Group 4 Metal-Based Metal—Organic Frameworks for Chemical Sensors
Abstract
:1. Introduction
2. Synthesis and Characterizations of Group 4 Metal-Based MOFs
3. Chemical Stabilities of Group 4 Metal-Based MOFs toward the Use in Chemosensors
4. Applications of Group 4 Metal-Based MOFs for Chemosensors
4.1. Optical Sensors
4.1.1. Zr-MOFs for Optical Ion Sensors
4.1.2. Zr-MOFs for Optical Sensors toward Organic Pollutants
4.1.3. Zr-MOFs for Optical Sensors toward Biomolecules
4.1.4. Zr-MOFs for Optical Sensors toward Inorganic Molecules
4.1.5. Zr-MOFs for Optical pH Sensors
4.1.6. Hf-MOFs for Optical Sensors
4.1.7. Ti-MOFs for Optical Sensors
4.1.8. Ce-MOFs for Optical Sensors
4.2. Electrochemical Sensors
4.2.1. Charge Transport in Group 4 Metal-Based MOFs
4.2.2. Progress in the Use of Group 4 Metal-Based MOFs for Electrochemical Sensors
4.3. Chemiresistive Sensors and Others
5. Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, P.; Li, G.; Liao, C.; Jiang, G. Applications of multifunctional zirconium-based metal-organic frameworks in analytical chemistry: Overview and perspectives. TrAC Trends Anal. Chem. 2020, 131, 116015. [Google Scholar] [CrossRef]
- Olorunyomi, J.F.; Geh, S.T.; Caruso, R.A.; Doherty, C.M. Metal–organic frameworks for chemical sensing devices. Mater. Horiz. 2021, 8, 2387–2419. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.J.; Dincă, M.; Long, J.R. Hydrogen storage in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Herm, Z.R.; Bloch, E.D.; Long, J.R. Hydrocarbon Separations in Metal–Organic Frameworks. Chem. Mater. 2014, 26, 323–338. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal–organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef] [PubMed]
- Konnerth, H.; Matsagar, B.M.; Chen, S.S.; Prechtl, M.H.G.; Shieh, F.-K.; Wu, K.C.W. Metal-organic framework (MOF)-derived catalysts for fine chemical production. Coord. Chem. Rev. 2020, 416, 213319. [Google Scholar] [CrossRef]
- Torad, N.L.; Li, Y.; Ishihara, S.; Ariga, K.; Kamachi, Y.; Lian, H.-Y.; Hamoudi, H.; Sakka, Y.; Chaikittisilp, W.; Wu, K.C.W.; et al. MOF-derived Nanoporous Carbon as Intracellular Drug Delivery Carriers. Chem. Lett. 2014, 43, 717–719. [Google Scholar] [CrossRef]
- Wu, M.X.; Yang, Y.W. Metal–organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Choi, K.M.; Jeong, H.M.; Park, J.H.; Zhang, Y.-B.; Kang, J.K.; Yaghi, O.M. Supercapacitors of Nanocrystalline Metal–Organic Frameworks. ACS Nano 2014, 8, 7451–7457. [Google Scholar] [CrossRef] [PubMed]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.-J.; Shao-Horn, Y.; Dincă, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2016, 16, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kaneti, Y.V.; Bando, Y.; Lin, J.; Liu, C.; Li, J.; Yamauchi, Y. Metal–organic framework-derived one-dimensional porous or hollow carbon-based nanofibers for energy storage and conversion. Mater. Horiz. 2018, 5, 394–407. [Google Scholar] [CrossRef] [Green Version]
- Majewski, M.B.; Peters, A.W.; Wasielewski, M.R.; Hupp, J.T.; Farha, O.K. Metal–Organic Frameworks as Platform Materials for Solar Fuels Catalysis. ACS Energy Lett. 2018, 3, 598–611. [Google Scholar] [CrossRef]
- Li, J.-H.; Wang, Y.-S.; Chen, Y.-C.; Kung, C.-W. Metal–Organic Frameworks Toward Electrocatalytic Applications. Appl. Sci. 2019, 9, 2427. [Google Scholar] [CrossRef] [Green Version]
- Stassen, I.; Burtch, N.; Talin, A.; Falcaro, P.; Allendorf, M.; Ameloot, R. An updated roadmap for the integration of metal-organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017, 46, 3185–3241. [Google Scholar] [CrossRef]
- Calvo, J.J.; Angel, S.M.; So, M.C. Charge transport in metal–organic frameworks for electronics applications. APL Mater. 2020, 8, 050901. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic Methods for the Functionalization of Metal–Organic Frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef]
- Islamoglu, T.; Goswami, S.; Li, Z.; Howarth, A.J.; Farha, O.K.; Hupp, J.T. Postsynthetic Tuning of Metal–Organic Frameworks for Targeted Applications. Acc. Chem. Res. 2017, 50, 805–813. [Google Scholar] [CrossRef]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water Stability and Adsorption in Metal–Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Yuan, S.; Qin, J.-S.; Lollar, C.T.; Zhou, H.-C. Stable Metal–Organic Frameworks with Group 4 Metals: Current Status and Trends. ACS Cent. Sci. 2018, 4, 440–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Wang, Y.; Zhao, D. The chemistry and applications of hafnium and cerium(IV) metal–organic frameworks. Chem. Soc. Rev. 2021, 50, 4629–4683. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Chen, Z.; Hanna, S.L.; Redfern, L.R.; Alezi, D.; Islamoglu, T.; Farha, O.K. Reticular chemistry in the rational synthesis of functional zirconium cluster-based MOFs. Coord. Chem. Rev. 2019, 386, 32–49. [Google Scholar] [CrossRef]
- Bon, V.; Senkovska, I.; Baburin, I.A.; Kaskel, S. Zr- and Hf-Based Metal–Organic Frameworks: Tracking Down the Polymorphism. Cryst. Growth Des. 2013, 13, 1231–1237. [Google Scholar] [CrossRef]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Férey, G. A New Photoactive Crystalline Highly Porous Titanium(IV) Dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef]
- Lammert, M.; Wharmby, M.T.; Smolders, S.; Bueken, B.; Lieb, A.; Lomachenko, K.A.; Vos, D.D.; Stock, N. Cerium-based metal organic frameworks with UiO-66 architecture: Synthesis, properties and redox catalytic activity. Chem. Commun. 2015, 51, 12578–12581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, J.; Ienco, A.; D’Amato, R.; Costantino, F.; Stock, N. The chemistry of Ce-based metal–organic frameworks. Dalton Trans. 2020, 49, 16551–16586. [Google Scholar] [CrossRef] [PubMed]
- Lammert, M.; Glißmann, C.; Reinsch, H.; Stock, N. Synthesis and Characterization of New Ce(IV)-MOFs Exhibiting Various Framework Topologies. Cryst. Growth Des. 2017, 17, 1125–1131. [Google Scholar] [CrossRef]
- Waitschat, S.; Fröhlich, D.; Reinsch, H.; Terraschke, H.; Lomachenko, K.A.; Lamberti, C.; Kummer, H.; Helling, T.; Baumgartner, M.; Henninger, S.; et al. Synthesis of M-UiO-66 (M = Zr, Ce or Hf) employing 2,5-pyridinedicarboxylic acid as a linker: Defect chemistry, framework hydrophilisation and sorption properties. Dalton Trans. 2018, 47, 1062–1070. [Google Scholar] [CrossRef]
