplicated process of electron transfer from the antioxidant molecule [46,50,51,52].
- The presence of two peaks of oxidation: the position of the first peak is in the potential range of 0.08 to 0.11 V and the position of the second peak is in a more positive range of potentials. These compounds include quercetin, dihidromyricetin, and baicalein (Figure 2). The compounds contain a catechol/pyrogall fragment. The presence of two peaks may indicate a stepwise oxidation process [50,51,52,53]. In this case, oxidation in the second stage can be difficult.
- The last group is characterized by the presence of an oxidation peak in a rather positive potential range (0.50 to 0.80 V) (Figure 3). These compounds include silybin, chrysin, genistein, apigenin, and 4-methyl-5,7-dihydroxycoumarin. The compounds of this group contain a resorcinol fragment, which is characterized by hindered electron transfer from functional OH groups of molecules and, as a rule, lack of antioxidant action by the electron transfer mechanism [46,50,51,52,53].
3.2. The Research of the Total Antioxidant Capacity by Potentiometric Sensor System
3.3. The Research of the Total Antiradical Capacity (ARC) by Potentiometric Sensor System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cheng, C.; Li, Z.Z.; Zhao, X.; Liao, C.L.; Quan, J.; Bode, A.M.; Cao, Y.; Luo, X.J. Natural alkaloid and polyphenol compounds targeting lipid metabolism: Treatment implications in metabolic diseases. Eur. J. Pharmacol. 2020, 870, 172922. [Google Scholar] [CrossRef] [PubMed]
- Dhir, A. Natural polyphenols in preclinical models of epilepsy. Phytother. Res. 2020, 34, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.M.; Pogacnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Wei, J.T.; Zhao, C.S.; Li, G.R. Natural Polyphenols Targeting Senescence: A Novel Prevention and Therapy Strategy for Cancer. Int. J. Mol. Sci. 2020, 21, 684. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Ozarowski, M.; Kujawski, R.; Mikołajczak, P.Ł.; Wielgus, K.; Klejewski, A.; Wolski, H.; Seremak-Mrozikiewicz, A. In vitro and in vivo activities of flavonoids—Apigenin, baicalin, chrysin, scutellarin—In regulation of hypertension—A review for their possible effects in pregnancy-induced hypertension. Herba Pol. 2019, 65, 55–70. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Zhang, H.L.; Zeng, T. Dihydromyricetin inhibits oxidative stress and apoptosis in oxygen and glucose deprivation/reoxygenation-induced HT22 cells by activating the Nrf2/HO-1 pathway. Mol. Med. Rep. 2021, 6, 397. [Google Scholar] [CrossRef]
- Ashaari, Z.; Hadjzadeh, M.A.R.; Hassanzadeh, G.; Alizamir, T.; Yousefi, B.; Keshavarzi, Z.; Mokhtari, T. The Flavone Luteolin Improves Central Nervous System Disorders by Different Mechanisms: A Review. J. Mol. Neurosci. 2018, 65, 491–506. [Google Scholar] [CrossRef]
- Gunache, R.O.; Apetrei, C. Estimation of Active Compounds Quantity from Pharmaceuticals Based on Ginkgo biloba. Chemosensors 2020, 4, 110. [Google Scholar] [CrossRef]
- Menshchikova, E.B.; Zenkov, N.K.; Kandalintseva, N.V. Phenolic Antioxidants in Biology and Medicine; Lap Lambert: Saarbrücken, Germany, 2012; p. 324. [Google Scholar]
- Gupta, A.; Birhman, K.; Raheja, I.; Kumar Sharma, S.; Kumar Kar, H. Quercetin: A wonder bioflvonoid with therapeutic potential in disease management. Asian Pac. J. Trop. Dis. 2016, 6, 248–252. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, S.J.; Kwon, M.J.; Jeong, T.S.; Bok, S.H.; Choi, W.Y.; Jeong, W.-I.; Ryu, S.-Y.; Do, S.-H.; Lee, C.-S.; et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaB pathway in lipopolysaccharidestimulated macrophage. Mol. Cell Biochem. 2003, 243, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Calamia, K.T. Current and future use of anti-TNF agents in the treatment of autoimmune, inflammatory disorders. Adv. Exp. Med. Biol. 2003, 528, 545–549. [Google Scholar]
