Hierarchical Porous Carbon Cobalt Nanocomposites-Based Sensor for Fructose
Abstract
1. Introduction
2. Experimental
2.1. Materials, Characterization Instruments, and Methods
2.2. Synthesis of Hierarchical Porous Carbon Cobalt and Cobalt Oxide Nanocomposites
2.3. Preparation of Modified Electrodes
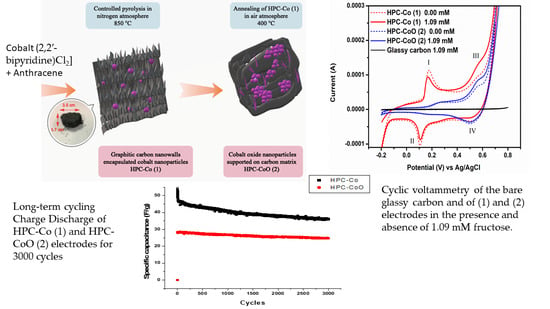
3. Results and Discussion
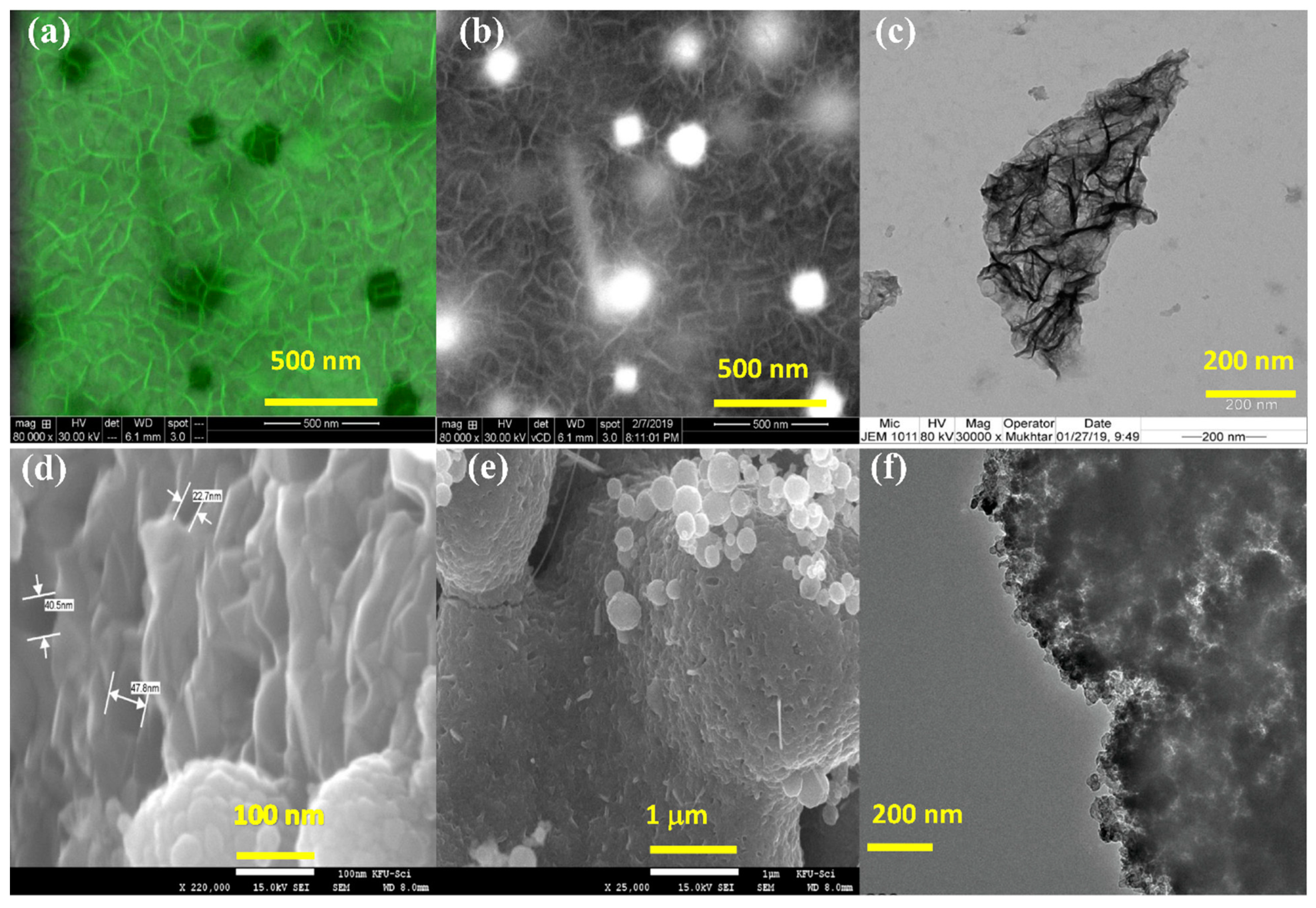
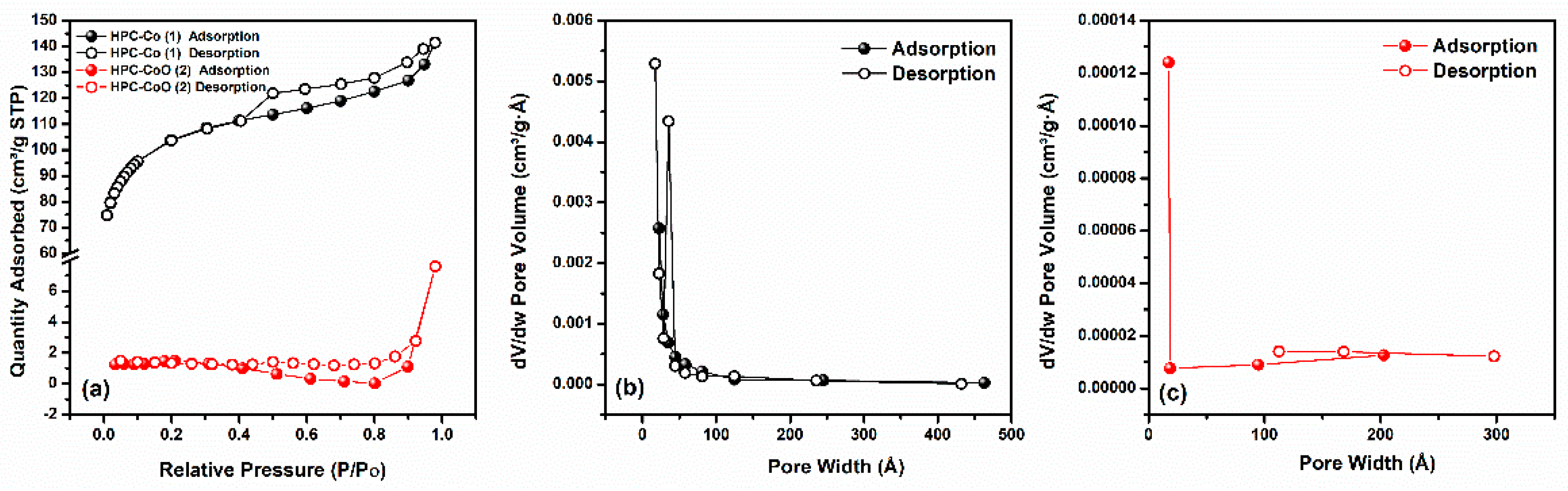
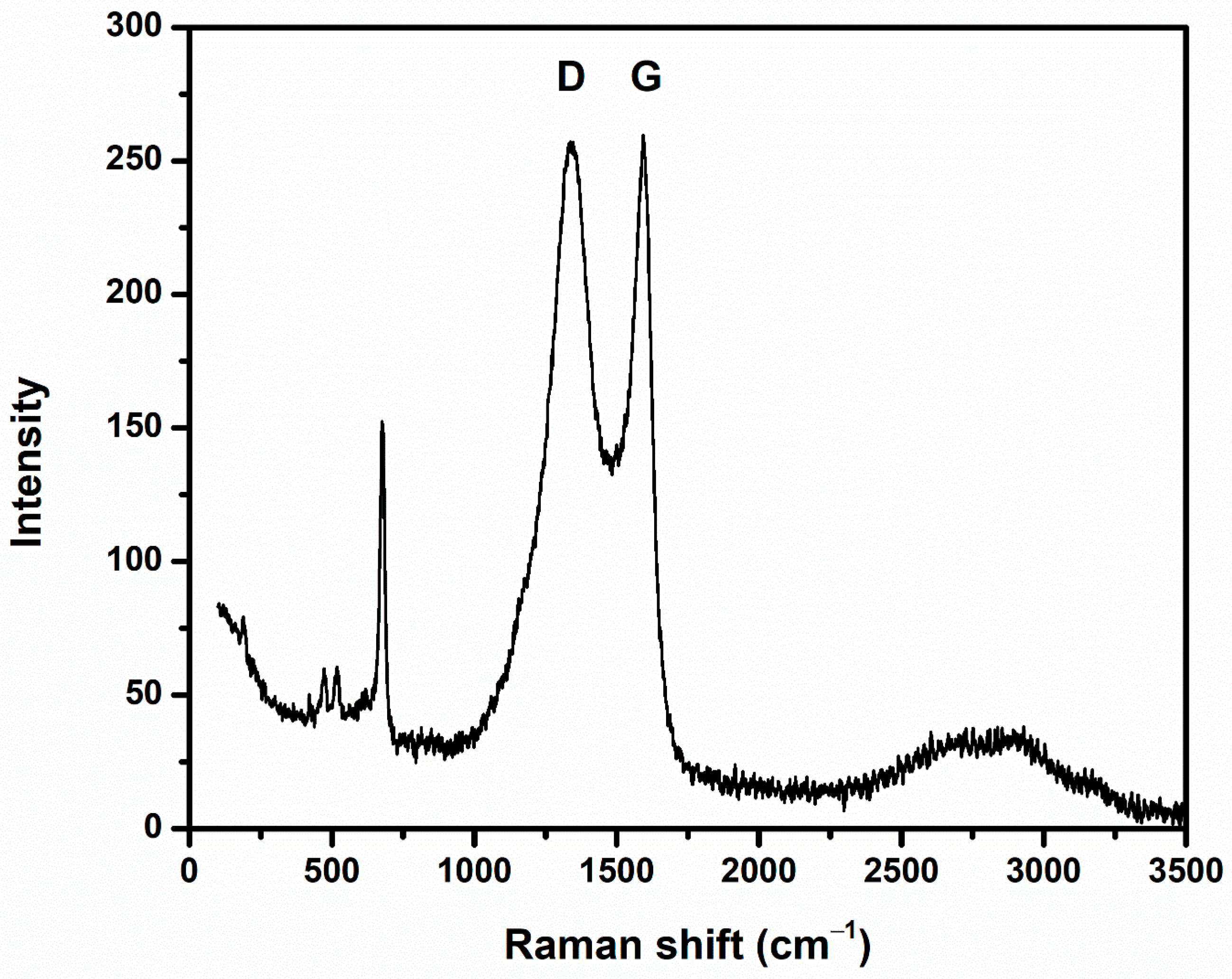
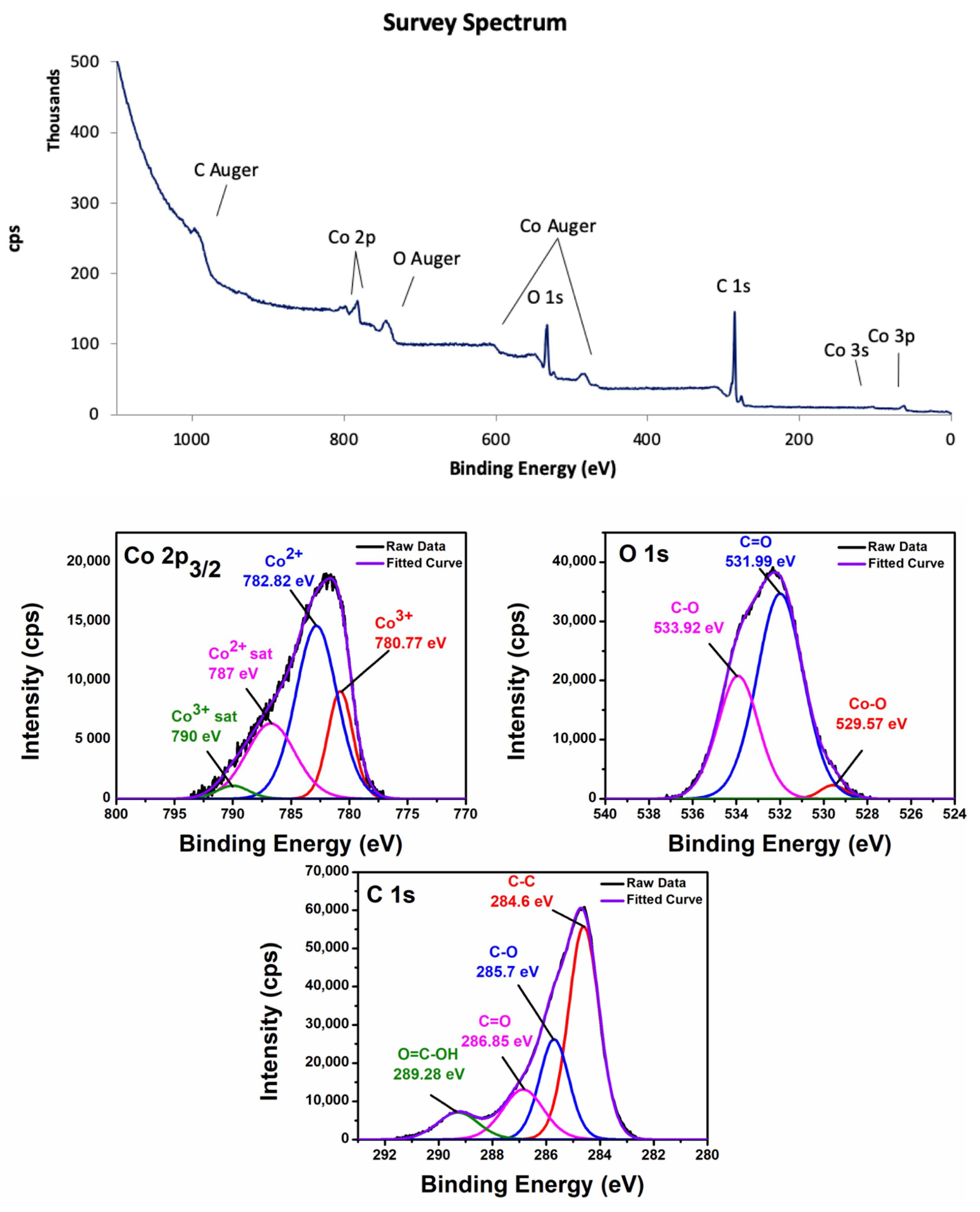
3.1. Characterization of Nanocomposites
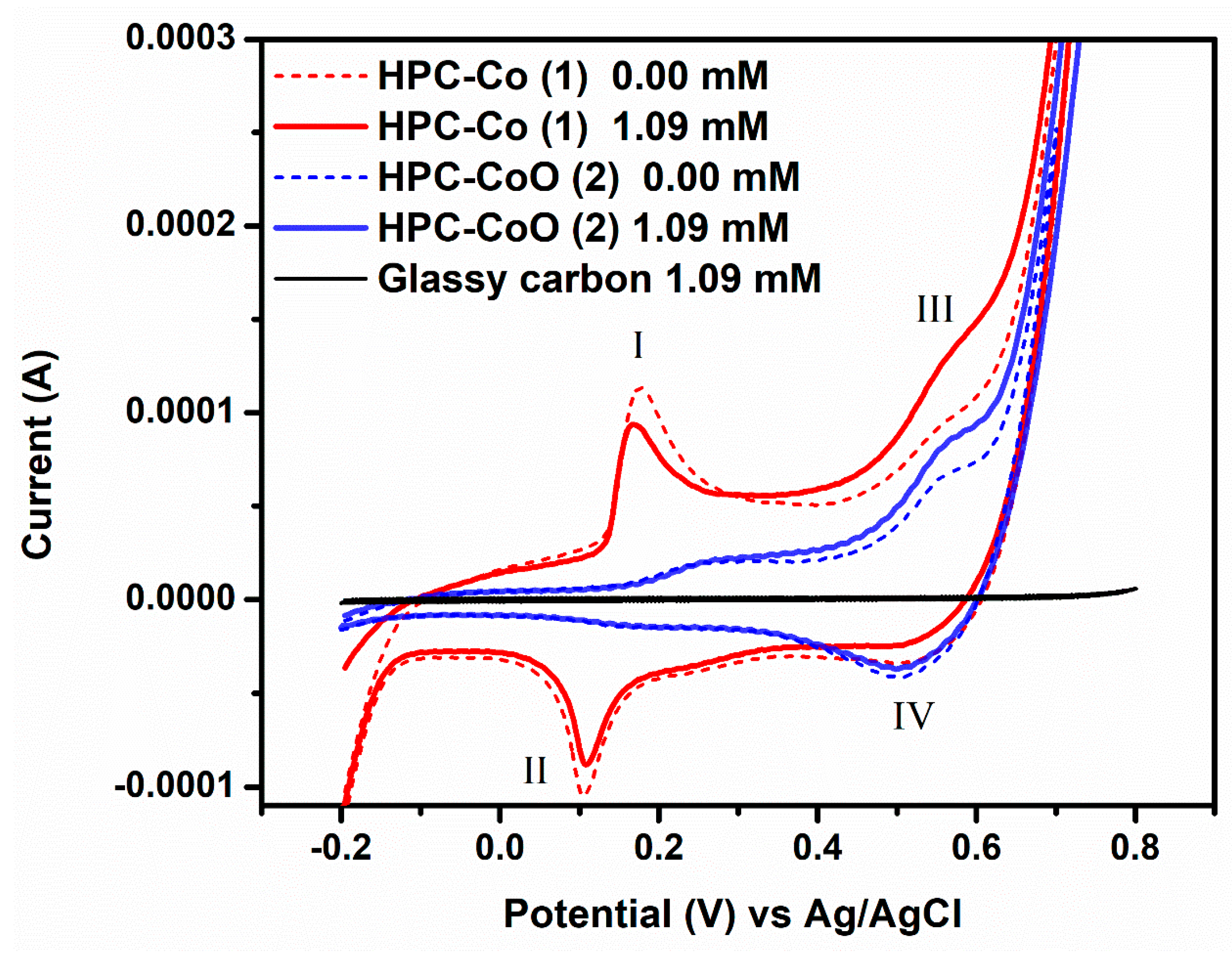
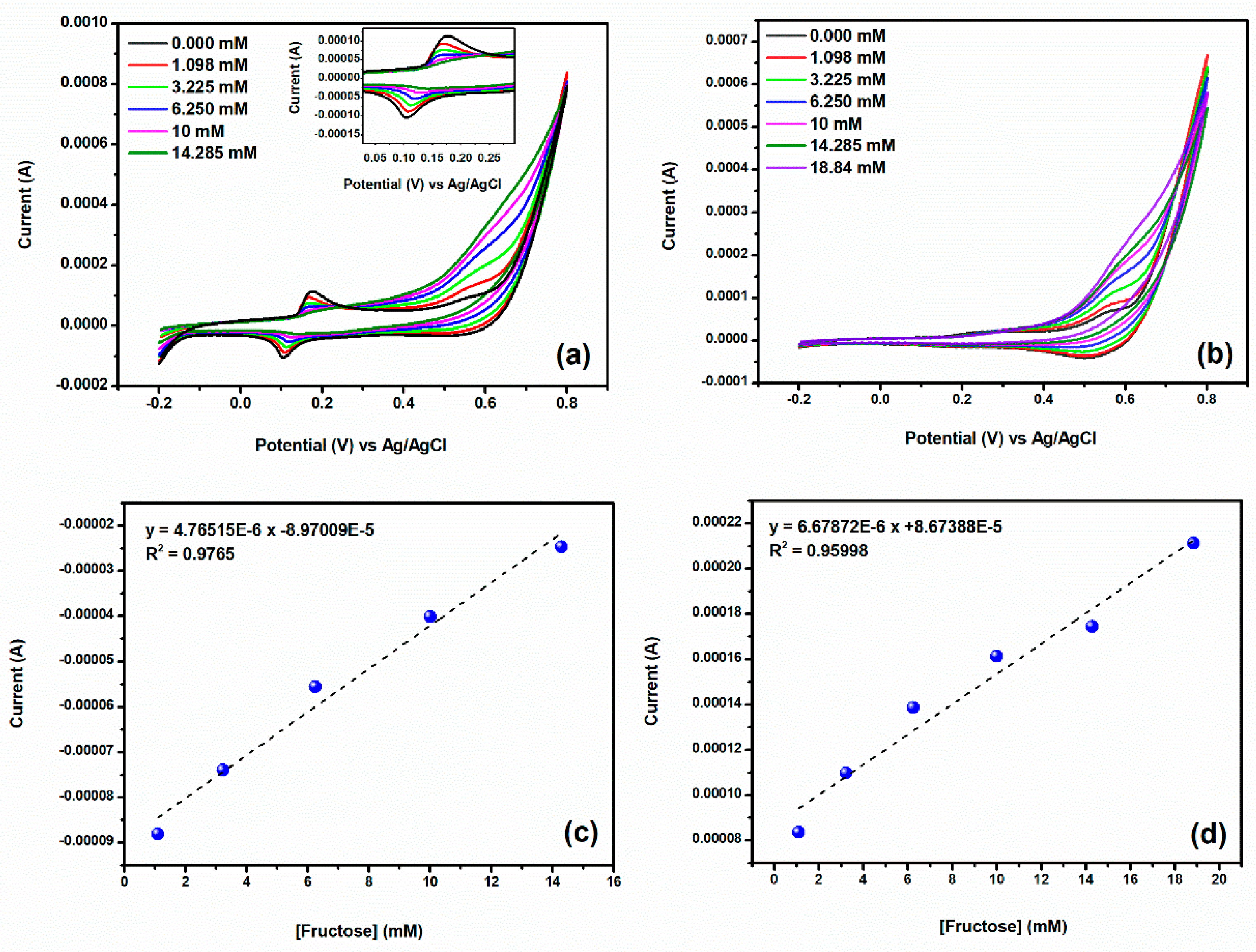
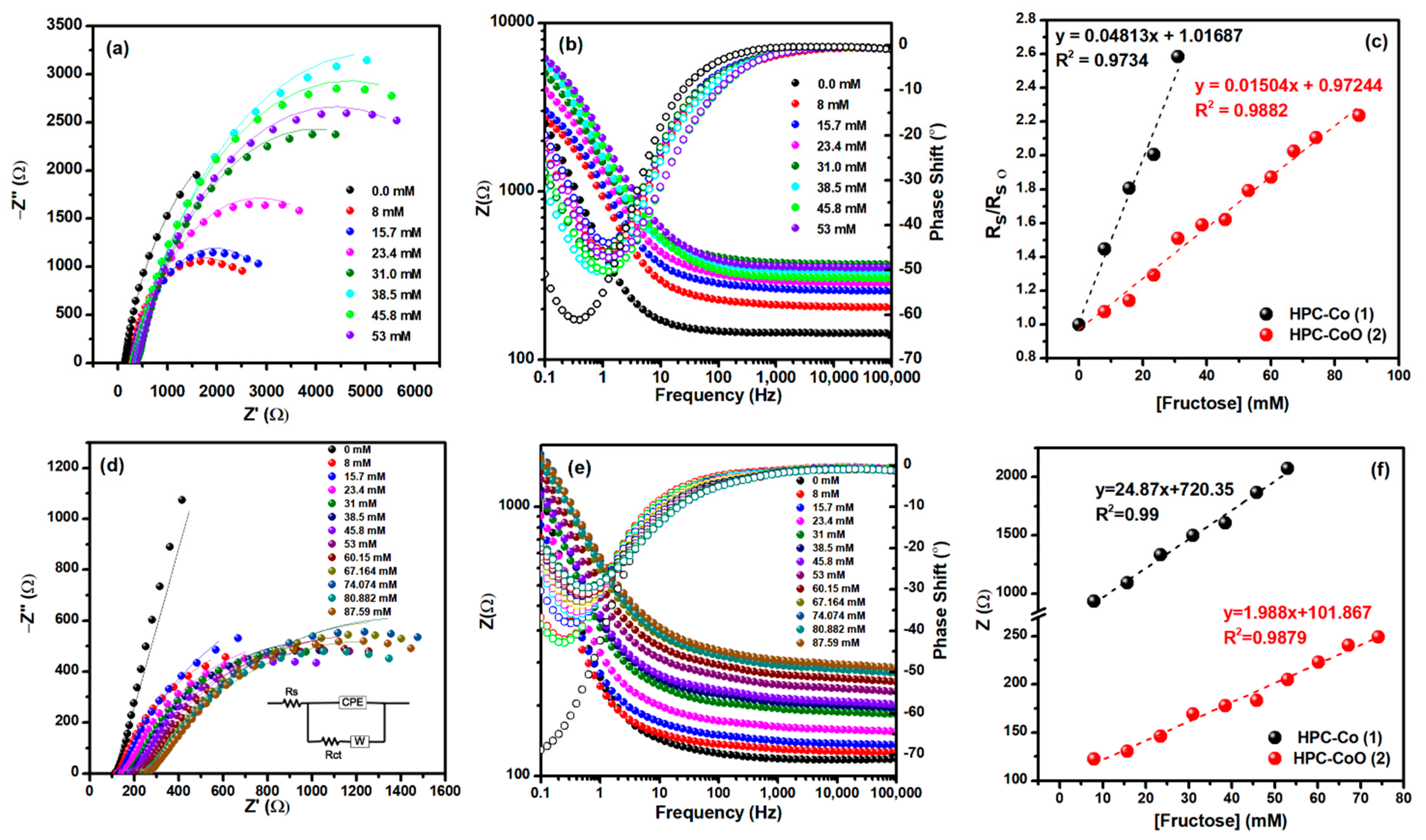
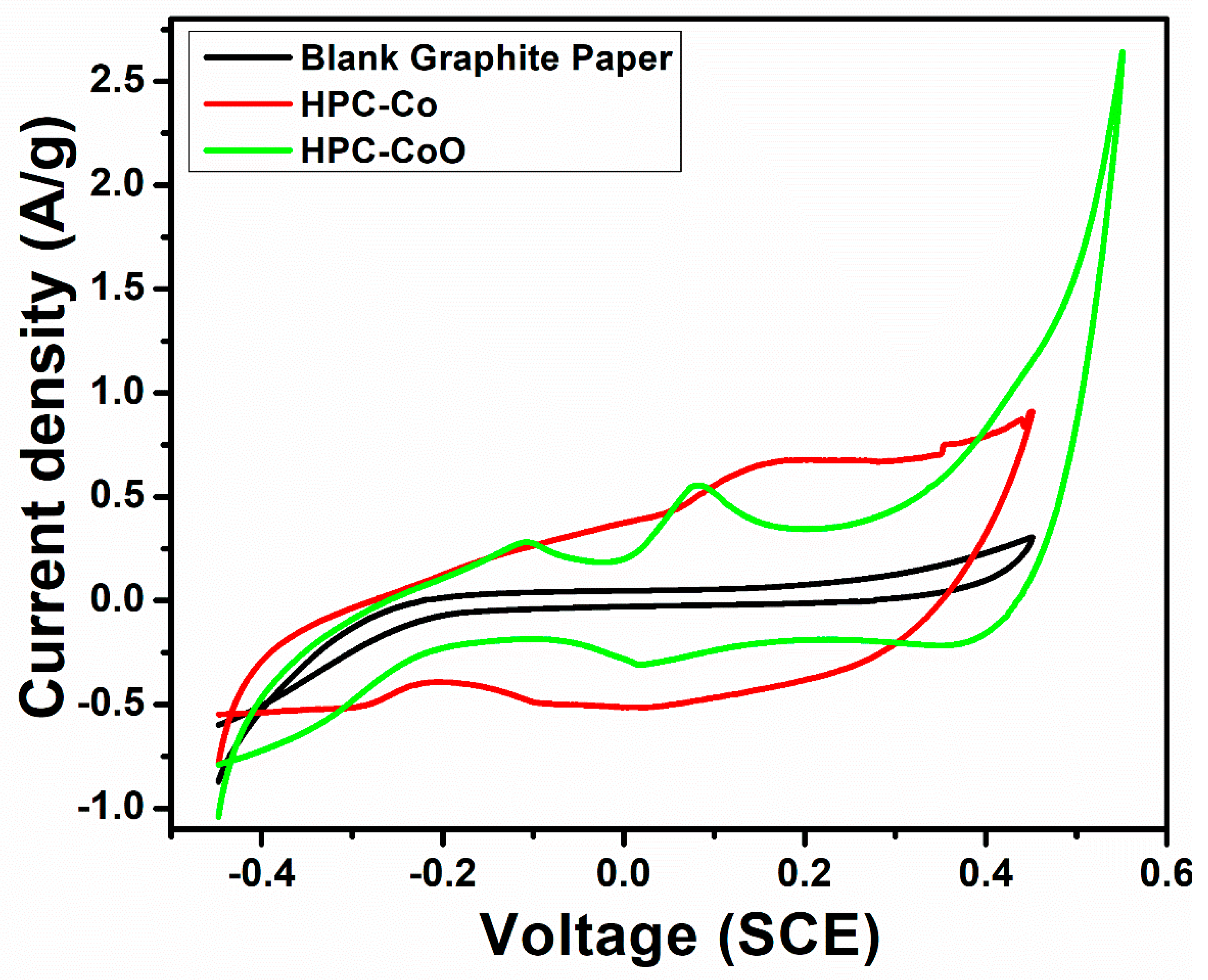
3.2. Electrochemical Behavior of HPC-Co and HPC-CoO Modified Electrodes
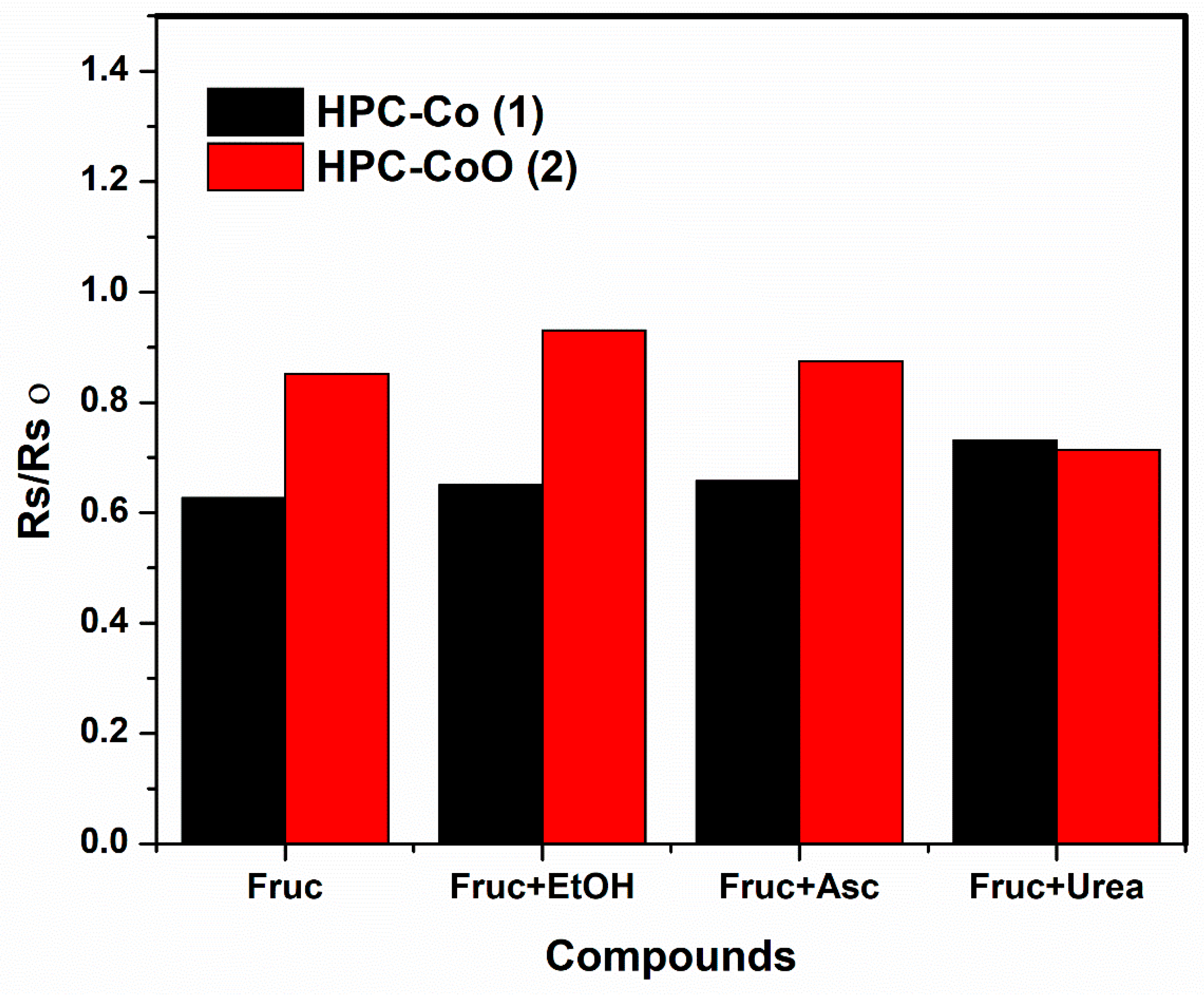
Interference and Real Sample Measurement
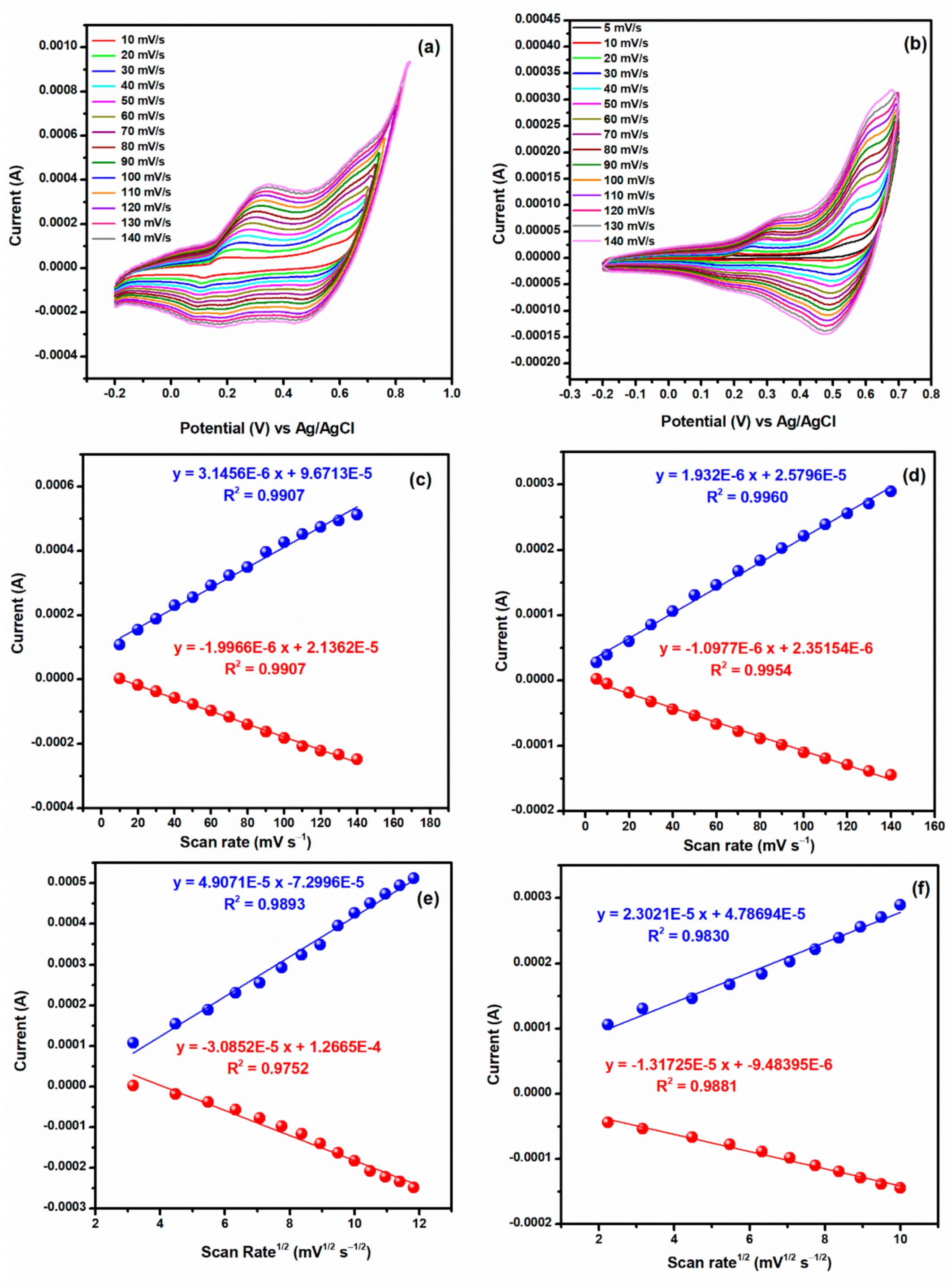
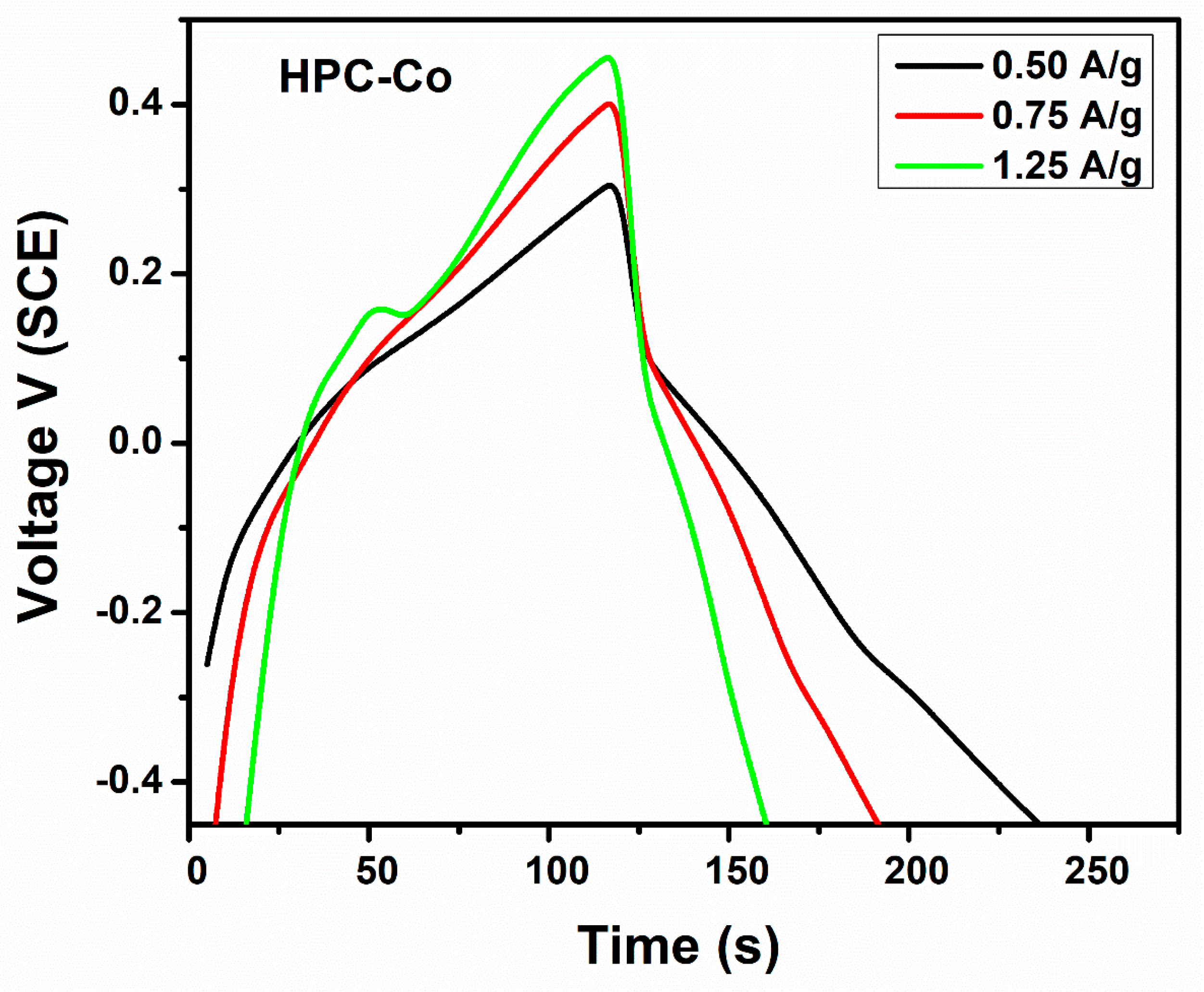
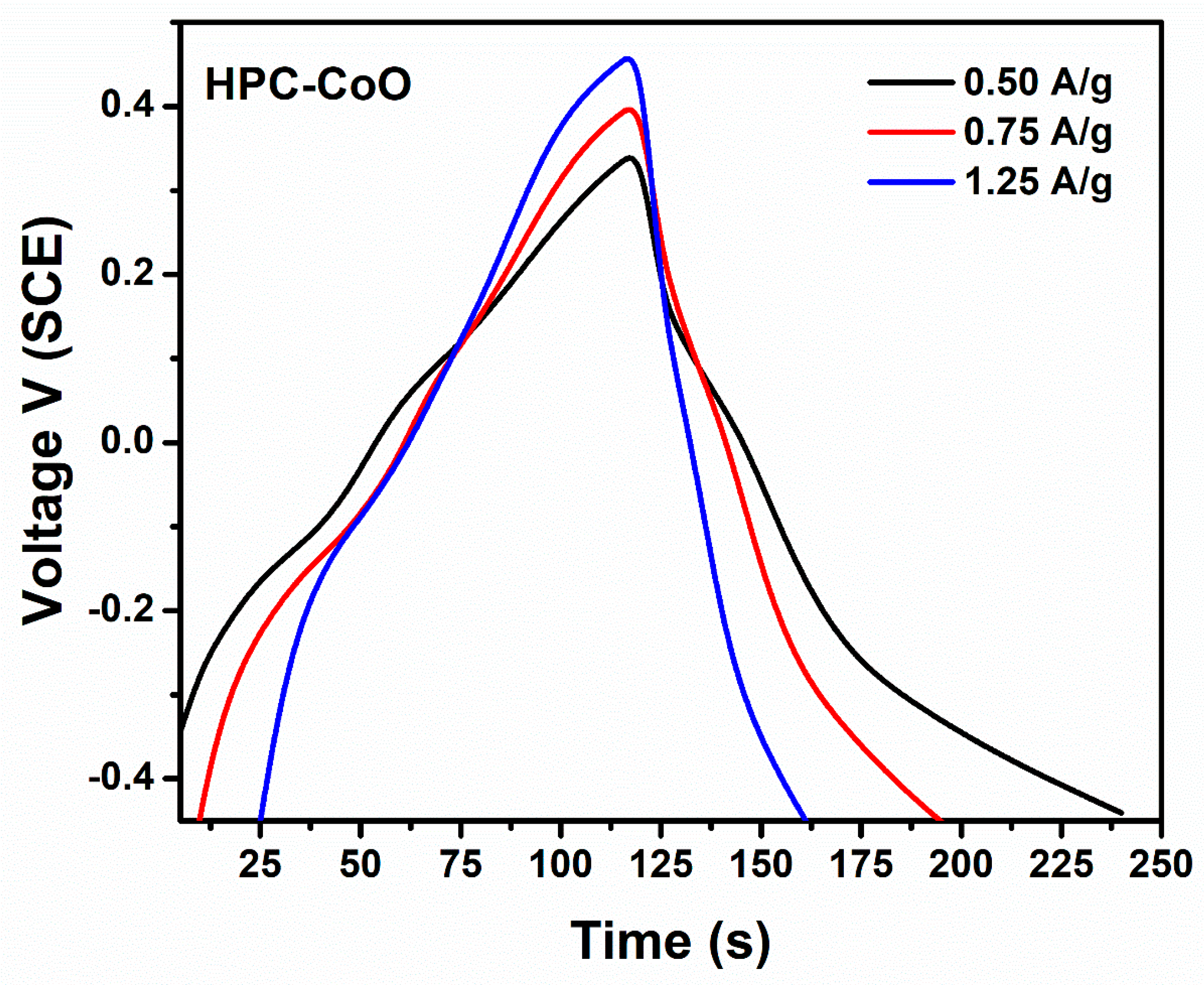
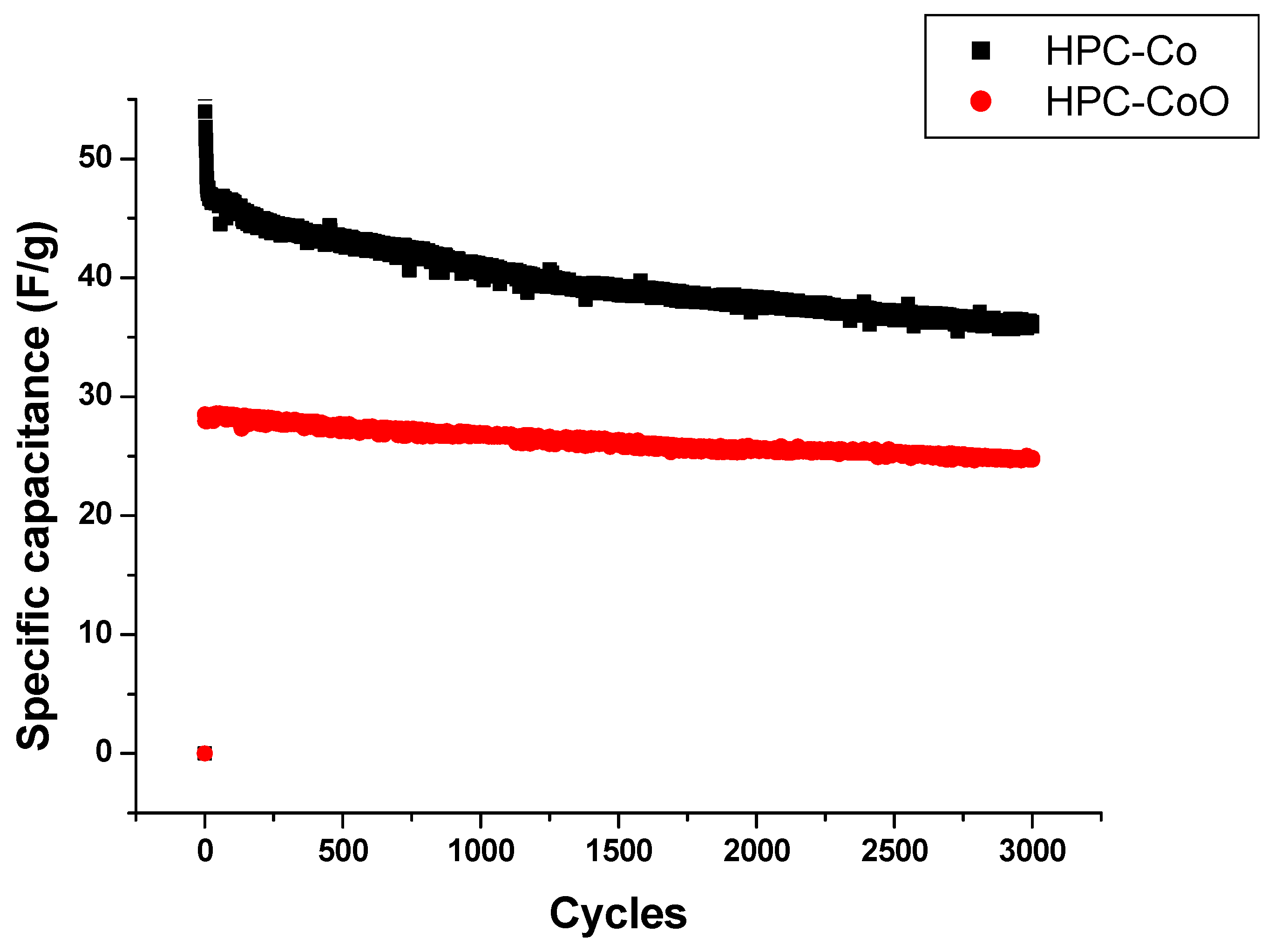
3.3. Capacitance Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Suzuki, Y.; Ikeda, A.; Ohno, K.; Fujihara, T.; Sugaya, T.; Ishihara, K. o-Azophenylboronic Acid-Based Colorimetric Sensors for d-Fructose: O-Azophenylboronic Acids with Inserted Protic Solvent Are the Key Species for a Large Color Change. J. Org. Chem. 2020, 85, 9680–9693. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, B.; Miao, H.; Tan, Y.; Bai, X.; Zhao, Y.-Y.; Wang, Y. Urine metabolomics reveals new insights into hyperlipidemia and the therapeutic effect of rhubarb. Anal. Methods 2015, 7, 3113–3123. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Peterson, K.E.; Gortmaker, S.L. Relation between consumption of sugar-sweetened drinks and childhood obesity: A prospective, observational analysis. Lancet 2001, 357, 505–508. [Google Scholar] [CrossRef]
- Schulze, M.B.; Manson, J.E.; Ludwig, D.S.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004, 292, 927–934. [Google Scholar] [CrossRef]
- Alam, M.M.; Asiri, A.M.; Rahman, M.M.; Islam, M.A. Fabrication of sensitive D-fructose sensor based on facile ternary mixed ZnO/CdO/SnO2 nanocomposites by electrochemical approach. Surfaces Interfaces 2020, 100540. [Google Scholar] [CrossRef]