- Dodson, R.A.; Wong-Foy, A.G.; Matzger, A.J. The Metal–Organic Framework Collapse Continuum: Insights from Two-Dimensional Powder X-ray Diffraction. Chem. Mater. 2018, 30, 6559–6565. [Google Scholar] [CrossRef]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best Practices for the Synthesis, Activation, and Characterization of Metal–Organic Frameworks. Chem. Mater. 2017, 29, 26–39. [Google Scholar] [CrossRef]
- Düren, T.; Millange, F.; Férey, G.; Walton, K.S.; Snurr, R.Q. Calculating Geometric Surface Areas as a Characterization Tool for Metal−Organic Frameworks. J. Phys. Chem. C 2007, 111, 15350–15356. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, M.; Lee, L.Y.S. Electrochemical Instability of Metal–Organic Frameworks: In Situ Spectroelectrochemical Investigation of the Real Active Sites. ACS Catal. 2020, 10, 81–92. [Google Scholar] [CrossRef]
- Miles, D.O.; Jiang, D.; Burrows, A.D.; Halls, J.E.; Marken, F. Conformal transformation of [Co(bdc)(DMF)] (Co-MOF-71, bdc = 1,4-benzenedicarboxylate, DMF = N,N-dimethylformamide) into porous electrochemically active cobalt hydroxide. Electrochem. Commun. 2013, 27, 9–13. [Google Scholar] [CrossRef]
- Qu, C.; Jiao, Y.; Zhao, B.; Chen, D.; Zou, R.; Walton, K.S.; Liu, M. Nickel-based pillared MOFs for high-performance supercapacitors: Design, synthesis and stability study. Nano Energy 2016, 26, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Babu, K.F.; Kulandainathan, M.A.; Katsounaros, I.; Rassaei, L.; Burrows, A.D.; Raithby, P.R.; Marken, F. Electrocatalytic activity of BasoliteTM F300 metal-organic-framework structures. Electrochem. Commun. 2010, 12, 632–635. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.-B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal–Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef]
- Feng, D.; Gu, Z.-Y.; Li, J.-R.; Jiang, H.-L.; Wei, Z.; Zhou, H.-C. Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal–Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.; Volosskiy, B.; Demir, S.; Gándara, F.; McGrier, P.L.; Furukawa, H.; Cascio, D.; Stoddart, J.F.; Yaghi, O.M. Synthesis, Structure, and Metalation of Two New Highly Porous Zirconium Metal–Organic Frameworks. Inorg. Chem. 2012, 51, 6443–6445. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Bury, W.; Fairen-Jimenez, D.; Kwon, S.; DeMarco, E.J.; Weston, M.H.; Sarjeant, A.A.; Nguyen, S.T.; Stair, P.C.; Snurr, R.Q.; et al. Vapor-Phase Metalation by Atomic Layer Deposition in a Metal–Organic Framework. J. Am. Chem. Soc. 2013, 135, 10294–10297. [Google Scholar] [CrossRef] [PubMed]
- Lammert, M.; Glißmann, C.; Stock, N. Tuning the stability of bimetallic Ce(IV)/Zr(IV)-based MOFs with UiO-66 and MOF-808 structures. Dalton Trans. 2017, 46, 2425–2429. [Google Scholar] [CrossRef]
- Peters, A.W.; Li, Z.; Farha, O.K.; Hupp, J.T. Atomically Precise Growth of Catalytically Active Cobalt Sulfide on Flat Surfaces and within a Metal–Organic Framework via Atomic Layer Deposition. ACS Nano 2015, 9, 8484–8490. [Google Scholar] [CrossRef] [Green Version]
- Noh, H.; Kung, C.-W.; Otake, K.-i.; Peters, A.W.; Li, Z.; Liao, Y.; Gong, X.; Farha, O.K.; Hupp, J.T. Redox-Mediator-Assisted Electrocatalytic Hydrogen Evolution from Water by a Molybdenum Sulfide-Functionalized Metal–Organic Framework. ACS Catal. 2018, 8, 9848–9858. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Modica, J.A.; Drout, R.J.; Farha, O.K. Acid-Resistant Mesoporous Metal–Organic Framework toward Oral Insulin Delivery: Protein Encapsulation, Protection, and Release. J. Am. Chem. Soc. 2018, 140, 5678–5681. [Google Scholar] [CrossRef]
- Bůžek, D.; Adamec, S.; Lang, K.; Demel, J. Metal–organic frameworks vs. buffers: Case study of UiO-66 stability. Inorg. Chem. Front. 2021, 8, 720–734. [Google Scholar] [CrossRef]
- Lin, S.; Pineda-Galvan, Y.; Maza, W.A.; Epley, C.C.; Zhu, J.; Kessinger, M.C.; Pushkar, Y.; Morris, A.J. Electrochemical Water Oxidation by a Catalyst-Modified Metal–Organic Framework Thin Film. ChemSusChem 2017, 10, 514–522. [Google Scholar] [CrossRef]
- Li, J.-H.; Chen, Y.-C.; Wang, Y.-S.; Ho, W.H.; Gu, Y.-J.; Chuang, C.-H.; Song, Y.-D.; Kung, C.-W. Electrochemical Evolution of Pore-Confined Metallic Molybdenum in a Metal–Organic Framework (MOF) for All-MOF-Based Pseudocapacitors. ACS Appl. Energy Mater. 2020, 3, 6258–6267. [Google Scholar] [CrossRef]
- McDonagh, C.; Burke, C.S.; MacCraith, B.D. Optical Chemical Sensors. Chem. Rev. 2008, 108, 400–422. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [Green Version]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, S.; Zhou, Y.; Zhang, Y.; Xu, M. Recent Progress in Metal–Organic Framework (MOF) Based Luminescent Chemodosimeters. Nanomaterials 2019, 9, 974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, T.; Zhang, Y.-Z.; Kong, X.-J.; Yu, J.; Lv, X.-L.; Wu, Y.; Guo, Z.-J.; Li, J.-R. Zr(IV)-Based Metal-Organic Framework with T-Shaped Ligand: Unique Structure, High Stability, Selective Detection, and Rapid Adsorption of Cr2O72– in Water. ACS Appl. Mater. Interfaces 2018, 10, 16650–16659. [Google Scholar] [CrossRef]
- Zhu, S.-Y.; Yan, B. A novel covalent post-synthetically modified MOF hybrid as a sensitive and selective fluorescent probe for Al3+ detection in aqueous media. Dalton Trans. 2018, 47, 1674–1681. [Google Scholar] [CrossRef]
- Fajal, S.; Samanta, P.; Dutta, S.; Ghosh, S.K. Selective and sensitive recognition of Fe3+ ion by a Lewis basic functionalized chemically stable metal-organic framework (MOF). Inorg. Chim. Acta 2020, 502, 119359. [Google Scholar] [CrossRef]
- Hibbard, H.A.J.; Burnley, M.J.; Rubin, H.N.; Miera, J.A.; Reynolds, M.M. Porphyrin-based metal-organic framework and polyvinylchloride composites for fluorescence sensing of divalent cadmium ions in water. Inorg. Chem. Commun. 2020, 115, 107861. [Google Scholar] [CrossRef]
- Gogoi, C.; Nagarjun, N.; Roy, S.; Mostakim, S.K.; Volkmer, D.; Dhakshinamoorthy, A.; Biswas, S. A Zr-Based Metal–Organic Framework with a DUT-52 Structure Containing a Trifluoroacetamido-Functionalized Linker for Aqueous Phase Fluorescence Sensing of the Cyanide Ion and Aerobic Oxidation of Cyclohexane. Inorg. Chem. 2021, 60, 4539–4550. [Google Scholar] [CrossRef]
