Second-Generation Phosgene and Diphosgene Detection Tube
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
2.3. Carrier Preparation
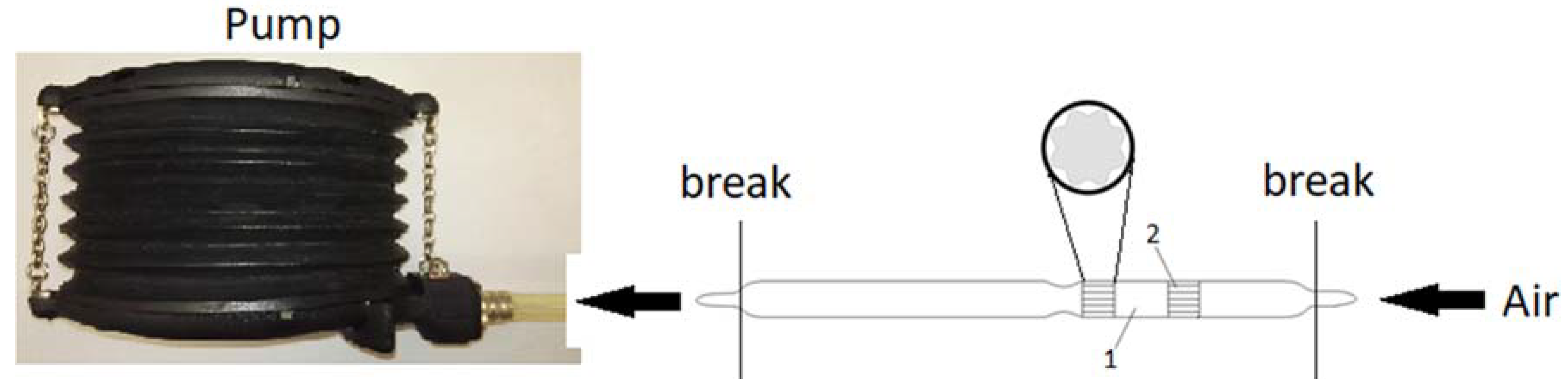
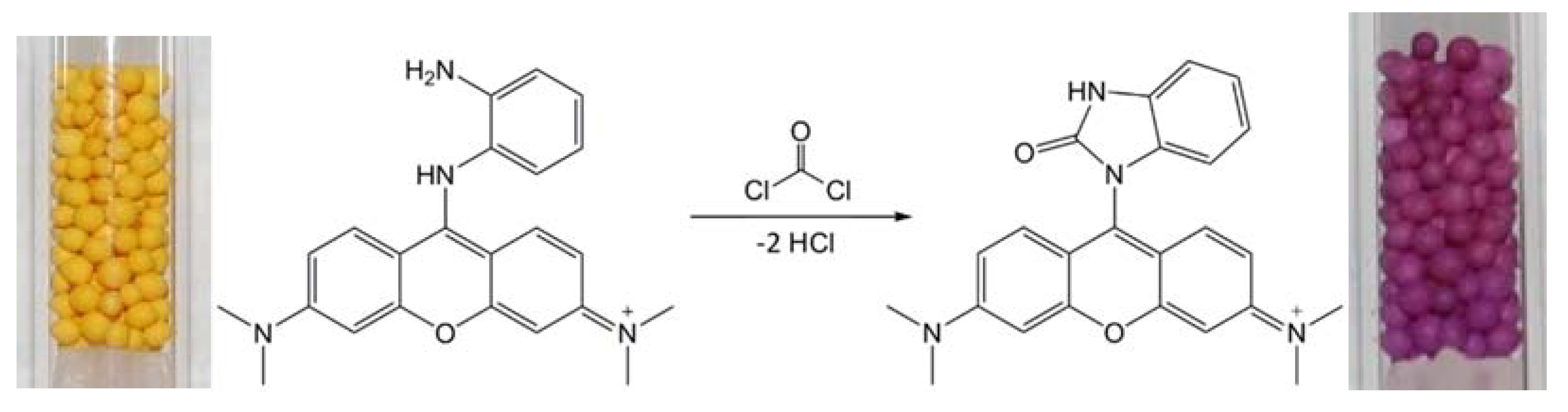
2.4. Carrier (Pellet) Impregnation and Detection Tube Preparation
2.5. Detection Tube Testing
3. Results and Discussion
3.1. Properties of the Carrier
3.2. Color Characteristics and Analytical Data
3.3. Selectivity and Interferences
3.4. Stability (Temperature, Ageing)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Potential Military Chemical (Biological) Agents and Compounds; Field Manual FM 3-11.9; Exidyne: Weantzeville, MO, USA, 2005.
