Abstract
Perovskite-modified electrodes have received increasing attention in the last decade, due to their electrocatalytic properties to undergo the sensitive and selective detection of bioactive molecules, such as hydrogen peroxide, glucose, and dopamine. In this review paper, different types of perovskites involved for their electrocatalytic properties are described, and the proposed mechanism of detection is presented. The analytical performances obtained for different electroactive molecules are listed and compared with those in terms of the type of perovskite used, its nanostructuration, and its association with other conductive nanomaterials. The analytical performance obtained with perovskites is shown to be better than those of Ni and Co oxide-based electrochemical sensors. Main trends and future challenges for enlarging and improving the use of perovskite-based electrochemical sensors are then discussed.
1. Introduction
For the integration in point-of-care testing (POCT) systems [1], such as commercial kits available for monitoring patients’ glucose levels (i.e., Accu-Chek® from Roche Diabetes Care Company) [2], there is an urgent need for precise, sensitive, portable, and cost-effective technologies for the detection of bioactive molecules. In this group of chemical compounds, hydrogen peroxide is a cancer biomarker, because, in comparison to normal cells, cancer cells are characterized by an increased H2O2 production rate and an impaired redox balance, thereby affecting the antitumoral immune response [3]. Diabetes mellitus is due to an abnormal level of glucose in the blood, and this level should be frequently monitored [4]. Dopamine (DA) is one of the most important catecholamines, present in the human central nervous system. Its depletion that should be monitored leads to neurodegenerative diseases such as Parkinson’s disease [5].
Electrochemical sensors are good candidates for their integration in POCT systems [6], due to their easy miniaturization and the low-cost instrumentation that could be interfaced with smart phones. All these bioactive molecules can be sensitively detected by enzymatic electrochemical sensors [7,8,9]. The main drawback of these enzymatic sensors is the stability of enzymes and their activities being modified by the immobilization procedure, by the pH value, or by the presence of inhibiting agents. The use of non-enzymatic electrochemical sensors should be highly required. During the last decade, perovskite nanomaterials have shown electrocatalytic properties, and bioactive molecules could be easily sensitively and selectively detected using perovskite-based electrodes, which present the advantage of stability of the sensors.
Perovskite oxides were discovered by Gustav Rose in the Ural mountains in 1839; the most common formula of perovskites is ABO3, with A being an alkali metal or a lanthanide, B being a transition metal, and O being the oxygen ion. The charges of A and B ions should be equivalent to the whole charge of the oxygen ions. The tolerance factor should be in the range of 0.8–1.0 with the radii of A and B ions greater than 0.090 nm and 0.051 nm, respectively. The cubic structure of the perovskite is stabilized by the 6-fold coordination of the B cation and the 12-fold coordination of the A cation (Figure 1).
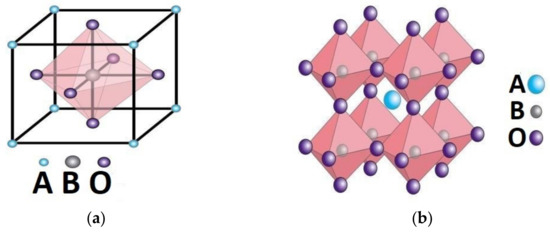
Figure 1.
(a) Cubic mesh of perovskite; (b) three-dimensional (3D) stacking in the ideal cubic structure.
Some distortions may occur in the ideal cubic structure, leading to orthorhombic, tetragonal, rhombohedral, or hexagonal structures.
Perovskite oxides present a diversity of electrical properties from insulating to semiconducting metallic and superconducting properties. They also present magnetic and optical properties [10]. A lot of devices are then conceived from perovskite oxides: phot-chromic, electro-chromic, image storage, switching, filtering, and surface acoustic wave signal-processing devices. In addition, they have been used as catalysts in different applications such as engine-exhaust gas treatment and hydrogen evolution reaction.
Several recent review papers were devoted to the sensory applications of perovskite based on their electrical and of optical properties [11,12,13,14,15,16,17]. This review paper is based on an exhaustive list of perovskite-based electrochemical sensors for the enzyme-free detection of hydrogen peroxide, glucose, and DA. In each case, the involved mechanism is described, and the analytical performance of the obtained sensor is presented. The perovskite formula leading to the lowest detection limit is highlighted, as well as the one leading to the most selective detection. The improvement brought by the association with other nanomaterials is also shown. The analytical performance of the perovskite-based sensors and those of nickel oxide and cobalt oxide-based sensors are compared. The main trends and future challenges are discussed.
2. Methods for the Synthesis and Characterization of Perovskites
2.1. Sol-Gel Synthesis
The sol-gel synthesis of precursors is one of the procedures used for the synthesis of perovskites [18]. It is based on the Pechini method that involves two chemical reactions: with nitrate salts of the metal ions being mixed according to the stoichiometry, the complexation of metal ions occurs with the addition of citric acid, and the polyesterification of the complexes is obtained with the addition of ethylene glycol [19]. A viscous solution is obtained after heating to 130 °C in an argon flow under stirring for about 10 h. The temperature is then raised to 150–200 °C to obtain a foam-dried mass which has to be ground in a mortar. This precursor is calcined at different temperatures of 600, 850, and 1000 °C in a muffle furnace, depending on the final targeted product.
2.2. Microwave Irradiation Process
The microwave irradiation process shows many advantages such as rapid reaction velocity, uniform heating, cleanness, and high energy efficiency. The conditions used for microwave preparation are 2.45 GHz, with a maximum output power of not less than 1 kW. Dielectric materials can absorb the microwave energy and transform it into heat energy directly through polarization and dielectric loss in the interior of the materials. Single-phase manganese-based perovskites are simply obtained from nitric solutions by a denitration process under microwave irradiation [20].
2.3. Coprecipitation Process
In the coprecipitation process [18], different types of precursors are employed: oxides, alkoxides, inorganic salts, and nitrates. The processing parameters (pH, coprecipitation rate, washing, drying, and temperature of synthesis) are controlled which results in homogeneous and weakly agglomerated nanopowders. They can be sintered at temperatures as low as 1250 °C and for short times (1–2 h) for the obtaining of perovskites of near the theoretical density.