- Li, J.; Yuan, S.; Qin, J.-S.; Pang, J.; Zhang, P.; Zhang, Y.; Huang, Y.; Drake, H.F.; Liu, W.R.; Zhou, H.-C. Stepwise Assembly of Turn-on Fluorescence Sensors in Multicomponent Metal–Organic Frameworks for in Vitro Cyanide Detection. Angew. Chem. Int. Ed. 2020, 59, 9319–9323. [Google Scholar] [CrossRef] [PubMed]
- Kalaj, M.; Cohen, S.M. Postsynthetic Modification: An Enabling Technology for the Advancement of Metal–Organic Frameworks. ACS Cent. Sci. 2020, 6, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-Y.; Yan, B. A novel sensitive fluorescent probe of S2O82− and Fe3+ based on covalent post-functionalization of a zirconium(IV) metal–organic framework. Dalton Trans. 2018, 47, 11586–11592. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, L.; Yan, B. A novel spectroscopic probe for detecting food preservative NO2−: Citric acid functionalized metal-organic framework and luminescence sensing. Microchem. J. 2020, 155, 104768. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, T.; Jiang, K.; Cui, Y.; Yang, Y.; Qian, G. Highly sensitive and selective detection of mercury (II) based on a zirconium metal-organic framework in aqueous media. J. Solid State Chem. 2017, 253, 277–281. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Z.; Meng, F.; Su, J.; Chen, D.; Guo, Z.; Xing, H. RhB-Embedded Zirconium–Naphthalene-Based Metal–Organic Framework Composite as a Luminescent Self-Calibrating Platform for the Selective Detection of Inorganic Ions. Chem. Eur. J. 2020, 26, 1661–1667. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Chen, L.; Guo, L.; Lei, Y.; Wang, L. Stable dual-emissive fluorescin@UiO-67 metal-organic frameworks for visual and ratiometric sensing of Al3+ and ascorbic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 120068. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, Z.; Zhang, Y.; Guo, Z.; Chen, D.; Jia, P.; Chen, P.; Xing, H. Dual-Emitting EY@Zr-MOF Composite as Self-Calibrating Luminescent Sensor for Selective Detection of Inorganic Ions and Nitroaromatics. ACS Sustain. Chem. Eng. 2019, 7, 6196–6203. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, Y.; Jia, P.; Wang, Q.; Liu, Y.; Bu, T.; Zhang, M.; Bai, F.; Wang, L. Dual-Emission Zr-MOF-Based Composite Material as a Fluorescence Turn-On Sensor for the Ultrasensitive Detection of Al3+. Inorg. Chem. 2020, 59, 18205–18213. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.-L.; Liu, C.-G.; Fu, Y.; Ye, F. A built-in self-calibrating luminescence sensor based on RhB@Zr-MOF for detection of cations, nitro explosives and pesticides. RSC Adv. 2020, 10, 19149–19156. [Google Scholar] [CrossRef]
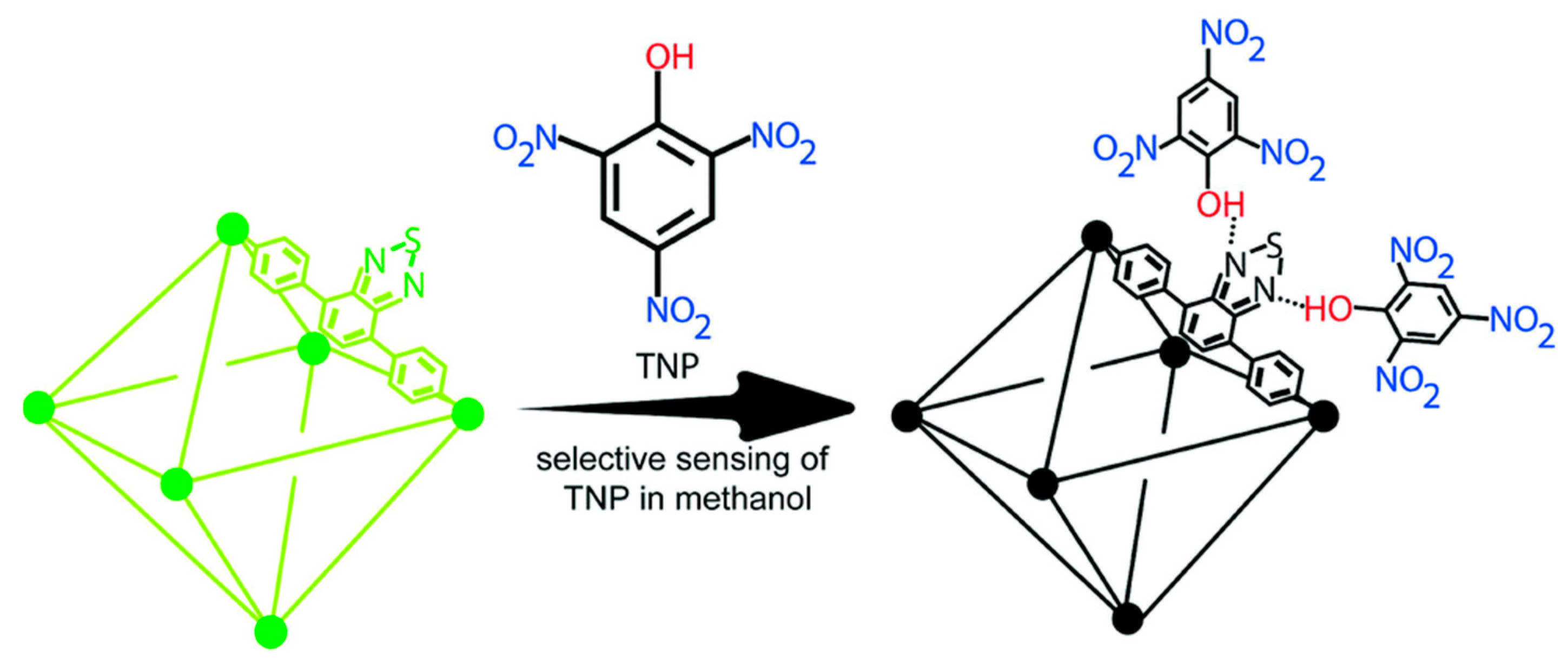
- Sk, M.; Biswas, S. A thiadiazole-functionalized Zr(IV)-based metal–organic framework as a highly fluorescent probe for the selective detection of picric acid. CrystEngComm 2016, 18, 3104–3113. [Google Scholar] [CrossRef]
- Li, Q.-Y.; Ma, Z.; Zhang, W.-Q.; Xu, J.-L.; Wei, W.; Lu, H.; Zhao, X.; Wang, X.-J. AIE-active tetraphenylethene functionalized metal–organic framework for selective detection of nitroaromatic explosives and organic photocatalysis. Chem. Commun. 2016, 52, 11284–11287. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.-L.; Feng, D.; Xie, L.-H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.-R.; Zhou, H.-C. Highly Stable Zr(IV)-Based Metal–Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef]
- Yang, J.-M.; Hu, X.-W.; Liu, Y.-X.; Zhang, W. Fabrication of a carbon quantum dots-immobilized zirconium-based metal-organic framework composite fluorescence sensor for highly sensitive detection of 4-nitrophenol. Microporous Mesoporous Mater. 2019, 274, 149–154. [Google Scholar] [CrossRef]
- Sen Bishwas, M.; Malik, M.; Poddar, P. Raman spectroscopy-based sensitive, fast and reversible vapour phase detection of explosives adsorbed on metal–organic frameworks UiO-67. N. J. Chem. 2021, 45, 7145–7153. [Google Scholar] [CrossRef]
- Ning, D.; Liu, Q.; Wang, Q.; Du, X.-M.; Li, Y.; Ruan, W.-J. Pyrene-based MOFs as fluorescent sensors for PAHs: An energetic pathway of the backbone structure effect on response. Dalton Trans. 2019, 48, 5705–5712. [Google Scholar] [CrossRef] [PubMed]
- Drache, F.; Bon, V.; Senkovska, I.; Adam, M.; Eychmüller, A.; Kaskel, S. Vapochromic Luminescence of a Zirconium-Based Metal–Organic Framework for Sensing Applications. Eur. J. Inorg. Chem. 2016, 2016, 4483–4489. [Google Scholar] [CrossRef]
- Zhang, W.-Q.; Li, Q.-Y.; Cheng, J.-Y.; Cheng, K.; Yang, X.; Li, Y.; Zhao, X.; Wang, X.-J. Ratiometric Luminescent Detection of Organic Amines Due to the Induced Lactam–Lactim Tautomerization of Organic Linker in a Metal–Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 31352–31356. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, J.; Zhu, H.; Liu, L.; Feng, Y.; Hu, G.; Yu, X. Dual-emitting fluorescence of Eu/Zr-MOF for ratiometric sensing formaldehyde. Sens. Actuators B Chem. 2017, 253, 275–282. [Google Scholar] [CrossRef]
- Rouschmeyer, P.; Guillou, N.; Serre, C.; Clavier, G.; Martineau, C.; Audebert, P.; Elkaïm, E.; Allain, C.; Devic, T. A Flexible Fluorescent Zr Carboxylate Metal–Organic Framework for the Detection of Electron-Rich Molecules in Solution. Inorg. Chem. 2017, 56, 8423–8429. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, D.; Guo, Z.; Jia, P.; Xing, H. Eosin Y-Embedded Zirconium-Based Metal–Organic Framework as a Dual-Emitting Built-In Self-Calibrating Platform for Pesticide Detection. Inorg. Chem. 2020, 59, 5386–5393. [Google Scholar] [CrossRef]
- Li, W.-J.; Chang, L.; Liu, Q.; Ning, D.; Yao, X.-Y.; Li, Y.; Ruan, W.-J. Enzyme-Assisted Metal–Organic Framework Sensing System for Diethylstilbestrol Detection. Chem. Eur. J. 2017, 23, 15498–15504. [Google Scholar] [CrossRef]
- Yang, H.; Wang, B.; Cheng, J.; Wang, R.; Zhang, S.; Dong, S.; Wei, S.; Wang, P.; Li, J.-R. Determination and removal of clenbuterol with a stable fluorescent zirconium(IV)-based metal organic framework. Microchim. Acta 2019, 186, 454. [Google Scholar] [CrossRef]
- Liu, S.; Bai, J.; Huo, Y.; Ning, B.; Peng, Y.; Li, S.; Han, D.; Kang, W.; Gao, Z. A zirconium-porphyrin MOF-based ratiometric fluorescent biosensor for rapid and ultrasensitive detection of chloramphenicol. Biosens. Bioelectron. 2020, 149, 111801. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Yan, B. Selective detection and controlled release of Aspirin over fluorescent amino-functionalized metal–organic framework in aqueous solution. Sens. Actuators B Chem. 2016, 230, 463–469. [Google Scholar] [CrossRef]