- Xu, J.; Wang, H.; Lu, X.; Ding, K.; Zhang, L.; He, J.; Wei, W.; Wu, Y. Posttraumatic administration of luteolin protects mice from traumatic brain injury: Implication of autophagy and inflammation. Brain Res. 2014, 1582, 237–246. [Google Scholar] [CrossRef]
- Sawmiller, D.; Li, S.; Shahaduzzaman, M.; Smith, A.J.; Obregon, D.; Giunta, B.; Borlongan, C.V.; Sanberg, P.R.; Tan, J. Luteolin reduces Alzheimer’s disease pathologies induced by traumatic brain injury. Int. J. Mol. Sci. 2014, 15, 895–904. [Google Scholar] [CrossRef]
- Sun, C.C.; Su, H.; Zheng, G.D.; Wang, W.J.; Yuan, E.; Zhang, Q.F. Fabrication and characterization of dihydromyricetin encapsulated zein-caseinate nanoparticles and its bioavailability in rat. Food Chem. 2020, 330, 127245. [Google Scholar] [CrossRef]
- Tong, H.; Zhang, X.; Tan, L.; Jin, R.; Huang, S.; Li, X. Multitarget and promising role of dihydromyricetin in the treatment of metabolic diseases. Eur. J. Pharmacol. 2020, 870, 172888. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, F.; Zhou, R.; Song, X.; Xie, M. Apigenin: A current review on its beneficial biological activities. J. Food Biochem. 2017, 41, e12376. [Google Scholar] [CrossRef]
- Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Ahad, A.; Ganai, A.A.; Mujeeb, M.; Siddiqui, W.A. Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol. Appl. Pharmacol. 2014, 279, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Veerappan, R.; Malarvili, T. Chrysin pretreatment improves angiotensin system, cGMP concentration in L-NAME induced hypertensive rats. Indian J. Clin. Biochem. 2018, 34, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Neuroprotective and cognitive enhancement potentials of baicalin: A review. Brain Sci. 2018, 8, 104. [Google Scholar] [CrossRef]
- Liang, W.; Huang, X.; Chen, W. The effects of baicalin and baicalein on cerebral ischemia: A review. Aging Dis. 2017, 8, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Gholampour, F.; Mohammadi, Z.; Karimi, Z.; Owji, S.M. Protective effect of genistein in a rat model of ischemic acute kidney injury. Gene 2020, 753, 144789. [Google Scholar] [CrossRef]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Chen, C.; Hu, Y.Y.; Feng, Q. Protective effect of genistein on nonalcoholic fatty liver disease (NAFLD). Biomed. Pharmacother. 2019, 117, 109047. [Google Scholar] [CrossRef] [PubMed]
- Diukendjieva, A.; Zaharieva, M.M.; Alov, P.; Tsakovska, I.; Pencheva, T.; Najdenski, H.; Kren, V.; Felici, C.; Bufalieri, F.; Di Marcotullio, L.; et al. Dual SMO/BRAF Inhibition by Flavonolignans from Silybum marianum. Antioxidants 2020, 9, 384. [Google Scholar] [CrossRef]
- Delmas, D.; Xiao, J.B.; Vejux, A.; Aires, V. Silymarin and Cancer: A Dual Strategy in Both in Chemoprevention and Chemosensitivity. Molecules 2009, 25, 2009. [Google Scholar] [CrossRef]
- Mastron, J.K.; Siveen, K.S.; Sethi, G.; Bishayee, A. Silymarin and hepatocellular carcinoma: A systematic, comprehensive, and critical review. Anticancer Drugs 2015, 26, 475–486. [Google Scholar] [CrossRef]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Effect of coumarins on HL-60 cell differentiation of coumarins on HL-60 cell differentiation. Anticancer Res. 2000, 20, 2505–2512. [Google Scholar] [PubMed]
