Gold and Silver Nanoparticle-Based Colorimetric Sensors: New Trends and Applications
Abstract
:1. Introduction
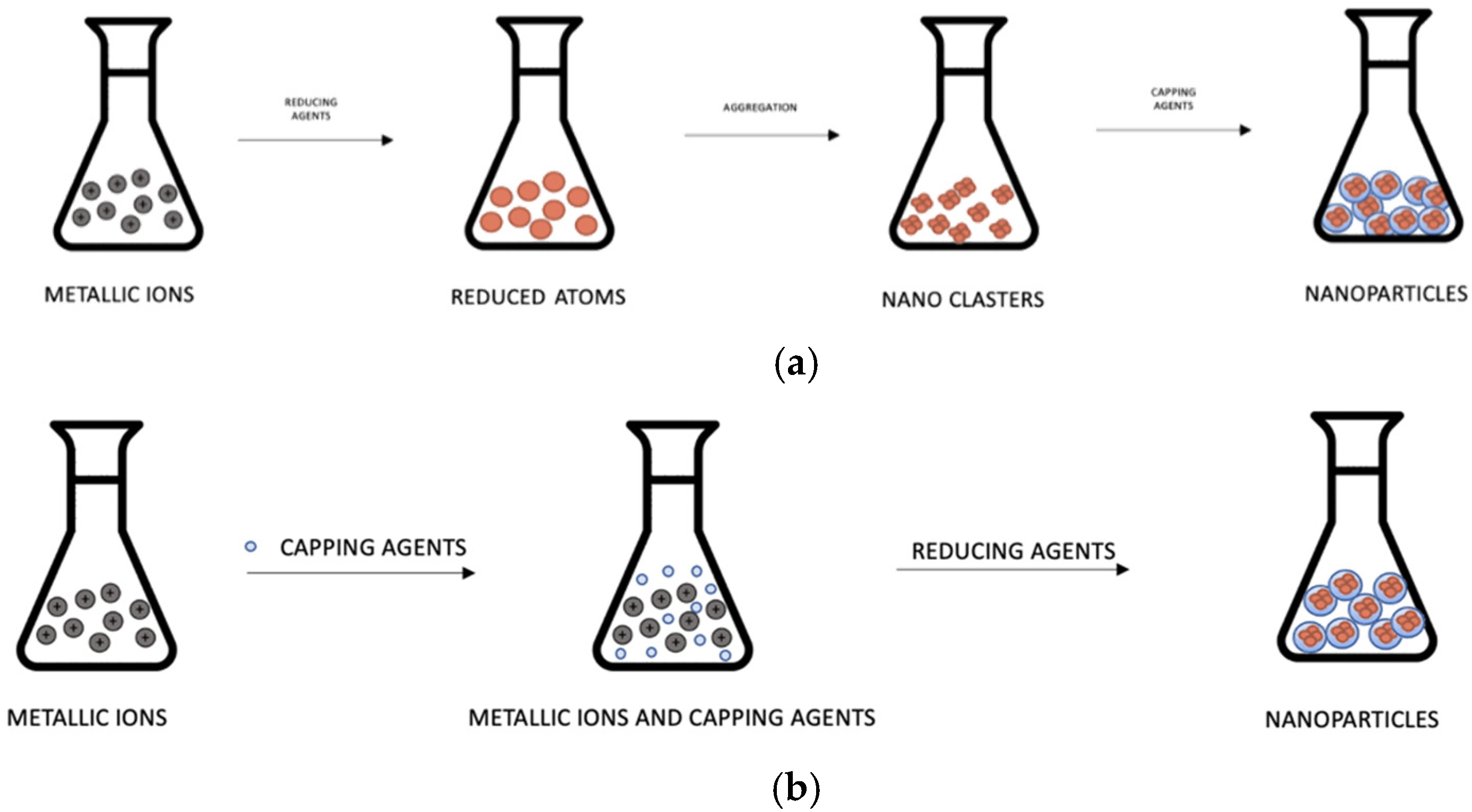
2. AgNPs and AuNPs: Synthesis and Properties
2.1. AgNPs Synthesis
2.1.1. Physical Methods
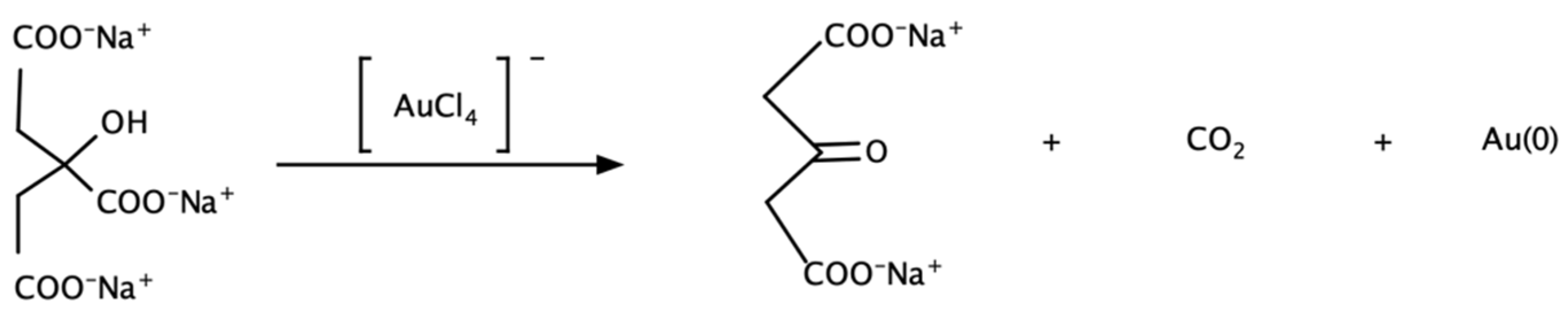
2.1.2. Chemical Methods
2.1.3. Green Methods
2.2. AuNPs Synthesis
2.2.1. Physical Methods
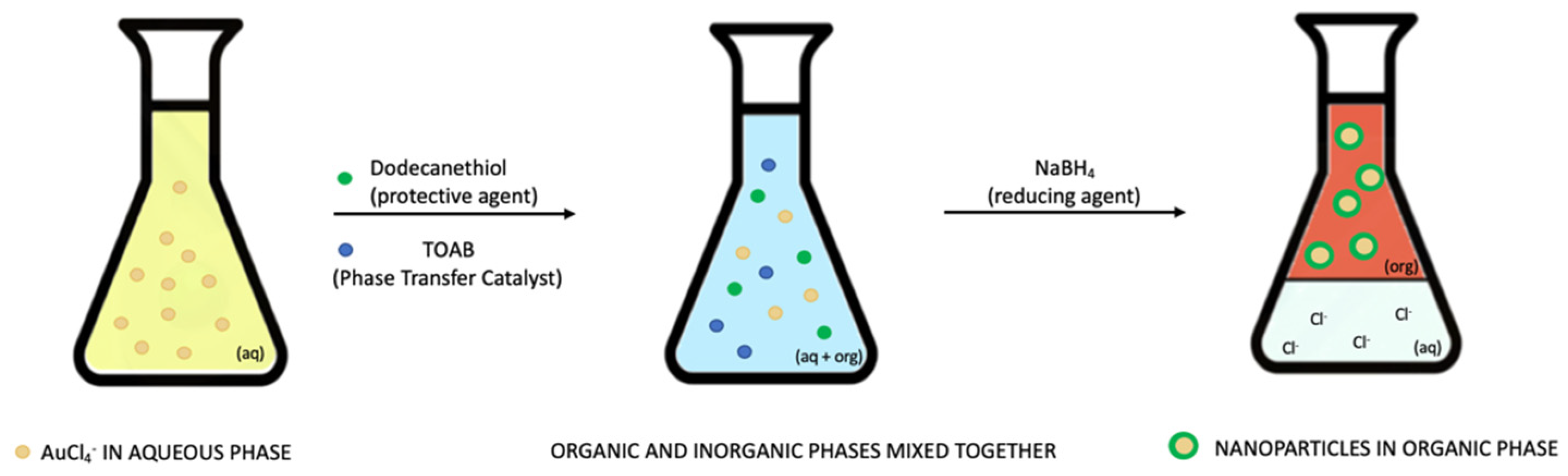
2.2.2. Chemical Methods
2.2.3. Green Methods
3. Ag Nanoparticles-Based Colorimetric Sensors
3.1. Colorimetric Sensors Based on AgNP Aggregation
3.1.1. Metal Ions Detection
3.1.2. Pharmaceutics, Biomolecules, and Environmental Contaminants Detection
3.2. Colorimetric Sensors Based on AgNPs Anti-Aggregation
3.3. Colorimetric Sensors Based on AgNPs Etching
3.4. Colorimetric Sensors Based on AgNPs Anti-Etching
3.5. Colorimetric Sensors Based on AgNP Growth
4. Au Nanoparticle-Based Colorimetric Sensors
4.1. Colorimetric Sensors Based on AuNPs Aggregation
4.2. Colorimetric Sensors Based on AuNP Anti-Aggregation
4.3. Colorimetric Sensors Based on AuNPs Etching
4.4. Colorimetric Sensors Based on AuNP Anti-Etching
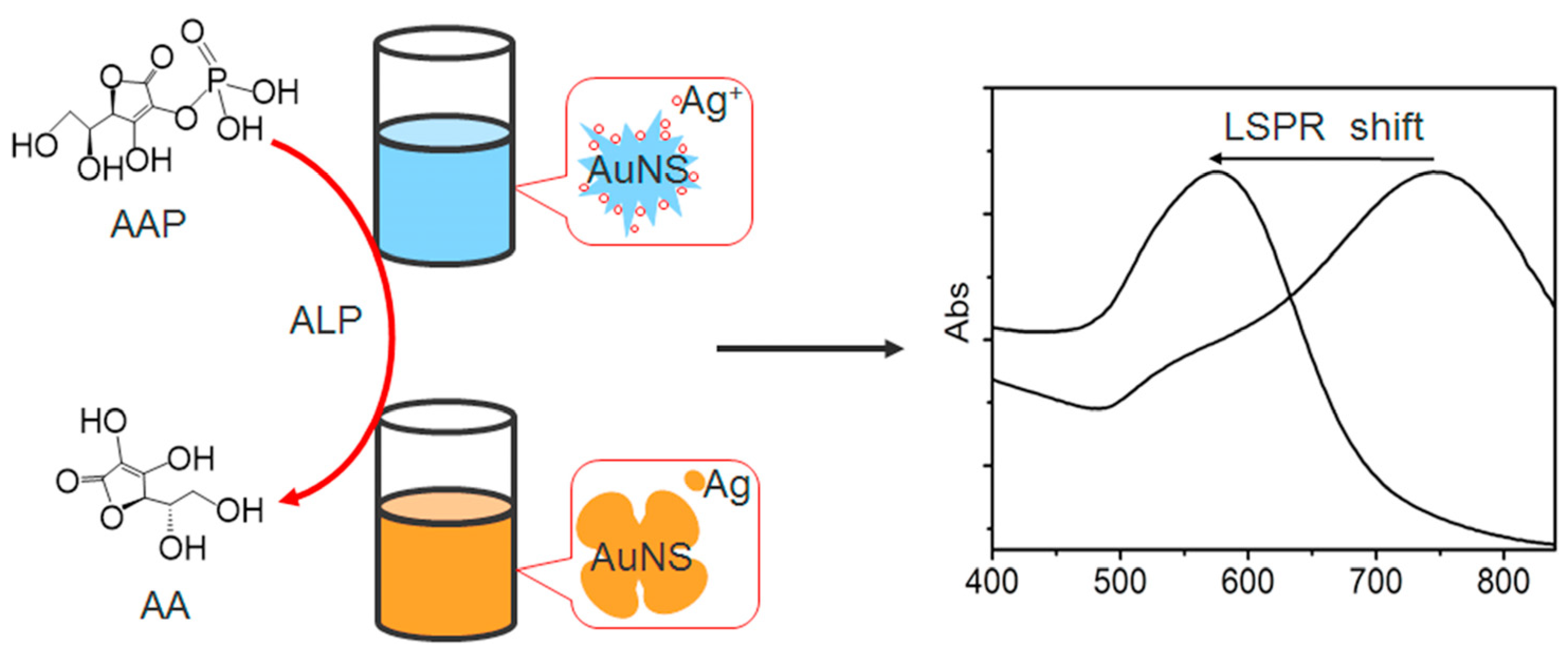
4.5. Colorimetric Sensors Based on AuNP Growth
5. Ag/Au Bimetallic NPs
5.1. Colorimetric Sensors Based on Ag/Au Bimetallic NP Aggregation
5.2. Colorimetric Sensors Based on Ag/Au Bimetallic NPs Etching
5.3. Colorimetric Sensors Based on Ag/Au Bimetallic NP Growth
6. Current Trends in Au and Ag Nanoparticle-Based Optical Devices
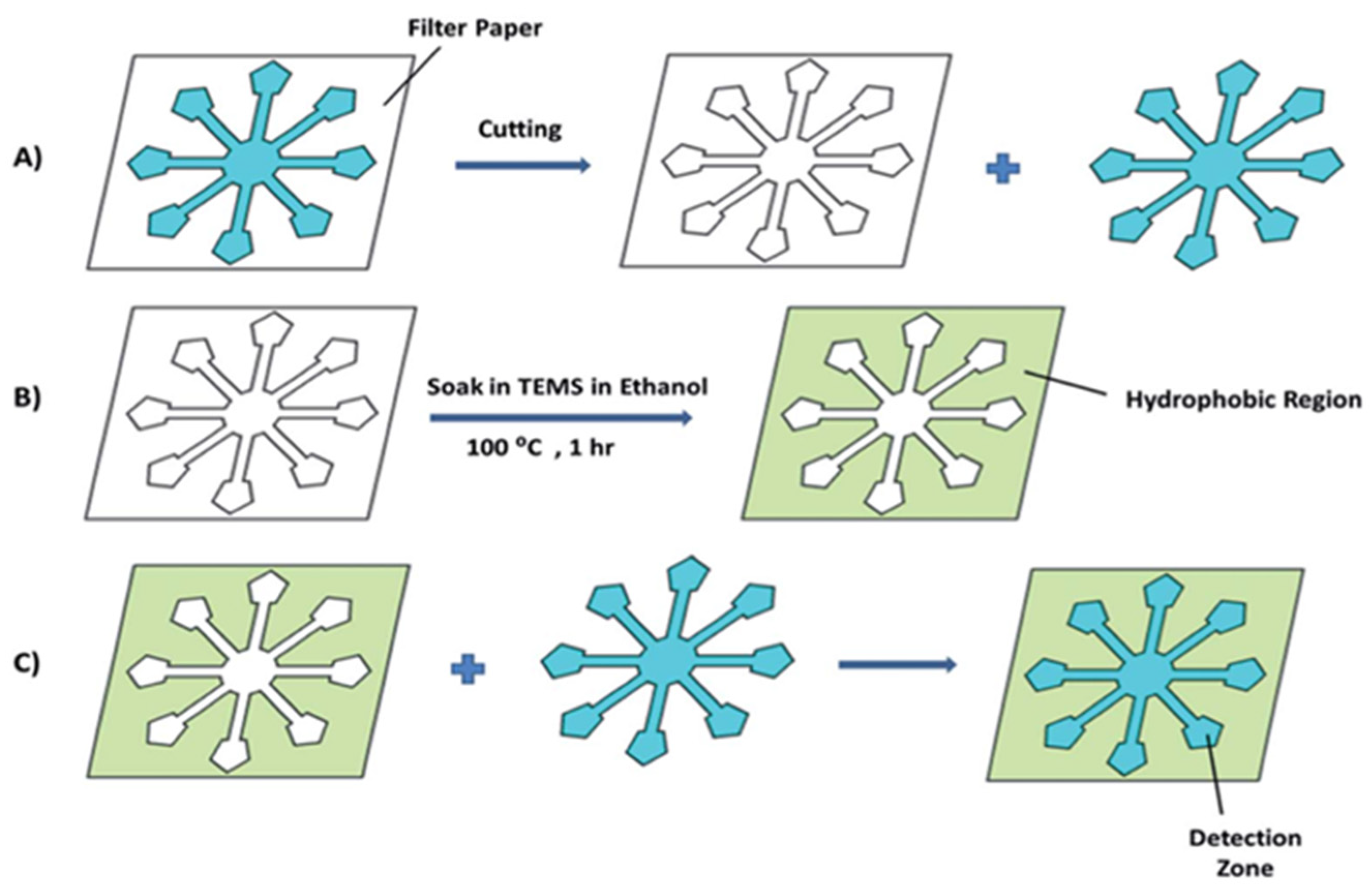
6.1. PAD Sensors
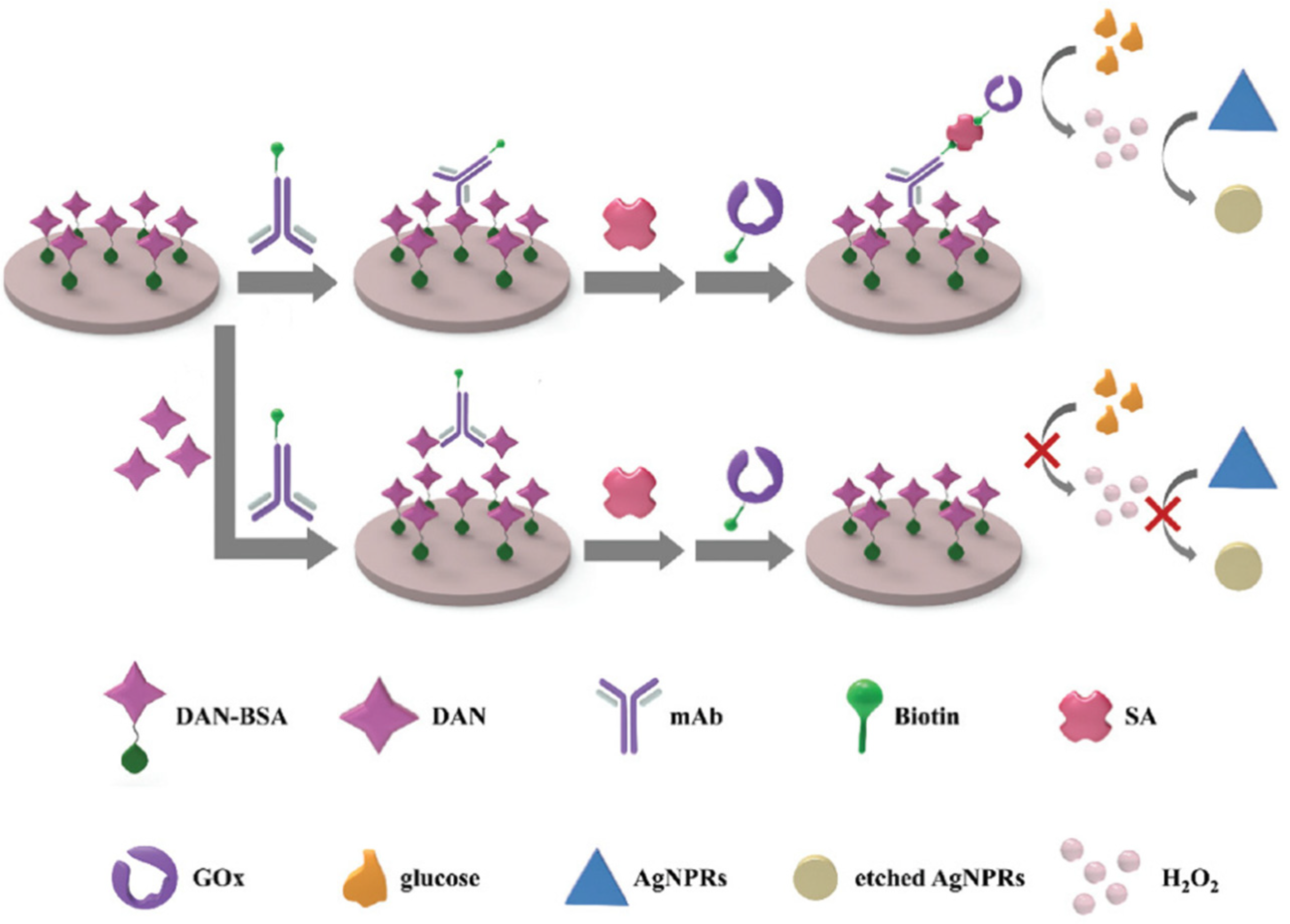
6.2. Biosensors
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Rao, H.; Luo, M.; Xue, X.; Xue, Z.; Lu, X. Noble metal nanoparticles growth-based colorimetric strategies: From monocolorimetric to multicolorimetric sensors. Co-ord. Chem. Rev. 2019, 398, 113003. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Disposable and Low-Cost Colorimetric Sensors for Environmental Analyses. Int. J. Environ. Res. Public Health 2020, 17, 8331. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Chen, Z.; Wang, X.; Choo, J.; Chen, L. Plasmonic colorimetric sensors based on etching and growth of noble metal nanoparticles: Strategies and applications. Biosens. Bioelectron. 2018, 114, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Kangas, M.J.; Burks, R.; Atwater, J.; Lukowicz, R.M.; Williams, P.; Holmes, A.E. Colorimetric Sensor Arrays for the Detection and Identification of Chemical Weapons and Explosives. Crit. Rev. Anal. Chem. 2016, 47, 138–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, X.-H.; Hahm, E.; Kim, T.H.; Kim, H.-M.; Lee, S.H.; Lee, Y.-S.; Jeong, D.H.; Jun, B.-H. Enzyme-catalyzed Ag Growth on Au Nanoparticle-assembled Structure for Highly Sensitive Colorimetric Immunoassay. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Shiping Song, D.L.; Fan, C. Target-Responsive Structural Switching for Nucleic Acid-Based Sensors. Acc. Chem. Res. 2010, 43, 631–641. [Google Scholar]
- Lei, J.; Ju, H. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012, 41, 2122–2134. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.Q.; Gao, Z. Plasmonic nanoparticles in biomedicine. Nano Today 2016, 11, 168–188. [Google Scholar] [CrossRef]
- Paterson, S.; de la Rica, R. Solution-based nanosensors for in-field detection with the naked eye. Analyst 2015, 140, 3308–3317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; He, S.; Qiu, B.; Luo, F.; Guo, L.; Lin, Z. Noble Metal Nanoparticle-Based Multicolor Immunoassays: An Approach toward Visual Quantification of the Analytes with the Naked Eye. ACS Sens. 2019, 4, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, H.; Wang, Y.; Wu, X.; Zhang, J. Electronic and optical properties of strained noble metals: Implications for applications based on LSPR. Nano Energy 2018, 53, 932–939. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.N.; Yadav, S.; Haque, M.H.; Munaz, A.; Islam, F.; Al Hossain, M.S.; Gopalan, V.; Lam, A.K.; Nguyen, N.-T.; Shiddiky, M.J. Optical biosensing strategies for DNA methylation analysis. Biosens. Bioelectron. 2017, 92, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, C.A.; Letsinger, R.L.; Mucic, R.C.; Storhoff, J.J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382, 607–609. [Google Scholar] [CrossRef]