- Johnson, R.J.; Perez-Pozo, S.E.; Sautin, Y.Y.; Manitius, J.; Sanchez-Lozada, L.; Feig, D.; Shafiu, M.; Segal, M.S.; Glassock, R.J.; Shimada, M.; et al. Hypothesis: Could excessive fructose intake and uric acid cause type 2 diabetes? Endocr. Rev. 2009, 30, 96–116. [Google Scholar] [CrossRef]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; de Souza, R.J.; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; Beyene, J.; Chiavaroli, L.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of fructose on body weight in controlled feeding trials: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 156, 291–304. [Google Scholar] [CrossRef]
- Vanessa Fiorentino, T.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef] [PubMed]
- Wahjudi, P.N.; Patterson, M.E.; Lim, S.; Yee, J.K.; Mao, C.S.; Lee, W.-N.P. Measurement of glucose and fructose in clinical samples using gas chromatography/mass spectrometry. Clin. Biochem. 2010, 43, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cousineau, C.; Xing, G.; Raha, N.; Clemens, S.; Mofikoya, M.; Kim, M.Y.; Zhang, J.Y. Development and validation of a quantitative ultra performance LC® hydrophilic interaction liquid chromatography MS/MS method to measure fructose and sorbitol in human plasma. Bioanalysis 2019, 11, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.A.; Jacksén, J.; Emmer, Å.; Centurión, M.E. Capillary electrophoresis method for the simultaneous determination of carbohydrates and proline in honey samples. Microchem. J. 2016, 129, 1–4. [Google Scholar] [CrossRef]
- Bollella, P.; Hibino, Y.; Kano, K.; Gorton, L.; Antiochia, R. Highly sensitive membraneless fructose biosensor based on fructose dehydrogenase immobilized onto aryl thiol modified highly porous gold electrode: Characterization and application in food samples. Anal. Chem. 2018, 90, 12131–12136. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Luo, L.; Ding, Y.; Jiang, L.; Zhang, Y.; Ouyang, X.; Liu, B. A novel nonenzymatic fructose sensor based on electrospun LaMnO3 fibers. J. Electroanal. Chem. 2014, 727, 21–26. [Google Scholar] [CrossRef]
- Gota, T.; Chowdhury, M.; Ojumu, T. Non-enzymatic Fructose Sensor Based on Co3O4 Thin Film. Electroanalysis 2017, 29, 2855–2862. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Maestre, E.; Katakis, I.; Narváez, A.; Domínguez, E. A multianalyte flow electrochemical cell: Application to the simultaneous determination of carbohydrates based on bioelectrocatalytic detection. Biosens. Bioelectron. 2005, 21, 774–781. [Google Scholar] [CrossRef]