- Yang, Q.; Hong, H.; Luo, Y. Heterogeneous nucleation and synthesis of carbon dots hybrid Zr-based MOFs for simultaneous recognition and effective removal of tetracycline. Chem. Eng. J. 2020, 392, 123680. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Q.; Zhang, D.; Gan, N.; Li, Q.; Cuan, J. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sens. Actuators B Chem. 2018, 262, 137–143. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Zhang, J.-W.; Huang, G.; Du, Z.-Y.; Jiang, H.-L. An amine-functionalized metal–organic framework as a sensing platform for DNA detection. Chem. Commun. 2014, 50, 12069–12072. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.V.; Samanta, P.; Manna, B.; Ghosh, S.K. Aqueous phase nitric oxide detection by an amine-decorated metal–organic framework. Chem. Commun. 2015, 51, 6111–6114. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Xie, M.; Wang, M.; Li, Z.; Su, X. UiO-66-NH2 MOF-based ratiometric fluorescent probe for the detection of dopamine and reduced glutathione. Talanta 2020, 220, 121352. [Google Scholar] [CrossRef]
- Li, Y.-A.; Zhao, C.-W.; Zhu, N.-X.; Liu, Q.-K.; Chen, G.-J.; Liu, J.-B.; Zhao, X.-D.; Ma, J.-P.; Zhang, S.; Dong, Y.-B. Nanoscale UiO-MOF-based luminescent sensors for highly selective detection of cysteine and glutathione and their application in bioimaging. Chem. Commun. 2015, 51, 17672–17675. [Google Scholar] [CrossRef]
- Gui, B.; Meng, Y.; Xie, Y.; Tian, J.; Yu, G.; Zeng, W.; Zhang, G.; Gong, S.; Yang, C.; Zhang, D.; et al. Tuning the Photoinduced Electron Transfer in a Zr-MOF: Toward Solid-State Fluorescent Molecular Switch and Turn-On Sensor. Adv. Mater. 2018, 30, 1802329. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Yang, J.; Li, Y.; Zhuang, Q.; Gu, J. Substitution-type luminescent MOF sensor with built-in capturer for selective cholesterol detection in blood serum. J. Mater. Chem. C 2019, 7, 12674–12681. [Google Scholar] [CrossRef]
- Xia, C.; Xu, Y.; Cao, M.-M.; Liu, Y.-P.; Xia, J.-F.; Jiang, D.-Y.; Zhou, G.-H.; Xie, R.-J.; Zhang, D.-F.; Li, H.-L. A selective and sensitive fluorescent probe for bilirubin in human serum based on europium(III) post-functionalized Zr(IV)-Based MOFs. Talanta 2020, 212, 120795. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Li, G.; Liu, Q.; Xu, Y.; Liu, X. A ratiometric fluorescent probe for determination of the anthrax biomarker 2,6-pyridinedicarboxylic acid based on a terbium(III)− functionalized UIO-67 metal-organic framework. Microchim. Acta 2020, 187, 122. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, S.; Ma, J.; Shi, H.; Wang, L.; Sheng, A.; Yin, T.; Sun, L.; Li, G. Colorimetric Sensor Array for Human Semen Identification Designed by Coupling Zirconium Metal–Organic Frameworks with DNA-Modified Gold Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 36316–36323. [Google Scholar] [CrossRef]
- Nickerl, G.; Senkovska, I.; Kaskel, S. Tetrazine functionalized zirconium MOF as an optical sensor for oxidizing gases. Chem. Commun. 2015, 51, 2280–2282. [Google Scholar] [CrossRef] [Green Version]
- Nandi, S.; Banesh, S.; Trivedi, V.; Biswas, S. A dinitro-functionalized metal–organic framework featuring visual and fluorogenic sensing of H2S in living cells, human blood plasma and environmental samples. Analyst 2018, 143, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, C.; Kumar, A.; Sk, M.; Biswas, S. Specific fluorescence sensing of hydrogen sulphide by an azide functionalized Zr(IV) MOF with DUT-52 topology. Microporous Mesoporous Mater. 2021, 311, 110725. [Google Scholar] [CrossRef]
- Guo, L.; Wang, M.; Cao, D. A Novel Zr-MOF as Fluorescence Turn-On Probe for Real-Time Detecting H2S Gas and Fingerprint Identification. Small 2018, 14, 1703822. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, X.; Yan, D.; Deng, P.; Guo, Z.; Zhan, H. Europium ion post-functionalized zirconium metal–organic frameworks as luminescent probes for effectively sensing hydrazine hydrate. RSC Adv. 2018, 8, 17471–17476. [Google Scholar] [CrossRef] [Green Version]
- Sk, M.; Khan, M.R.U.Z.; Das, A.; Nandi, S.; Trivedi, V.; Biswas, S. A phthalimide-functionalized UiO-66 metal–organic framework for the fluorogenic detection of hydrazine in live cells. Dalton Trans. 2019, 48, 12615–12621. [Google Scholar] [CrossRef] [PubMed]
- Sk, M.; Banesh, S.; Trivedi, V.; Biswas, S. Selective and Sensitive Sensing of Hydrogen Peroxide by a Boronic Acid Functionalized Metal–Organic Framework and Its Application in Live-Cell Imaging. Inorg. Chem. 2018, 57, 14574–14581. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, A.; Biswas, S. A functionalized UiO-66 MOF acting as a luminescent chemosensor for selective and sensitive turn-on detection of superoxide and acetylacetone. Microporous Mesoporous Mater. 2021, 323, 111251. [Google Scholar] [CrossRef]
- Das, A.; Anbu, N.; Sk, M.; Dhakshinamoorthy, A.; Biswas, S. A functionalized UiO-66 MOF for turn-on fluorescence sensing of superoxide in water and efficient catalysis for Knoevenagel condensation. Dalton Trans. 2019, 48, 17371–17380. [Google Scholar] [CrossRef]
- Li, Q.-Y.; Li, Y.-A.; Guan, Q.; Li, W.-Y.; Dong, X.-J.; Dong, Y.-B. UiO-68-PT MOF-Based Sensor and Its Mixed Matrix Membrane for Detection of HClO in Water. Inorg. Chem. 2019, 58, 9890–9896. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Feng, D.; Wang, K.; Gu, Z.-Y.; Wei, Z.; Chen, Y.-P.; Zhou, H.-C. An Exceptionally Stable, Porphyrinic Zr Metal–Organic Framework Exhibiting pH-Dependent Fluorescence. J. Am. Chem. Soc. 2013, 135, 13934–13938. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Shan, D.; Chen, J.; Zhang, S.; Lu, X. Dual-Emitting Fluorescent Metal–Organic Framework Nanocomposites as a Broad-Range pH Sensor for Fluorescence Imaging. Anal. Chem. 2018, 90, 7056–7063. [Google Scholar] [CrossRef] [PubMed]
- Deibert, B.J.; Li, J. A distinct reversible colorimetric and fluorescent low pH response on a water-stable zirconium–porphyrin metal–organic framework. Chem. Commun. 2014, 50, 9636–9639. [Google Scholar] [CrossRef]