- Citartan, M.; Tang, T.-H. Recent developments of aptasensors expedient for point-of-care (POC) diagnostics. Talanta 2019, 199, 556–566. [Google Scholar] [CrossRef]
- Howes, P.; Rana, S.; Stevens, M.M. Plasmonic nanomaterials for biodiagnostics. Chem. Soc. Rev. 2013, 43, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Ajay, P.V.S.; Printo, J.; Kiruba, D.S.C.G.; Susithra, L.; Takatoshi, K.; Sivakumar, M. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. 2017, 78, 1231–1245. [Google Scholar]
- Cao, J.; Sun, T.; Grattan, K. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B Chem. 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Sun, L.; Liu, L.; Xu, C.; Kuang, H. Nanoparticle-based sensors for food contaminants. TrAC Trends Anal. Chem. 2019, 113, 74–83. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Schaming, D.; Remita, H. Nanotechnology: From the ancient time to nowadays. Found. Chem. 2015, 17, 187–205. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metals nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug. Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Hartlieb, K.J.; Saunders, M.; Jachuck, R.J.J.; Raston, C. Continuous flow synthesis of small silver nanoparticles involving hydrogen as the reducing agent. Green Chem. 2010, 12, 1012–1017. [Google Scholar] [CrossRef]
- Agunloye, E.; Panariello, L.; Gavriilidis, A.; Mazzei, L. A model for the formation of gold nanoparticles in the citrate synthesis method. Chem. Eng. Sci. 2018, 191, 318–331. [Google Scholar] [CrossRef]
- Sun, K.; Qiu, J.; Liu, J.; Miao, Y. Preparation and characterization of gold nanoparticles using ascorbic acid as reducing agent in reverse micelles. J. Mater. Sci. 2009, 44, 754–758. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Kalla, D.; Uppuluri, K.B.; Anbazhagan, V. Green synthesis of silver and gold nanoparticles employing levan, a biopolymer from Acetobacter xylinum NCIM 2526, as a reducing agent and capping agent. Carbohydr. Polym. 2014, 112, 539–545. [Google Scholar] [CrossRef] [PubMed]
- García-Barrasa, J.; López-De-Luzuriaga, J.M.; Monge, M. Silver nanoparticles: Synthesis through chemical methods in solution and biomedical applications. Open Chem. 2011, 9, 7–19. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, S.; Gokhale, R.; Burgess, D.J. Physical stability of nanosuspensions: Investigation of the role of stabilizers on Ostwald ripening. Int. J. Pharm. 2011, 406, 145–152. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhao, D. Manipulating the Size and Dispersibility of Zerovalent Iron Nanoparticles by Use of Carboxymethyl Cellulose Stabilizers. Environ. Sci. Technol. 2007, 41, 6216–6221. [Google Scholar] [CrossRef] [PubMed]
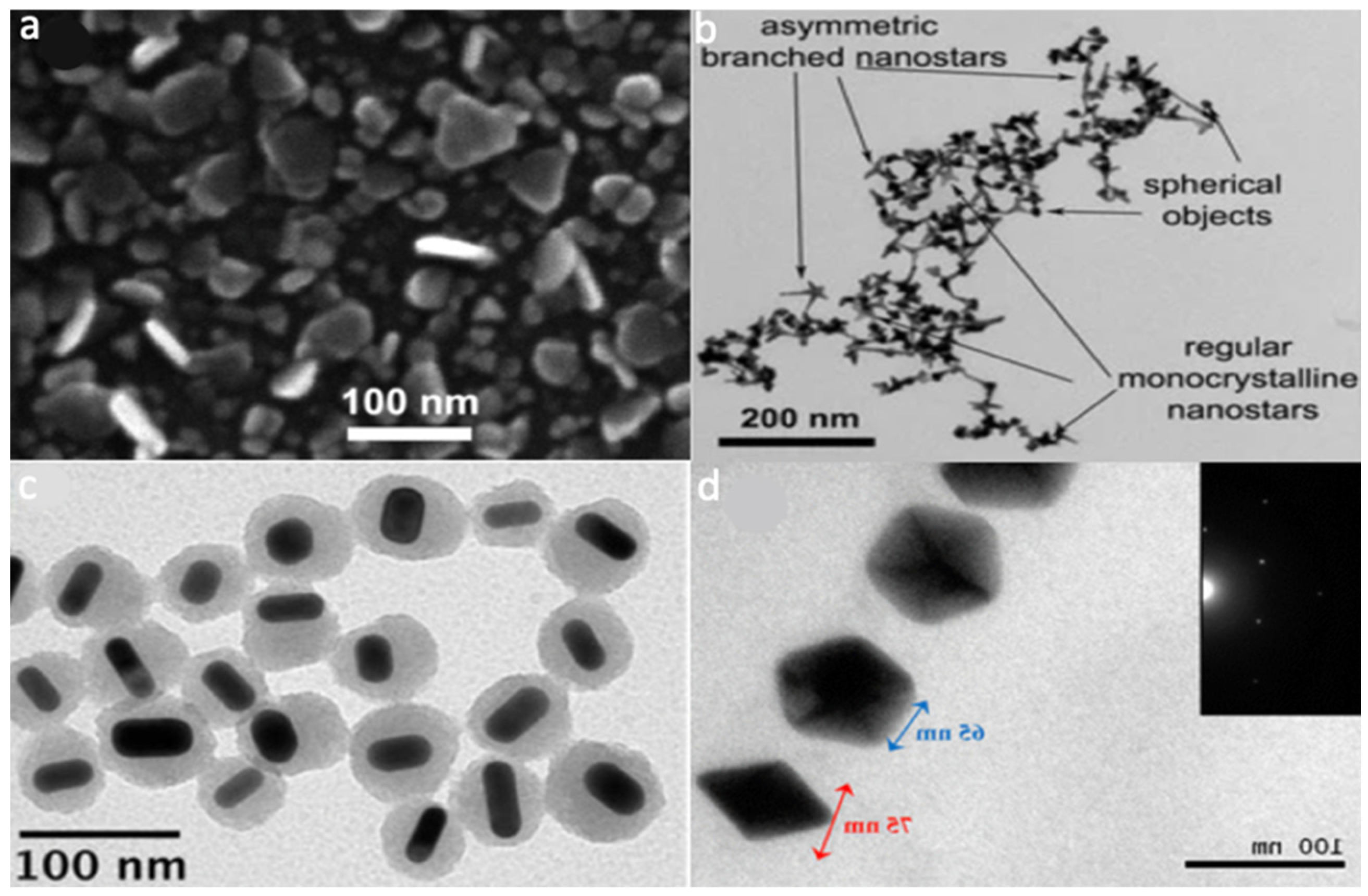
- Casu, A.; Cabrini, E.; Donà, A.; Falqui, A.; Diaz-Fernandez, Y.; Milanese, C.; Taglietti, A.; Pallavicini, P. Controlled Synthesis of Gold Nanostars by Using a Zwitterionic Surfactant. Chem.—A Eur. J. 2012, 18, 9381–9390. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, J.Y.; Wang, D.I.C.; Ting, Y.P. Silver Nanoplates: From Biological to Biomimetic Synthesis. ACS Nano 2007, 1, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, L.M.; Shifrin, Z.B. Dendrimers as Encapsulating, Stabilizing, or Directing Agents for Inorganic Nanoparticles. Chem. Rev. 2011, 111, 5301–5344. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Preciado-Flores, S.; Wheeler, D.A.; Tran, T.M.; Tanaka, Z.; Jiang, C.; Barboza-Flores, M.; Qian, F.; Li, Y.; Chen, B.; Zhang, J.Z. SERS spectroscopy and SERS imaging of Shewanella oneidensis using silver nanoparticles and nanowires. Chem. Commun. 2011, 47, 4129–4131. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Guo, S.; Goebl, J.; Yin, Y. Seeded Growth of Uniform Ag Nanoplates with High Aspect Ratio and Widely Tunable Surface Plasmon Bands. Nano Lett. 2010, 10, 5037–5042. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Kim, N.H.; Kim, M.; Lee, K.Y.; Han, S.W.H. Anisotropic Assembly of Ag Nanoprisms. J. Am. Chem. Soc. 2008, 130, 5432–5433. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, A.; Giovannozzi, A.M.; Mandrile, L.; Sacco, A.; Rossi, A.M.; Taglietti, A. In situ seed-growth synthesis of silver nanoplates on glass for the detection of food contaminats by surface enhaced Raman scattering. Talanta 2020, 216, 120936. [Google Scholar] [CrossRef] [PubMed]