- Kato, A.; Kobayashi, K.; Narukawa, K.; Minoshima, Y.; Adachi, I.; Hirono, S.; Nash, R.J. 6,7-Dihydroxy-4-phenylcoumarin as inhibitor of aldose reductase 2. Bioorg. Med. Chem. Lett. 2010, 20, 5630–5633. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.J.; Lu, L.L.; Zeng, S.; Luo, F.F.; Dai, P.M.; Wu, P.; Wang, Y.; Liu, L.; Hu, M.; Liu, Z.Q. UDP-Glucuronosyltransferases 1A6 and 1A9 are the Major Isozymes Responsible for the 7-O-Glucuronidation of Esculetin and 4-Methylesculetin in Human Liver Microsomes. Drug Metab. Dispos. 2015, 43, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Hadjipavlou-Litina, D.J.; Litinas, K.E.; Kontogiorgis, C. The Anti-inflammatory Effect of Coumarin and its Derivatives. Antiinflamm. Antiallergy Agents Med. Chem. 2007, 6, 293–306. [Google Scholar] [CrossRef]
- Menshchikova, E.B.; Zenkov, N.K.; Lankin, V.Z.; Bondar, I.A.; Trufakin, V.A. Oxidative Stress: Pathologic States and Diseases; Sibirskoe Univ. Izd.: Novosibirsk, Russia, 2008; p. 534. [Google Scholar]
- Menschikova, E.B.; Zenkov, N.K.; Lankin, V.Z.; Bondar, I.A.; Trufakin, V.A. Oxidative stress: Pathological Conditions and Diseases; ARTA: Novosibirsk, Russia, 2008; p. 435. [Google Scholar]
- Galaris, D.; Barbouti, A.; Korantzopoulos, P. Oxidative Stress in Hepatic Ischemia-Reperfusion Injury: The Role of Antioxidants and Iron Chelating Compounds. Curr. Pharm. Des. 2006, 12, 2875–2890. [Google Scholar] [CrossRef]
- Apak, R.; Capanoglu, E.; Shahidi, F. Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2018; p. 337. [Google Scholar]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT), and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Huang, D.; Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Brainina, K.Z. Potentiometric Study of Antioxidant Activity: Development and Prospects. Crit. Rev. Anal. Chem. 2015, 45, 311–322. [Google Scholar] [CrossRef]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E.; Popova, K.G.; Matern, A.I. Study of the antioxidant activity and total polyphenol concentration of medicinal plants. J. Anal. Chem. 2017, 72, 415. [Google Scholar] [CrossRef]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E.; Borisova, M.; Drokin, R.; Gorbunov, E.; Ulomskiy, E.; Rusinov, V. The antioxidant screening of potential materials for drugs based on 6-nitro-1,2,4-triazoloazines containing natural polyphenol fragments. Anal. Bioanal. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.V.; Gerasimova, E.L.; Gazizullina, E.R. New antiradical capacity assay with the use potentiometric method. Anal. Chim. Acta 2019, 1046, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.Z.; Ivanova, A.V.; Sharafutdinova, E.N.; Lozovskaya, E.L.; Shkarina, E.I. Potentiometry as a method of antioxidant activity investigation. Talanta 2007, 71, 13. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and Flavonols’ Antiradical Structure-Activity Relationship—A Quantum Chemical Study. Antioxidants 2020, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ma, Z.; Zhu, X.; Zeng, R.; Tie, S.; Nan, J. Electrochemical behavior and simultaneous determination of catechol, resorcinol, and hydroquinone using thermally reduced carbonnano-fragment modified glassy carbon electrode. Anal. Methods 2016, 8, 605. [Google Scholar] [CrossRef]
- Peng, J.; Gao, Z.N. Influence of micelles on the electrochemical behaviors of catechol and hydroquinone and their simultaneous determination. Anal. Bioanal. Chem. 2006, 384, 1525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chu, L.; Liu, Y.X.; Wang, A.L.; Ji, B.P.; Wu, W.; Zhou, F.; Wei, Y.; Cheng, Q.; Cai, S.B.; et al. Analysis of the Antioxidant Capacities of Flavonoids under Different Spectrophotometric Assays Using Cyclic Voltammetry and Density Functional Theory. J. Agric. Food Chem. 2011, 59, 10277–10285. [Google Scholar] [CrossRef]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827. [Google Scholar]