- Sousaraei, A.; Queirós, C.; Moscoso, F.G.; Silva, A.M.G.; Lopes-Costa, T.; Pedrosa, J.M.; Cunha-Silva, L.; Cabanillas-Gonzalez, J. Reversible Protonation of Porphyrinic Metal-Organic Frameworks Embedded in Nanoporous Polydimethylsiloxane for Colorimetric Sensing. Adv. Mater. Interfaces 2021, 8, 2001759. [Google Scholar] [CrossRef]
- Moon, S.-Y.; Howarth, A.J.; Wang, T.; Vermeulen, N.A.; Hupp, J.T.; Farha, O.K. A visually detectable pH responsive zirconium metal–organic framework. Chem. Commun. 2016, 52, 3438–3441. [Google Scholar] [CrossRef]
- Aguilera-Sigalat, J.; Bradshaw, D. A colloidal water-stable MOF as a broad-range fluorescent pH sensor via post-synthetic modification. Chem. Commun. 2014, 50, 4711–4713. [Google Scholar] [CrossRef] [Green Version]
- Sk, M.; Nandi, S.; Singh, R.K.; Trivedi, V.; Biswas, S. Selective Sensing of Peroxynitrite by Hf-Based UiO-66-B(OH)2 Metal–Organic Framework: Applicability to Cell Imaging. Inorg. Chem. 2018, 57, 10128–10136. [Google Scholar] [CrossRef]
- Dalapati, R.; Nandi, S.; Biswas, S. Post-synthetic modification of a metal–organic framework with a chemodosimeter for the rapid detection of lethal cyanide via dual emission. Dalton Trans. 2020, 49, 8684–8692. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Biswas, S. A diamino functionalized metal-organic framework for fluorometric recognition of free chlorine in environmental water samples. Microporous Mesoporous Mater. 2020, 299, 110116. [Google Scholar] [CrossRef]
- Wu, K.; Zheng, J.; Huang, Y.-L.; Luo, D.; Li, Y.Y.; Lu, W.; Li, D. Cr2O72− inside Zr/Hf-based metal–organic frameworks: Highly sensitive and selective detection and crystallographic evidence. J. Mater. Chem. C 2020, 8, 16974–16983. [Google Scholar] [CrossRef]
- Xing, K.; Fan, R.-Q.; Liu, X.-Y.; Gai, S.; Chen, W.; Yang, Y.-L.; Li, J. A self-calibrating dual responsive platform for the sensitive detection of sulfite and sulfonic derivatives based on a robust Hf(IV) metal–organic framework. Chem. Commun. 2020, 56, 631–634. [Google Scholar] [CrossRef]
- Xu, H.; Gao, J.; Qian, X.; Wang, J.; He, H.; Cui, Y.; Yang, Y.; Wang, Z.; Qian, G. Metal–organic framework nanosheets for fast-response and highly sensitive luminescent sensing of Fe3+. J. Mater. Chem. A 2016, 4, 10900–10905. [Google Scholar] [CrossRef]
- Zhong, F.; Li, C.; Xie, Y.; Xu, H.; Gao, J. Titanium metal-organic framework nanorods for highly sensitive nitroaromatic explosives detection and nanomolar sensing of Fe3+. J. Solid State Chem. 2019, 278, 120892. [Google Scholar] [CrossRef]
- Kaur, M.; Mehta, S.K.; Kansal, S.K. Amine-functionalized titanium metal-organic framework (NH2-MIL-125(Ti)): A novel fluorescent sensor for the highly selective sensing of copper ions. Mater. Chem. Phys. 2020, 254, 123539. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Y.; Chen, F.; Bai, G.; Xu, H.; Xu, S. A Fluorescent Titanium-based Metal-Organic Framework Sensor for Nitro-aromatics Detection. Z. Anorg. Allg. Chem. 2021, 647, 759–763. [Google Scholar] [CrossRef]
- Jia, C.; Bai, J.; Liu, Z.; Gao, S.; Han, Y.; Yan, H. Application of a titanium-based metal-organic framework to protein kinase activity detection and inhibitor screening. Anal. Chim. Acta 2020, 1128, 99–106. [Google Scholar] [CrossRef]
- Rogge, S.M.J.; Yot, P.G.; Jacobsen, J.; Muniz-Miranda, F.; Vandenbrande, S.; Gosch, J.; Ortiz, V.; Collings, I.E.; Devautour-Vinot, S.; Maurin, G.; et al. Charting the Metal-Dependent High-Pressure Stability of Bimetallic UiO-66 Materials. ACS Mater. Lett. 2020, 2, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Islamoglu, T.; Atilgan, A.; Moon, S.-Y.; Peterson, G.W.; DeCoste, J.B.; Hall, M.; Hupp, J.T.; Farha, O.K. Cerium(IV) vs Zirconium(IV) Based Metal–Organic Frameworks for Detoxification of a Nerve Agent. Chem. Mater. 2017, 29, 2672–2675. [Google Scholar] [CrossRef]
- He, X.; Looker, B.G.; Dinh, K.T.; Stubbs, A.W.; Chen, T.; Meyer, R.J.; Serna, P.; Román-Leshkov, Y.; Lancaster, K.M.; Dincă, M. Cerium(IV) Enhances the Catalytic Oxidation Activity of Single-Site Cu Active Sites in MOFs. ACS Catal. 2020, 10, 7820–7825. [Google Scholar] [CrossRef]
- Yue, D.; Zhao, D.; Zhang, J.; Zhang, L.; Jiang, K.; Zhang, X.; Cui, Y.; Yang, Y.; Chen, B.; Qian, G. A luminescent cerium metal–organic framework for the turn-on sensing of ascorbic acid. Chem. Commun. 2017, 53, 11221–11224. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, W.; Sun, B.; Hu, H.; Li, X.; Bao, S.; Su, Z. Two fluorescent cerium metal-organic frameworks for the “turn-on” sensing of AA with high sensitivity as well as biological and electrochemical properties. J. Solid State Chem. 2021, 302, 122376. [Google Scholar] [CrossRef]
- Dalapati, R.; Sakthivel, B.; Ghosalya, M.K.; Dhakshinamoorthy, A.; Biswas, S. A cerium-based metal–organic framework having inherent oxidase-like activity applicable for colorimetric sensing of biothiols and aerobic oxidation of thiols. CrystEngComm 2017, 19, 5915–5925. [Google Scholar] [CrossRef]
- Wang, C.; Tang, G.; Tan, H. Colorimetric determination of mercury(II) via the inhibition by ssDNA of the oxidase-like activity of a mixed valence state cerium-based metal-organic framework. Microchim. Acta 2018, 185, 475. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Glucose Biosensors: 40 Years of Advances and Challenges. Electroanalysis 2001, 13, 983–988. [Google Scholar] [CrossRef]
- Murray, R.W. Chemically modified electrodes. Acc. Chem. Res. 1980, 13, 135–141. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Y.; Liu, S.; Xu, M. The Applications of Metal−Organic Frameworks in Electrochemical Sensors. ChemElectroChem 2018, 5, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Kempahanumakkagari, S.; Vellingiri, K.; Deep, A.; Kwon, E.E.; Bolan, N.; Kim, K.-H. Metal–organic framework composites as electrocatalysts for electrochemical sensing applications. Coord. Chem. Rev. 2018, 357, 105–129. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Kung, C.-W. Metal−Organic Frameworks toward Electrochemical Sensors: Challenges and Opportunities. Electroanalysis 2020, 32, 1885–1895. [Google Scholar] [CrossRef]
- Kung, C.-W.; Goswami, S.; Hod, I.; Wang, T.C.; Duan, J.; Farha, O.K.; Hupp, J.T. Charge Transport in Zirconium-Based Metal–Organic Frameworks. Acc. Chem. Res. 2020, 53, 1187–1195. [Google Scholar] [CrossRef]
