Charge Transfer on the Surface-Enhanced Raman Scattering of Ag/4-MBA/PEDOT:PSS System: Intermolecular Hydrogen Bonding
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
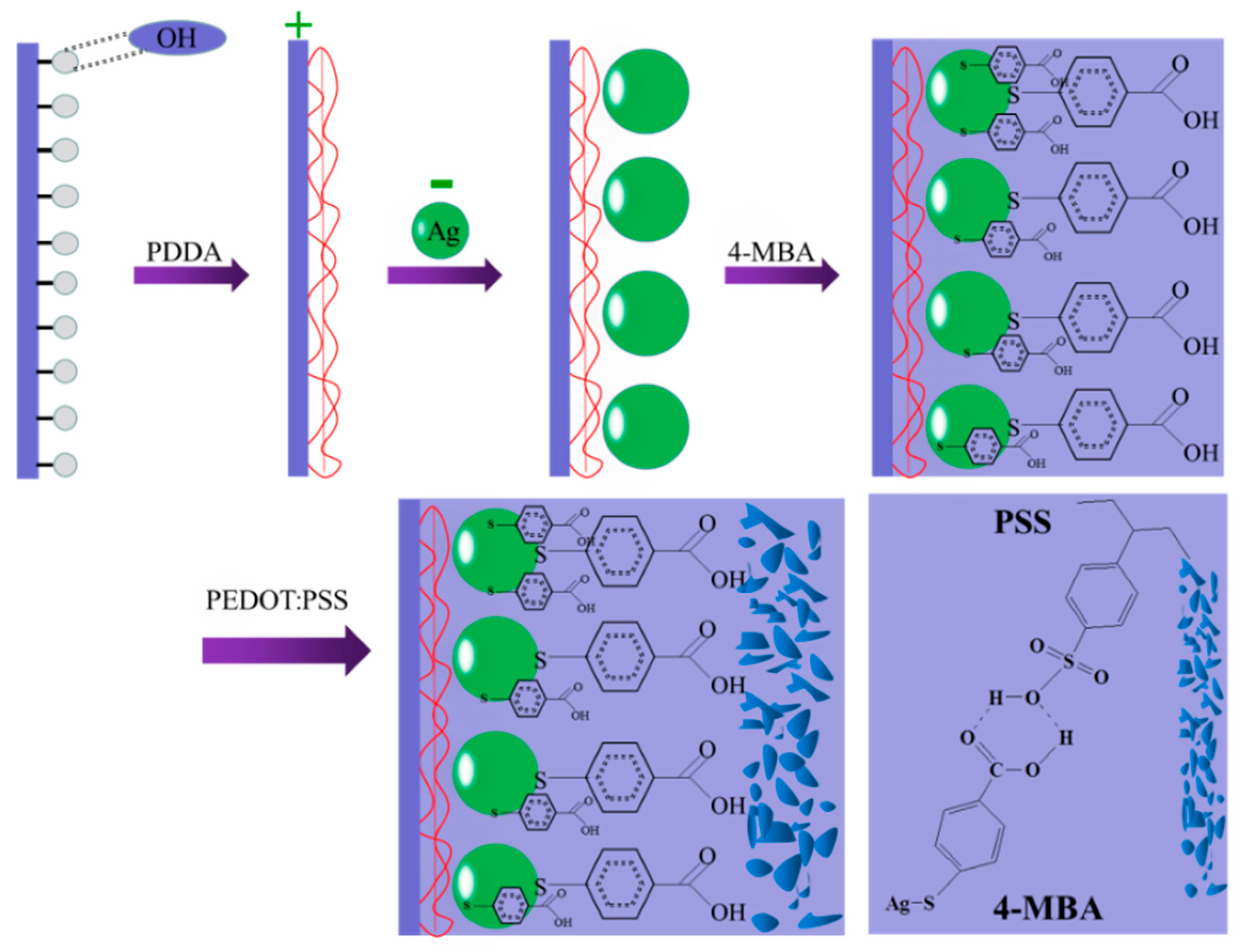
2.2. Preparation of Ag/4-MBA/ PEDOT:PSS ComposiZtes
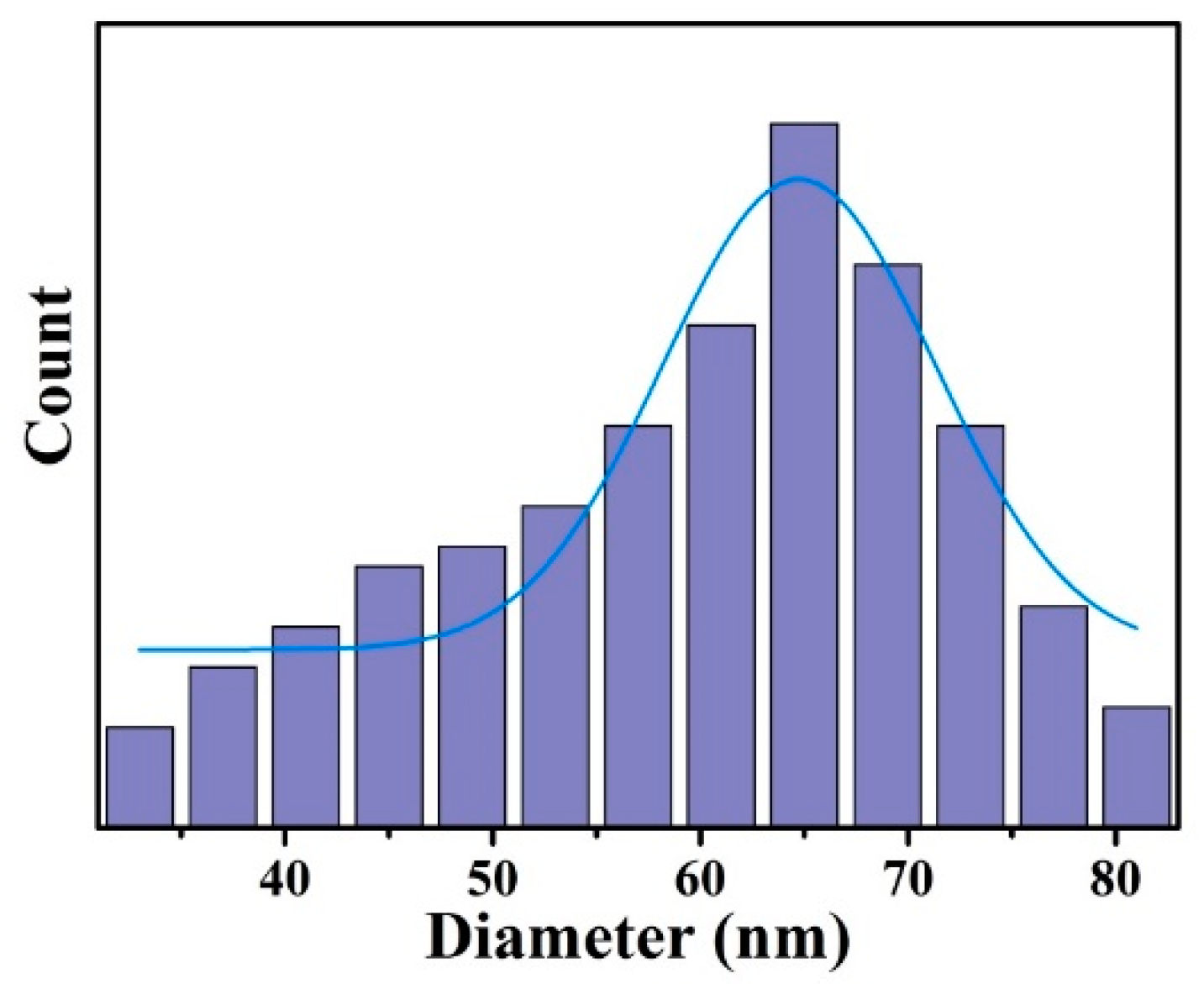
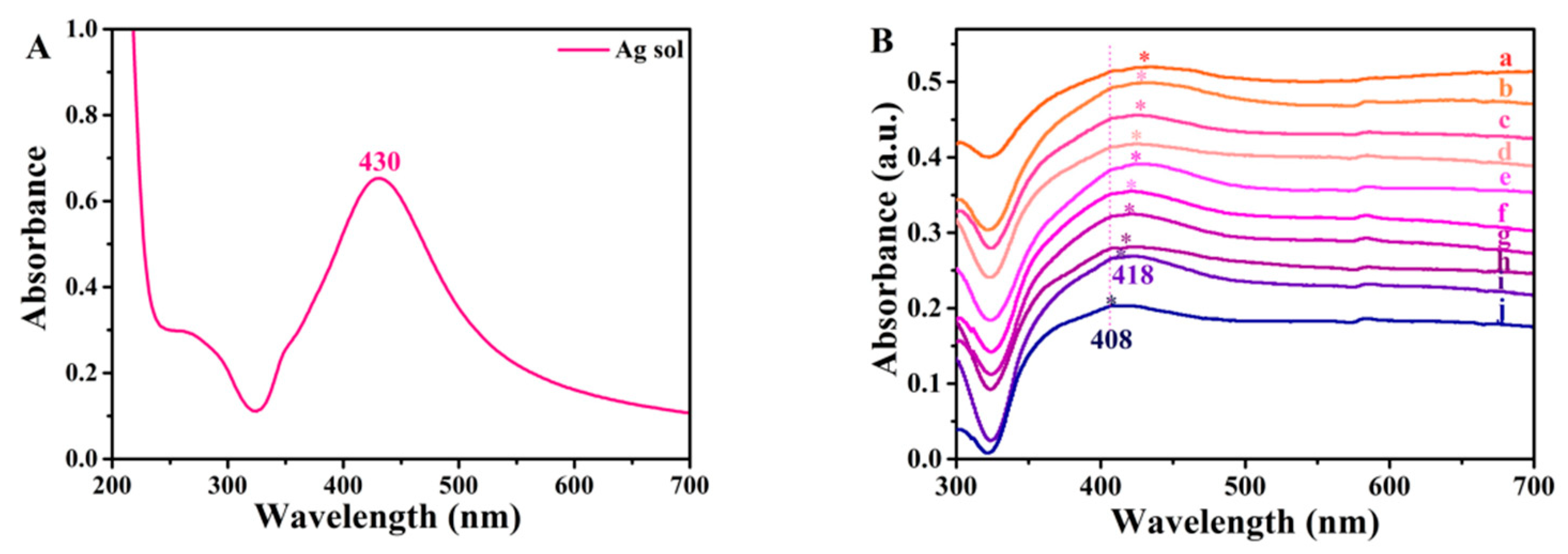
2.2.1. Synthesis of Ag NPs
2.2.2. Preparation of Ag/4-MBA System
2.2.3. PEDOT:PSS Deposition Procedure on the Ag/4-MBA System
2.3. Characterization and SERS Measurements
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, J.; He, L. Surface-enhanced Raman spectroscopy for the chemical analysis of food. Compr. Rev. Food Sci. F 2014, 13, 317–328. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, B.; Hou, R.; Zhang, Z.; Kinchla, A.J.; Clark, J.M.; He, L. Evaluation of the penetration of multiple classes of pesticides in fresh produce using surface-enhanced Raman scattering mapping. J. Food. Sci. 2016, 81, T2891–T2901. [Google Scholar] [CrossRef]
- Lee, C.H.; Tian, L.; Singamaneni, S. Paper-based SERS swab for rapid trace detection on real-world surfaces. ACS Appl. Mater. Interfaces 2010, 2, 3429–3435. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.W.; Zhao, B.; Xu, W.Q.; Li, B.F.; Fan, Y.G. Surface-enhanced Raman spectroscopy study on the structure changes of 4-mercaptopyridine adsorbed on silver substrates and silver colloids. Spectrochim. Acta A 2002, 58, 2827–2834. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.L.; Choi, J.Y.; Shin, D.; Shin, K.S. Effect of volatile organic chemicals on surface-enhanced Raman scattering of 4-aminobenzenethiol on Ag: Comparison with the potential dependence. Phys. Chem. Chem. Phys. 2011, 13, 15603–15609. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, C.; Yuan, Z.; Zoric, I.; Kasemo, B. Plasmonic properties of supported Pt and Pd nanostructures. Nano Lett. 2006, 6, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cui, X.; Yeo, B.-S.; Schmid, T.; Hafner, C.; Zenobi, R. Nanoscale roughness on metal surfaces can increase tip-enhanced Raman scattering by an order of magnitude. Nano Lett. 2007, 7, 1401–1405. [Google Scholar] [CrossRef]
- Popp, J.; Mayerhoefer, T. Surface-enhanced Raman spectroscopy. Anal. Bioanal. Chem. 2009, 394, 1717–1718. [Google Scholar] [CrossRef]
- Campion, A.; Kambhampati, P. Surface-enhanced Raman scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Haynes, C.L.; McFarland, A.D.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Anal. Chem. 2005, 77, 338A–346A. [Google Scholar] [CrossRef]
- Otto, A. The ‘chemical’ (electronic) contribution to surface-enhanced Raman scattering. J. Raman Spectrosc. 2005, 36, 497–509. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Fateixa, S.; Nogueira, H.I.S.; Trindade, T. Hybrid nanostructures for SERS: Materials development and chemical detection. Phys. Chem. Chem. Phys. 2015, 17, 21046–21071. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, J.R.; Birke, R.L. A unified approach to surface-enhanced Raman spectroscopy. J. Phys. Chem. C 2008, 12, 5605–5617. [Google Scholar] [CrossRef]