- He, J.; Zhong, Y.; Xu, Q.; Sun, H.; Zhou, W.; Shao, Z. Nitrogen-doped graphic carbon protected Cu/Co/CoO nanoparticles for ultrasensitive and stable non-enzymatic determination of glucose and fructose in wine. J. Electrochem. Soc. 2018, 165, B543. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Song, W. Binary CuO/Co3O4 nanofibers for ultrafast and amplified electrochemical sensing of fructose. Electrochim. Acta. 2011, 56, 10191–10196. [Google Scholar] [CrossRef]
- Alotaibi, N.; Hammud, H.H.; Al Otaibi, N.; Prakasam, T. Electrocatalytic Properties of 3D Hierarchical Graphitic Carbon–Cobalt Nanoparticles for Urea Oxidation. ACS Omega 2020, 5, 26038–26048. [Google Scholar] [CrossRef]
- Sudhakar, P.; Soni, H. Catalytic reduction of Nitrophenols using silver nanoparticles-supported activated carbon derived from agro-waste. J. Environ. Chem. Eng. 2018, 6, 28–36. [Google Scholar] [CrossRef]
- Cao, R.; Xia, T.; Zhu, R.; Liu, Z.; Guo, J.; Chang, G.; Zhang, Z.; Liu, X.; He, Y. Novel synthesis of core-shell Au-Pt dendritic nanoparticles supported on carbon black for enhanced methanol electro-oxidation. Appl. Surf. Sci. 2018, 433, 840–846. [Google Scholar] [CrossRef]
- Morales, D.M.; Kazakova, M.; Dieckhofer, S.; Selyutin, A.G.; Golubtsov, G.; Schuhmann, W.; Masa, J. Trimetallic Mn-Fe-Ni Oxide Nanoparticles Supported on Multi-Walled Carbon Nanotubes as High-Performance Bifunctional ORR/OER Electrocatalyst in Alkaline Media. Adv. Funct. Mater. 2020, 30, 1905992. [Google Scholar] [CrossRef]
- Khoshroo, A.; Hosseinzadeh, L.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Ehrlich, H. Development of electrochemical sensor for sensitive determination of oxazepam based on silver-platinum core–shell nanoparticles supported on graphene. J. Electroanal. Chem. 2018, 823, 61–66. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Wei, Y.; Yang, P. Ni/NiO nanoparticles embedded inporous graphite nanofibers towards enhanced electrocatalytic performance. Int. J. Hydrog. Energy 2019, 44, 19792–19804. [Google Scholar] [CrossRef]