- Rovati, D.; Albini, B.; Galinetto, P.; Grisoli, P.; Bassi, B.; Pallavicini, P.; Dacarro, G.; Taglietti, A. High Stability Thiol-Coated Gold Nanostars Monolayers with Photo-Thermal Antibacterial Activity and Wettability Control. Nanomaterials 2019, 9, 1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Lodeiro, A.; Djafari, J.; Fernández-Lodeiro, J.; Duarte, M.; Mauricio, E.M.; Capelo-Martínez, J.; Lodeiro, C. Synthesis of Mesoporous Silica Coated Gold Nanorods Loaded with Methylene Blue and Its Potentials in Antibacterial Applications. Nanomaterials 2021, 11, 1338. [Google Scholar] [CrossRef] [PubMed]
- Naveenraj, S.; Mangalaraja, R.V.; Wu, J.J.; Asiri, A.M.; Anandan, S. Gold Triangular Nanoprisms and Nanodecahedra: Synthesis and Interaction Studies with Luminol toward Biosensor Applications. Langmuir 2016, 32, 11854–11860. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Nachane, R.P.; Balasubramanya, R.H.; Varadarajan, P.V. A novel one-pot ‘green’ synthesis of stable silver nanoparticles using soluble starch. Carbohydr. Res. 2006, 341, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, M.; Nikkhah, V.; Sarafraz, M.M.; Shoja, S. Green synthesis of silver nanoparticles using green tea leaves: Experimental study on the morphological, rheological and antibacterial behaviour. Heat Mass Transf. 2017, 53, 3201–3209. [Google Scholar] [CrossRef]
- Daizy, P. Honey mediated green synthesis of gold nanoparticles. Spectrom. Acta Part A 2009, 73, 650–653. [Google Scholar]
- Harlepp, S.; Robert, J.; Darnton, N.C.; Chatenay, D. Subnanometric measurements of evanescent wave penetration depth using total internal reflection microscopy combined with fluorescent correlation spectroscopy. Appl. Phys. Lett. 2004, 85, 3917. [Google Scholar] [CrossRef]
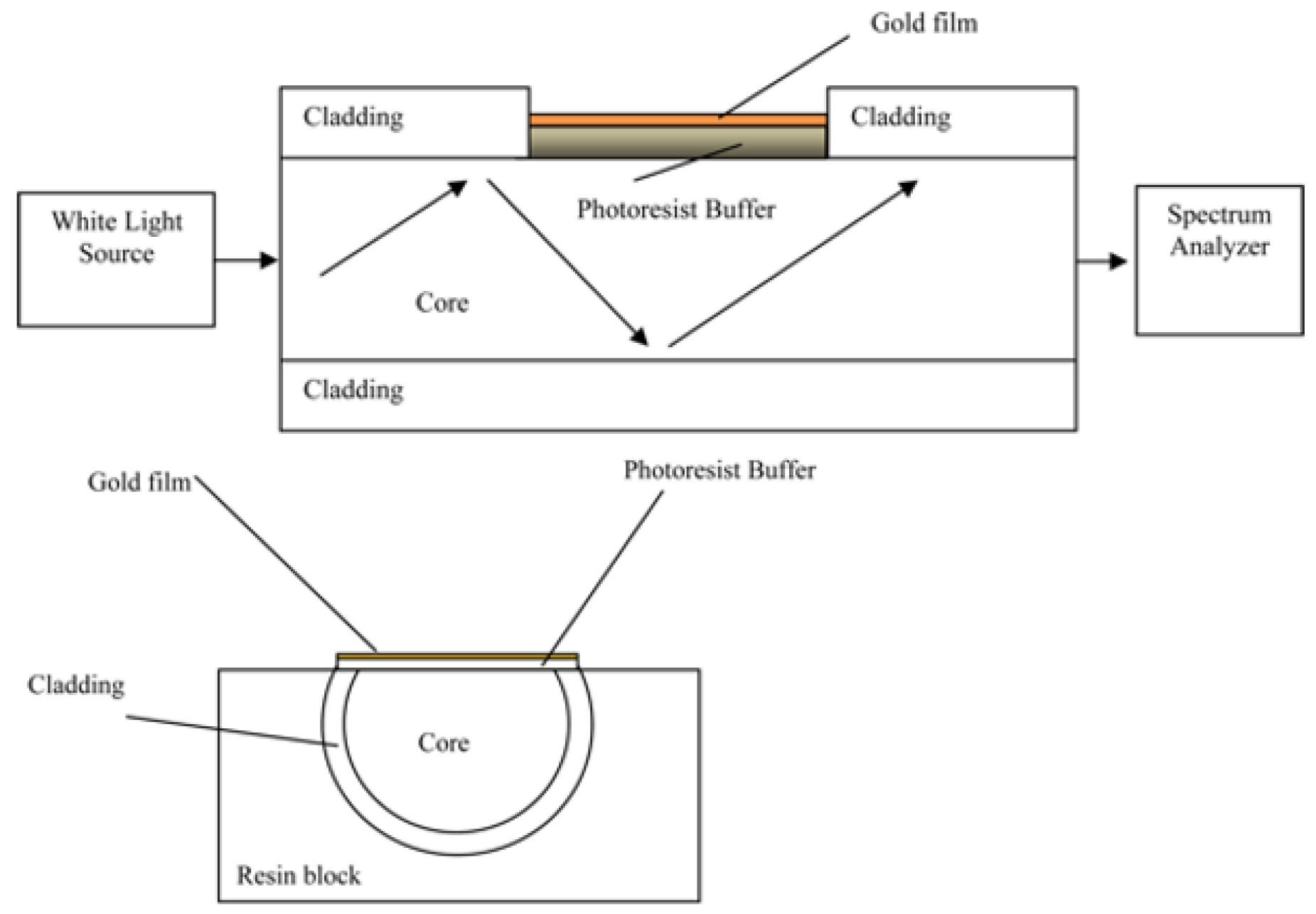
- Cennamo, N.; Pesavento, M.; Zeni, L. A review on simple and highly sensitive plastic optical fiber probes for bio-chemical sensing. Sens. Actuators B Chem. 2020, 331, 129393. [Google Scholar] [CrossRef]
- Cennamo, N.; Massarotti, D.; Conte, L.; Zeni, L. Low Cost Sensors Based on SPR in a Plastic Optical Fiber for Biosensor Implementation. Sensors 2011, 11, 11752–11760. [Google Scholar] [CrossRef]
- Dwivedi, Y.S.; Sharma, A.K.; Gupta, B.D. Influence of Design Parameters on the Performance of a Surface Plasmon Sensor Based Fiber Optic Sensor. Plasmonics 2008, 3, 79–86. [Google Scholar] [CrossRef]
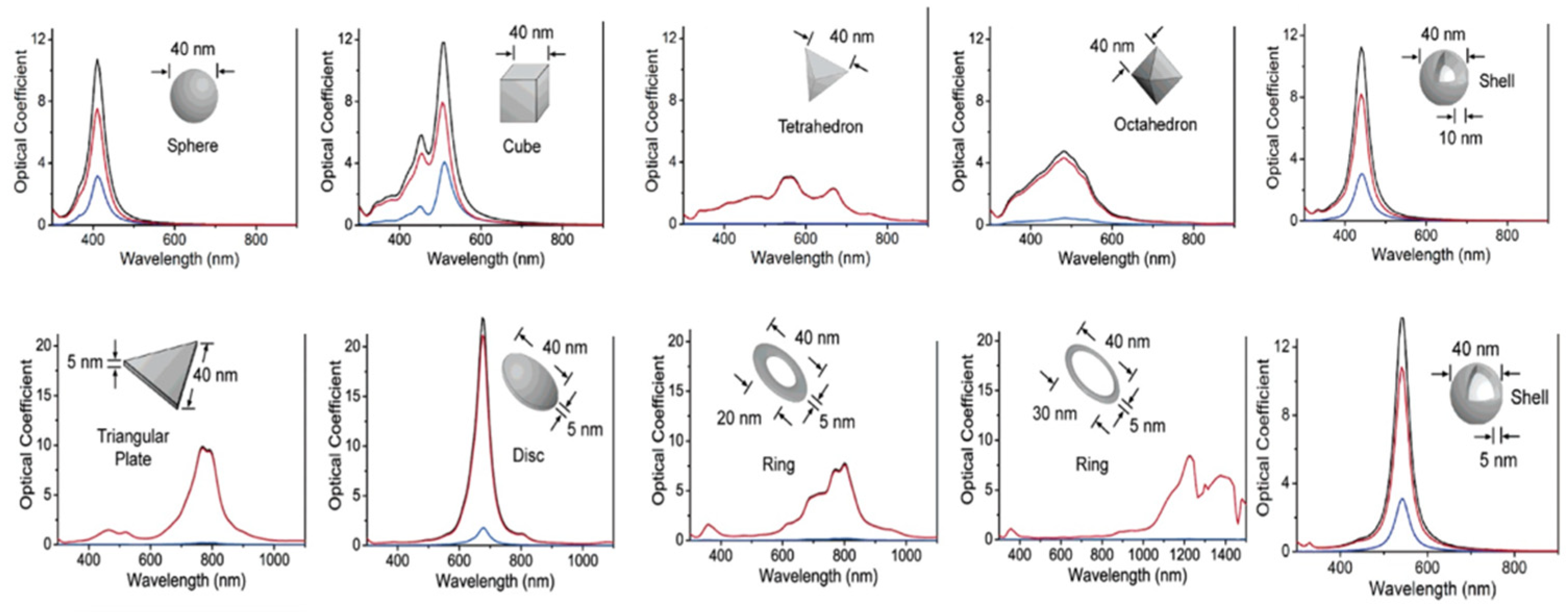
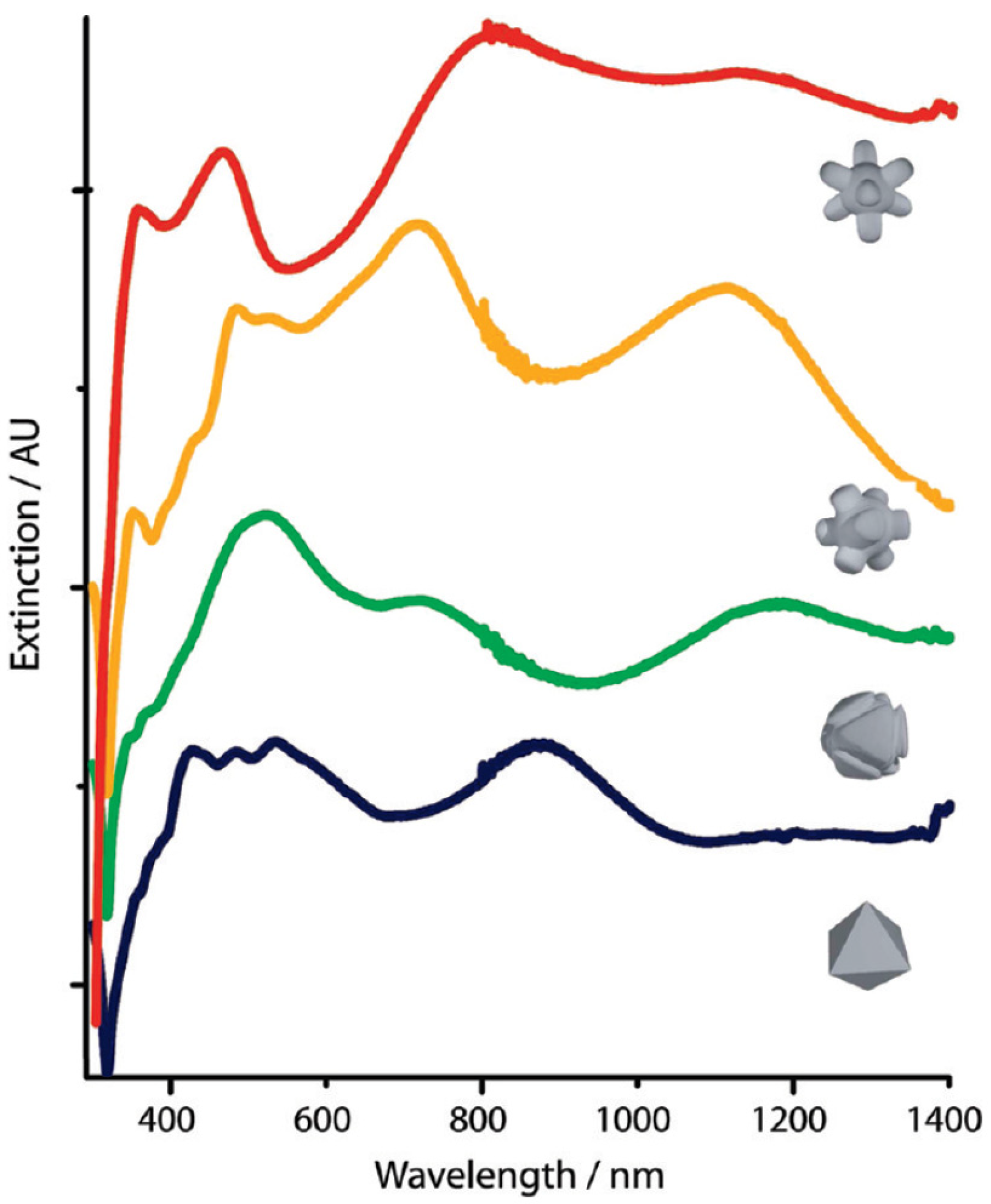
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the Synthesis and Assembly of Silver Nanostructures for Plasmonic Applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Colloidal silver nanoplates. State of the art and future challenges. J. Mater. Chem. 2008, 18, 1724–1737. [Google Scholar] [CrossRef]
- Wiley, B.J.; Im, S.H.; Li, Z.-Y.; McLellan, J.; Siekkinen, A.A.; Xia, Y. Maneuvering the Surface Plasmon Resonance of Silver Nanostructures through Shape-Controlled Synthesis. J. Phys. Chem. B 2006, 110, 15666–15675. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer: New York, NY, USA, 1995. [Google Scholar]
- Olson, J.; Dominguez-Medina, S.; Hoggard, A.; Wang, L.Y.; Chang, W.S.; Link, S. Optical Characterization of Single Plasmonic Nanoparticles. Chem. Soc. Rev. 2015, 44, 40–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amendola, V.; Meneghetti, M. Size Evaluation of Gold Nanoparticles by UV-vis Spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Jain, P.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Accounts Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications—A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, A.H.; Jose-Yacaman, M. Alfalfa Sprouts: A Natural Source for the Synthesis of Silver Nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2015, 7, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.F.; Varca, G.H.C.; Batista, J.G.D.S.; Lugão, A.B. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengani, M.; Grumezescu, A.M.; Rajeswari, V.D. Recent trends and methodologies in gold nanoparticle synthesis—A prospective review on drug delivery aspect. OpenNano 2017, 2, 37–46. [Google Scholar] [CrossRef]