- Halámek, E.; Kobliha, Z.; Pitschmann, V. Analysis of Chemical Warfare Agents; University of Defence: Brno, Czech Republic, 2009. [Google Scholar]
- Moureu, H.; Chovin, P.; Truffert, L. The photochemical transformation of chloropicrin and phosgene characterization of the two corps. Compt. Rend. 1949, 228, 1954–1956. [Google Scholar]
- Spěvák, A.; Kratochvíl, V. Rapid semiquantitative determination of concentration of phosgene in air. Chem. Prům. 1965, 15, 682–685. (In Czech) [Google Scholar]
- Vargas, A.P.; Gámez, F.; Roales, J.; Lopes-Costa, T.; Pedrosa, J.M. An optical dosimeter for the selective detection of gaseous phosgene with ultralow detection limit. ACS Sensors 2018, 3, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Linch, A.L.; Lord, S.S.; Kubitz, K.A.; De Brunner, M.R. Phosgene in air—Development of improved detection procedures. Am. Ind. Hyg. Assoc. J. 1965, 26, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Witten, B.; Prostak, A. Sensitive detector crayons for phosgene, hydrogen cyanide, cyanogen chloride, and lewisite. Anal. Chem. 1957, 29, 885–887. [Google Scholar] [CrossRef]
- Dixon, B.E.; Hands, G.C. A field method for the determination of phosgene. Analyst 1959, 84, 463–464. [Google Scholar] [CrossRef]
- Pitschmann, V.; Tusarová, I.; Kobliha, Z.; Vetchý, D. Detection tube with composite carrier for detection of phosgene and diphosgene in air. Chem. Ind. 2012, 66, 79–84. [Google Scholar] [CrossRef][Green Version]
- Forostyan, Y.N.; Efimova, E.I.; Oleinik, A.P. Products of the reaction of anabasine with phosgene. Chem. Nat. Compd. 1970, 6, 587–589. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Jung, H.; Zeng, Y.; Lee, S.; Swamy, K.M.K.; Zhou, X.; Kim, M.H.; Yoon, J. Effective strategy for colorimetric and fluorescence sensing of phosgene based on small organic dyes and nanofiber platforms. ACS Appl. Mater. Interfaces 2016, 8, 22246–22252. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, Y.; Lilyan, C.; Wu, X.; Yoon, J. A fluorescent sensor for dual-chanel discrimination between phosgene and a nerve-gas mimic. Angew. Chem. Int. Ed. 2016, 55, 4729–4733. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, X.; Jung, H.; Nam, S.-J.; Kim, M.H.; Yoon, J. Colorimetric and fluorescent detecting phosgene by a second-generation chemosensor. Anal. Chem. 2018, 90, 3382–3386. [Google Scholar] [CrossRef]
- Wang, S.-L.; Zhong, L.; Song, Q.H. A ratiometric fluorescent chemosensor for selective and visual detection of phosgene in solutions and in the gas phase. Chem. Commun. 2017, 53, 1530–1533. [Google Scholar] [CrossRef]
- Liu, P.; Liu, N.; Liu, C.; Jia, Y.; Huang, L.; Zhou, G.; Li, C.; Wang, S. A colorimetric and ratiometric fluorescent probe with ultralow detection limit and high selectivity for phosgene sensing. Dye. Pigment. 2019, 163, 489–495. [Google Scholar] [CrossRef]
- Feng, W.; Gong, S.; Zhou, E.; Yin, X.; Feng, G. Readily prepared iminocoumarin for rapid, colorimetric and ratiometric fluorescent detection of phosgene. Anal. Chim. Acta 2018, 1029, 97–103. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Z.; Yang, Y.; Asakura, T. A highly sensitive fluorogenic chemodosimeter for rapid visual detection of phosgene. Chem. Commun. 2012, 48, 1895–1897. [Google Scholar] [CrossRef]