- Sun, M.T.; Fang, Y.R.; Yang, Z.L.; Xu, H.X. Chemical and electromagnetic mechanisms of tip-enhanced Raman scattering. Phys. Chem. Chem. Phys. 2009, 11, 9412–9419. [Google Scholar] [CrossRef]
- Hu, X.; Meng, G.; Huang, Q.; Xu, W.; Han, F.; Sun, K.; Xu, Q.; Wang, Z. Large-scale homogeneously distributed Ag-NPs with sub-10 nm gaps assembled on a two-layered honeycomb-like TiO2 film as sensitive and reproducible SERS substrates. Nanotechnology 2012, 23, 385705. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Dieringer, J.A.; McFarland, A.D.; Shah, N.C.; Stuart, D.A.; Whitney, A.V.; Yonzon, C.R.; Young, M.A.; Zhang, X.Y.; Van Duyne, R.P. Surface enhanced Raman spectroscopy: New materials, concepts, characterization tools, and applications. Faraday Discuss. 2006, 132, 9–26. [Google Scholar] [CrossRef]
- Whitney, A.V.; Myers, B.D.; Van Duyne, R.P. Sub-100 nm triangular nanopores fabricated with the reactive ion etching variant of nanosphere lithography and angle-resolved nanosphere lithography. Nano Lett. 2004, 4, 1507–1511. [Google Scholar] [CrossRef]
- Ren, B.; Lin, X.F.; Yang, Z.L.; Liu, G.K.; Aroca, R.F.; Mao, B.W.; Tian, Z.Q. Surface-enhanced Raman scattering in the ultraviolet spectral region: UV-SERS on rhodium and ruthenium electrodes. J. Am. Chem. Soc. 2003, 125, 9598–9599. [Google Scholar] [CrossRef]