- Nune, S.K.; Chanda, N.; Shukla, R.; Katti, K.; Kulkarni, R.R.; Thilakavathi, S.; Mekapothula, S.; Kannan, R.; Katti, K.V. Green Nanotechnology from Tea: Phytochemicals in Tea as Building Blocks for Production of Biocompatible Gold Nanoparticles. J. Mater. Chem. 2009, 19, 2912–2920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katti, K.K.; Chanda, N.; Shukla, R.; Zambre, A.; Suibramanian, T.; Kulkarni, R.R.; Kannan, R.; Katti, K.V. Green Nanotechnology from Cumin Phytochemicals: Generation of Biocompatible Gold Nanoparticles. Int. J. Nanotechnol. Biomed. 2009, 1, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Pinnaka, A.K.; Raje, M.; Fnu, A.; Bhattacharyya, M.S.; Choudhury, A.R. Exploitation of marine bacteria for production of gold nanoparticles. Microb. Cell Factories 2012, 11, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.; Senapati, S.; Mandal, D.; Ahmad, A.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Synthesis of Gold Nanoparticles by the Fungus Fusarium oxysporum. ChemBioChem 2002, 3, 461–463. [Google Scholar] [CrossRef]
- Vilela, D.; González, M.C.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay. A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Roto, R.; Mellisani, B.; Kuncaka, A.; Mudasir, M.; Suratman, A. Colorimetric Sensing of Pb2+ Ion by Using Ag Nanoparticles in the Presence of Dithizone. Chemosensors 2019, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Niaz, A.; Zaman, M.I.; Khan, F.A.; Nisar-Ul-Haq, M.; Tariq, M. Sensitive and selective colorimetric detection of Pb2+ by silver nanoparticles synthesized from Aconitum violaceum plant leaf extract. Mater. Res. Bull. 2018, 102, 330–336. [Google Scholar] [CrossRef]
- Shrivas, K.; Sahu, B.; Deb, M.K.; Thakur, S.S.; Sahu, S.; Kurrey, R.; Kant, T.; Patle, T.K.; Jangde, R. Colorimetric and paper-based detection of lead using PVA capped silver nanoparticles: Experimental and theoretical approach. Microchem. J. 2019, 150. [Google Scholar] [CrossRef]
- Ahmed, F.; Kabir, H.; Xiong, H. Dual Colorimetric Sensor for Hg2+/Pb2+ and an Efficient Catalyst Based on Silver Nanoparticles Mediating by the Root Extract of Bistorta amplexicaulis. Front. Chem. 2020, 8, 591958. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, D.K.; Mohan, S.; Bano, D.; Gundampati, R.K.; Hasan, S.H. Green synthesis of silver nanoparticle for the selective and sensitive colorimetric detection of mercury (II) ion. J. Photochem. Photobiol. B Biol. 2017, 168, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ding, L.; Jin, X.; Zhu, N. Silver nanoparticles capped with chalcon carboxylic acid as a probe for colorimetric determination of cadmium(II). Microchim. Acta 2017, 184, 3357–3362. [Google Scholar] [CrossRef]
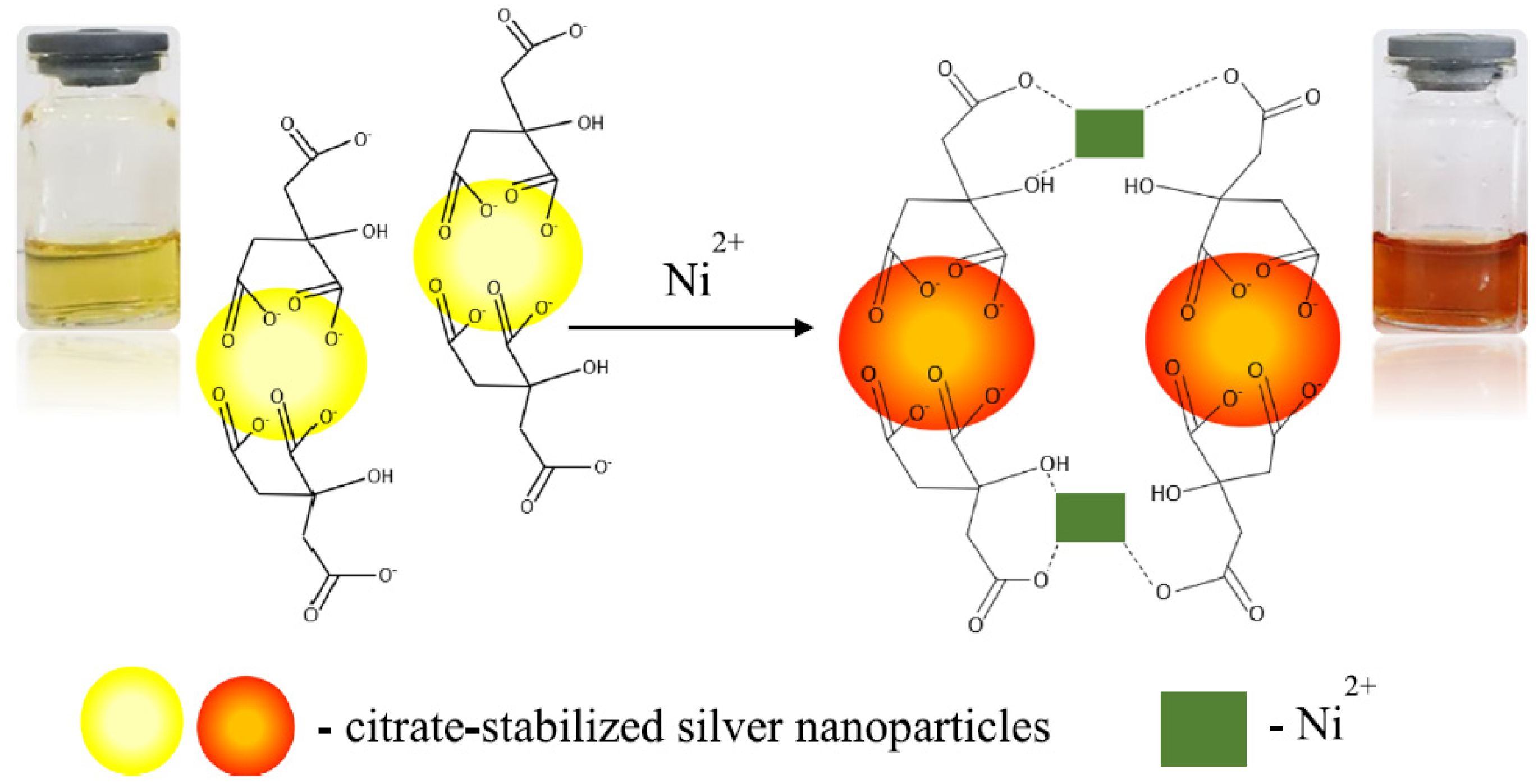
- Almaquer, F.E.P.; Ricacho, J.S.Y.; Ronquillo, R.L.G. Simple and rapid colorimetric sensing of Ni(II) ions in tap water based on aggregation of citrate-stabilized silver nanoparticles. Sustain. Environ. Res. 2019, 29, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Dhillon, A.; Kumar, D. Mentha-Stabilized Silver Nanoparticles for High-Performance Colorimetric Detection of Al(III) in Aqueous Systems. Sci. Rep. 2018, 8, 5189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastian, M.; Aravind, A.; Mathew, B. Green Silver Nanoparticles Based Multi-Technique Sensor for Environmental Hazardous Cu(II) Ion. BioNanoScience 2019, 9, 373–385. [Google Scholar] [CrossRef]
- Chavada, V.D.; Bhatt, N.M.; Sanyal, M.; Shrivastav, P.S. Dual Fluorescence-colorimetric Silver Nanoparticles Based Sensor for Determination of Olanzapine: Analysis in Rat Plasma and Pharmaceuticals. J. Fluoresc. 2020, 30, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, K.; Sasikumar, T.; Campos, C.H.; Ilanchelian, M.; Mangalaraja, R.V.; Torres, C.C. Colorimetric determination of cysteamine based on the aggregation of polyvinylpyrrolidone-stabilized silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 236, 118281. [Google Scholar] [CrossRef]
- Alula, M.T.; Karamchand, L.; Hendricks, N.R.; Blackburn, J.M. Citrate-capped silver nanoparticles as a probe for sensitive and selective colorimetric and spectrophotometric sensing of creatinine in human urine. Anal. Chim. Acta 2018, 1007, 40–49. [Google Scholar] [CrossRef]
- Balasurya, S.; Syed, A.; Thomas, A.M.; Bahkali, A.H.; Elgorban, A.M.; Raju, L.L.; Khan, S.S. Highly sensitive and selective colorimetric detection of arginine by polyvinylpyrrolidone functionalized silver nanoparticles. J. Mol. Liq. 2019, 300, 112361. [Google Scholar] [CrossRef]
