Ultrasensitive Leaky Surface Acoustic Wave Immunosensor for Real-Time Detection of Alpha-Fetoprotein in Biological Fluids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
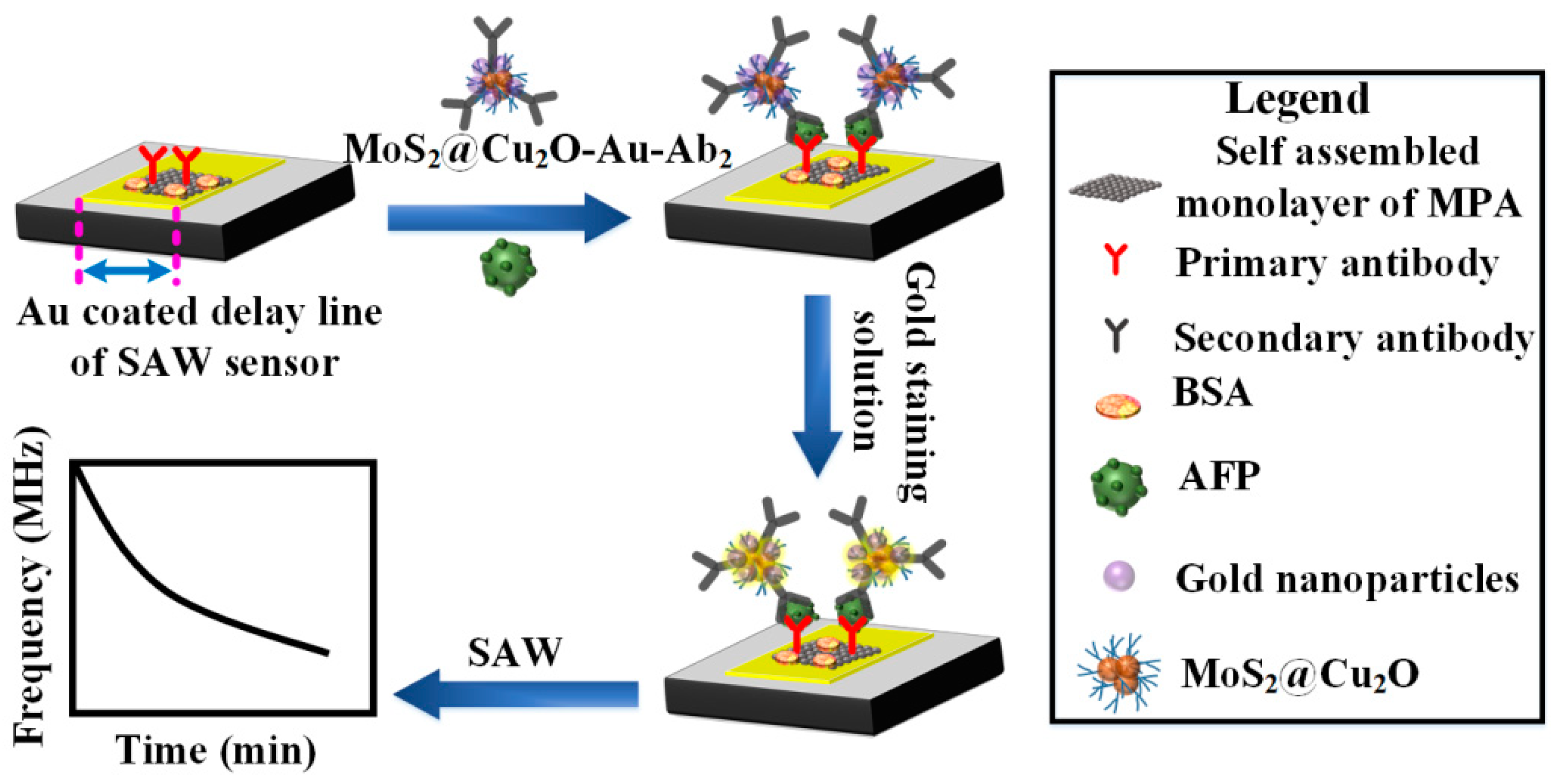
2.2. Preparation of MoS2@Cu2O-Au-Ab2 Conjugate
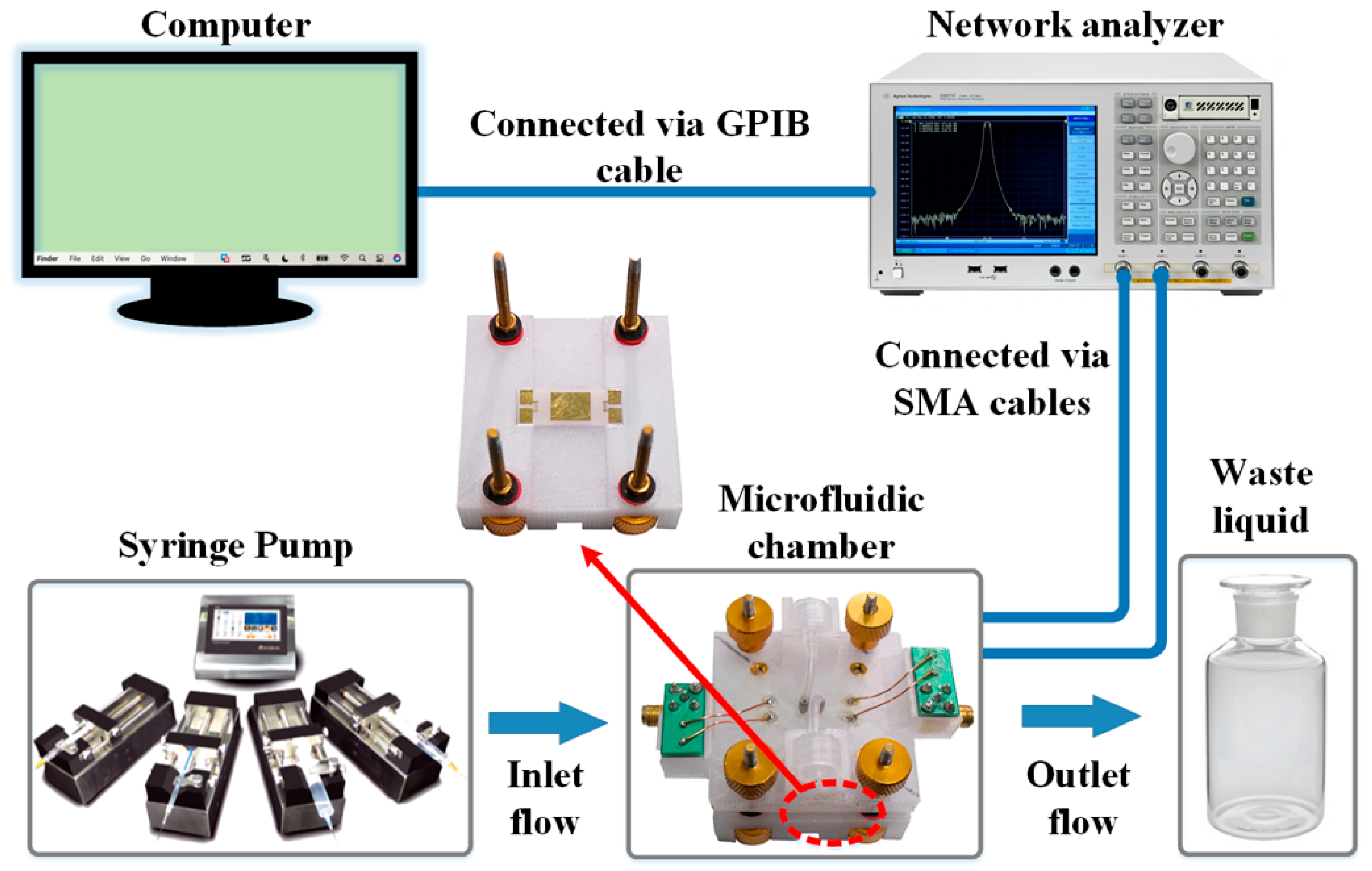
2.3. Fabrication of SAW Device
2.4. Immobilization of Ab1 on Delay Line of SAW
2.5. SAW-Based Sandwich Immunoassay
3. Results and Discussion
3.1. Characterization of MoS2@Cu2O-Au
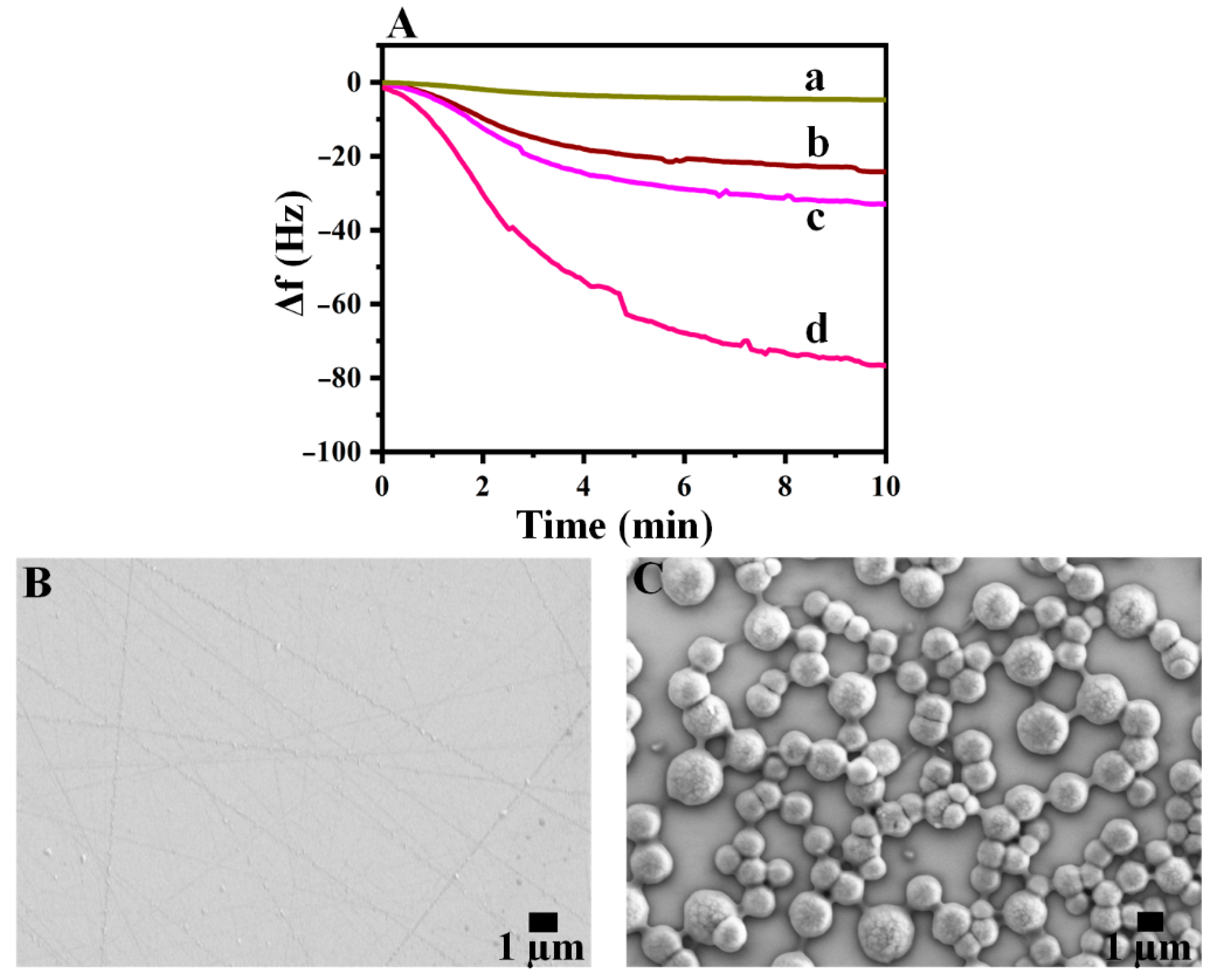
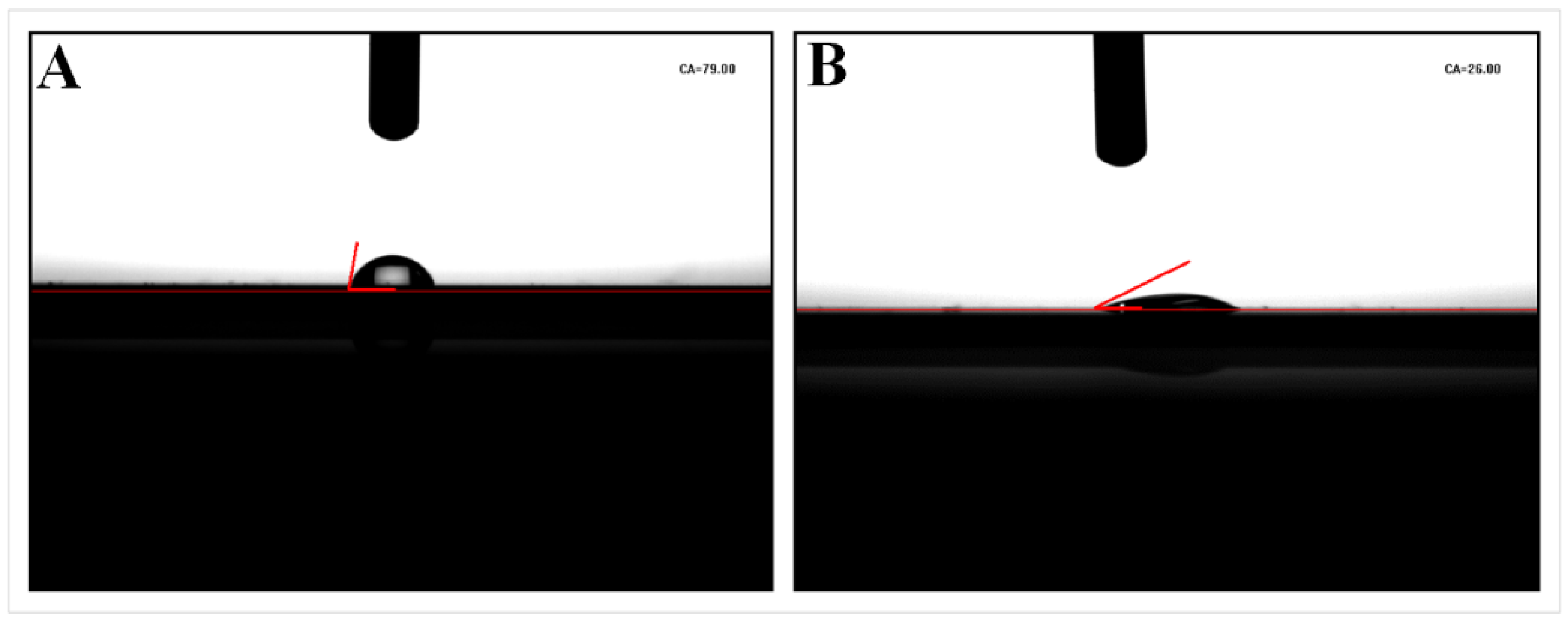
3.2. Preparation and Characterization of Self-Assembled Monolayer (SAM)
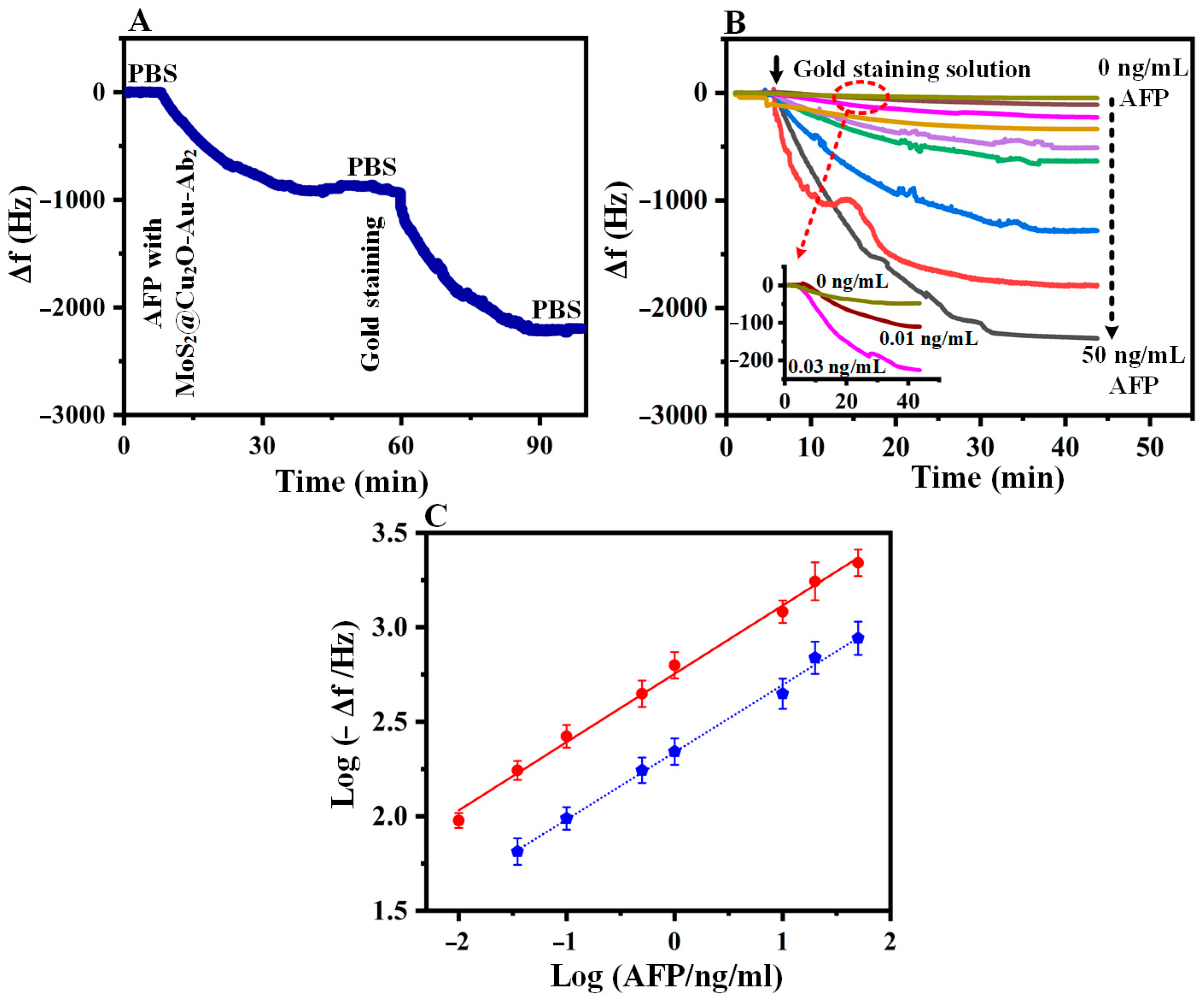
3.3. Real-Time Detection Studies with SAW Immunosensor
3.4. Quantification of AFP
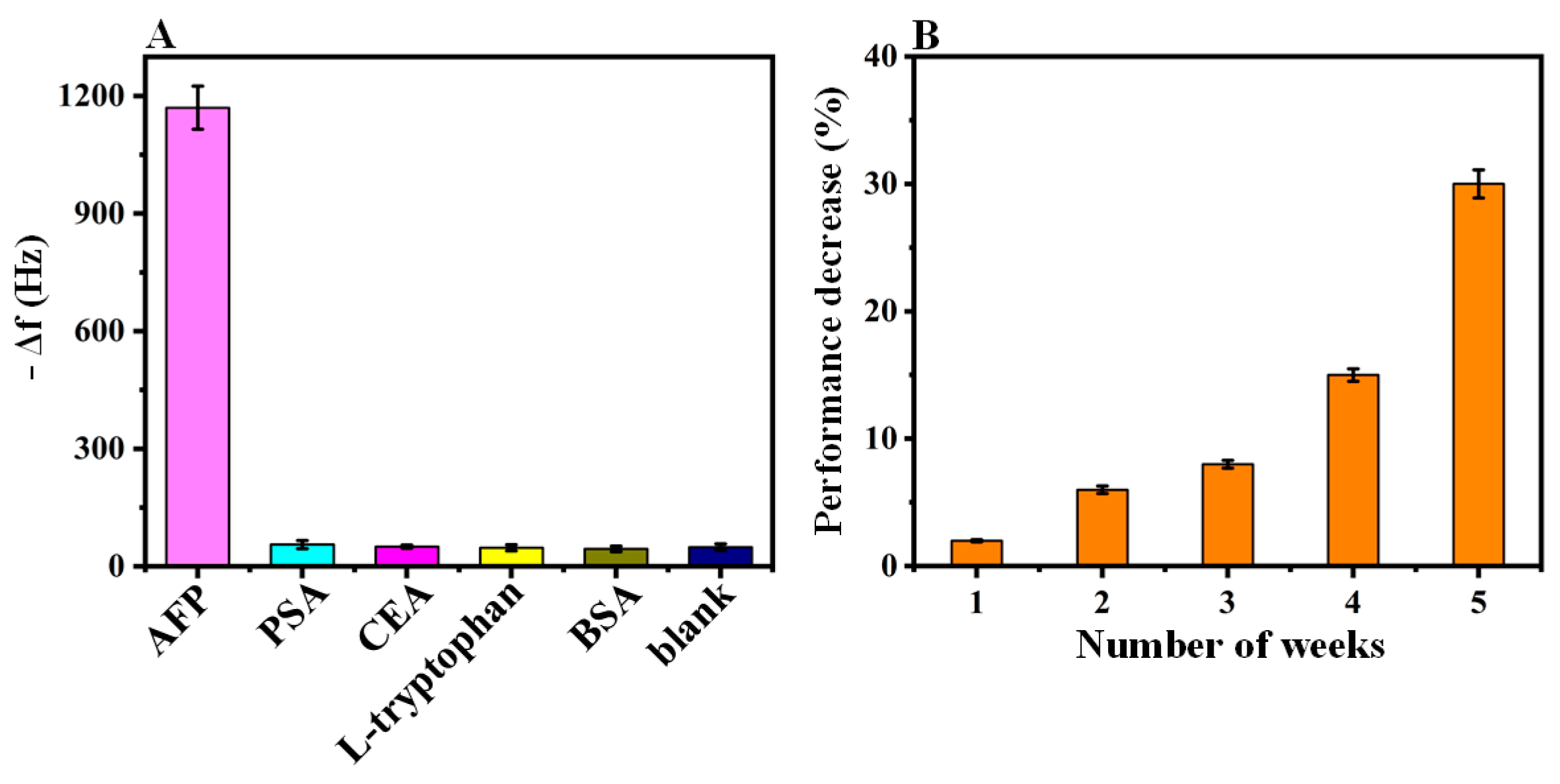
3.5. Immunosensor Selectivity
3.6. Detection of AFP in Biological Fluids
3.7. Long-Term Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, L.M.; Kreikemeier, J.T.; Fimmel, C.J. A concise review of serum markers for hepatocellular cancer. Cancer Detect. Prev. 2007, 31, 35–44. [Google Scholar] [CrossRef]
- Gong, X.; Yan, H.; Yang, J.; Wu, Y.; Zhang, J.; Yao, Y.; Liu, P.; Wang, H.; Hu, Z.; Chang, J. High-performance fluorescence-encoded magnetic microbeads as microfluidic protein chip supports for AFP detection. Anal. Chim. Acta 2016, 939, 84–92. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Li, N.; Zhang, Y.; Yan, T.; Ma, H.; Wei, Q. Sandwich-type electrochemical immunosensor for the detection of AFP based on Pd octahedral and APTES-M-CeO2-GS as signal labels. Biosens. Bioelectron. 2016, 79, 482–487. [Google Scholar]