- Kim, T.I.; Hwang, B.; Bouffard, J.; Kim, Y. Instaneous colorimetric and fluorogenic detection of phosgene with a meso-oxime-BODIPY. Anal. Chem. 2017, 89, 12837–12842. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, D.; Yoon, J. Recent advances in the development of chromofore-based chemosensors for nerve agents and phosgene. ACS Sens. 2018, 3, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.C.; Xu, X.H.; Song, Q.H. A fluorescent chemosensor for selective detetion of phosgene in solutions and in gas phase. ACS Sens. 2017, 2, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Li, C.; Song, Q.H. Fluorescent chemosensor for dual-chanel discrimination between phosgene and triphosgene. Anal. Chem. 2019, 91, 5690–5697. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.; Wang, B.; Fan, P.; Jiu, Y.; Zhang, M.; Jiang, L.; Hou, J.-T. A highly selective phenothiazine-based fluorescent chemosensor for phosgene. Dye. Pigment. 2020, 173, 107933. [Google Scholar] [CrossRef]
- Zeng, L.; Zeng, H.; Wang, S.; Wang, S.; Hou, J.T.; Yoon, J. A paper-based chemosensor for highly specific, ultrasensitive, and instantaneous visual detection of toxic phosgene. Chem. Commun. 2019, 55, 13753–13756. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Hwang, K.C. Rational design of fluorescent phosgene sensors. Anal. Chem. 2012, 84, 4594–4597. [Google Scholar] [CrossRef]
- Chen, L.; Wu, D.; Kim, J.-M.; Yoon, J. An ESIPT-based fluorescence probe for colorimetric, ratiometric, and selective detection of phosgene in solutions and the gas phase. Anal. Chem. 2017, 89, 12569–12601. [Google Scholar] [CrossRef] [PubMed]
- Pitschmann, V.; Matějovský, L.; Lunerová, K.; Dymák, M.; Urban, M.; Králík, L. Detection papers with chromogenic chemosensors for direct visual detection and distinction of liquid chemical warfare agents. Chemosensors 2019, 7, 30. [Google Scholar] [CrossRef]
- Zeman, J.; Pavloková, S.; Vetchý, D.; Pitschmann, V. Double-coated pellets with semipermeable ethylcellulose coating for the detection of cholinesterase inhibitors. Ceska Slov. Farm. 2020, 69, 24–32. [Google Scholar] [PubMed]
- Zeman, J.; Vetchý, D.; Pavloková, S.; Franc, A.; Pitschmann, V.; Dominik, M.; Urbanová, M.; Šeděnková, I. Tubes for detection of cholinesterase inhibitors—Unique effects of Neusilin on the stability of butyrylcholinesterase-impregnated carrier. Enzyme Microb. Technol. 2019, 128, 26–33. [Google Scholar] [CrossRef]
- Zeman, J.; Vetchý, D.; Pavloková, S.; Franc, A.; Pitschmann, V. Unique coated neusilin pellets with a more distinct and fast visual detection of nerve agents and other cholinesterase inhibitors. J. Pharm. Biomed. Anal. 2020, 179, 113004. [Google Scholar] [CrossRef]
- Oritest Detection Accessories, CHP-5. Available online: https://www.oritest.cz/en/products/detection/accessories/chp-5-en/ (accessed on 29 October 2020).