- Yousefi, S.; Saraji, M. Optical aptasensor based on silver nanoparticles for the colorimetric detection of adenosine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, Z.; Shi, J.; Huang, X.; Sun, Z.; Zhang, D.; Zou, X.; Sun, Y.; Zhang, J.; Holmes, M.; et al. A colorimetric hydrogen sulfide sensor based on gellan gum-silver nanoparticles bionanocomposite for monitoring of meat spoilage in intelligent packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Heal. 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-C.; Lin, A.-Y.; Hu, C.-C.; Chiu, T.-C. Functionalized silver nanoparticles as colorimetric probes for sensing tricyclazole. Food Chem. 2021, 347, 129044. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, C.; Wang, Y.; Wei, W.; Ma, S.; Sun, X.; He, J. Green synthesis of carbon dots functionalized silver nanoparticles for the colorimetric detection of phoxim. Talanta 2018, 185, 309–315. [Google Scholar] [CrossRef]
- Ragam, P.N.; Mathew, B. Unmodified silver nanoparticles for dual detection of dithiocarbamate fungicide and rapid degradation of water pollutants. Int. J. Environ. Sci. Technol. 2019, 17, 1739–1752. [Google Scholar] [CrossRef]
- Motahhari, A.; Abdolmohammad-Zadeh, H.; Farhadi, K. Development of a New Fluoride Colorimetric Sensor Based on Anti-aggregation of Modified Silver Nanoparticles. Anal. Bioanal. Chem. Res. 2021, 8, 79–89. [Google Scholar] [CrossRef]
- Chavada, V.D.; Bhatt, N.M.; Sanyal, M.; Shrivastav, P.S. Pyrophosphate functionalized silver nanoparticles for colorimetric determination of deferiprone via competitive binding to Fe(III). Microchim. Acta 2017, 184, 4203–4208. [Google Scholar] [CrossRef]
- Zheng, L.; Qi, P.; Zhang, D. A simple, rapid and cost-effective colorimetric assay based on the 4-mercaptophenylboronic acid functionalized silver nanoparticles for bacteria monitoring. Sens. Actuators B Chem. 2018, 260, 983–989. [Google Scholar] [CrossRef]
- Mulvihill, M.; Ling, X.Y.; Henzie, J.; Yang, P. Anisotropic Etching of Silver Nanoparticles for Plasmonic Structures Capable of Single-Particle SERS. J. Am. Chem. Soc. 2009, 132, 268–274. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Chen, Z. Colorimetric determination of uric acid based on the suppression of oxidative etching of silver nanoparticles by chloroauric acid. Microchim. Acta 2019, 187, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, L.; Zeng, J.; Tan, J.; Long, Y.; Wang, Y. Colorimetric captopril assay based on oxidative etching-directed morphology control of silver nanoprisms. Microchim. Acta 2020, 187, 107. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Xiong, Q.; Zhang, G.; Xiong, Z.; Liu, D.; Duan, H.; Lai, W. Silver nanoprism-based plasmonic ELISA for sensitive detection of fluoroquinolones. J. Mater. Chem. B 2020, 8, 3667–3675. [Google Scholar] [CrossRef]
- Tran, H.V.; Nguyen, T.V.; Nguyen, L.T.; Hoang, H.S.; Huynh, C.D. Silver nanoparticles as a bifunctional probe for label-free and reagentless colorimetric hydrogen peroxide chemosensor and cholesterol biosensor. J. Sci. Adv. Mater. Devices 2020, 5. [Google Scholar] [CrossRef]
- Fang, X.; Ren, H.; Zhao, H.; Li, Z. Ultrasensitive visual and colorimetric determination of dopamine based on the prevention of etching of silver nanoprisms by chloride. Microchim. Acta 2016, 184, 415–421. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Zhao, Y.; Chen, Z. Colorimetric detection of Hg(II) by measurement the color alterations from the “before” and “after” RGB images of etched triangular silver nanoplates. Microchim. Acta 2018, 185, 235. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lee, S.M.; Kim, H.Y.; Kim, S.; Park, S.; Park, K.S.; Park, H.G. Colorimetric detection of individual biothiols by tailor made reactions with silver nanoprisms. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Afsharipour, R.; Shabani, A.M.H.; Dadfarnia, S.; Kazemi, E. Selective fluorometric determination of sulfadiazine based on the growth of silver nanoparticles on graphene quantum dots. Microchim. Acta 2019, 187, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.M.; Moon, B.-S.; Kim, D.-H. Selective and sensitive colorimetric detection of p-aminophenol in human urine and paracetamol drugs based on seed-mediated growth of silver nanoparticles. Environ. Technol. Innov. 2021, 22, 101517. [Google Scholar] [CrossRef]
- Megarajan, S.; Veerappan, A. A selective pink-to-purple colorimetric sensor for aluminium via the aggregation of gold nanoparticles. Opt. Mater. 2020, 108, 110177. [Google Scholar] [CrossRef]