- Wang, Y.; Zhao, G.; Wang, H.; Cao, W.; Du, B.; Wei, Q. Sandwich-type electrochemical immunoassay based on Co3O4@MnO2-thionine and pseudo-ELISA method toward sensitive detection of alpha fetoprotein. Biosens. Bioelectron. 2018, 106, 179–185. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, M.; Liu, L.; Pang, Y.; Long, Y.; Zheng, H. GTP as a peroxidase-mimic to mediate enzymatic cascade reaction for alkaline phosphatase detection and alkaline phosphatase-linked immunoassay. Sens. Actuators B Chem. 2018, 275, 43–49. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Jin, D.; Yu, Y.; Yang, F.; Zhang, Y.; Yao, Q.; Zhang, G.-J. AuNP-Amplified surface acoustic wave sensor for the quantification of exosomes. ACS Sens. 2020, 5, 362–369. [Google Scholar] [CrossRef]
- Jandas, P.J.; Luo, J.; Quan, A.; Qiu, C.; Cao, W.; Fu, C.; Fu, Y.Q. Highly selective and label-free Love-mode surface acoustic wave biosensor for carcinoembryonic antigen detection using a self-assembled monolayer bioreceptor. Appl. Surf. Sci. 2020, 518, 146061. [Google Scholar] [CrossRef]
- Xu, Z.; Yuan, Y.J. Implementation of guiding layers of surface acoustic wave devices: A review. Biosens. Bioelectron. 2018, 99, 500–512. [Google Scholar] [PubMed]
- Matatagui, D.; Fontecha, J.L.; Fernández, M.J.; Gràcia, I.; Cané, C.; Santos, J.P.; Horrillo, M.C. Love-wave sensors combined with microfluidics for fast detection of biological warfare agents. Sensors 2014, 14, 12658–12669. [Google Scholar] [CrossRef] [Green Version]