2.4. Solid-State Synthesis Technique
LaFeO3 nanoparticles are prepared through the solid-state synthesis technique using mechanical ball milling [21]. Stoichiometric amounts of La2O3 and Fe2O3 metal oxide precursors (molar ratio: 1:1) are transferred to a planetary ball mill. Wet milling is carried out for 20 h (with toluene as the process control agent). Based on thermal analysis, the ball milled powder is calcined at 900 °C for 2 h and then ground into fine powders in an agate mortar and pestle.
2.5. Other Synthesis Techniques
LaNiO3 electrospun nanofibers are prepared by mixing metal salts with polyvinylpyrrolidone (PVP) followed by sequential calcinations [22]. Thin films of La0.5Sr0.5CoO3-δ are obtained by the pulsed laser deposition (PLD) technique [23].
2.6. Characterization Methods
The different phases of the prepared perovskites can be differentiated using X-ray powder diffraction (XRD). In addition, the structure of perovskite can be characterized using single-crystal XRD analysis. Thermal analysis techniques such as thermogravimetry (TGA), differential thermal analysis (DTA), and differential scanning calorimetry (DSC) can be used to test the thermal stability of the prepared perovskites. The different morphological characteristics of the prepared perovskites can be studied using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). In addition, the surface area measurement of the prepared perovskites can be carried out using surface area analysis (BET). Raman spectroscopy allows the determination of vibration modes in relation with molecular dynamics. In addition, the surface chemical groups of the prepared perovskites can be identified using Fourier-transform infrared spectroscopy (FTIR) and X-ray photo-electron spectroscopy (XPS). The frequency-dependent conductivity spectra are determined by using impedance spectroscopy.
3. Perovskite-Based Electrochemical Sensors for the Detection of Hydrogen Peroxide
Table 1 shows a summary of Perovskite-based electrochemical sensors for the detection of hydrogen peroxide.

Table 1.
Perovskite-based electrochemical sensors for the detection of hydrogen peroxide.
The first study about the electrochemical detection of hydrogen peroxide using a perovskite was published in 1996 by Shimizu [26]. Among ABO3 perovskites used for the electrochemical detection of hydrogen peroxide (Table 1), the more commonly used are lanthanum-based perovskites, certain being substituted with alkaline earth ions such as calcium and strontium. The frequency of the use of B ions for the H2O2 detection, among all the published papers (Table 1), is as follows: Co > Ni = Mn > Fe > Ti. A-site ion is mainly La that can be substituted by alkaline ions such as strontium [20,23,27,31,32] or calcium [26].
Figure 2 illustrates the overall steps of the H2O2 electro-oxidation on the perovskite surface, which involves diffusion, adsorption/desorption, and electro-oxidation reaction.
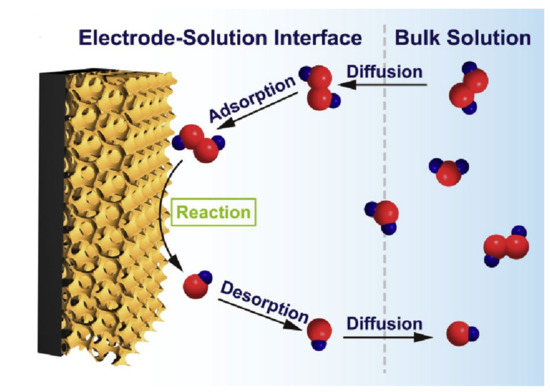
Figure 2.
Schematic diagram of the overall process steps on a perovskite/glassy carbon electrode (GCE) [21]. Reproduced with the permission from Elsevier.
The complex mechanisms involved in the electrocatalytic oxidation of hydrogen peroxide were deeply analyzed [32]. The partial substitution of the A-site cations by divalent cations such as Ca2+, Sr2+, and Ba2+ can lead to an oxidation of the B-site cation as B3+ [24,25,27,32]. The increasing of this substitution also leads to the reduction in oxygen vacancy formation energy which is consistent with the existence of highly oxidative oxygen species [32]. The most possible mechanism of the oxidation of H2O2 on La0.6Sr0.4CoO3-δ in 0.1 M NaOH at a potential of 0.3V/Ag/AgCl is presented in Figure 3. Two parallel pathways are involved: Co3+/Co4+ redox couple (Figure 3A) and oxygen vacancies formation which allows the transfer of lattice oxygen to the adsorbed intermediates, generating ion superoxide (defined as the lattice-oxygen-mediated oxygen evolution reaction (LOM-OER); Figure 3B).
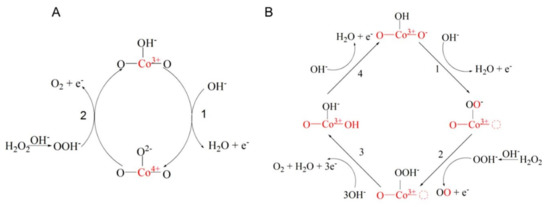
Figure 3.
Electro-oxidation mechanisms of H2O2 on La0.6Sr0.4CoO3-δ that occur simultaneously via the Co3+/Co4+ redox couple (A) and lattice-oxygen-mediated oxygen evolution reaction (LOM-OER) (B) involving oxygen vacancies and superoxide ion (O22−/O−) [32]. Reproduced with the permission from Elsevier.
Regarding the analytical performance of the perovskite-based electrochemical sensors for H2O2, the obtained lower detection limits are 1 nM and 2 nM, obtained with LaNi0.6Co0.4O3 and with LaCo0.4Fe0.6O3, respectively [28,29].
The specific surface area of the perovskite can be increased by different preparation procedures, showing that this parameter is also of importance for the analytical performance. Electrospun nanofibers are prepared by mixing La0.7Sr0.3Mn0.75Co0.25O3 [27] and with LaNiO3 [22] in PVP. Higher sensitivities of detection (more than 1000 µA/mM/cm2) and large dynamic ranges until 1000 µM are obtained. A three-dimensional (3D) ordered microporous SmCoO3 perovskite is prepared using a poly(methylmethacrylate) colloidal crystal template route [25]. A detection limit of 4 nM and a dynamic range from 0.1 to 5000 µM are obtained.