- Yoshida, K.; Itoh, T.; Biju, V.; Ishikawa, M.; Ozaki, Y. Spectral shapes of surface-enhanced resonance Raman scattering sensitive to the refractive index of media around single Ag nanoaggregates. Appl. Phys. Lett. 2009, 95, 263104. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Ren, B.; Wu, D.Y. Surface-enhanced Raman scattering: From noble to transition metals and from rough surfaces to ordered nanostructures. J. Phys. Chem. B 2002, 106, 9463–9483. [Google Scholar] [CrossRef]
- Yilmaz, M.; Babur, E.; Ozdemir, M.; Gieseking, R.L.; Dede, Y.; Tamer, U.; Schatz, G.C.; Facchetti, A.; Usta, H.; Demirel, G. Nanostructured organic semiconductor films for molecular detection with surface-enhanced Raman spectroscopy. Nat. Mater. 2017, 16, 918. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.; Usta, H.; Yilmaz, M.; Celik, M.; Alidagi, H.A.; Buyukserin, F. Surface-enhanced Raman spectroscopy (SERS): An adventure from plasmonic metals to organic semiconductors as SERS platforms. J. Mater. Chem. C 2018, 6, 5314–5335. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption And Surface-Enhanced Raman of Dyes on Silver and Gold Sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Liang, Z.; Mao, Q.; Wang, Y.-P.; Zhu, F.-C.; Li, J.-X.; Yao, X.; Gao, F.; Wu, X.; Xu, M.; Wang, J.-Z. Progress on the research and development of inactivated EV71 whole-virus vaccines. Hum. Vaccin. Immunother. 2013, 9, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Nakamura, H. Roles of electrostatic interaction in proteins. Q. Rev. Biophys. 1996, 29, 1–90. [Google Scholar] [CrossRef]