- Neves, M.A.D.; Blaszykowski, C.; Bokhari, S.; Thompson, M. Ultra-high frequency piezoelectric aptasensor for the label-free detection of cocaine. Biosens. Bioelectron. 2015, 72, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Gammoudi, I.; Blanc, L.; Moroté, F.; Grauby-Heywang, C.; Boissière, C.; Kalfat, R.; Rebière, D.; Cohen-Bouhacina, T.; Dejous, C. High sensitive mesoporous TiO2-coated love wave device for heavy metal detection. Biosens. Bioelectron. 2014, 57, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Rana, L.; Gupta, R.; Tomar, M.; Gupta, V. Highly sensitive Love wave acoustic biosensor for uric acid. Sens. Actuators B Chem. 2018, 261, 169–177. [Google Scholar] [CrossRef]
- Bröker, P.; Lücke, K.; Perpeet, M.; Gronewold, T.M.A. A nanostructured SAW chip-based biosensor detecting cancer cells. Sens. Actuators B Chem. 2012, 165, 1–6. [Google Scholar] [CrossRef]
- Kong, H.; Li, C.; Guo, Z.; Zhang, W.; Yao, J.; Zhu, H.; Yan, R.; Wang, L.; Li, J.; Wei, W.; et al. Sensitivity improved with Parylene-C passivized on Lamb wave sensor for aPTT measurement through monitoring whole blood reaction. Sens. Actuators B Chem. 2019, 285, 479–486. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef] [Green Version]
- Ten, S.T.; Hashim, U.; Gopinath, S.C.B.; Liu, W.W.; Foo, K.L.; Sam, S.T.; Rahman, S.F.A.; Voon, C.H.; Nordin, A.N. Highly sensitive escherichia coli shear horizontal surface acoustic wave biosensor with silicon dioxide nanostructures. Biosens. Bioelectron. 2017, 93, 146–154. [Google Scholar] [CrossRef]