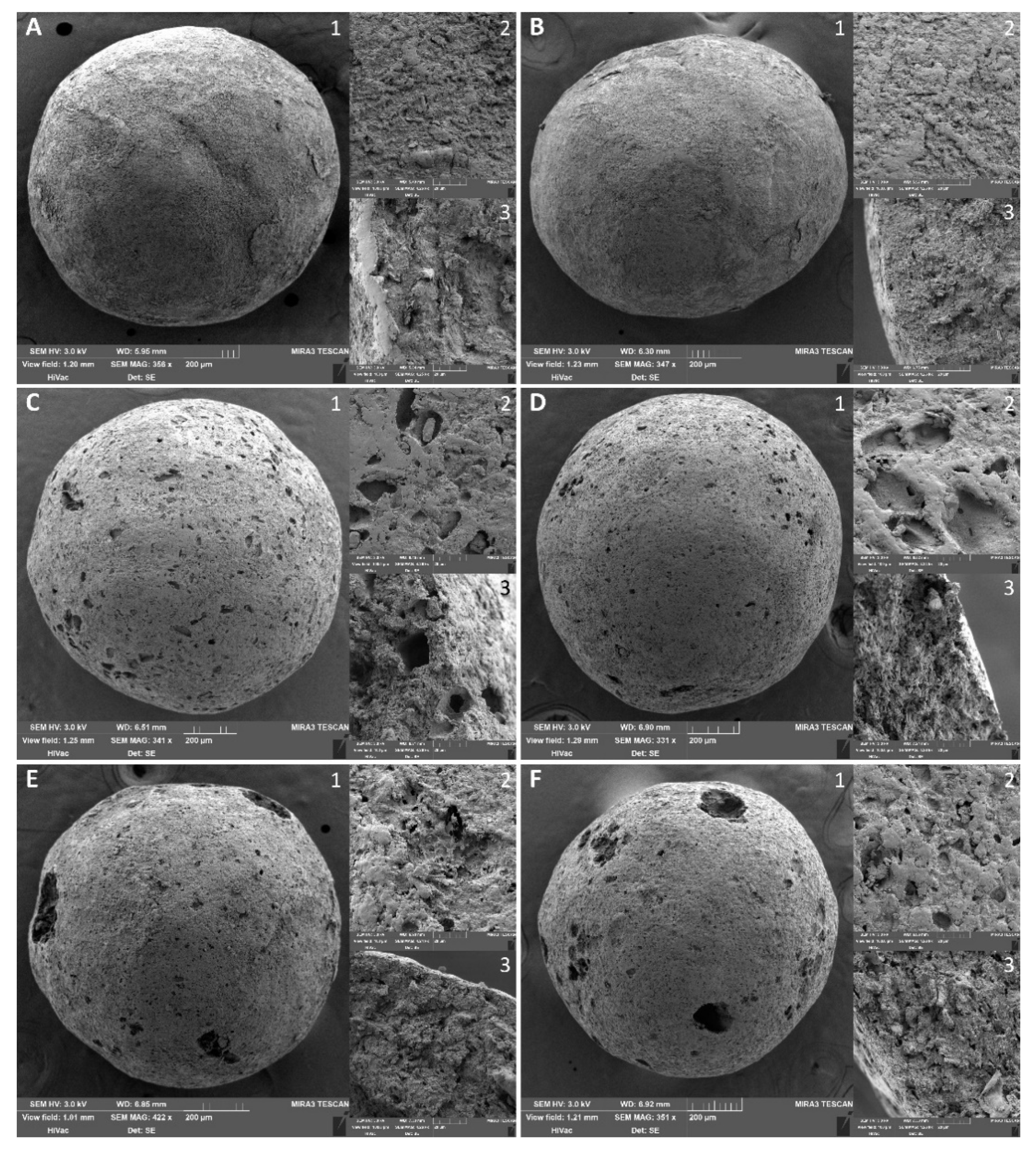
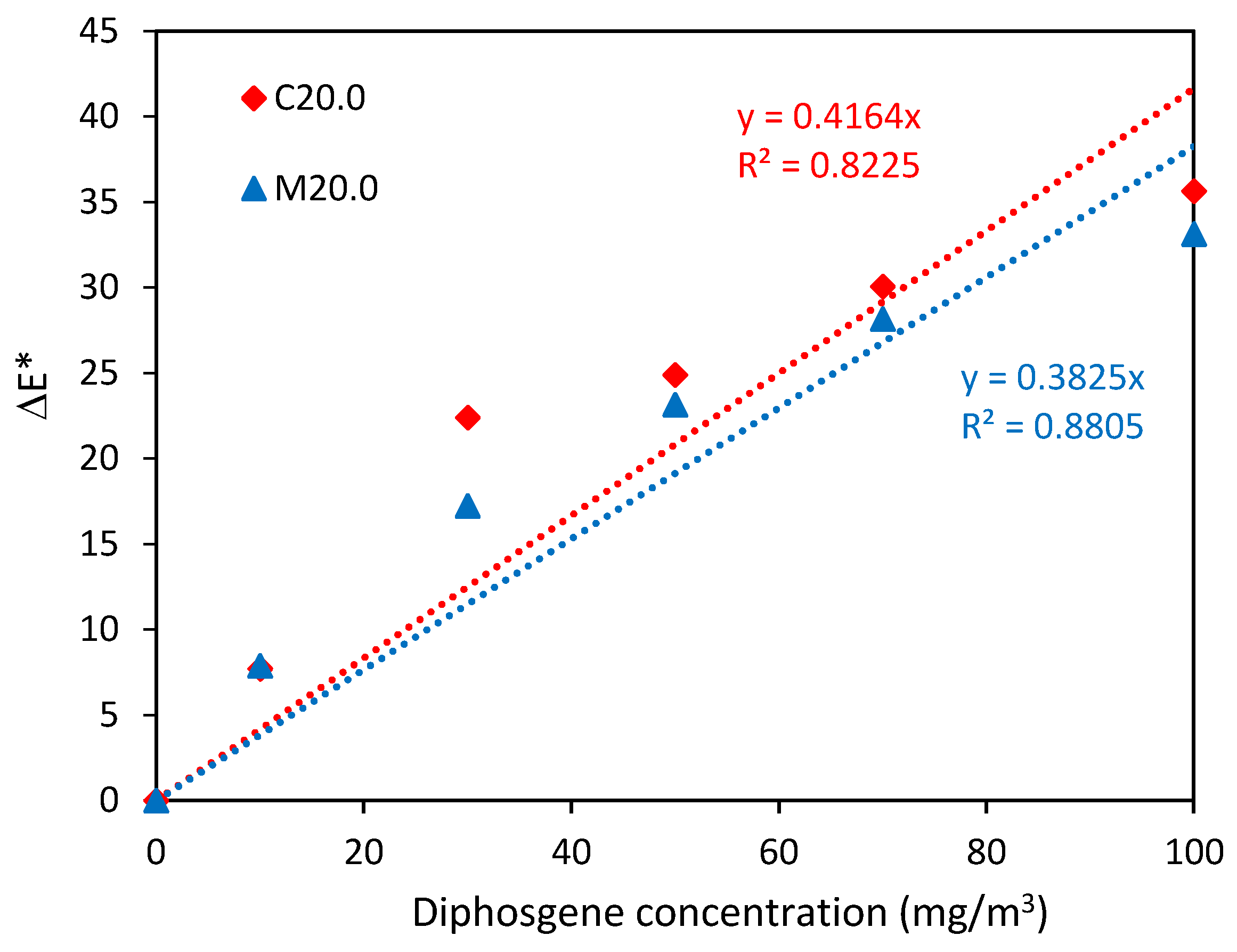
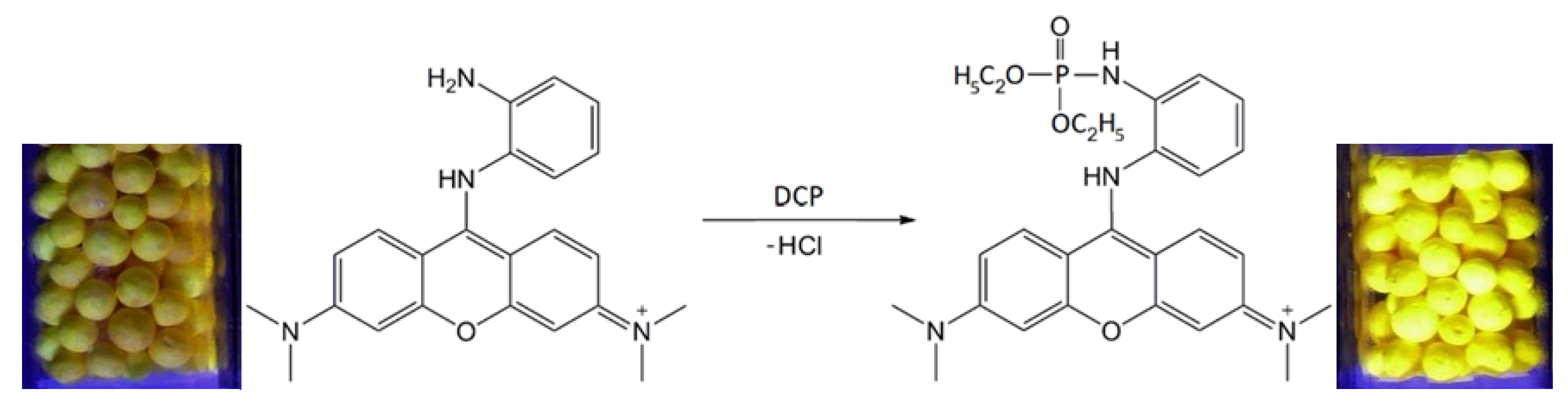
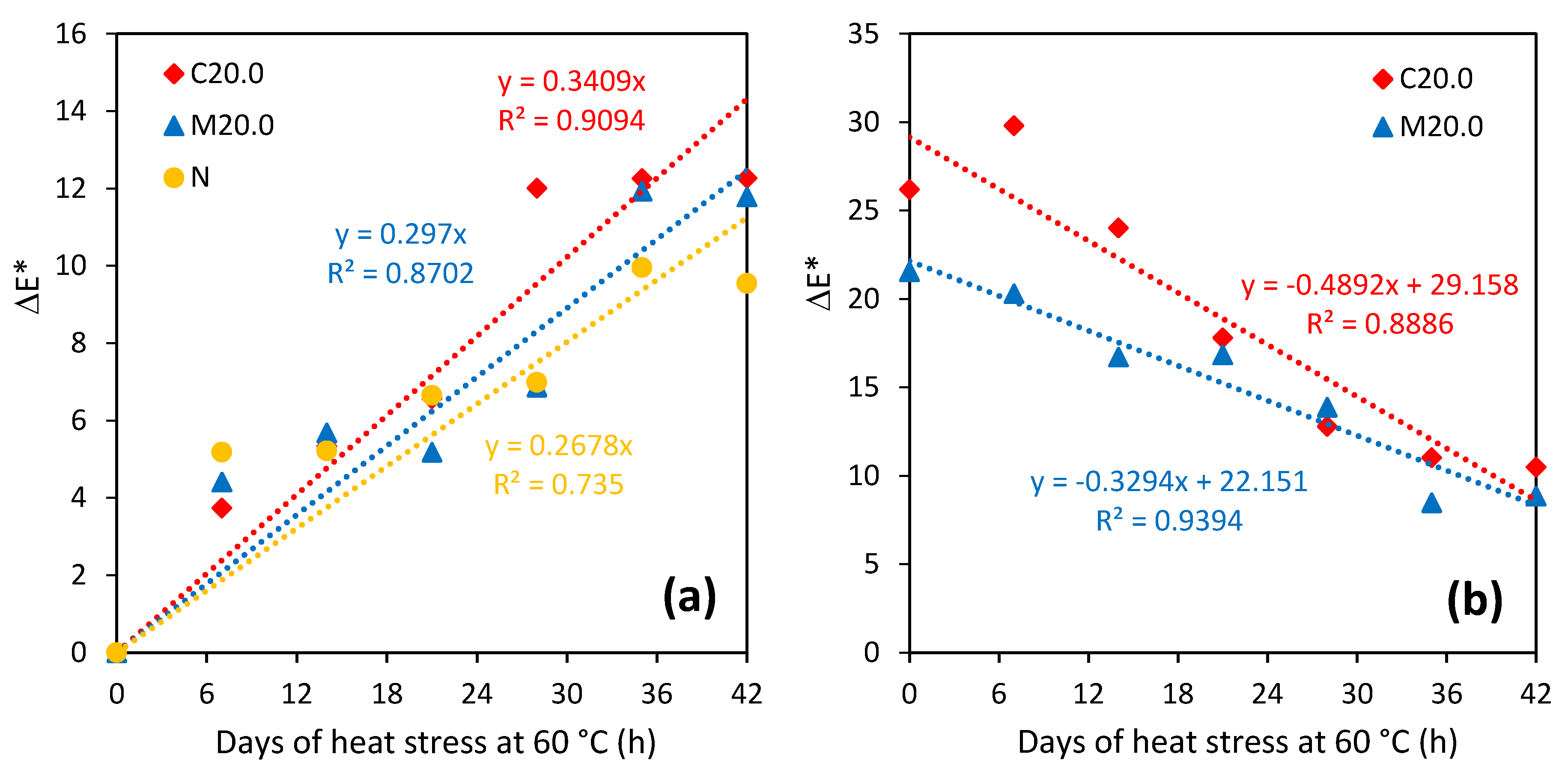
| Property | N | N/PY-OPD | M20.0 | M20.0/PY-OPD | C20.0 | C20.0/PY-OPD |
|---|---|---|---|---|---|---|
| Bulk density, g/cm3 | 0.777 ± 0.003 | 0.758 ± 0.006 | 0.500 ± 0.005 | 0.502 ± 0.001 | 0.583 ± 0.004 | 0.588 ± 0.000 |
| Tapped density, g/cm3 | 0.796 ± 0.004 | 0.790 ± 0.007 | 0.509 ± 0.001 | 0.520 ± 0.002 | 0.596 ± 0.004 | 0.624 ± 0.002 |
| Hausner ratio | 1.024 ± 0.000 | 1.042 ± 0.005 | 1.019 ± 0.008 | 1.036 ± 0.005 | 1.024 ± 0.000 | 1.060 ± 0.004 |
| Hardness, N | 11.99 ± 1.57 | 12.28 ± 1.59 | 3.64 ± 0.48 | 3.58 ± 0.55 | 6.99 ± 1.12 | 7.03 ± 1.11 |
| Friability, % | 0.14 ± 0.03 | 0.13 ± 0.03 | 0.19 ± 0.04 | 0.18 ± 0.04 | 0.17 ± 0.04 | 0.17 ± 0.05 |