The association of perovskites with conductive nanomaterials allows a decrease of the detection limit. When perovskite LaMnO3 is intimately mixed with a conductive carbon black, forming a composite, a very low detection limit of 0.805 nM is obtained. [33]. It was also noticed that the mixture of La0.6Sr0.4CoO3-δ with reduced graphene oxide [32] improves the sensitivity by a factor of 2 and decreases the detection limit also by a factor of 2.
The storage stability of these perovskite-based sensors is in the range of one month.
4. Perovskite-Based Electrochemical Sensors for the Detection of Glucose
As for hydrogen peroxide detection, numerous works on the detection of glucose (Table 2) are carried out using La-based perovskites. In some of them, La is substituted by alkaline earth such as strontium [31,32].

Table 2.
Perovskite-based electrochemical sensors for the detection of glucose.
The complex mechanism for the electrooxidation of glucose on La0.6Sr0.4CoO3-δ at 0.06 V/Ag/AgCl, in 0.1 M NaOH is presented in [32] and is similar to the proposed mechanism for the electrooxidation of H2O2, according to two pathways via the Co3+/Co4+ redox couple and via the LOM-OER.
The lower detection limit obtained with La-based perovskite (8 nM) is obtained with LaNi0.6Co0.4O3 [28]. The perovskite gives also a lower detection limit for H2O2 (Table 1), showing the higher catalytic effect of B-site ions Ni and Co. Two La-based perovskites are associated with conductive nanomaterials such as AgNPs [41,42], leading to a very low detection limit (2.5 nM) with an Electrochemiluminescence (ECL) sensor [41].
Other lanthanide A-site ions are used, such as praseodynme [31] and neodyme [33], substituted by alkaline earth such as Ba [35]. Another used A-site ion is an alkaline earth ion, strontium [36,38,43]. The lower detection limit of 2.11 nM was obtained with Sr2Pd0.7Au0.3O3 [38]. SrPdO3 is also associated with conductive nanomaterials such as AuNPs [43], which allows the selective detection of glucose in the presence of other bioactive interfering species ascorbic acid (AA), uric acid (UA), N-acetyl-para-aminophenol or paracetamol (APAP), and DA to be shown through linear sweep voltammetry (LSV) in the case of SrPdO3/AuNPs (Figure 4) [43].
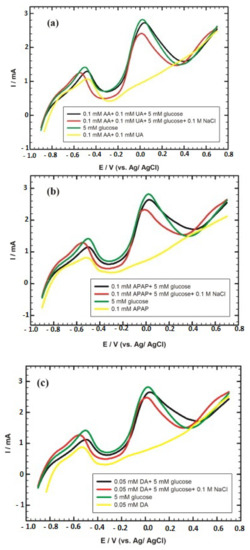
Figure 4.
(a) Linear sweep voltammetry (LSV) curves of 5 mM glucose/0.1 M NaOH in the absence and the presence of 0.1 mM ascorbic acid (AA), 0.1 mM uric acid (UA) and 0.1 M NaCl at graphite/SrPdO3/AuNPs. (b) LSV curves of 5 mM/0.1 M NaOH in the absence and the presence of 0.1 mM N-acetyl-para-aminophenol or paracetamol (APAP) and 0.1 M NaCl at graphite/SrPdO3/AuNPs. (c) LSV curves of 5 mM glucose/0.1 M NaOH in the absence and the presence of 0.05 mM dopamine (DA) and 0.1 M NaCl at graphite/SrPdO3/AuNPs [43]. Reproduced with the permission from Elsevier.
5. Perovskite-Based Electrochemical Sensors for the Detection of DA
Seventeen papers from 2014 to 2021 are devoted to the electrocatalytic detection of DA using perovskite nanomaterials (Table 3).

Table 3.
Perovskite-based electrochemical sensors for the detection of DA and other bioactive molecules.
The electrooxidation of DA on FeTiO3 is depicted in Figure 5 [52]. The adsorption of DA on the FeTiO3 surface (including the oxygen deficient sites) occurs, due to the electrostatic force of attraction. The oxidation process, with respect to the applied potential, takes place according to two pathways via the Ti3+/Ti4+ and Fe2+/Fe3+ redox couples and via the LOM-OER.
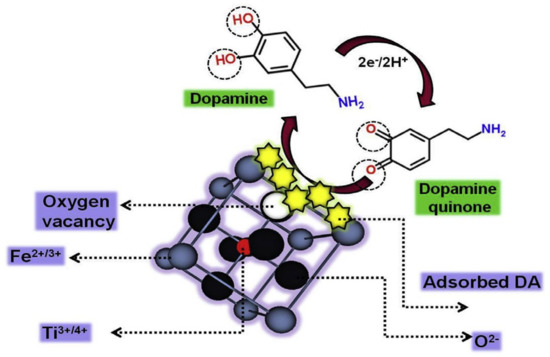
Figure 5.
Electrooxidation mechanism of DA on FeTiO3 [52]. Reproduced with the permission from Elsevier.
With La-based perovskites, the B-site ions are, as for hydrogen peroxide and glucose detection, Fe, Co, Mn, or Ni. In [49], the electrochemical detection of DA is performed with LaFeCO3, LaCoO3, and LaNiO3 for the comparison of the influence of the B-site cations. The lower detection limit of 9 nM is obtained with LaNiO3. This result was explained by the fact that the energy of the 3d electron present in Ni3+ is higher than those in Co3+ and Fe3+. Then, the energy of the 3d electron may become higher than that of the orbital energy (LUMO) of the analyte in the solution. Thermodynamically favorable energy transfer is then possible towards the LaNiO3-modified electrode, better than towards the other perovskite-modified electrodes. Among other lanthanide A-site ions, it was shown that between neodyme and samarium, Nd-based perovskite (NdFeO3) presents the lower detection limit of 270 nM [56]. Other A-site ions are alkali ions such as sodium [53,55], cesium [59], alkaline-earth ions such as strontium [50,54,58], iron ions [52], or zinc ions [57]. Associated B-site ions, involved in one of the pathways of oxidation of DA to DA hydroquinone (Figure 5), are palladium [50], titanium [52,54], niobium [53,59], iron [55], tin [57], and lead [59]. The lower detection limits (1–3 nM) are obtained with FeTiO3 [53], β-NaFeO2 [55], ZnSnO3 [57], and CsPbBr3 [59].