- Trogadas, P.; Fuller, T.F.; Strasser, P. Carbon as catalyst and support for electrochemical energy conversion. Carbon N. Y. 2014, 75, 5–42. [Google Scholar] [CrossRef]
- Hammud, H.H.; El Hamaoui, B.; Noubani, N.H.; Feng, X.; Wu, Z.-S.; Mullen, K.; Ayub, K. Carbon-Cobalt nanostructures as an efficient adsorbent of malachite green. Nanosci. Nanotechnol. Asia 2018, 8, 263–280. [Google Scholar] [CrossRef]
- Alotaibi, N.; Hammud, H.H.; Karnati, R.K.; Hussain, S.G.; Mazher, J.; Prakasam, T. Cobalt–carbon/silica nanocomposites prepared by pyrolysis of a cobalt 2,2′-bipyridine terephthalate complex for remediation of cationic dyes. RSC Adv. 2020, 10, 17660–17672. [Google Scholar] [CrossRef]
- Alotaibi, N.; Hammud, H.H.; Al Otaibi, N.; Hussain, S.G.; Prakasam, T. Novel cobalt–carbon@silica adsorbent. Sci. Rep. 2020, 10, 18652. [Google Scholar] [CrossRef]
- Alramadhan, S.A.; Hammud, H.H. Graphene nickel silica supported nanocomposites as an efficient purifier for water treatment. Appl. Nanosci. 2020. [Google Scholar] [CrossRef]
- Kader, M.S.; Chusuei, C.C. A Cobalt (II) Oxide Carbon Nanotube Composite to Assay Dopamine. Chemosensors 2020, 8, 22. [Google Scholar] [CrossRef]
- Jiao, L.; Zhou, Y.-X.; Jiang, H.-L. Metal–organic framework-based CoP/reduced graphene oxide: High-performance bifunctional electrocatalyst for overall water splitting. Chem. Sci. 2016, 7, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Urbańczyk, E.; Maciej, A.; Simka, W. Electrocatalytic properties of Co decorated graphene and graphene oxide for small organic molecules oxidation. Int. J. Hydrog. Energy 2020, 45, 1769–1783. [Google Scholar] [CrossRef]
- Chen, B.; Ma, G.; Zhu, Y.; Xia, Y. Metal-organic-frameworks derived cobalt embedded in various carbon structures as bifunctional electrocatalysts for oxygen reduction and evolution reactions. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal–organic framework-derived nanoporous metal oxides toward supercapacitor applications: Progress and prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef] [PubMed]
- Kaneti, Y.V.; Tang, J.; Salunkhe, R.R.; Jiang, X.; Yu, A.; Wu, K.C.; Yamauchi, Y. Nanoarchitectured design of porous materials and nanocomposites from metal-organic frameworks. Adv. Mater. 2017, 29, 1604898. [Google Scholar] [CrossRef]