| Pycnometric pellet density, g/cm3 | 1.6467 ± 0.0065 | 1.6459 ± 0.0082 | 1.6227 ± 0.008 | 1.6159 ± 0.0019 | 1.6596 ± 0.0029 | 1.6528 ± 0.0015 |
| Interparticular porosity, % | 52.80 ± 0.21 | 53.97 ± 0.35 | 69.19 ± 0.31 | 68.95 ± 0.09 | 64.90 ± 0.24 | 64.41 ± 0.00 |
| Intraparticular porosity, % | 2.76 ± 0.38 | 2.81 ± 0.48 | 4.02 ± 0.05 | 4.42 ± 0.11 | 2.29 ± 0.17 | 2.69 ± 0.09 |
| Sphericity | 0.982 ± 0.010 | 0.977 ± 0.017 | 0.986 ± 0.010 | 0.980 ± 0.018 | 0.982 ± 0.012 | 0.980 ± 0.014 |
| Specific surface area, m2/g | 65.90 ± 0.84 | 63.73 ± 0.19 | 121.14 ± 5.32 | 127.72 ± 0.55 | 113.44 ± 3.44 | 119.94 ± 6.94 |
| Chemical | Color Change/Daylight | Fluorescence |
|---|---|---|
| Acetone | No | No |
| Carbon disulphide | No | No |