Apart from the sensitivity of detection, the simultaneous detection of DA in the presence of other bioactive molecules is another concern. A simultaneous detection of two neurotransmitters, DA and serotonin, in the presence of acetaminophen and tyrosine is obtained with LaNiO3 (Figure 6) [49].
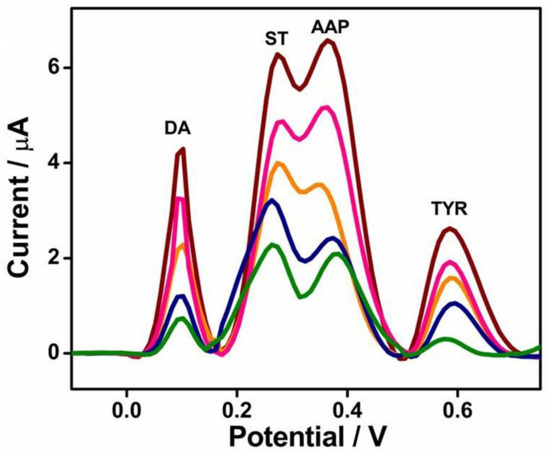
Figure 6.
Interference measurement of LaNiO3/carbon paste electrode (CPE) sensor using diffential pulse voltammetry (DPV) technique in 0.1 M PBS of pH 7 containing varying concentrations of DA (from green to garnet curve 0.005 to 0.045 mM), serotonine (from green to garnet curve 0.03 to 0.17 mM), acetaminophen (from green to garnet curve 0.03 to 0.09 mM), and tyrosine (from green to garnet curve 0.0008 to 0.04 mM) [49]. Reproduced with the permission from Elsevier.
Inadequate levels of DA in human blood sera leads to neurological desorders such as Parkinson’s disease, while abnormal levels of UA, xanthine, and hypoxanthine result in gout, pneumonia, and others. Therefore, the simultaneous detection of these biomarkers is of high interest. It has been obtained using electrocatalysis with β-NaFeO2 perovskite (Figure 7) [55].
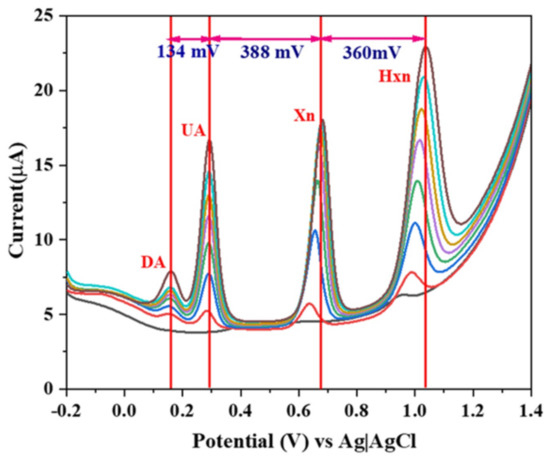
Figure 7.
DPV response of a β-NaFeO2-modified carbon paste electrode at different concentrations of DA (from red to black curve 10 nM to 70 nM), UA (from red to black curve 5 µM to 200 µM), Xn (from red to black curve 5 µM to 200 µM), and Hxn (from red to black curve 5 µM to 200 µM) [55]. Reproduced with the permission from Elsevier.
6. Comparison of Perovskite-Based Electrochemical Sensors with Ni and Co Oxide-Based Electrochemical Sensors
Nickel oxide nanomaterials and cobalt oxide nanomaterials are also used due to their electrocatalytic properties for the detection of electroactive molecules as hydrogen peroxide, glucose, and DA. The works published in the five last years, where the oxide nanomaterials were not associated with other nanomaterials such as graphene, carbon nanotubes, or metallic nanoparticles, are presented in Table 4.

Table 4.
Nickel oxide and cobalt oxide-based electrochemical sensors for the detection of bioactive molecules.
Hydrogen peroxide is detected with a 3D porous Co3O4/Nafion-modified GCE at 0.31 V/SCE, in a 0.1 M NaOH solution, and a detection limit of 0.24 µM is obtained [68]. A detection limit of 1 nM is obtained with a LaNi0.6Co0.4O3 carbon paste electrode [28], and a detection limit of 2 nM is obtained with a Co0.4Fe0.6LaO3 carbon paste electrode [29], with the voltammetric signal being obtained at 0.55 V/SCE in 0.1 M NaOH. A detection limit of 23 nM is obtained with a LaNi0.5Ti0.5O3/CoFe2O3-modified GCE [30], and a detection limit of 35 nM is obtained with a LaNiO3/Nafion-modified GCE [34], with the voltammetric signal being obtained at 0.6 V/SCE [30] and at −0.5 V/SCE (cathodic peak) [34] in 0.1 M NaOH. When the experimental conditions are similar, the detection limit for hydrogen peroxide obtained with lanthanum-based perovskite is lower than that obtained with cobalt oxide nanomaterials.
When glucose is detected with NiO-modified electrodes, the obtained detection limits are between 0.1 µM and 8.1 µM [60,61,62,63,64], with glucose being detected between 0.48 V/SCE and 0.58 V/SCE, in 10−4 M–0.5 M NaOH. When glucose is detected with Co3O4-modified electrodes, the obtained detection limits are between 0.1 µM to 5 µM [68,69,70], with glucose being detected between 0.47 V/SCE and 0.6 V/SCE, in 0.1–0.3 M NaOH. With a Sr2Pd0.7Au0.3O3-modified graphite electrode, a detection limit of 2.11 nM is obtained [38], and with a LaNi0.6Co0.4O3 carbon paste electrode, a detection limit of 8.0 nM is obtained [28], with glucose being detected in a 0.1 M NaOH solution at −76 mV/SCE (with Sr2Pd0.7Au0.3O3) and at 0.55 V/SCE (with LaNi0.6Co0.4O3). The experimental conditions for glucose detection in the presence of lanthanum-based perovskites are very close to those for glucose detection in the presence of oxide-modified electrodes, but the obtained detection limit is lower. In the presence of Pd-Au-B site strontium perovskite, the detection conditions are quite different (very low oxidation potential), and the detection limit is quite lower [38].