- Brewer, B.; Brooks, N.R.; Abdul-Halim, S.; Sykes, A.G. Differential metathesis reactions of 2,2′-bipyridine and 1,10-phenanthroline complexes of cobalt(II) and nickel(II): Cocrystallization of ionization isomers {[cis-Ni(phen)2(H2O)2][cis-Ni(phen)2(H2O)Cl]} (PF6)3·4.5H2O, and a synthetic route to asymmetric t. J. Chem. Crystallogr. 2003, 33, 651–662. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Yang, Z.C.; Li, X.; Wang, J. Intrinsically fluorescent nitrogen-containing carbon nanoparticles synthesized by a hydrothermal process. Carbon N. Y. 2011, 49, 5207–5212. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of n-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Maruyama, S.; Fukutsuka, T.; Miyazaki, K.; Abe, T. Observation of the intercalation of dimethyl sulfoxide-solvated lithium ion into graphite and decomposition of the ternary graphite intercalation compound using in situ Raman spectroscopy. Electrochim. Acta. 2018, 265, 41–46. [Google Scholar] [CrossRef]
- Ji, D.; Wan, Y.; Yang, Z.; Li, C.; Xiong, G.; Li, L.; Han, M.; Guo, R.; Luo, H. Nitrogen-doped graphene enwrapped silicon nanoparticles with nitrogen-doped carbon shell: A novel nanocomposite for lithium-ion batteries. Electrochim. Acta 2016, 192, 22–29. [Google Scholar] [CrossRef]
- Allaedini, G.; Tasirin, S.M.; Aminayi, P.; Yaakob, Z.; Talib, M.Z.M. Bulk production of bamboo-shaped multi-walled carbon nanotubes via catalytic decomposition of methane over tri-metallic Ni.Co.Fe catalyst. React. Kinet. Mech. Catal. 2015, 116, 385–396. [Google Scholar] [CrossRef]
- Srinivas, G.; Zhu, Y.; Piner, R.; Skipper, N.; Ellerby, M.; Ruoff, R. Synthesis of graphene-like nanosheets and their hydrogen adsorption capacity. Carbon N. Y. 2010, 48, 630–635. [Google Scholar] [CrossRef]
- Long, J.Y.; Yan, Z.S.; Gong, Y.; Lin, J.H. MOF-derived Cl/O-doped C/CoO and C nanoparticles for high performance supercapacitor. Appl. Surf. Sci. 2018, 448, 50–63. [Google Scholar] [CrossRef]
- Leroy, S.; Martinez, H.; Dedryvère, R.; Lemordant, D.; Gonbeaua, D. Influence of the lithium salt nature over the surface film formation on a graphite electrode in Li-ion batteries: An XPS study. Appl. Surf. Sci. 2007, 253, 11. [Google Scholar] [CrossRef]
- Alegre, C.; Modica, E.; Blasi, A.D.; Blasi, O.D.; Busacca, C.; Ferraro, M.; Arico, A.; Antonucci, V.; Baglio, V. NiCo-loaded carbon nanofibers obtained by electrospinning: Bifunctional behavior as air electrodes. Renew. Energy 2018, 125, 250–259. [Google Scholar] [CrossRef]