- Belovickis, J.; Rimeika, R.; Čiplys, D. Acousto-optic interaction with leaky surface acoustic waves in Y-cut LiTaO3 crystals. Ultrasonics 2012, 52, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Cai, F.; Zhang, Z.; Niu, L.; Jin, Q.; Yan, F.; Wu, J.; Wang, Z.; Zheng, H. Transportation of single cell and microbubbles by phase-shift introduced to standing leaky surface acoustic waves. Biomicrofluidics 2011, 5, 044104. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.; Wang, F.; Ding, Y.; Pan, F.; Li, F.; Jia, S.; Lu, W.; Deng, S.; Shi, J.; Chen, M. Development and validation of a novel leaky surface acoustic wave immunosensor array for label-free and high-sensitive detection of cyclosporin A in whole-blood samples. Biosens. Bioelectron. 2014, 54, 151–157. [Google Scholar] [CrossRef]
- Chang, K.; Pi, Y.; Lu, W.; Wang, F.; Pan, F.; Li, F.; Jia, S.; Shi, J.; Deng, S.; Chen, M. Label-free and high-sensitive detection of human breast cancer cells by aptamer-based leaky surface acoustic wave biosensor array. Biosens. Bioelectron. 2014, 60, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chang, K.; Lu, W.; Chen, W.; Ding, Y.; Jia, S.; Zhang, K.; Li, F.; Shi, J.; Cao, L.; et al. Detection of single-nucleotide polymorphisms with novel leaky surface acoustic wave biosensors, DNA ligation and enzymatic signal amplification. Biosens. Bioelectron. 2012, 33, 274–278. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Chen, M.; Luo, Y.; Deng, K.; Chen, D.; Fu, W. A new system for the amplification of biological signals: RecA and complimentary single strand DNA probes on a leaky surface acoustic wave biosensor. Biosens. Bioelectron. 2014, 60, 259–264. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Zhang, L.; Ding, Y.; Luo, Y.; Xu, Q.; Shi, J.; Cao, L.; Fu, W. Rapid detection of human papilloma virus using a novel leaky surface acoustic wave peptide nucleic acid biosensor. Biosens. Bioelectron. 2009, 24, 3455–3460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, S.; Cao, K.; Wang, P.; Su, Y.; Zhu, X.; Wan, Y. A microfluidic love-wave biosensing device for PSA detection based on an aptamer beacon probe. Sensors 2015, 15, 13839–13850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wan, Y.; Su, Y.; Fan, C.; Bhethanabotla, V.R. Gold nanoparticle-based low limit of detection Love wave biosensor for carcinoembryonic antigens. Biosens. Bioelectron. 2017, 95, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Chi, L.; Xu, C.; Li, S.; Wang, X.; Tang, D.; Xue, F. In Situ amplified QCM immunoassay for carcinoembryonic antigen with colorectal cancer using horseradish peroxidase nanospheres and enzymatic biocatalytic precipitation. Analyst 2020, 145, 6111–6118. [Google Scholar] [CrossRef]