When DA is detected with NiO-modified electrodes, the obtained detection limits are between 85 nM and 690 nM [65,66,67], with DA being detected at 0.2 V/SCE, at pH between 6 and 7.4. When DA is detected with a LaNiO3 carbon paste electrode, a detection limit of 9 nM is obtained [49], with the detection being obtained at very similar conditions at 0.1 V/SCE and pH 7. When DA is detected with a FeTiO3-modified GCE, a detection limit of 1.3 nM is obtained [52], with the detection being obtained at very similar conditions at 0.15 V/SCE in PBS. Besides the detection limit, another point is the simultaneous detection of DA with possible interfering molecules such as AA and UA. With a LaFeO3-modified GCE, the detection limit of DA is 10 nM, and the positions of the different anodic peaks are 0.25 V/SCE, −0.05 V/SCE, and 0.38 V/SCE for DA, AA, and UA, respectively. With NiO-modified electrodes, the anodic peak of DA is at 0.21 V/SCE, and the anodic peaks of AA and UA are at 0.02 V/SCE and at 0.32 V/SCE, respectively. When comparing the DA–AA peak distance, it comes that with a LaFeO3-modified GCE, it is 0.30 V/SCE and with a NiO-modified electrode, it is between 0.19 V/SCE and 0.23 V/SCE, which is lower. When comparing the DA–UA peak distance, it comes that with a LaFeO3-modified GCE, it is 0.13 V/SCE and with a NiO-modified electrode, it is 0.11 V/SCE. It appears that with a LaFeO3-modified GCE, the DA anodic peak is more separated from the interfering peaks.
All these examples show that with lanthanum-based perovskites, with Ni or Co B-site, lower detection limits compared to with nickel and cobalt oxides are obtained and much lower detection limits could be obtained with, for instance, Sr-Pd and Fe-Ti-based perovskites, without any other nanomaterials. Moreover, with LaFeO3, the distance between the anodic peaks of the interfering compounds can be larger.
7. Conclusions and Perspectives
A lot of ABO3 perovskites are used for their electrocatalytic properties applied for the sensitive electrochemical detection of bioactive molecules: hydrogen peroxide, glucose, and DA. La-based perovskites, containing Ni and Co ions as B-site, present a higher sensitivity of detection, with the detection limit being in the range of 1–10 nM. The proposed mechanism for the electrooxidation of the bioactive molecules takes place in two pathways via the B3+/B4+ redox couple and via the LOM-OER. The experimental conditions for the hydrogen peroxide, glucose, and DA detection in the presence of lanthanum-based perovskites are very close to those for their detection in the presence of oxide-modified electrodes, but the obtained detection limit is lower due to the involved complex mechanism of detection.
The main trends for improving the analytical performance are the nanostructuration of perovskite materials (nanocrystals, nanoneedles, etc.) for increasing their specific surface areas and the association of perovskites with conductive nanomaterials such as carbon nanotubes, graphene, and metallic nanomaterials. Some perovskites are commercially available; for instance, CsPbBr3 is in form of quantum dots or others are in form of powders or can be used for sputtering. They could also be easily used due to their electrocatalytic properties.
The applications of perovskite-modified electrodes are numerous, due to the large range of detectable molecules and the concerned biomedical, environmental, and agrifood fields. For limiting the environmental impact of perovskite nanomaterials and due to the scarcity and the toxicity of rare earth elements, it is necessary to recycle these elements as well as heavy metals (Ni, Co, and Pb), using solid-state extraction, for instance graphite/magnetite nanocomposites.
Author Contributions
Conceptualization, A.Z. and I.B.; resources, A.E.; writing—original draft preparation, I.B.; writing—review and editing, N.J.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CAMPUS FRANCE, through PHC Maghreb #39382RE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
I.Boubezari thanks the government of Algeria for her work-study grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H.T. Emerging Technologies for Next-Generation Point-of-Care Testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Lockhart, M.J.; Smith, D. Should continuous glucose monitoring systems be offered to all patients with type 1 diabetes mellitus? Ir. J. Med. Sci. 2021, 1–14, Online ahead of print. [Google Scholar] [CrossRef]
- Sackner-Bernstein, J. Estimates of Intracellular Dopamine in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Parkinsons Dis. 2021, 11, 1011–1018. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, N. Electrochemical Biosensors Based on Micro-fabricated Devices for Point-of-care Testing: A Review. Electroanalysis 2021, 33, 1–17. [Google Scholar]
- Mass, M.; Veiga, L.S.; Garate, O.; Longinotti, G.; Moya, A.; Ramón, E.; Villa, R.; Ybarra, G.; Gabriel, G. Fully Inkjet-Printed Biosensors Fabricated with a Highly Stable Ink Based on Carbon Nanotubes and Enzyme-Functionalized Nanoparticles. Nanomaterials 2021, 11, 1645. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, G.; Xu, T.; Su, L.; Yan, D.; Zhang, X. Ruthenium-based Conjugated Polymer and Metal-organic Framework Nanocomposites for Glucose Sensing. Electroanalysis 2021, 33, 1902–1910. [Google Scholar] [CrossRef]