| Nitromethane | No | No |
| Pyridine | No | No |
| 2-(Butylamino)ethanethiol | Slight yellowing | No |
| 2-Chloroethyl ethyl ether | No | No |
| 2-Chloroethyl ethyl sulphide | No | No |
| N,N-Dimethylformamide | Slight yellowing | No |
| Ethylenediamine | Slight yellowing | No |
| N,N-Dimethylethanolamine | Slight yellowing | No |
| Hydrogen chloride | Deep orange color | Yellow |
| Ammonia | Green-yellow color | No |
| DCP | Deep orange color | Yellow |
| Fuel petrol | No | No |
| Nitric acid | Red-brown color | Yellow |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitschmann, V.; Matějovský, L.; Zeman, J.; Vetchý, D.; Dymák, M.; Lobotka, M.; Pavloková, S.; Moravec, Z. Second-Generation Phosgene and Diphosgene Detection Tube. Chemosensors 2020, 8, 107. https://doi.org/10.3390/chemosensors8040107
Pitschmann V, Matějovský L, Zeman J, Vetchý D, Dymák M, Lobotka M, Pavloková S, Moravec Z. Second-Generation Phosgene and Diphosgene Detection Tube. Chemosensors. 2020; 8(4):107. https://doi.org/10.3390/chemosensors8040107
Chicago/Turabian StylePitschmann, Vladimír, Lukáš Matějovský, Jiří Zeman, David Vetchý, Michal Dymák, Martin Lobotka, Sylvie Pavloková, and Zdeněk Moravec. 2020. "Second-Generation Phosgene and Diphosgene Detection Tube" Chemosensors 8, no. 4: 107. https://doi.org/10.3390/chemosensors8040107
APA StylePitschmann, V., Matějovský, L., Zeman, J., Vetchý, D., Dymák, M., Lobotka, M., Pavloková, S., & Moravec, Z. (2020). Second-Generation Phosgene and Diphosgene Detection Tube. Chemosensors, 8(4), 107. https://doi.org/10.3390/chemosensors8040107