- Ye, A.Q. Complexation between milk proteins and polysaccharides via electrostatic interaction: Principles and applications—A review. Int. J. Food. Sci. Tech. 2008, 43, 406–415. [Google Scholar] [CrossRef]
- Kolny, J.; Kornowski, A.; Weller, H. Self-organization of cadmium sulfide and gold nanoparticles by electrostatic interaction. Nano Lett. 2002, 2, 361–364. [Google Scholar] [CrossRef]
- Demirel, G.; Gieseking, R.L.M.; Ozdemir, R.; Kahmann, S.; Loi, M.A.; Schatz, G.C.; Facchetti, A.; Usta, H. Molecular engineering of organic semiconductors enables noble metal-comparable SERS enhancement and sensitivity. Nat. Commun. 2019, 10, 9. [Google Scholar] [CrossRef]
- Bishnoi, S.W.; Rozell, C.J.; Levin, C.S.; Gheith, M.K.; Johnson, B.R.; Johnson, D.H.; Halas, N.J. All-optical nanoscale pH meter. Nano Lett. 2006, 6, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Moskovits, M. Visualizing chromatographic separation of metal ions on a surface-enhanced Raman active medium. Nano Lett. 2011, 1, 145–150. [Google Scholar] [CrossRef]
- Scaffidi, J.P.; Gregas, M.K.; Seewaldt, V.; Vo-Dinh, T. SERS-based plasmonic nanobiosensing in single living cells. Anal. Bioanal. Chem. 2009, 393, 1135–1141. [Google Scholar] [CrossRef]