- Yu, L.; Song, Z.; Peng, J.; Yang, M.; Zhi, H.; He, H. Progress of gold nanomaterials for colorimetric sensing based on different strategies. TrAC Trends Anal. Chem. 2020, 127, 115880. [Google Scholar] [CrossRef]
- Kalluri, J.R.; Arbneshi, T.; Afrin Khan, S.; Neely, A.; Candice, P.; Varisli, B.; Washington, M.; McAfee, S.; Robinson, B.; Banerjee, S.; et al. Use of gold nanoparticles in a simple colorimetric and ultrasensitivedynamic light scattering assay: Selective detection of arsenic in groundwater. Angew. Chem. Int. Ed. 2009, 48, 1–5. [Google Scholar] [CrossRef]
- Rawat, K.A.; Kailasa, S.K. 4-Amino nicotinic acid mediated synthesis of gold nanoparticles for visual detection of arginine, histidine, methionine and tryptophan. Sens. Actuators B Chem. 2016, 222, 780–789. [Google Scholar] [CrossRef]
- Song, J.; Li, Z.; Cheng, Y.; Liu, C. Self-aggregation of oligonucleotide-functionalized gold nanoparticles and its applications for highly sensitive detection of DNA. Chem. Commun. 2010, 46, 5548–5550. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, C.; Ma, H.; Zhou, T.; Jiang, B.; Chen, M.; Chen, X. A non-aggregation spectrometric determination for mercury ions based on gold nanoparticles and thiocyanuric acid. Talanta 2015, 134, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-C.; Gao, N.; Li, J.-F.; Wu, F.-Y. Colorimetric detection of methionine based on anti-aggregation of gold nanoparticles in the presence of melamine. Sens. Actuators B Chem. 2018, 255, 2779–2784. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Z.; Wang, S.; Qu, C.; Chen, L.; Wang, Z. Colorimetric sensing of copper(II) based on catalytic etching of gold nanoparticles. Talanta 2013, 112, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nam, Y.-S.; Choi, S.-H.; Lee, Y.; Lee, K.-B. Highly sensitive photometric determination of cyanide based on selective etching of gold nanorods. Microchim. Acta 2016, 183, 3035–3041. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Guan, M.; Zhu, S.; Yan, X.; Lei, Y.; Shen, X.; Luo, L.; He, H. A multicolor colorimetric assay for sensitive detection of sulfide ions based on anti-etching of triangular gold nanoplates. Microchem. J. 2020, 159, 105429. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, R.; Wang, Y.; Zhu, S.; Yan, X.; Lei, Y.; Sun, Y.; He, H.; Luo, L. Sequential colorimetric sensing of cupric and mercuric ions by regulating the etching process of triangular gold nanoplates. Microchim. Acta 2020, 187, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Li, Z.; Song, Z.; Chen, H.; Guo, L.; Fu, F.; Wu, Z. Visual and colorimetric detection of p-aminophenol in environmental water and human urine samples based on anisotropic growth of Ag nanoshells on Au nanorods. Talanta 2016, 148, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, P.; Mao, X.; Fu, W.; Liu, C. Seed-mediated growth of bimetallic nanoparticles as an effective strategy for sensitive detection of vitamin C. Sens. Actuators B Chem. 2016, 231, 95–101. [Google Scholar] [CrossRef]
- Jafarinejad, S.; Ghazi-Khansari, M.; Ghasemi, F.; Sasanpour, P.; Hormozi-Nezhad, M.R. Colorimetric Fingerprints of Gold Nanorods for Discriminating Catecholamine Neurotransmitters in Urine Samples. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zeng, Y.; Fu, W.; Zhang, P.; Li, L.; Ye, C.; Yu, L.; Zhu, X.; Zhao, S. Seed-mediated growth of Au@Ag core-shell nanorods for the detection of ellagic acid in whitening cosmetics. Anal. Chim. Acta 2018, 1002, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kuai, L.; Qin, Q.; Geng, B. Ag–Au bimetallic nanostructures: Co-reduction synthesis and their component-dependent performance for enzyme-free H2O2 sensing. J. Mater. Chem. A 2013, 1, 7111–7117. [Google Scholar] [CrossRef]
- Tokonami, S.; Morita, N.; Takasaki, K.; Toshima, N. Novel Synthesis, Structure, and Oxidation Catalysis of Ag/Au Bimetallic Nanoparticles. J. Phys. Chem. C 2010, 114, 10336–10341. [Google Scholar] [CrossRef]
- Huang, H.; Li, H.; Feng, J.-J.; Wang, A.-J. One-step green synthesis of fluorescent bimetallic Au/Ag nanoclusters for temperature sensing and in vitro detection of Fe3+. Sens. Actuators B Chem. 2015, 223, 550–556. [Google Scholar] [CrossRef]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-Based Plasmonic Nanoparticles for and Their Use in Biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Okuni, J.; Toshima, N. One-pot synthesis of Ag–Au bimetallic nanoparticles with Au shell and their high catalytic activity for aerobic glucose oxidation. J. Colloid Interface Sci. 2011, 354, 131–138. [Google Scholar] [CrossRef]