- Xie, L.S.; Skorupskii, G.; Dincă, M. Electrically Conductive Metal–Organic Frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, S.; Slater, B. Unusually Large Band Gap Changes in Breathing Metal–Organic Framework Materials. J. Phys. Chem. C 2015, 119, 16667–16677. [Google Scholar] [CrossRef]
- Hendrickx, K.; Vanpoucke, D.E.P.; Leus, K.; Lejaeghere, K.; Van Yperen-De Deyne, A.; Van Speybroeck, V.; Van Der Voort, P.; Hemelsoet, K. Understanding Intrinsic Light Absorption Properties of UiO-66 Frameworks: A Combined Theoretical and Experimental Study. Inorg. Chem. 2015, 54, 10701–10710. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.-W.; Han, P.-C.; Chuang, C.-H.; Wu, K.C.-W. Electronically conductive metal–organic framework-based materials. APL Mater. 2019, 7, 110902. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.C.; Hod, I.; Audu, C.O.; Vermeulen, N.A.; Nguyen, S.T.; Farha, O.K.; Hupp, J.T. Rendering High Surface Area, Mesoporous Metal–Organic Frameworks Electronically Conductive. ACS Appl. Mater. Interfaces 2017, 9, 12584–12591. [Google Scholar] [CrossRef]
- Goswami, S.; Ray, D.; Otake, K.-i.; Kung, C.-W.; Garibay, S.J.; Islamoglu, T.; Atilgan, A.; Cui, Y.; Cramer, C.J.; Farha, O.K.; et al. A porous, electrically conductive hexa-zirconium(IV) metal-organic framework. Chem. Sci. 2018, 9, 4477–4482. [Google Scholar] [CrossRef]
- Kung, C.-W.; Otake, K.; Buru, C.T.; Goswami, S.; Cui, Y.; Hupp, J.T.; Spokoyny, A.M.; Farha, O.K. Increased Electrical Conductivity in a Mesoporous Metal–Organic Framework Featuring Metallacarboranes Guests. J. Am. Chem. Soc. 2018, 140, 3871–3875. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chiang, W.-H.; Kurniawan, D.; Yeh, P.-C.; Otake, K.-i.; Kung, C.-W. Impregnation of Graphene Quantum Dots into a Metal–Organic Framework to Render Increased Electrical Conductivity and Activity for Electrochemical Sensing. ACS Appl. Mater. Interfaces 2019, 11, 35319–35326. [Google Scholar] [CrossRef]
- Ray, D.; Goswami, S.; Duan, J.; Hupp, J.T.; Cramer, C.J.; Gagliardi, L. Tuning the Conductivity of Hexa-Zirconium(IV) Metal–Organic Frameworks by Encapsulating Heterofullerenes. Chem. Mater. 2021, 33, 1182–1189. [Google Scholar] [CrossRef]
- Blauch, D.N.; Saveant, J.M. Dynamics of electron hopping in assemblies of redox centers. Percolation and diffusion. J. Am. Chem. Soc. 1992, 114, 3323–3332. [Google Scholar] [CrossRef]
- Kung, C.-W.; Wang, T.C.; Mondloch, J.E.; Fairen-Jimenez, D.; Gardner, D.M.; Bury, W.; Klingsporn, J.M.; Barnes, J.C.; Van Duyne, R.; Stoddart, J.F.; et al. Metal–Organic Framework Thin Films Composed of Free-Standing Acicular Nanorods Exhibiting Reversible Electrochromism. Chem. Mater. 2013, 25, 5012–5017. [Google Scholar] [CrossRef]
- Lin, S.; Usov, P.M.; Morris, A.J. The role of redox hopping in metal–organic framework electrocatalysis. Chem. Commun. 2018, 54, 6965–6974. [Google Scholar] [CrossRef]
- Usov, P.M.; Fabian, C.; D’Alessandro, D.M. Rapid determination of the optical and redox properties of a metal–organic framework via in situ solid state spectroelectrochemistry. Chem. Commun. 2012, 48, 3945–3947. [Google Scholar] [CrossRef]
- Ahrenholtz, S.R.; Epley, C.C.; Morris, A.J. Solvothermal Preparation of an Electrocatalytic Metalloporphyrin MOF Thin Film and its Redox Hopping Charge-Transfer Mechanism. J. Am. Chem. Soc. 2014, 136, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Hod, I.; Sampson, M.D.; Deria, P.; Kubiak, C.P.; Farha, O.K.; Hupp, J.T. Fe-Porphyrin-Based Metal–Organic Framework Films as High-Surface Concentration, Heterogeneous Catalysts for Electrochemical Reduction of CO2. ACS Catal. 2015, 5, 6302–6309. [Google Scholar] [CrossRef]
- Fei, H.; Pullen, S.; Wagner, A.; Ott, S.; Cohen, S.M. Functionalization of robust Zr(IV)-based metal-organic framework films via a postsynthetic ligand exchange. Chem. Commun. 2015, 51, 66–69. [Google Scholar] [CrossRef]
- Johnson, B.A.; Bhunia, A.; Fei, H.; Cohen, S.M.; Ott, S. Development of a UiO-Type Thin Film Electrocatalysis Platform with Redox-Active Linkers. J. Am. Chem. Soc. 2018, 140, 2985–2994. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, R.; He, W.; Liberman, I.; Hod, I. Tuning of Redox Conductivity and Electrocatalytic Activity in Metal–Organic Framework Films Via Control of Defect Site Density. J. Phys. Chem. C 2019, 123, 5531–5539. [Google Scholar] [CrossRef]
- Maindan, K.; Li, X.; Yu, J.; Deria, P. Controlling Charge-Transport in Metal–Organic Frameworks: Contribution of Topological and Spin-State Variation on the Iron–Porphyrin Centered Redox Hopping Rate. J. Phys. Chem. B 2019, 123, 8814–8822. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-H.; Li, J.-H.; Chen, Y.-C.; Wang, Y.-S.; Kung, C.-W. Redox-Hopping and Electrochemical Behaviors of Metal–Organic Framework Thin Films Fabricated by Various Approaches. J. Phys. Chem. C 2020, 124, 20854–20863. [Google Scholar] [CrossRef]
- Shen, C.-H.; Chuang, C.-H.; Gu, Y.-J.; Ho, W.H.; Song, Y.-D.; Chen, Y.-C.; Wang, Y.-C.; Kung, C.-W. Cerium-Based Metal–Organic Framework Nanocrystals Interconnected by Carbon Nanotubes for Boosting Electrochemical Capacitor Performance. ACS Appl. Mater. Interfaces 2021, 13, 16418–16426. [Google Scholar] [CrossRef] [PubMed]
- Amombo Noa, F.M.; Abrahamsson, M.; Ahlberg, E.; Cheung, O.; Göb, C.R.; McKenzie, C.J.; Öhrström, L. A unified topology approach to dot-, rod-, and sheet-MOFs. Chem 2021, 7, 2491–2512. [Google Scholar] [CrossRef]
- Kung, C.-W.; Chang, T.-H.; Chou, L.-Y.; Hupp, J.T.; Farha, O.K.; Ho, K.-C. Porphyrin-based metal–organic framework thin films for electrochemical nitrite detection. Electrochem. Commun. 2015, 58, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Kung, C.-W.; Li, Y.-S.; Lee, M.-H.; Wang, S.-Y.; Chiang, W.-H.; Ho, K.-C. In situ growth of porphyrinic metal-organic framework nanocrystals on graphene nanoribbons for the electrocatalytic oxidation of nitrite. J. Mater. Chem. A 2016, 4, 10673–10682. [Google Scholar] [CrossRef]
- Su, C.-H.; Kung, C.-W.; Chang, T.-H.; Lu, H.-C.; Ho, K.-C.; Liao, Y.-C. Inkjet-printed porphyrinic metal–organic framework thin films for electrocatalysis. J. Mater. Chem. A 2016, 4, 11094–11102. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Chen, Y.-C.; Chuang, W.-S.; Li, J.-H.; Wang, Y.-S.; Chuang, C.-H.; Chen, C.-Y.; Kung, C.-W. Pore-Confined Silver Nanoparticles in a Porphyrinic Metal–Organic Framework for Electrochemical Nitrite Detection. ACS Appl. Nano Mater. 2020, 3, 9440–9448. [Google Scholar] [CrossRef]