- Tan, E.Z.; Yin, P.G.; You, T.T.; Wang, H.; Guo, L. Three dimensional design of large-scale TiO2 nanorods scaffold decorated by silver nanoparticles as SERS sensor for ultrasensitive malachite green detection. ACS Appl. Mater. Interfaces 2012, 4, 3432–3437. [Google Scholar] [CrossRef]
- Levitt, M.; Perutz, M.F. Aromatic rings act as hydrogen-bond acceptors. J. Mol. Biol. 1988, 201, 751–754. [Google Scholar] [CrossRef]
- Bell, D.A.; Anslyn, E.V. Complexation of carbonyl-compounds with an organic salt dominated by acid-base interactions in dichloromethane. J. Org. Chem. 1994, 59, 512–514. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A unified view of surface-enhanced Raman scattering. Acc. Chem. Res. 2009, 42, 734–742. [Google Scholar] [CrossRef]
- Ratajczak, H.; Orvillethomas, W.J. Charge-transfer properties of hydrogen-bond 0.6. charge-transfer theory and dipole-moments of hydrogen-bonded complexes. J. Mol. Struct. 1975, 26, 387–391. [Google Scholar] [CrossRef]
- Ratajczak, H.; Orvillet, W.J. Charge-transfer properties of hydrogen-bonds 0.3. charge-transfer theory and relation between energy and enhancement of dipole-moment of hydrogen-bonded complexes. J. Chem. Phys. 1973, 58, 911–919. [Google Scholar] [CrossRef]
- Zhang, X.L.; Yu, Z.; Ji, W.; Sui, H.M.; Gong, Q.; Wang, X.; Zhao, B. Charge-transfer effect on surface-enhanced Raman scattering (SERS) in an ordered Ag NPs/4-mercaptobenzoic acid/TiO2 system. J. Phys. Chem. C 2015, 119, 22439–22444. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, F.; Deng, X.Y.; Xue, X.X.; Wang, L.; Sun, Y.T.; Feng, J.D.; Zhang, Y.J.; Wang, Y.X.; Jung, Y.M. SERS study of surface plasmon resonance induced carrier movement in Au@Cu2O core-shell nanoparticles. Spectrochim. Acta A 2018, 189, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Iwan, A.; Caballero-Briones, F.; Filapek, M.; Boharewicz, B.; Tazbir, I.; Hreniak, A.; Guerrero-Contreras, J. Electrochemical and photocurrent characterization of polymer solar cells with improved performance after GO addition to the PEDOT:PSS hole transporting layer. Sol. Energy 2017, 146, 230–242. [Google Scholar] [CrossRef]
- Wu, X.J.; Li, Q.X.; Huang, J.; Yang, J.L. Nonequilibrium electronic transport of 4,4′-bipyridine molecular junction. J. Chem. Phys. 2015, 123, 184712. [Google Scholar] [CrossRef]

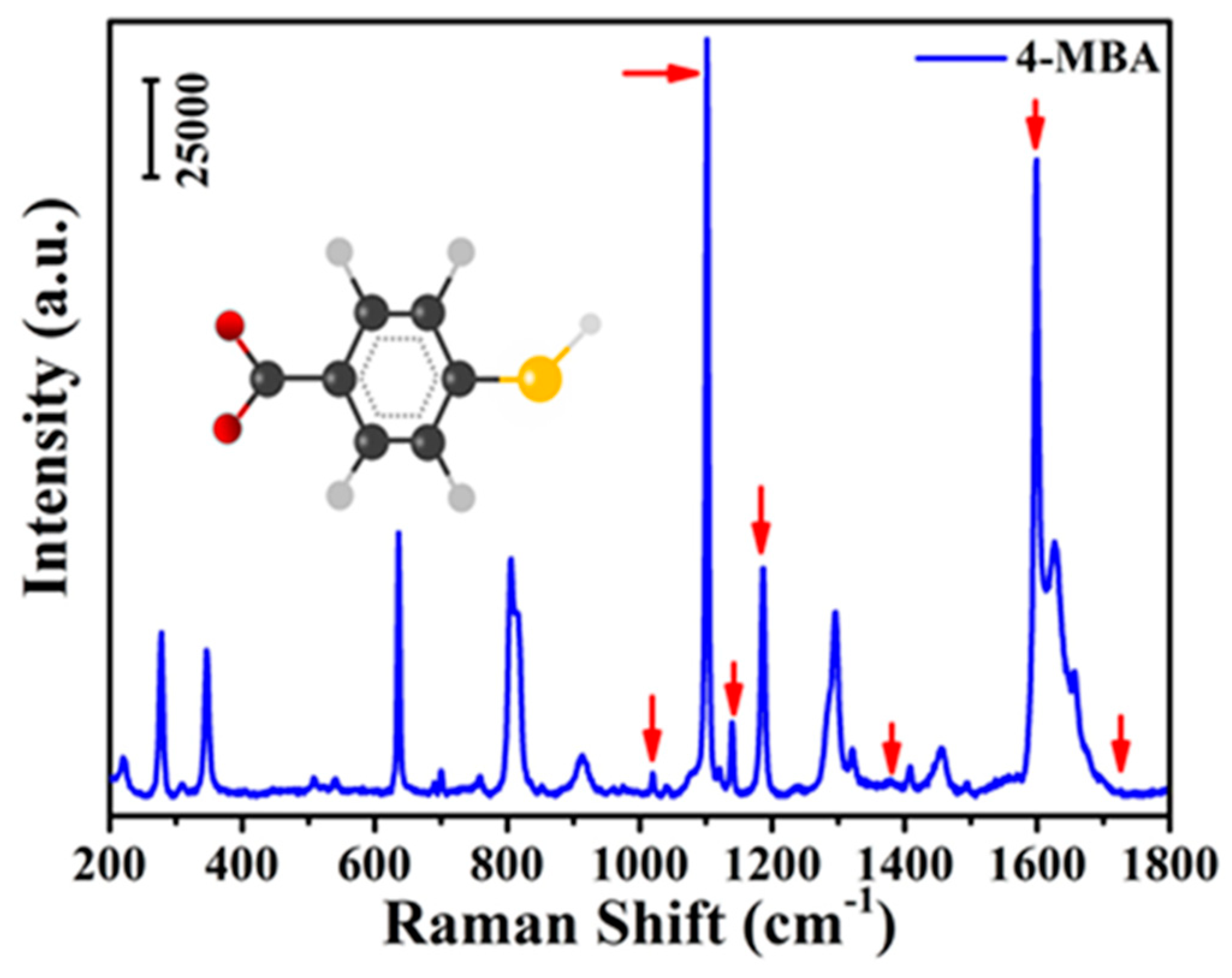
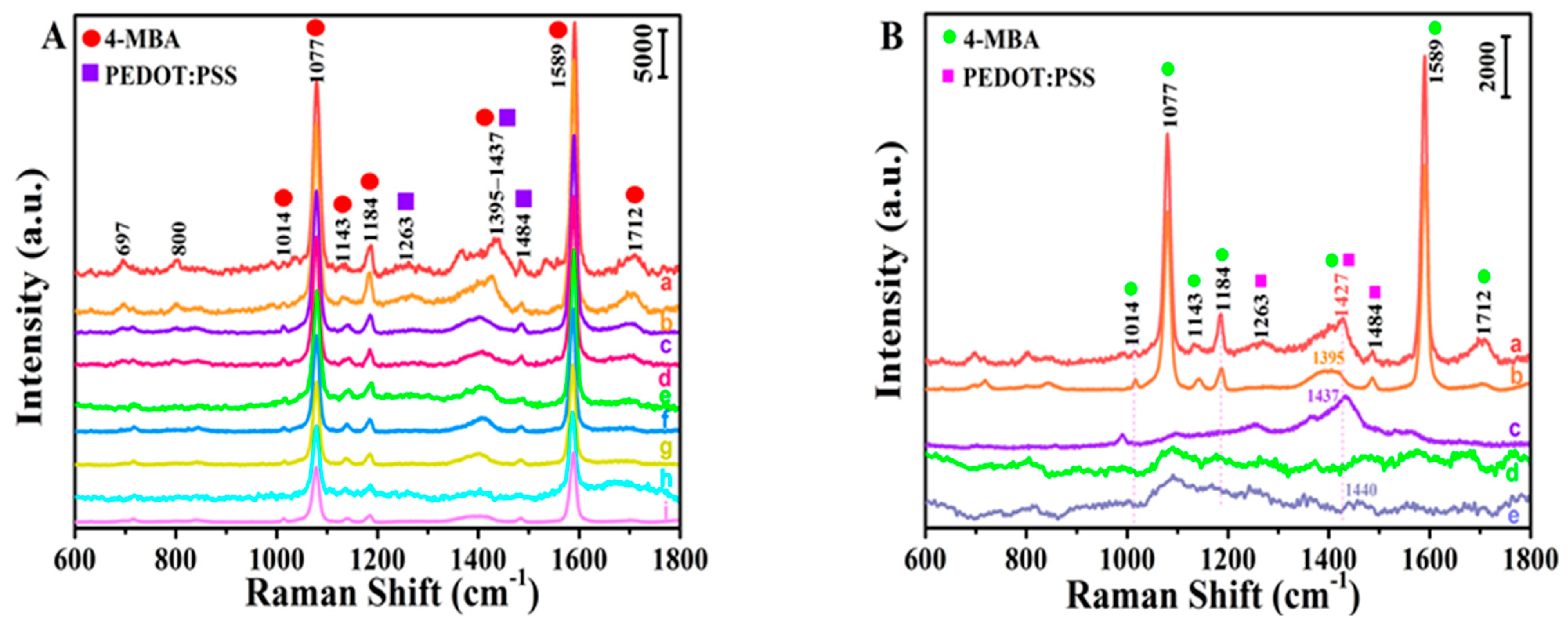
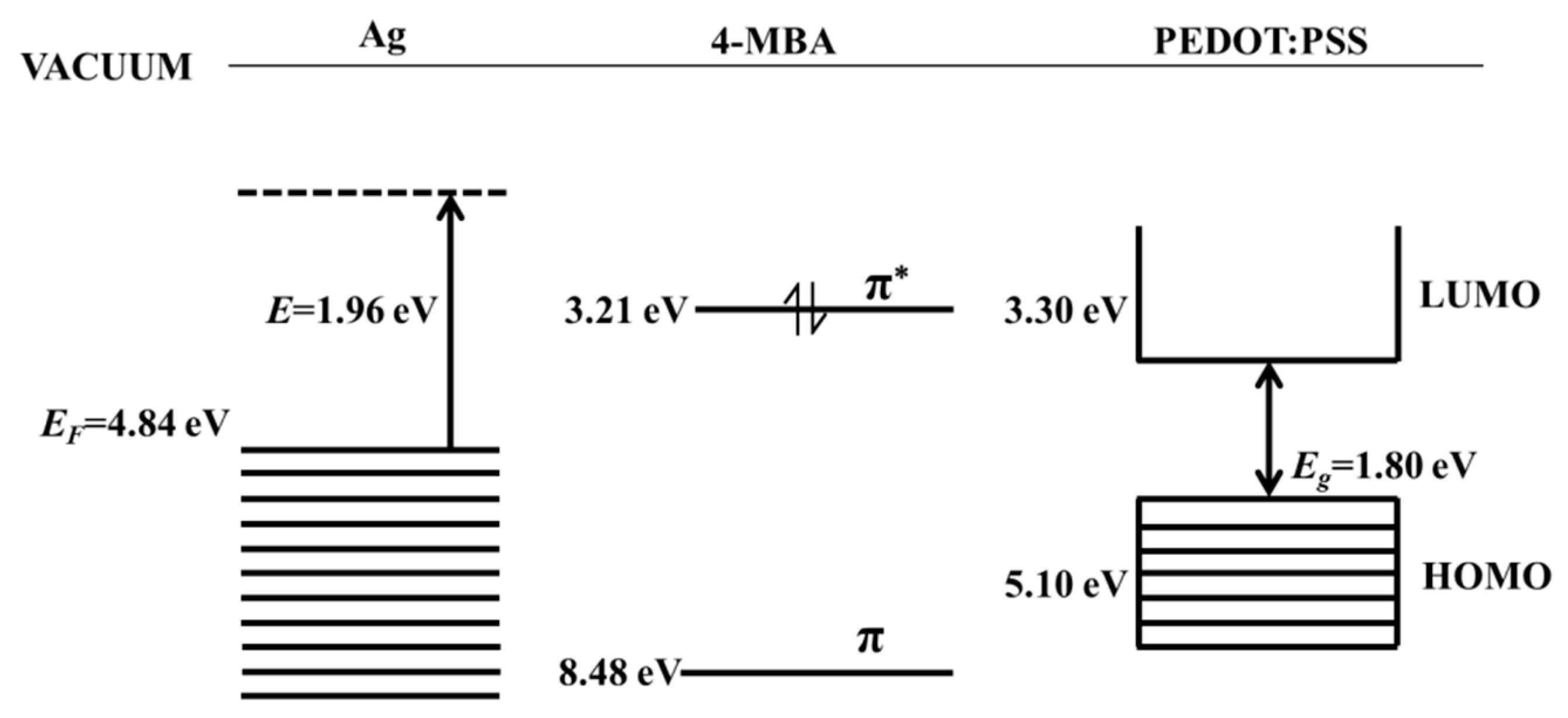
| Wavenumber (cm−1) | Band Assignments | Corresponding Molecule |
|---|---|---|
| 1014 | In-plane ring breathing, b2 | 4-MBA |
| 1077 | In-plane ring breathing + υ(CS) | 4-MBA |
| 1143 | C-H deformation modes, b2 | 4-MBA |
| 1184 | C-H deformation modes, a1 | 4-MBA |
| 1263 | υ(Cα-Cα’) inter-ring | PEDOT:PSS |
| 1395 | β(O-H) + υ(C-ph) + in-plane υ(CC) + | 4-MBA and |
| 1437 | asymmetry α(CO2), b2 and υ(Cβ-Cβ’) inter-ring | PEDOT:PSS |
| 1484 | υsym(Cα=Cβ) | PEDOT:PSS |
| 1589 | Totally symmetric υ(CC), a1 | 4-MBA |
| 1712 | C=O stretching | 4-MBA |