- Du, J.; Hu, X.; Zhang, G.; Wu, X.; Gong, D. Colorimetric detection of cadmium in water using L-cysteine Functionalized gold–silver nanoparticles. Anal. Lett. 2018, 51, 2906–2919. [Google Scholar] [CrossRef]
- Hu, X.; Du, J.; Pan, J.; Wang, F.; Gong, D. Zhang, G. Colorimetric detection of the β-agonist ractopamine in animal feed, tissue and urine samples using gold–silver alloy nanoparticles modified with sulfanilic acid. Food Addit. Contam. Part. A 2019, 36, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hu, Z.; Wu, J.; Ning, Z.; Jian, J.; Zhao, T.; Liang, X.; Yang, X.; Yang, Z.; Zhao, Q.; et al. Size-tunable Au@Ag nanoparticles for colorimetric and SERS dual-mode sensing of palmatine in traditional Chinese medicine. J. Pharm. Biomed. Anal. 2019, 174, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Bi, N.; Zhang, Y.; Xi, Y.; Hu, M.; Song, W.; Xu, J.; Jia, L. Colorimetric response of lysine-caped gold/silver alloy nanocomposites for mercury(II) ion detection. Colloids Surf. B Biointerfaces 2021, 205, 111846. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, B.-Z.; Qi, Y.; Li, J.-J.; Li, X.; Zhao, J.-W. Colorimetric determination of Hg(II) by combining the etching and aggregation effect of cysteine-modified Au-Ag core-shell nanorods. Sens. Actuators B Chem. 2018, 255, 2927–2935. [Google Scholar] [CrossRef]
- George, J.M.; Priyanka, R.N.; Mathew, B. Bimetallic Ag–Au nanoparticles as pH dependent dual sensing probe for Mn(II) ion and ciprofloxacin. Microchem. J. 2020, 155, 104686. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zoua, C.; Zhouab, J.; Yanga, M.; Zhangab, S.; Huoa, D.; Houac, C. Colorimetric detection of Cr6+ ions based on surface plasma resonance using the catalytic etching of gold nano-double cone @ silver nanorods. Anal. Chim. Acta 2020, 1149, 238141. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Zhang, Y.; Zhou, X.; Hu, Z.; Liao, X.; Sheng, B.; Yuan, K.; Wu, X.; Cai, H.; et al. Colorimetric and SERS dual-mode sensing of mercury (II) based on controllable etching of Au@Ag core/shell nanoparticles. Sens. Actuators B Chem. 2020, 330, 129364. [Google Scholar] [CrossRef]
- Chen, J.-K.; Zhao, S.-M.; Zhu, J.; Li, J.-J.; Zhao, J.-W. Colorimetric determination and recycling of Hg2+ based on etching-induced morphology transformation from hollow AuAg nanocages to nanoboxes. J. Alloys Compd. 2020, 828, 154392. [Google Scholar] [CrossRef]
- He, H.; Xu, X.; Wu, H.; Jin, Y. Enzymatic Plasmonic Engineering of Ag/Au Bimetallic Nanoshells and Their Use for Sensitive Optical Glucose Sensing. Adv. Mater. 2012, 24, 1736–1740. [Google Scholar] [CrossRef]
- Liu, A.; Li, M.; Wang, J.; Feng, F.; Zhang, Y.; Qiu, Z.; Chen, Y.; Meteku, B.E.; Wen, C.; Yan, Z.; et al. Ag@Au core/shell triangular nanoplates with dual enzyme-like properties for the colorimetric sensing of glucose. Chin. Chem. Lett. 2020, 31, 1133–1136. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, J.; Li, J.; Ju, H. A plasmonic colorimetric strategy for biosensing through enzyme guided growth of silver nanoparticles on gold nanostars. Biosens. Bioelectron. 2015, 78, 267–273. [Google Scholar] [CrossRef]
- Lin, T.; Wu, Y.; Li, Z.; Song, Z.; Guo, L.; Fu, F. Visual Monitoring of Food Spoilage Based on Hydrolysis-Induced Silver Metallization of Au Nanorods. Anal. Chem. 2016, 88, 11022–11027. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Monisha; Kant, T.; Karbhal, I.; Kurrey, R.; Sahu, B.; Sinha, D.; Patra, G.K.; Deb, M.K.; Pervez, S. Smartphone coupled with paper-based chemical sensor for on-site determination of iron(III) in environmental and biological samples. Anal. Bioanal. Chem. 2020, 412, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Shariati, S.; Khayatian, G. The colorimetric and microfluidic paper-based detection of cysteine and homocysteine using 1,5-diphenylcarbazide-capped silver nanoparticles. RSC Adv. 2021, 11, 3295–3303. [Google Scholar] [CrossRef]
- Shrivas, K.; Patel, S.; Sinha, D.; Thakur, S.S.; Patle, T.K.; Kant, T.; Dewangan, K.; Satnami, M.L.; Nirmalkar, J.; Kumar, S. Colorimetric and smartphone-integrated paper device for on-site determination of arsenic (III) using sucrose modified gold nanoparticles as a nanoprobe. Microchim. Acta 2020, 187, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Monisha; Shrivas, K.; Kant, T.; Patel, S.; Devi, R.; Dahariya, N.S.; Pervez, S.; Deb, M.K.; Rai, M.K.; Rai, J. Inkjet-printed paper-based colorimetric sensor coupled with smartphone for determination of mercury (Hg2+). J. Hazard. Mater. 2021, 414, 125440. [Google Scholar] [CrossRef] [PubMed]