- Arteaga, J.F.; Ruiz-Montoya, M.; Palma, A.; Alonso-Garrido, G.; Pintado, S.; Rodríguez-Mellado, J.M. Comparison of the Simple Cyclic Voltammetry (CV) and DPPH Assays for the Determination of Antioxidant Capacity of Active Principles. Molecules 2012, 5, 5126–5138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Türke, A.; Kilmartin, P. Electrochemistry of White Wine Polyphenols Using PEDOT Modified Electrodes. Beverages 2017, 3, 28. [Google Scholar] [CrossRef]
- Tarantul, V.Z. Explanatory Dictionary of Molecular and Cellular Biotechnology; Russian Academy of Sciences: Moscow, Russia, 2015; p. 984. [Google Scholar]
- Cao, S.J.; Lv, Z.Q.; Guo, S.; Jiang, G.P.; Liu, H.L. An update—Prolonging the action of protein and peptide drugs. J. Drug Deliv. Sci. Technol. 2021, 61, 102124. [Google Scholar] [CrossRef]
- Jungbluth, G.; Ruhling, I.; Ternes, W. Oxidation of flavonols with Cu(II), Fe(II) and Fe(III) in aqueous media. J. Chem. Soc. Perkin Trans. 2 2000, 9, 1946–1952. [Google Scholar] [CrossRef]
- Tang, Z.M.; Zhao, P.R.; Wang, H.; Liu, Y.Y.; Bu, W.B. Biomedicine Meets Fenton Chemistry. Chem. Rev. 2021, 121, 1981. [Google Scholar] [CrossRef]
- Hippeli, S.; Elstner, E.F. Transition metal ion-catalyzed oxygen activation during pathogenic processes. FEBS Lett. 1999, 443, 1–7. [Google Scholar] [CrossRef]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef]
- Lurie, J. Handbook of Analytical Chemistry; Mir Publishers: Moscow, Russia, 1975; p. 654. [Google Scholar]
- Rusina, I.F.; Karpukhin, O.N.; Kasaikina, O.T. Chemiluminescent methods for studying inhibited oxidation. Russ. J. Phys. Chem. 2013, 7, 463–477. [Google Scholar] [CrossRef]
- Mellado-Ortega, E.; Zabalgogeazcoa, I.; de Aldana, B.R.V.; Arellano, J.B. Solutions to decrease a systematic error related to AAPH addition in the fluorescence-based ORAC assay. Anal. Biochem. 2017, 519, 27–29. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedrielli, P.; Pedulli, G.F.; Valginigli, L.; Gigmes, D.; Tordo, P. Bond dissociation energies of the N-H bond and rate constants for the reaction with alkyl, alkoxyl, and peroxyl radicals of phenothiazines and related compounds. J. Am. Chem. Soc. 1999, 121, 11546–11553. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Gazizullina, E.R. An integrated approach to the investigation of antioxidant properties by potentiometry. Anal. Chim. Acta 2020, 1111, 83. [Google Scholar] [CrossRef] [PubMed]







| Compounds | Source | Biological Activity |
|---|---|---|
Quercetin | Red grapes | circulation system protection, anti-allergic, anti-inflammatory, anti-cancer, anti-diabetes, cataract prevention, cardiovascular protection, anti-ulcer, anti-diabetes [13,14,15] |
Luteolin | anti-inflammatory, neuroprotective, anti-cancer [10,16,17] | |
Dihydromyricetin | anti-cancer, anti-inflammatory, anti-microbial, hepatoprotective, lipid and glucose metabolism regulation [18,19] | |
Apigenin | Scutellaria baicalensis Georgi | vasorelaxant effects, decrease in heart pressure, dose-dependent lowering concentrations of low-density lipoprotein cholesterol, triglycerides and total content of cholesterol in their serum, anti-inflammatory [8,20,21] |
Chrysin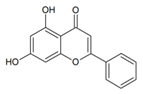 | anti-inflammatory, antihypertensive activity, neuroprotective, anticancer properties, possessed protective effects against toxic agents [8,22,23] | |
Baicalein | anti-viral, neuroprotective and enhancing cognitive functions, anti-inflammatory, anti-cancer, hepatoprotection, anti-hypertensive, cardioprotective, antiplatelet, anticoagulant, and profibrinolytic activities [8,24,25]. | |
Genistein | Genista tinctorial | anti-carcinogenic effects, nephroprotective activity against kidney injury induced by cisplatin and its hepatoprotective activity against “nonalcoholic fatty liver disease”, anti-inflammatory activity [26,27,28] |