- Pila, T.; Chirawatkul, P.; Piyakeeratikul, P.; Somjit, V.; Sawangphruk, M.; Kongpatpanich, K. Metalloporphyrin-Based Metal–Organic Frameworks on Flexible Carbon Paper for Electrocatalytic Nitrite Oxidation. Chem. Eur. J. 2020, 26, 17399–17404. [Google Scholar] [CrossRef]
- Ling, P.; Lei, J.; Ju, H. Porphyrinic metal-organic framework as electrochemical probe for DNA sensing via triple-helix molecular switch. Biosens. Bioelectron. 2015, 71, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-Y.; Zhuang, Y.-H.; Shan, D.; Su, G.-F.; Cosnier, S.; Zhang, X.-J. Zirconium-Based Porphyrinic Metal–Organic Framework (PCN-222): Enhanced Photoelectrochemical Response and Its Application for Label-Free Phosphoprotein Detection. Anal. Chem. 2016, 88, 11207–11212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Chen, H.; Hu, X.; Ma, S. Fabrication of Highly Sensitive and Stable Hydroxylamine Electrochemical Sensor Based on Gold Nanoparticles and Metal–Metalloporphyrin Framework Modified Electrode. ACS Appl. Mater. Interfaces 2016, 8, 18173–18181. [Google Scholar] [CrossRef]
- Huang, T.Y.; Kung, C.W.; Liao, Y.T.; Kao, S.Y.; Cheng, M.; Chang, T.H.; Henzie, J.; Alamri Hatem, R.; Alothman Zeid, A.; Yamauchi, Y.; et al. Enhanced Charge Collection in MOF-525–PEDOT Nanotube Composites Enable Highly Sensitive Biosensing. Adv. Sci. 2017, 4, 1700261. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Yin, X.; Bo, X.; Guo, L. High-performance electrocatalyst based on metal-organic framework/macroporous carbon composite for efficient detection of luteolin. J. Electroanal. Chem. 2018, 824, 153–160. [Google Scholar] [CrossRef]
- Yu, G.; Song, X.; Zheng, S.; Zhao, Q.; Yan, D.; Zhao, J. A facile and sensitive tetrabromobisphenol-A sensor based on biomimetic catalysis of a metal–organic framework: PCN-222(Fe). Anal. Methods 2018, 10, 4275–4281. [Google Scholar] [CrossRef]
- Zhang, J.; Qiang, Y.; Xu, X. An Ultrasensitive Electrochemical Aptasensor for Thrombin Detection Using MoS2 Nanoparticles Loaded Iron-Porphyrinic Metal-Organic Framework as Signal Amplifier. J. Electrochem. Soc. 2020, 167, 087503. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Qiang, Y. Ultrasensitive electrochemical aptasensor for ochratoxin A detection using AgPt bimetallic nanoparticles decorated iron-porphyrinic metal-organic framework for signal amplification. Sens. Actuators B Chem. 2020, 312, 127964. [Google Scholar] [CrossRef]
- Wei, C.; Zhou, H.; Liu, Q. PCN-222 MOF decorated conductive PEDOT films for sensitive electrochemical determination of chloramphenicol. Mater. Chem. Phys. 2021, 270, 124831. [Google Scholar] [CrossRef]
- Zhou, Z.; Mukherjee, S.; Hou, S.; Li, W.; Elsner, M.; Fischer, R.A. Porphyrinic MOF Film for Multifaceted Electrochemical Sensing. Angew. Chem. Int. Ed. 2021, 60, 20551–20557. [Google Scholar] [CrossRef]
- Su, F.; Zhang, S.; Ji, H.; Zhao, H.; Tian, J.-Y.; Liu, C.-S.; Zhang, Z.; Fang, S.; Zhu, X.; Du, M. Two-Dimensional Zirconium-Based Metal–Organic Framework Nanosheet Composites Embedded with Au Nanoclusters: A Highly Sensitive Electrochemical Aptasensor toward Detecting Cocaine. ACS Sens. 2017, 2, 998–1005. [Google Scholar] [CrossRef]
- Deng, M.; Lin, S.; Bo, X.; Guo, L. Simultaneous and sensitive electrochemical detection of dihydroxybenzene isomers with UiO-66 metal-organic framework/mesoporous carbon. Talanta 2017, 174, 527–538. [Google Scholar] [CrossRef]
- Deng, M.; Bo, X.; Guo, L. Encapsulation of platinum nanoparticles into a series of zirconium-based metal-organic frameworks: Effect of the carrier structures on electrocatalytic performances of composites. J. Electroanal. Chem. 2018, 815, 198–209. [Google Scholar] [CrossRef]
- Liu, H.; Hassan, M.; Bo, X.; Guo, L. Fumarate-based metal-organic framework/mesoporous carbon as a novel electrochemical sensor for the detection of gallic acid and luteolin. J. Electroanal. Chem. 2019, 849, 113378. [Google Scholar] [CrossRef]
- Du, L.; Chen, W.; Wang, J.; Cai, W.; Kong, S.; Wu, C. Folic acid-functionalized zirconium metal-organic frameworks based electrochemical impedance biosensor for the cancer cell detection. Sens. Actuators B Chem. 2019, 301, 127073. [Google Scholar] [CrossRef]
- Li, Y.; Hu, M.; Huang, X.; Wang, M.; He, L.; Song, Y.; Jia, Q.; Zhou, N.; Zhang, Z.; Du, M. Multicomponent zirconium-based metal-organic frameworks for impedimetric aptasensing of living cancer cells. Sens. Actuators B Chem. 2020, 306, 127608. [Google Scholar] [CrossRef]
- Fang, X.; Chen, X.; Liu, Y.; Li, Q.; Zeng, Z.; Maiyalagan, T.; Mao, S. Nanocomposites of Zr(IV)-Based Metal–Organic Frameworks and Reduced Graphene Oxide for Electrochemically Sensing Ciprofloxacin in Water. ACS Appl. Nano Mater. 2019, 2, 2367–2376. [Google Scholar] [CrossRef]
- Wang, H.; Bo, X.; Zhou, M.; Guo, L. DUT-67 and tubular polypyrrole formed a cross-linked network for electrochemical detection of nitrofurazone and ornidazole. Anal. Chim. Acta 2020, 1109, 1–8. [Google Scholar] [CrossRef]
- Wang, Q.; Gu, C.; Fu, Y.; Liu, L.; Xie, Y. Ultrasensitive Electrochemical Sensor for Luteolin Based on Zirconium Metal-Organic Framework UiO-66/Reduced Graphene Oxide Composite Modified Glass Carbon Electrode. Molecules 2020, 25, 4557. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Lan, Q.; Xie, Y.; Sun, X.; Wang, Y. Label-Free Electrochemical Immunosensor for Ultrasensitive Detection of Carbohydrate Antigen 125 Based on Antibody-Immobilized Biocompatible MOF-808/CNT. ACS Appl. Mater. Interfaces 2021, 13, 3295–3302. [Google Scholar] [CrossRef]
- Ho, W.H.; Chen, T.-Y.; Otake, K.-i.; Chen, Y.-C.; Wang, Y.-S.; Li, J.-H.; Chen, H.-Y.; Kung, C.-W. Polyoxometalate adsorbed in a metal–organic framework for electrocatalytic dopamine oxidation. Chem. Commun. 2020, 56, 11763–11766. [Google Scholar] [CrossRef]
- Chang, T.-E.; Chuang, C.-H.; Kung, C.-W. An iridium-decorated metal–organic framework for electrocatalytic oxidation of nitrite. Electrochem. Commun. 2021, 122, 106899. [Google Scholar] [CrossRef]