- Yakoh, A.; Rattanarat, P.; Siangproh, W.; Chailapakul, O. Simple and selective paper-based colorimetric sensor for determination of chloride ion in environmental samples using label-free silver nanoprisms. Talanta 2018, 178, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, S.; Zhuo, S.; Ji, Y.; Li, R. A carbon dots functionalized paper coupled with AgNPs composites platform: Application as a sensor for hydrogen peroxide detection based on surface plasmon-enhanced energy transfer. New J. Chem. 2021. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, H.; Wang, X. Functionalized gold nanomaterials as biomimetic nanozymes and biosensing actuators. TrAC Trends Anal. Chem. 2021, 143, 116376. [Google Scholar] [CrossRef]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2013, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Dai, H.; Wang, Y.; Sun, Y.; Shi, Y.; Hu, J.; Li, Z. Visual detection of melamine based on the peroxidase-like activity enhancement of bare gold nanoparticles. Biosens. Bioelectron. 2014, 60, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.-X.; Wu, X.-M.; Dong, Y.-M.; Li, Z.-J.; Wang, G.-L. Colorimetric determination of melamine based on the reversal of the mercury(II) induced inhibition of the light-triggered oxidase-like activity of gold nanoclusters. Microchim. Acta 2015, 183, 441–448. [Google Scholar] [CrossRef]
- He, W.; Zhou, Y.-T.; Wamer, W.G.; Hu, X.; Wu, X.; Zheng, Z.; Boudreau, M.D.; Yin, J.-J. Intrinsic catalytic activity of Au nanoparticles with respect to hydrogen peroxide decomposition and superoxide scavenging. Biomaterials 2012, 34, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.-W.; Chen, Y.-C.; Chang, H.-T.; Huang, C.-C. Logical regulation of the enzyme-like activity of gold nanoparticles by using heavy metal ions. Nanoscale 2013, 5, 8227–8234. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ye, K.; Jia, Z.; Xue, T.; Nie, A.; Xiang, J.; Mu, C.; Wang, B.; Wen, F.; Zhai, K.; et al. High-sensitivity and versatile plasmonic biosensor based on grain boundaries in polycrystalline 1L WS2 films. Biosens. Bioelectron. 2021, 194, 113596. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sun, T.; Liu, L.; Xia, N.; Zhao, Y.; Yi, X. Surface plasmon resonance biosensor for the detection of miRNAs by combining the advantages of homogeneous reaction and heterogeneous detection. Talanta 2021, 234, 122622. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.L.; de Lima, L.F.; Moraes, A.S.; Rubira, R.J.; Constantino, C.J.; Leite, F.L.; Delgado-Silva, A.O.; Ferreira, M. Development of a novel biosensor for Creatine Kinase (CK-MB) using Surface Plasmon Resonance (SPR). Appl. Surf. Sci. 2021, 554, 149565. [Google Scholar] [CrossRef]
- Bereli, N.; Bakhshpour, M.; Topçu, A.A.; Denizli, A. Surface Plasmon Resonance-Based Immunosensor for Igm Detection with Gold Nanoparticles. Micromachines 2021, 12, 1092. [Google Scholar] [CrossRef] [PubMed]
- Huong, V.T.; Phuong, N.T.T.; Tai, N.T.; An, N.T.; Lam, V.D.; Manh, D.H.; Chi, T.T.K.; Mai, N.X.D.; Phung, V.-D.; Tran, N.H.T. Gold Nanoparticles Modified a Multimode Clad-Free Fiber for Ultrasensitive Detection of Bovine Serum Albumin. J. Nanomater. 2021, 2021, 1–6. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Gold and Silver Nanoparticle-Based Colorimetric Sensors: New Trends and Applications. Chemosensors 2021, 9, 305. https://doi.org/10.3390/chemosensors9110305
Alberti G, Zanoni C, Magnaghi LR, Biesuz R. Gold and Silver Nanoparticle-Based Colorimetric Sensors: New Trends and Applications. Chemosensors. 2021; 9(11):305. https://doi.org/10.3390/chemosensors9110305
Chicago/Turabian StyleAlberti, Giancarla, Camilla Zanoni, Lisa Rita Magnaghi, and Raffaela Biesuz. 2021. "Gold and Silver Nanoparticle-Based Colorimetric Sensors: New Trends and Applications" Chemosensors 9, no. 11: 305. https://doi.org/10.3390/chemosensors9110305
APA StyleAlberti, G., Zanoni, C., Magnaghi, L. R., & Biesuz, R. (2021). Gold and Silver Nanoparticle-Based Colorimetric Sensors: New Trends and Applications. Chemosensors, 9(11), 305. https://doi.org/10.3390/chemosensors9110305