Silybin | Silybum marianum | anti-inflammatory, anti-cancer and anti-viral activities, its potential usefulness in the treatment of chronic liver diseases, cirrhosis and hepatocellular carcinoma, chemopreventive activity [29,30,31] |
Nordalbergin | Dalbergia sissoo | possesses antineoplasmic activity and can reduce diabetic complications as an inhibitor of aldose reductase [32,33] |
4-Methylesculetin | Synthetic | anti-inflammatory, neuroprotective properties [34] |
4-Methyldaphnetin | inhibit selectively the proinflammatory 5-lipoxygenase enzyme [35] | |
4-Methyl-5,7-dihydroxycoumarin | high potency against cyclooxygenase [35] | |
7,8-Dihydroxy-4-methyl-chroman-3-toluene-2-one | in the process of research |
| Group Number | Name | Eox, V | Ereox, V |
|---|---|---|---|
| 1 | Luteolin | 0.25 | 0.21 |
| Nordalbergin | 0.28 | 0.18 | |
| 4-Methylesculetin | 0.28 | 0.21 | |
| 4-methyldaphnetin | 0.22 | 0.16 | |
| 7,8-dihydroxy-4-methyl-chroman-3-toluene-2-one | 0.21 | 0.16 | |
| 2 | Quercetin | 0.11 0.46 | - |
| Dihydromyricetin | 0.11 0.80 | - | |
| Baicalein | 0.07 0.68 | - | |
| 3 | Silybin | 0.50 0.77 | - |
| Chrysin | 0.80 | - | |
| Genistein | 0.58 0.56 | - | |
| Apigenin | 0.66 | - | |
| 4-methyl-5,7-dihydroxycoumarin | 0.59 | - |
| No. | Name | AOC, 10−4 M-eq | RSD, % | τ1/2, c |
|---|---|---|---|---|
| 1 | Quercetin | 5.26 ± 0.26 | 4.66 | 10 |
| 2 | 4-Methylesculetin | 3.56 ± 0.12 | 3.53 | 4 |
| 3 | Chrysin | not found | ||
| 4 | Genistein | not found | ||
| 5 | Silybin | not found | ||
| 6 | Luteolin | 3.90 ± 0.12 | 3.43 | 4 |
| 7 | Apigenin | not found | ||
| 8 | 4-methyldaphnetin | 5.00 ± 0.09 | 1.75 | 363 |
| 9 | 7,8-dihydroxy-4-methyl-chroman-3-toluene-2-one | 4.10 ± 0.08 | 2.44 | 4 |
| 10 | 4-methyl-5,7-dihydroxycoumarin | not found | ||
| 11 | Nordalbergin | 3.69 ± 0.06 | 1.56 | 1 |
| 12 | Baicalein | 2.46 ± 0.02 | 0.65 | 1 |
| 13 | Dihydromyricetin | 5.67 ± 0.06 | 1.09 | 6 |
 | AOC, 10−4 M-eq |  | ARC, 10−4 M-eq | ||
| Dihydromyricetin | 5.67 ± 0.06 | 4-Methylesculetin | 6.71 ± 0.27 | ||
| Quercetin | 5.26 ± 0.26 | Chrysin | 6.48 ± 0.06 | ||
| 4-Methyldaphetin | 5.00 ± 0.10 | Luteolin | 5.42 ± 0.05 | ||
| 7,8-DH-4-M-Ch-3-T-2 | 4.10 ± 0.10 | Dihydromyricetin | 5.38 ± 0.16 | ||
| Luteolin | 3.90 ± 0.10 | Apigenin | 4.63 ± 0.09 | ||
| Nordalbergin | 3.69 ± 0.06 | Nordalbergin | 4.52 ± 0.15 | ||
| 4-Methylesculetin | 3.56 ± 0.12 | Genistein | 4.36 ± 0.13 | ||
| Baicalein | 2.46 ± 0.02 | 7,8-DH-4-M-Ch-3-T-2 | 3.89 ± 0.12 | ||
| Silybin | - | 4-Methyldaphetin | 3.60 ± 0.10 | ||
| Chrysin | - | Quercetin | 3.41 ± 0.07 | ||
| Genistein | - | 4-M-5,7-DH-Co | 3.36 ± 0.17 | ||
| Apigenin | - | Baicalein | 2.9 ± 0.03 | ||
| 4-M-5,7-DH-Co | - | Silybin | 2.44 ± 0.12 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimova, E.; Gazizullina, E.; Radosteva, E.; Ivanova, A. Antioxidant and Antiradical Properties of Some Examples of Flavonoids and Coumarins—Potentiometric Studies. Chemosensors 2021, 9, 112. https://doi.org/10.3390/chemosensors9050112
Gerasimova E, Gazizullina E, Radosteva E, Ivanova A. Antioxidant and Antiradical Properties of Some Examples of Flavonoids and Coumarins—Potentiometric Studies. Chemosensors. 2021; 9(5):112. https://doi.org/10.3390/chemosensors9050112
Chicago/Turabian StyleGerasimova, Elena, Elena Gazizullina, Ekaterina Radosteva, and Alla Ivanova. 2021. "Antioxidant and Antiradical Properties of Some Examples of Flavonoids and Coumarins—Potentiometric Studies" Chemosensors 9, no. 5: 112. https://doi.org/10.3390/chemosensors9050112
APA StyleGerasimova, E., Gazizullina, E., Radosteva, E., & Ivanova, A. (2021). Antioxidant and Antiradical Properties of Some Examples of Flavonoids and Coumarins—Potentiometric Studies. Chemosensors, 9(5), 112. https://doi.org/10.3390/chemosensors9050112