- Xu, W.; Lyu, F.; Bai, Y.; Gao, A.; Feng, J.; Cai, Z.; Yin, Y. Porous Cobalt Oxide Nanoplates Enriched with Oxygen Vacancies for Oxygen Evolution Reaction. Nano Energy 2018, 43, 110–116. [Google Scholar] [CrossRef]
- Sun, H.; Qu, X.; Zhao, A.; Gao, N.; Li, K.; Ren, J. Deciphering a Nanocarbon-Based Artificial Peroxidase: Chemical Identification of the Catalytically Active and Substrate-Binding Sites on Graphene Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 1–6. [Google Scholar]
- Gabe, A.; García-Aguilar, J.; Berenguer-Murcia, Á.; Morallón, E.; Cazorla-Amorós, D. Key Factors Improving Oxygen Reduction Reaction Activity in Cobalt Nanoparticles Modified Carbon Nanotubes. Appl. Catal. B Environ. 2017, 217, 303–312. [Google Scholar] [CrossRef]
- Majima, T.; Kono, E.; Ogo, S.; Sekine, Y. Pre-reduction and K loading effects on noble metal free Co-system catalyst for water gas shift reaction. Appl. Catal. A Gen. 2016, 523, 92–96. [Google Scholar] [CrossRef]
- Yu, J.; Ni, Y.; Zhai, M. Highly selective non-enzyme glucose detection based on Co-CoO-Co3O4 nanocomposites prepared via a solution-combustion and subsequent heat-treating route. J. Alloys Compd. 2017, 723, 904–911. [Google Scholar] [CrossRef]
- Batool, R.; Hayat, A.; Han, D.; Akhtar, M.A.; Niu, L.; Ahmad, M.A.; Nawaz, M.H. A nanocomposite prepared from magnetite nanoparticles, polyaniline and carboxy-modified graphene oxide for non-enzymatic sensing of glucose. Microchim. Acta 2019, 186, 267. [Google Scholar] [CrossRef] [PubMed]
- Tamiji, T.; Nezamzadeh-Ejhieh, A. Sensitive voltammetric determination of bromate by using ion-exchange property of a Sn (II)-clinoptilolite-modified carbon paste electrode. J. Solid State Electrochem. 2019, 23, 143–157. [Google Scholar] [CrossRef]
- Ghasemi, N.; Nezamzadeh-Ejhieh, A. Study of the interactions of influencing parameters on electrocatalytic determination of dopamine by a carbon paste electrode based on Fe (ii)–clinoptilolite nanoparticles. New J. Chem. 2018, 42, 520–527. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Guix, M.; Madrid, R.E.; Merkoçi, A. Bimetallic nanowires as electrocatalysts for nonenzymatic real-time impedancimetric detection of glucose. Chem. Commun. 2012, 48, 1686–1688. [Google Scholar] [CrossRef]