- Baluta, S.; Zaja, D.; Szyszka, A.; Malecha, K.; Cabaj, J. Enzymatic Platforms for Sensitive Neurotransmitter Detection. Sensors 2020, 20, 423. [Google Scholar] [CrossRef] [Green Version]
- Wolfram, T.; Ellialtioglu, S. Electronic and Optical Properties of d-Band Perovskites; Cambridge University Press: Cambridge, UK, 2009; ISBN 9780511541292. [Google Scholar]
- Szafraniak, B.; Fusnik, L.; Xu, J.; Gao, F.; Brudnik, A.; Rydosz, A. Semiconducting metal oxides: SrTiO3, BaTiO3 and BaSrTiO3 in gas-sensing applications: A review. Coatings 2021, 11, 185. [Google Scholar] [CrossRef]
- Atta, N.F.; El-Ads, E.H.; Galal, A. Perovskites: Smart Nanomaterials for Sensory Applications in Designing Nanosensors for Chemical and Biological Applications; Atta, N.F., Ed.; International Frequency Sensor Association Publishing: Barcelona, Spain, 2017; pp. 149–205. ISBN 978-84-697-3290-8. [Google Scholar]
- Assirey, E.A.R. Perovskite synthesis, properties and their related biochemical and industrial application. Saudi Pharm. J. 2019, 27, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.W.; Ramachandran, R.; Chen, S.M.; Kavitha, N.; Dinakaran, K.; Kannan, R.; Anushya, G.; Bhuvana, N.; Jeyapragasam, T.; Mariyappan, V.; et al. Developing low-cost, high performance, robust and sustainable perovskite electrocatalytic materials in the electrochemical sensors and energy sectors: “An overview”. Catalysts 2020, 10, 938. [Google Scholar] [CrossRef]
- Chen, T.W.; Ramachandran, R.; Chen, S.M.; Anushya, G.; Ramachandran, K. Graphene and perovskite-based nanocomposite for both electrochemical and gas sensor applications: An overview. Sensors 2020, 20, 6755. [Google Scholar] [CrossRef] [PubMed]
- Thatikayala, D.; Ponnamma, D.; Sadasivuni, K.K.; Cabibihan, J.J.; Al-Ali, A.K.; Malik, R.A.; Min, B. Progress of advanced nanomaterials in the non-enzymatic electrochemical sensing of glucose and H2O2. Biosensors 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Jesna, G.K.; Halali, V.V.; Sanjayan, C.G.; Suvina, V.; Sakar, M.; Balakrishna, R.G. Perovskite nanomaterials as optical and electrochemical sensors. Inorg. Chem. Front. 2020, 7, 2702–2725. [Google Scholar]
- Moure, C.; Pena, O. Recent advances in perovskites: Processing and properties. Prog. Solid State Chem. 2015, 43, 123–148. [Google Scholar] [CrossRef]
- Deganello, F.; Marcì, G.; Deganello, G. Citrate—nitrate auto-combustion synthesis of perovskite-type nanopowders: A systematic approach. J. Eur. Ceram. Soc. 2009, 29, 439–450. [Google Scholar]
- Luque, G.L.; Ferreyra, N.F.; Leyva, A.G.; Rivas, G.A. Characterization of carbon paste electrodes modified with manganese based perovskites-type oxides from the amperometric determination of hydrogen peroxide. Sens. Actuat. B Chem. 2009, 142, 331–336. [Google Scholar] [CrossRef]
- Vijayaraghavan, T.; Sivasubramanian, R.; Shamima Hussain, S.; Ashok, A. A facile synthesis of LaFeO3-based perovskites and their application towards sensing of neurotransmitters. Chem. Select 2017, 2, 5570–5577. [Google Scholar] [CrossRef]
- Wang, B.; Gu, S.; Ding, Y.; Chu, Y.; Zhang, Z.; Ba, X.; Zhang, Q.; Li, X. A novel route to prepare LaNiO3 perovskite-type oxide nanofibers by electrospinning for glucose and hydrogen peroxide sensing. Analyst 2013, 138, 362–367. [Google Scholar] [CrossRef]
- Anh, D.T.V.; Olthuis, W.; Bergveld, P. Sensing properties of perovskite oxide La1-xSrxCoO3-δ obtained by using pulsed laser deposition. Sens. Actuat. B Chem. 2004, 103, 165–168. [Google Scholar] [CrossRef]
- Deganello, F.; Liotta, L.F.; Leonardi, S.G.; Neri, G. Electrochemical properties of Ce-doped SrFeO3 perovskites-modified electrodes towards hydrogen peroxide oxidation. Electrochim. Acta 2016, 190, 939–947. [Google Scholar] [CrossRef]
- He, J.; Zhou, W.; Sunarso, J.; Xu, X.; Zhong, Y.; Shao, Z.; Chen, X.; Zhu, H. 3D ordered macroporous SmCoO3 perovskite for highly active and selective hydrogen peroxide detection. Electrochim. Acta 2018, 260, 372–383. [Google Scholar] [CrossRef]
- Shimizu, Y.; Komatsu, H.; Michishita, S.; Miura, N.; Yamazo, N. Sensing characteristics of hydrogen peroxide sensor using carbon-based electrode loaded with perovskite-type oxide. Sens. Actuat. B Chem. 1996, 34, 493–498. [Google Scholar] [CrossRef]
- Xu, D.; Li, L.; Ding, Y.; Cui, S. Electrochemical hydrogen peroxide sensors based on electrospun La0.7Sr0.3Mn0.75Co0.25O3 nanofiber modified electrodes. Anal. Methods 2015, 7, 6083–6088. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, S.; Ding, Y.; Jin, J. A novel nonenzymatic sensor based on LaNi0.6Co0.4O3 modified electrode for hydrogen peroxide and glucose. Anal. Chim. Acta 2012, 745, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gu, S.; Ding, Y.; Zhang, F.; Jin, J. Determination of hydrogen peroxide and glucose using a novel sensor platform based on Co0.4Fe0.6LaO3 nanoparticles. Microchim. Acta 2013, 180, 1043–1049. [Google Scholar] [CrossRef]