- Atar, N.; Yola, M.L. A novel QCM immunosensor development based on gold nanoparticles functionalized sulfur-doped graphene quantum dot and h-ZnS-CdS NC for Interleukin-6 detection. Anal. Chim. Acta 2021, 1148, 338202. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Feng, J.; Gao, Z.; Lv, H.; Ren, X.; Wei, Q. Facile synthesis of MoS2@Cu2O-Pt nanohybrid as enzyme-mimetic label for the detection of the Hepatitis B surface antigen. Biosens. Bioelectron. 2018, 100, 512–518. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Guo, Y.; Chen, Y.; Su, Z.; Zhang, P. Coral-Like MoS2/Cu2O Porous Nanohybrid with Dual-Electrocatalyst Performances. Adv. Mater. Interfaces 2016, 3, 1600658. [Google Scholar] [CrossRef]
- Zou, L.; Tian, Y.; Zhang, X.; Fang, J.; Hu, N.; Wang, P. A competitive love wave immunosensor for detection of okadaic acid based on immunogold staining method. Sens. Actuators B Chem. 2017, 238, 1173–1180. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Park, J.-Y.; Seo, H.; Lee, T.; Lee, W.; Kim, S.K.; Hahn, Y.K.; Jung, J.Y.; Kim, S.; et al. Sensitive and reproducible detection of cardiac troponin I in human plasma using a surface acoustic wave immunosensor. Sens. Actuators B Chem. 2013, 178, 19–25. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.-S.; Lee, Y.; Lee, H.J.; Lee, J.N.; Kim, S.K.; Han, K.Y.; Cho, E.C.; Park, J.C.; Lee, S.S. Sensitive and Simultaneous Detection of Cardiac Markers in Human Serum Using Surface Acoustic Wave Immunosensor. Anal. Chem. 2011, 83, 8629–8635. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.; Lee, S.S. Highly sensitive piezoelectric immunosensors employing signal amplification with gold nanoparticles. Nanotechnology 2019, 30, 445502. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Bain, C.D.; Troughton, E.B.; Tao, Y.T.; Evall, J.; Whitesides, G.M.; Nuzzo, R.G. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J. Am. Chem. Soc. 1989, 111, 321–335. [Google Scholar] [CrossRef]
- Ding, P.; Liu, R.; Liu, S.; Mao, X.; Hu, R.; Li, G. Reusable gold nanoparticle enhanced QCM immunosensor for detecting C-reactive protein. Sens. Actuators B Chem. 2013, 188, 1277–1283. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of detection. A closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar]
- Tsai, W.-C.; Lin, I.C. Development of a piezoelectric immunosensor for the detection of alpha-fetoprotein. Sens. Actuators B Chem. 2005, 106, 455–460. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, J.; Wang, H.; Shen, G.; Yu, R. A piezoelectric immunosensor for the detection of α-fetoprotein using an interface of gold/hydroxyapatite hybrid nanomaterial. Biomaterials 2007, 28, 2147–2154. [Google Scholar] [CrossRef]
- Xu, T.; Miao, J.; Wang, Z.; Yu, L.; Li, C.M. Micro-piezoelectric immunoassay chip for simultaneous detection of Hepatitis B virus and α-fetoprotein. Sens. Actuators B Chem. 2011, 151, 370–376. [Google Scholar] [CrossRef]

| Sample | Added (ng/mL) | Found (ng/mL) | Recovery (%) n = 3 |
|---|---|---|---|
| Human serum | 0.50 | 0.53 (±0.03) | 106 |
| 2.0 | 2.17 (±0.13) | 108 | |
| 5.0 | 5.11 (±0.45) | 102 | |
| Human saliva | 0.5 | 0.47 (±0.02) | 94 |
| 2.0 | 2.07 (±0.15) | 103 | |
| 5.0 | 4.75 (±0.31) | 95 |