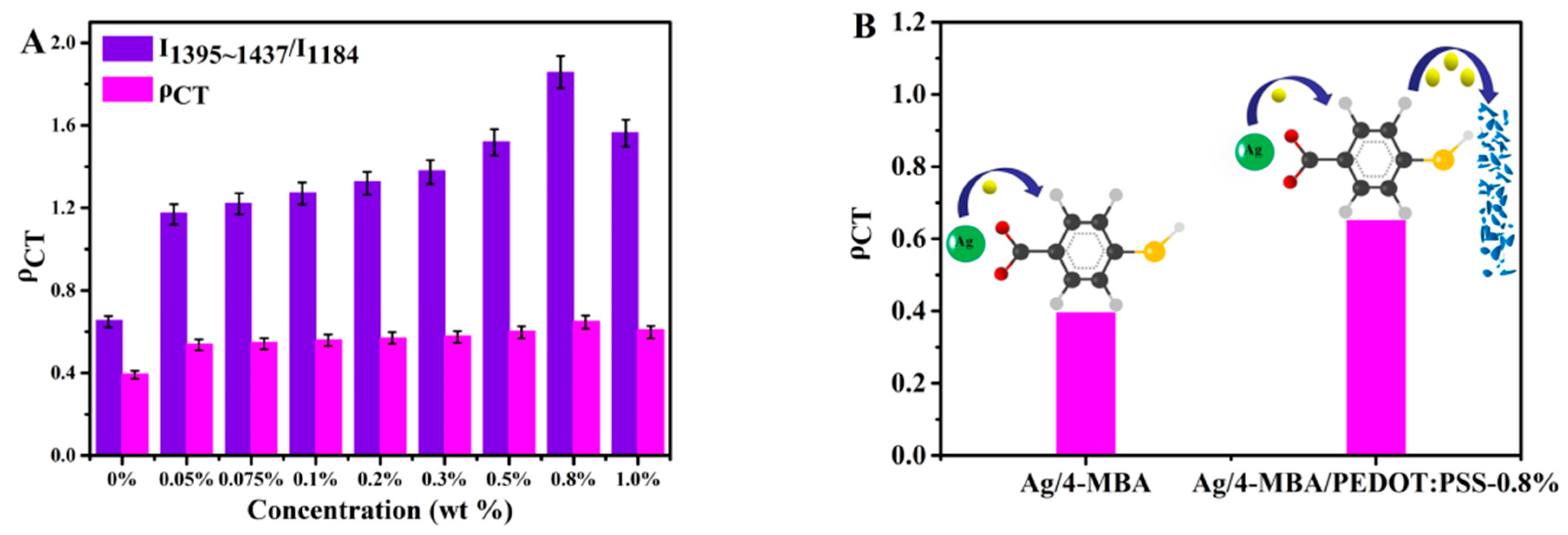
| Ag/4-MBA/PEDOT:PSS Composite Systems with Different Concentration of PEDOT:PSS | ρCT |
|---|---|
| 0% | 0.395 |
| 0.05% | 0.540 |
| 0.075% | 0.549 |
| 0.1% | 0.560 |
| 0.2% | 0.570 |
| 0.3% | 0.579 |
| 0.5% | 0.603 |
| 0.8% | 0.650 |
| 1.0% | 0.610 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Wang, W.; Guo, S.; Jin, S.; Park, E.; Sun, Y.; Chen, L.; Jung, Y.M. Charge Transfer on the Surface-Enhanced Raman Scattering of Ag/4-MBA/PEDOT:PSS System: Intermolecular Hydrogen Bonding. Chemosensors 2021, 9, 111. https://doi.org/10.3390/chemosensors9050111
Pan Y, Wang W, Guo S, Jin S, Park E, Sun Y, Chen L, Jung YM. Charge Transfer on the Surface-Enhanced Raman Scattering of Ag/4-MBA/PEDOT:PSS System: Intermolecular Hydrogen Bonding. Chemosensors. 2021; 9(5):111. https://doi.org/10.3390/chemosensors9050111
Chicago/Turabian StylePan, Yuenan, Wei Wang, Shuang Guo, Sila Jin, Eungyeong Park, Yantao Sun, Lei Chen, and Young Mee Jung. 2021. "Charge Transfer on the Surface-Enhanced Raman Scattering of Ag/4-MBA/PEDOT:PSS System: Intermolecular Hydrogen Bonding" Chemosensors 9, no. 5: 111. https://doi.org/10.3390/chemosensors9050111
APA StylePan, Y., Wang, W., Guo, S., Jin, S., Park, E., Sun, Y., Chen, L., & Jung, Y. M. (2021). Charge Transfer on the Surface-Enhanced Raman Scattering of Ag/4-MBA/PEDOT:PSS System: Intermolecular Hydrogen Bonding. Chemosensors, 9(5), 111. https://doi.org/10.3390/chemosensors9050111