- Yang, J.; Yang, L.; Ye, H.; Zhao, F.; Zeng, B. Highly dispersed AuPd alloy nanoparticles immobilized on UiO-66-NH2 metal-organic framework for the detection of nitrite. Electrochim. Acta 2016, 219, 647–654. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Huang, W.; Zhang, T.; Hu, X.; Perman, J.A.; Ma, S. A metal–organic framework and conducting polymer based electrochemical sensor for high performance cadmium ion detection. J. Mater. Chem. A 2017, 5, 8385–8393. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Li, J.-H.; Chen, Y.-C.; Ho, W.H.; Song, Y.-D.; Kung, C.-W. Electrodeposition of pore-confined cobalt in metal–organic framework thin films toward electrochemical H2O2 detection. Electrochim. Acta 2020, 347, 136276. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Jin, G.; Jin, D.; Li, K.; Mao, A.; Hu, X. Photooxidation assisted sensitive detection of trace Mn2+ in tea by NH2-MIL-125 (Ti) modified carbon paste electrode. Sens. Actuators B Chem. 2014, 201, 274–280. [Google Scholar] [CrossRef]
- Jin, D.; Xu, Q.; Yu, L.; Hu, X. Photoelectrochemical detection of the herbicide clethodim by using the modified metal-organic framework amino-MIL-125(Ti)/TiO2. Microchim. Acta 2015, 182, 1885–1892. [Google Scholar] [CrossRef]
- Dong, P.; Zhu, L.; Huang, J.; Ren, J.; Lei, J. Electrocatalysis of cerium metal-organic frameworks for ratiometric electrochemical detection of telomerase activity. Biosens. Bioelectron. 2019, 138, 111313. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Y.; Shan, J.; Zhou, H.; Li, M. Electrochemical sensor for determination of bisphenol A based on MOF-reduced graphene oxide composites coupled with cetyltrimethylammonium bromide signal amplification. Ionics 2020, 26, 3135–3146. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Y.; Chen, Z.; Chen, J.; Hu, Y.; Zhu, J.-J. Electrochemical sensor based on Ce-MOF/carbon nanotube composite for the simultaneous discrimination of hydroquinone and catechol. J. Hazard. Mater. 2021, 416, 125895. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.-L.; Guo, Z.; Huang, X.-J. Graphene-based hybrids for chemiresistive gas sensors. TrAC Trends Anal. Chem. 2015, 68, 37–47. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive polyaniline-based gas sensors: A mini review. Sens. Actuators B Chem. 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Campbell, M.G.; Dincă, M. Metal–Organic Frameworks as Active Materials in Electronic Sensor Devices. Sensors 2017, 17, 1108. [Google Scholar] [CrossRef]
- Koo, W.-T.; Jang, J.-S.; Kim, I.-D. Metal-Organic Frameworks for Chemiresistive Sensors. Chem 2019, 5, 1938–1963. [Google Scholar] [CrossRef]
- Chen, X.; Li, G. Proton conductive Zr-based MOFs. Inorg. Chem. Front. 2020, 7, 3765–3784. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Zhang, Y.; Cong, H.; Fu, B.; Wen, S.; Ruan, S. A novel humidity sensor based on NH2-MIL-125(Ti) metal organic framework with high responsiveness. J. Nanopart. Res. 2013, 15, 2014. [Google Scholar] [CrossRef]
- Kung, C.-W.; Platero-Prats, A.E.; Drout, R.J.; Kang, J.; Wang, T.C.; Audu, C.O.; Hersam, M.C.; Chapman, K.W.; Farha, O.K.; Hupp, J.T. Inorganic “Conductive Glass” Approach to Rendering Mesoporous Metal–Organic Frameworks Electronically Conductive and Chemically Responsive. ACS Appl. Mater. Interfaces 2018, 10, 30532–30540. [Google Scholar] [CrossRef]
- Lee, J.-H.; Nguyen, T.T.T.; Nguyen, L.H.T.; Phan, T.B.; Kim, S.S.; Doan, T.L.H. Functionalization of zirconium-based metal–organic frameworks for gas sensing applications. J. Hazard. Mater. 2021, 403, 124104. [Google Scholar] [CrossRef] [PubMed]
- Dmello, M.E.; Sundaram, N.G.; Singh, A.; Singh, A.K.; Kalidindi, S.B. An amine functionalized zirconium metal–organic framework as an effective chemiresistive sensor for acidic gases. Chem. Commun. 2019, 55, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.T.; Tran, M.T.; Feller, J.-F.; Castro, M.; Van Ngo, T.; Hassan, K.; Nine, M.J.; Losic, D. Graphene and metal organic frameworks (MOFs) hybridization for tunable chemoresistive sensors for detection of volatile organic compounds (VOCs) biomarkers. Carbon 2020, 159, 333–344. [Google Scholar] [CrossRef]
- Moghadam, B.H.; Hasanzadeh, M.; Simchi, A. Self-Powered Wearable Piezoelectric Sensors Based on Polymer Nanofiber–Metal–Organic Framework Nanoparticle Composites for Arterial Pulse Monitoring. ACS Appl. Nano Mater. 2020, 3, 8742–8752. [Google Scholar] [CrossRef]
- Tran, H.; Feig, V.R.; Liu, K.; Zheng, Y.; Bao, Z. Polymer Chemistries Underpinning Materials for Skin-Inspired Electronics. Macromolecules 2019, 52, 3965–3974. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shi, G.; Qin, J.; Lowe, S.E.; Zhang, S.; Zhao, H.; Zhong, Y.L. Recent Progress of Direct Ink Writing of Electronic Components for Advanced Wearable Devices. ACS Appl. Electron. Mater. 2019, 1, 1718–1734. [Google Scholar] [CrossRef]
- Lawson, S.; Snarzyk, M.; Hanify, D.; Rownaghi, A.A.; Rezaei, F. Development of 3D-Printed Polymer-MOF Monoliths for CO2 Adsorption. Ind. Eng. Chem. Res. 2020, 59, 7151–7160. [Google Scholar] [CrossRef]
- Evans, K.A.; Kennedy, Z.C.; Arey, B.W.; Christ, J.F.; Schaef, H.T.; Nune, S.K.; Erikson, R.L. Chemically Active, Porous 3D-Printed Thermoplastic Composites. ACS Appl. Mater. Interfaces 2018, 10, 15112–15121. [Google Scholar] [CrossRef]
- Dhainaut, J.; Bonneau, M.; Ueoka, R.; Kanamori, K.; Furukawa, S. Formulation of Metal–Organic Framework Inks for the 3D Printing of Robust Microporous Solids toward High-Pressure Gas Storage and Separation. ACS Appl. Mater. Interfaces 2020, 12, 10983–10992. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, S.; Yu, S.-S.; Kung, C.-W. Group 4 Metal-Based Metal—Organic Frameworks for Chemical Sensors. Chemosensors 2021, 9, 306. https://doi.org/10.3390/chemosensors9110306
Pal S, Yu S-S, Kung C-W. Group 4 Metal-Based Metal—Organic Frameworks for Chemical Sensors. Chemosensors. 2021; 9(11):306. https://doi.org/10.3390/chemosensors9110306
Chicago/Turabian StylePal, Souvik, Sheng-Sheng Yu, and Chung-Wei Kung. 2021. "Group 4 Metal-Based Metal—Organic Frameworks for Chemical Sensors" Chemosensors 9, no. 11: 306. https://doi.org/10.3390/chemosensors9110306
APA StylePal, S., Yu, S.-S., & Kung, C.-W. (2021). Group 4 Metal-Based Metal—Organic Frameworks for Chemical Sensors. Chemosensors, 9(11), 306. https://doi.org/10.3390/chemosensors9110306