- Rinaldi, A.L.; Carballo, R. Impedimetric non-enzymatic glucose sensor based on nickel hydroxide thin film onto gold electrode. Sens. Actuators B Chem. 2016, 228, 43–52. [Google Scholar] [CrossRef]
- Tashkhourian, J.; Nami-Ana, S.F.; Shamsipur, M. A new bifunctional nanostructure based on Two-Dimensional nanolayered of Co (OH) 2 exfoliated graphitic carbon nitride as a high performance enzyme-less glucose sensor: Impedimetric and amperometric detection. Anal. Chim. Acta 2018, 1034, 63–73. [Google Scholar] [CrossRef]
- Aksorn, J.; Teepoo, S. Development of the simultaneous colorimetric enzymatic detection of sucrose, fructose and glucose using a microfluidic paper-based analytical device. Talanta 2020, 207, 120302. [Google Scholar] [CrossRef]
- Silva-Carrillo, C.; Reynoso-Soto, E.A.; Paraguay-Delgado, F.; Alonso-Núñez, G.; Félix-Navarro, R.M. Synthesis of PtNPs/MWCNT functionalized with 4-mercaptophenylboronic acid for an electrochemical sensor of fructose. J. Electrochem. Soc. 2017, 164, B86. [Google Scholar] [CrossRef]
- Raj, V.; Vijayan, A.N.; Joseph, K. Naked eye detection of infertility using fructose blue–A novel gold nanoparticle based fructose sensor. Biosens. Bioelectron. 2014, 54, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Seraj, S.; Rouhani, S.; Faridbod, F. Fructose recognition using new ‘Off–On’ fluorescent chemical probes based on boronate-tagged 1,8-naphthalimide. New J. Chem. 2018, 42, 19872–19880. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To be or not to be pseudocapacitive. J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Nagamuthu, S.; Vijayakumar, S.; Muralidharan, G. Ag incorporated Mn 3 O 4/AC nanocomposite based supercapacitor devices with high energy density and power density. Dalt. Trans. 2014, 43, 17528–17538. [Google Scholar] [CrossRef]
- Iqbal, M.; Haider, S.S.; Zakar, S.; Alzaid, M.; Afzal, A.M.; Aftab, M. Cobalt-oxide/carbon composites for asymmetric solid-state supercapacitors. Mater. Res. Bull 2020, 131, 110974. [Google Scholar] [CrossRef]
- Wei, F.; Jiang, J.; Yu, G.; Sui, Y. A novel cobalt–carbon composite for the electrochemical supercapacitor electrode material. Mater. Lett. 2015, 146, 20–22. [Google Scholar] [CrossRef]