- Ye, D.; Xu, Y.; Luo, L.; Ding, Y.; Wang, Y.; Liu, X.; Xing, L.; Peng, J. A novel non-enzymatic hydrogen peroxide sensor based on LaNi0.5Ti0.5O3/CoFe2O4 modified electrode. Colloid Surf. B Biointerfaces 2012, 89, 10–14. [Google Scholar] [CrossRef]
- Liotta, L.F.; Puleo, F.; La Parola, V.; Leonardi, S.G.; Donato, N.; Aloisio, D.; Neri, G. La0.6Sr0.4FeO3-δ and La0.6Sr0.4Co0.2Fe0.8O3-δ Perovskite materials for H2O2 and glucose electrochemical sensors. Electroanalysis 2015, 27, 684–692. [Google Scholar] [CrossRef]
- He, J.; Sunarso, J.; Zhu, Y.; Zhong, Y.; Miao, J.; Zhou, W.; Shao, Z. High-performance non-enzymatic perovskite sensor for hydrogen peroxide and glucose electrochemical detection. Sens. Actuat. B Chem. 2017, 244, 482–491. [Google Scholar] [CrossRef]
- Karuppiah, C.; Kohila rani, K.; Wang, S.F.; Devasenathipathy, R.; Yang, C.C. Dry particle coating preparation of highly conductive LaMnO3@C composite for the oxygen reduction reaction and hydrogen peroxide sensing. J. Taiwan Inst. Chem. Eng. 2018, 93, 94–102. [Google Scholar] [CrossRef]
- Ahmadi, E.; Mohammad Bagher Gholivand, M.B.; Karamic, C. Enzyme-less amperometric sensor manufactured using a Nafion–LaNiO3 nanocomposite for hydrogen peroxide. RSC Adv. 2020, 10, 23457–23465. [Google Scholar] [CrossRef]
- Boubezari, I.; Zazoua, A.; Bessueille, F.; Errachid, A.; Jaffrezic-Renault, N. Design of a new non-enzymatic sensor based on a substituted A2BO4+δ perovskite for the voltammetric detection of glucose. Electroanalysis 2020, 32, 1642–1650. [Google Scholar] [CrossRef]
- El Ads, E.H.; Galal, A.; Atta, N.F. The effect of A-site doping in a strontium palladium perovskite and its applications for non-enzymatic glucose sensing. RSC Adv. 2016, 6, 16183–16196. [Google Scholar] [CrossRef]
- Sivakumar, M.; Pandi, K.; Chen, S.M.; Cheng, Y.H.; Sakthivel, M. Facile synthesis of perovskite-type NdNiO3 nanoparticles for an effective electrochemical non-enzymatic glucose biosensor. New J. Chem. 2017, 41, 11201–11207. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; El-Ads, E.H. Effect of B-site doping on Sr2PdO3 perovskite catalyst activity for non-enzymatic determination of glucose in biological fluids. J. Electroanal. Chem. 2019, 852, 113523. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Luo, L.; Ding, Y.; Liu, X.; Huang, A. A novel sensitive nonenzymatic glucose sensor based on perovskite LaNi0.5Ti0.5O3-modified carbon paste electrode. Sens. Actuat. B Chem. 2010, 151, 65–70. [Google Scholar] [CrossRef]
- Xu, D.; Luo, L.; Ding, Y.; Xu, P. Sensitive electrochemical detection of glucose based on electrospun La0.88Sr0.12MnO3 nanofibers modified electrode. Anal. Biochem. 2015, 489, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.F.; Zhong, H.; Zhang, W.G.; Li, X.R.; Wang, G.Y.; Song, J.; Cheng, Z.P.; Yin, J.Z.; Guo, L.P. A novel nonenzymatic ECL glucose sensor based on perovskite LaTiO3-Ag0.1 nanomaterials. Sens. Actuat. B Chem. 2015, 212, 174–182. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhong, H.; Li, X.M.; Jia, F.F.; Shi, Y.X.; Zhang, W.G.; Cheng, Z.P.; Zhang, L.L.; Wang, J.K. Perovskite LaTiO3–Ag0.2 nanomaterials for non-enzymatic glucose sensor with high performance. Biosens. Bioelectron. 2013, 48, 56–60. [Google Scholar] [CrossRef] [PubMed]
- El Ads, E.H.; Galal, A.; Atta, N.F. Electrochemistry of glucose at gold nanoparticles modified graphite/SrPdO3 electrode–towards a novel non-enzymatic glucose sensor. J. Electroanal. Chem. 2015, 749, 42–52. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Mastelaro, V.R.; Ganesh, V.; Ponpandian, N. Detection of the neurotransmitter dopamine by a glassy carbon electrode modified with self-assembled perovskite LaFeO3 microspheres made up of nanospheres. RSC Adv. 2014, 4, 25957–25962. [Google Scholar] [CrossRef]
- Priyatharshni, S.; Tamilselvan, A.; Viswanathan, C.; Ponpandian, N. LaCoO3 nanostructures modified glassy carbon electrode for simultaneous electrochemical detection of dopamine, ascorbic acid and uric Acid. J. Electrochem. Soc. 2017, 164, B152–B158. [Google Scholar] [CrossRef]
- Tohidinia, M.; Sabbaghi, N.; Shaybani, S.; Noroozifar, M. Simultaneous determination of dopamine, acetaminophen and xanthine by modified carbon ceramic micro-electrode with nanosized LaFe0.2Ni0.8O3 perovskite. Anal. Bioanal. Electrochem. 2018, 10, 1525–1537. [Google Scholar]
- Kumar, Y.; Pramanik, S.; Das, D.P. Lanthanum ortho-ferrite (LaFeO3) nano-particles based electrochemical sensor for the detection of dopamine. Biointerface Res. Appl. Chem. 2020, 10, 6182–6188. [Google Scholar]
- Shafi, P.M.; Joseph, N.; Karthik, R.; Shim, J.J.; Bose, A.C.; Ganesh, V. Lemon juice-assisted synthesis of LaMnO3 perovskite nanoparticles for electrochemical detection of dopamine. Microchem. J. 2021, 164, 105945. [Google Scholar] [CrossRef]
- Thomas, J.; Anitha, P.K.; Thomas, T.; Thomas, N. The influence of B-site cation in LaBO3 (B = Fe, Co, Ni) perovskites on the nanomolar sensing of neurotransmitters. Sens. Actuat. B Chem. 2021, 332, 129362. [Google Scholar] [CrossRef]