| Sample | Elisa (ng/mL) | Proposed Method (ng/mL) |
|---|---|---|
| Human serum | 0.47 (± 0.01) | 0.53 (± 0.03) |
| 2.13 (± 0.17) | 2.09 (± 0.12) | |
| 5.42 (± 0.37) | 5.25 (± 0.47) | |
| Human saliva | 0.52 (± 0.04) | 0.49 (± 0.02) |
| 2.19 (± 0.12) | 2.18 (± 0.14) | |
| 5.38 (± 0.40) | 4.69 (± 0.30) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauf, S.; Qazi, H.I.A.; Luo, J.; Fu, C.; Tao, R.; Rauf, S.; Yang, L.; Li, H.; Fu, Y. Ultrasensitive Leaky Surface Acoustic Wave Immunosensor for Real-Time Detection of Alpha-Fetoprotein in Biological Fluids. Chemosensors 2021, 9, 311. https://doi.org/10.3390/chemosensors9110311
Rauf S, Qazi HIA, Luo J, Fu C, Tao R, Rauf S, Yang L, Li H, Fu Y. Ultrasensitive Leaky Surface Acoustic Wave Immunosensor for Real-Time Detection of Alpha-Fetoprotein in Biological Fluids. Chemosensors. 2021; 9(11):311. https://doi.org/10.3390/chemosensors9110311
Chicago/Turabian StyleRauf, Sana, Hafiz Imran Ahmad Qazi, Jingting Luo, Chen Fu, Ran Tao, Sajid Rauf, Lei Yang, Honglang Li, and Yongqing Fu. 2021. "Ultrasensitive Leaky Surface Acoustic Wave Immunosensor for Real-Time Detection of Alpha-Fetoprotein in Biological Fluids" Chemosensors 9, no. 11: 311. https://doi.org/10.3390/chemosensors9110311
APA StyleRauf, S., Qazi, H. I. A., Luo, J., Fu, C., Tao, R., Rauf, S., Yang, L., Li, H., & Fu, Y. (2021). Ultrasensitive Leaky Surface Acoustic Wave Immunosensor for Real-Time Detection of Alpha-Fetoprotein in Biological Fluids. Chemosensors, 9(11), 311. https://doi.org/10.3390/chemosensors9110311