- Wang, S.; Wang, T.; Shi, Y.; Liu, G.; Li, J. Mesoporous Co3O4@carbon composites derived from microporous cobalt-based porous coordination polymers for enhanced electrochemical properties in supercapacitors. RSC Adv. 2016, 6, 18465–18470. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Zhang, S.; Wang, D. Preparation of Cobalt/Coal–Based Activated Carbon Composites with Synergistic Electrochemical Performance. Int. J. Electrochem. Sci. 2017, 12, 3991–4000. [Google Scholar] [CrossRef]




| Material | Detection Method | Linear Range (mM) | Limit of Detection LOD (mM) | Reference |
|---|---|---|---|---|
| Enzyme-based paper biosensor | Colorimetric | 2–20 | 0.8 | [60] |
| PtNPs/MWCNT Functionalized with 4-Mercaptophenylboronic Acid | Cyclic voltammetry Impedimetric | 2.5–10 0.5–10 | 0.06 0.00637 | [61] |
| AuNP-based sensor | Colorimetric | 2.8–33.3 | 1.7 | [62] |
| Naphthalimide-based sensor | Flourescence | 0.34–50 | 0.1 | [63] |
| HPC-Co HPC-CoO HPC-Co HPC-CoO | Impedimetric Cyclic Voltammetry | 8.0–53.0 8.0–87.6 1.1–14.3 1.1–18.8 | 3 4 0.5 0.5 | This study |
| Current Density (A/g) | Specific Capacitance (F/g) | |
|---|---|---|
| HPC-Co | HPC-CoO | |
| 0.25 | 49.64 | 45.96 |
| 0.75 | 41.75 | 39.31 |
| 1.25 | 31.60 | 29.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammud, H.H.; Alotaibi, N.; Al Otaibi, N.; Aljaafari, A.; Ahmed, F.; Azam, A.; Prakasam, T. Hierarchical Porous Carbon Cobalt Nanocomposites-Based Sensor for Fructose. Chemosensors 2021, 9, 6. https://doi.org/10.3390/chemosensors9010006
Hammud HH, Alotaibi N, Al Otaibi N, Aljaafari A, Ahmed F, Azam A, Prakasam T. Hierarchical Porous Carbon Cobalt Nanocomposites-Based Sensor for Fructose. Chemosensors. 2021; 9(1):6. https://doi.org/10.3390/chemosensors9010006
Chicago/Turabian StyleHammud, Hassan H., Nusaybah Alotaibi, Nasreen Al Otaibi, Abdullah Aljaafari, Faheem Ahmed, Ameer Azam, and Thirumurugan Prakasam. 2021. "Hierarchical Porous Carbon Cobalt Nanocomposites-Based Sensor for Fructose" Chemosensors 9, no. 1: 6. https://doi.org/10.3390/chemosensors9010006
APA StyleHammud, H. H., Alotaibi, N., Al Otaibi, N., Aljaafari, A., Ahmed, F., Azam, A., & Prakasam, T. (2021). Hierarchical Porous Carbon Cobalt Nanocomposites-Based Sensor for Fructose. Chemosensors, 9(1), 6. https://doi.org/10.3390/chemosensors9010006