- Atta, N.F.; Ali, S.M.; El Ads, E.H.; Galal, A. Nano-perovskite carbon paste composite electrode for the simultaneous determination of dopamine, ascorbic acid and uric acid. Electrochim. Acta 2014, 128, 16–24. [Google Scholar] [CrossRef]
- Anajafi, Z.; Naseri, M.; Marini, S.; Espro, C.; Iannazzo, D.; Leonardi, S.G.; Neri, G. NdFeO3 as a new electrocatalytic material for the electrochemical monitoring of dopamine. Anal. Bioanal. Chem. 2019, 411, 7681–7688. [Google Scholar] [CrossRef]
- Aparna, T.K.; Sivasubramanian, R. FeTiO3 nanohexagons based electrochemical sensor for the detection of dopamine in presence of uric acid. Mater. Chem. Phys. 2019, 233, 319–328. [Google Scholar] [CrossRef]
- Durai, L.; Badhulika, S. A facile, solid-state reaction assisted synthesis of a berry-like NaNbO3 perovskite structure for binder-free, highly selective sensing of dopamine in blood samples. New J. Chem. 2019, 43, 11994–12003. [Google Scholar] [CrossRef]
- Gopi, P.K.; Muthukutty, B.; Chen, S.M.; Chen, T.W.; Liu, X.; Alothman, A.A.; Ali, M.A.; Wabaidur, S.M. Platelet-structured strontium titanate perovskite decorated on graphene oxide as a nanocatalyst for electrochemical determination of neurotransmitter dopamine. New J. Chem. 2020, 44, 18431–18441. [Google Scholar] [CrossRef]
- Durai, L.; Badhulika, S. Facile synthesis of large area pebble-like β-NaFeO2 perovskite for simultaneous sensing of dopamine, uric acid, xanthine and hypoxanthine in human blood. Mater. Sci. Eng. C Biomimetic Supramol. 2020, 109, 110631. [Google Scholar] [CrossRef]
- Anajafi, Z.; Naseri, M.; Neri, G. Gas sensing and electrochemical properties of rare earthferrite, LnFeO3 (Ln = Nd, Sm). Ceram. Int. 2020, 46, 26682–26688. [Google Scholar] [CrossRef]
- Durai, L.; Badhulika, S. One pot hydrothermal synthesis of large area nano cube like ZnSnO3 perovskite for simultaneous sensing of uric acid and dopamine using differential pulse voltammetry. IEEE Sensors J. 2020, 20, 13212–13219. [Google Scholar] [CrossRef]
- Wu, S.; Sun, T.; Wang, H.; Fan, Z.; Li, L.; Fan, B.; Liu, L.; Ma, J.; Tong, Z. A sandwich-structured, layered CoTMPyP/Sr2Nb3O10 nanocomposite for simultaneous voltammetric determination of dopamine and ascorbic acid. J. Electroanal. Chem. 2020, 873, 114403. [Google Scholar] [CrossRef]
- Li, Z.; Kang, Q.; Chen, L.; Zhang, B.; Zou, G.; Shen, D. Enhancing aqueous stability and radiative-charge-transfer efficiency of CsPbBr3 perovskite nanocrystals via conductive silica gel coating. Electrochim. Acta 2020, 330, 135332. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Nafady, A.; Soomro, R.A.; Sirajuddin Shrazi, S.T.H.; Abro, M.I.; Willander, M. Glycine-assisted synthesis of NiO hollow cage-like nanostructures for sensitive non-enzymatic glucose sensing. RSC Adv. 2015, 5, 18773–18781. [Google Scholar] [CrossRef]
- Mishra, S.; Yogi, P.; Sagdeo, P.R.; Kumar, R. Mesoporous nickel oxide (NiO) nanopetals for ultrasensitive glucose sensing. Nanoscale Res. Lett. 2018, 13, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, N.; Banerjee, S.; Bhaumik, A. A facile route for the syntheses of Ni(OH)2 and NiO nanostructures as potential candidates for non-enzymatic glucose sensor. J. Colloid Interface Sci. 2018, 516, 121–127. [Google Scholar] [CrossRef]
- Heyser, C.; Schrebler, R.; Grez, P. New route for the synthesis of nickel (II) oxide nanostructures and its application as non-enzymatic glucose sensor. J. Electroanal. Chem. 2019, 832, 189–195. [Google Scholar] [CrossRef]
- Ahmad, R.; Khan, M.; Tripathy, N.; Khan, M.I.R.; Khosla, A. Hydrothermally synthesized nickel oxide nanosheets for non-enzymatic electrochemical glucose. J. Electrochem. Soc. 2020, 167, 107504. [Google Scholar] [CrossRef]
- Reddy, S.; Swany, B.E.K.; Ramakrishana, S.; He, L.; Jayadevappa, H. NiO nanoparticles based carbon paste as a sensor for detection of dopamine. Int. J. Electrochem. Sci. 2018, 13, 5748–5761. [Google Scholar] [CrossRef]
- Emran, M.Y.; Shenashen, M.A.; Mekawy, M.; Azzam, A.M.; Akhtar, N.; Gomaa, H.; Selim, M.M.; Faheem, A.; El-Safty, S.A. Ultrasensitive in-vitro monitoring of monoamine neurotranmetters from dopaminergic cells. Sens. Actuat. B Chem. 2018, 259, 114–124. [Google Scholar] [CrossRef]
- Althagafi, Z.T.; Althakafy, J.T.; Al Jahdaly, B.A.; Awad, M.I. Differential electroanalysis of dopamine in the presence of a large excess of ascorbic acid at a nickel oxide nanoparticle-modified glassy carbon electrode. J. Sensors 2020, 2020, 8873930. [Google Scholar] [CrossRef]
- Han, L.; Yang, D.P.; Liu, A. Leaf-templated synthesis of 3D hierarchical porous cobalt oxide nanostructure as direct electrochemical biosensing interface with enhanced electrocatalysis. Biosens. Bioelectron. 2015, 63, 145–152. [Google Scholar] [CrossRef]
- Kang, L.; He, D.; Bie, L.; Jiang, P. Nanoporous cobalt oxide nanowires for non-enzymatic electrochemical glucose detection. Sens. Actuat. B Chem. 2015, 220, 888–894. [Google Scholar] [CrossRef]
- Soomro, R.A.; Nafady, A.; Ibupoto, Z.H.; Sirajuddin; Sherazi, S.T.H.; Willander, M.; Abro, M.I. Development of sensitive non-enzymatic glucose sensor using complex nanostructures of cobalt oxide. Mat. Sci. Semicond. Process. 2015, 34, 373–381. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).