Sensitive Electrochemical Detection of Tryptophan Using a Hemin/G-Quadruplex Aptasensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Synthesis of the Fe3O4@SiO2/DABCO
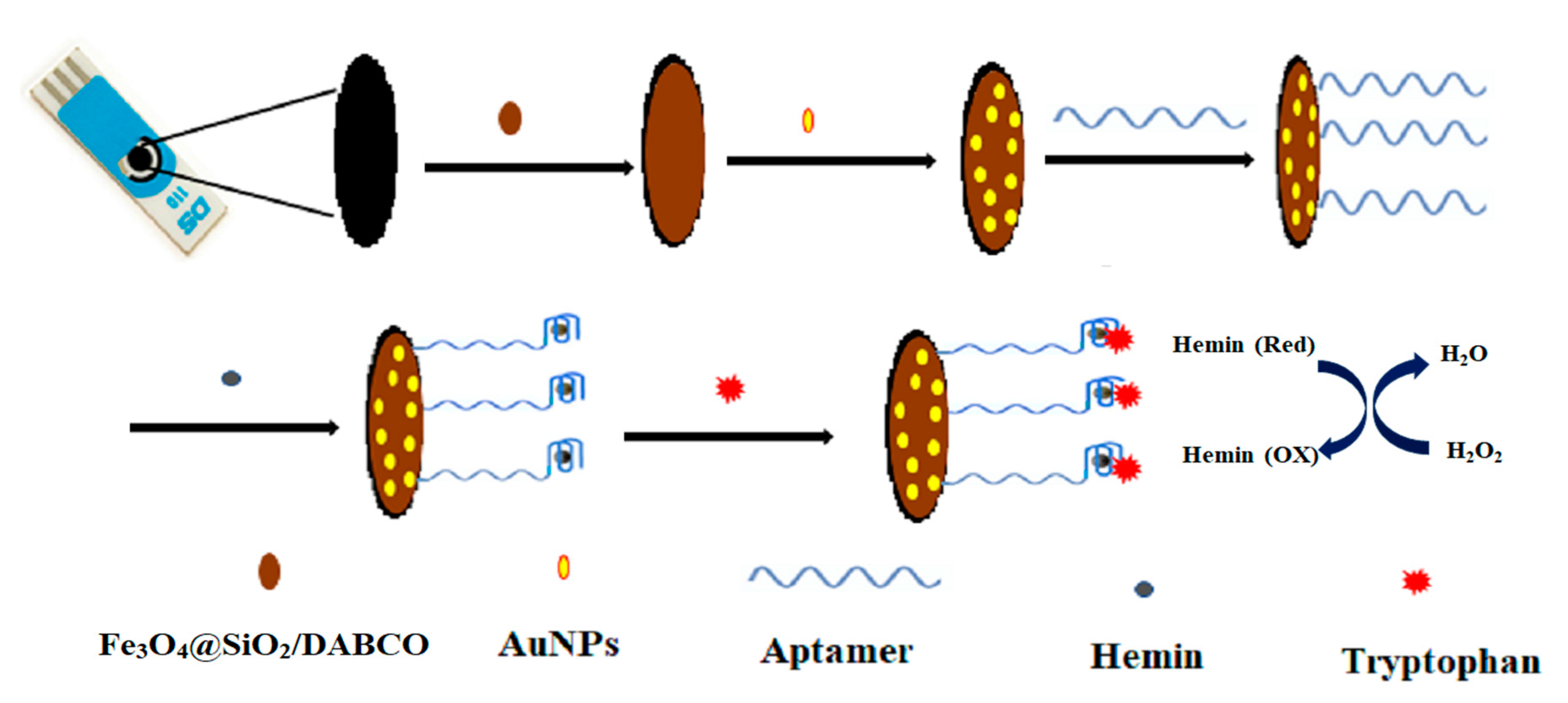
2.3. Preparation of Trp Aptasensor
2.4. Determination of Trp in Human Serum
3. Results
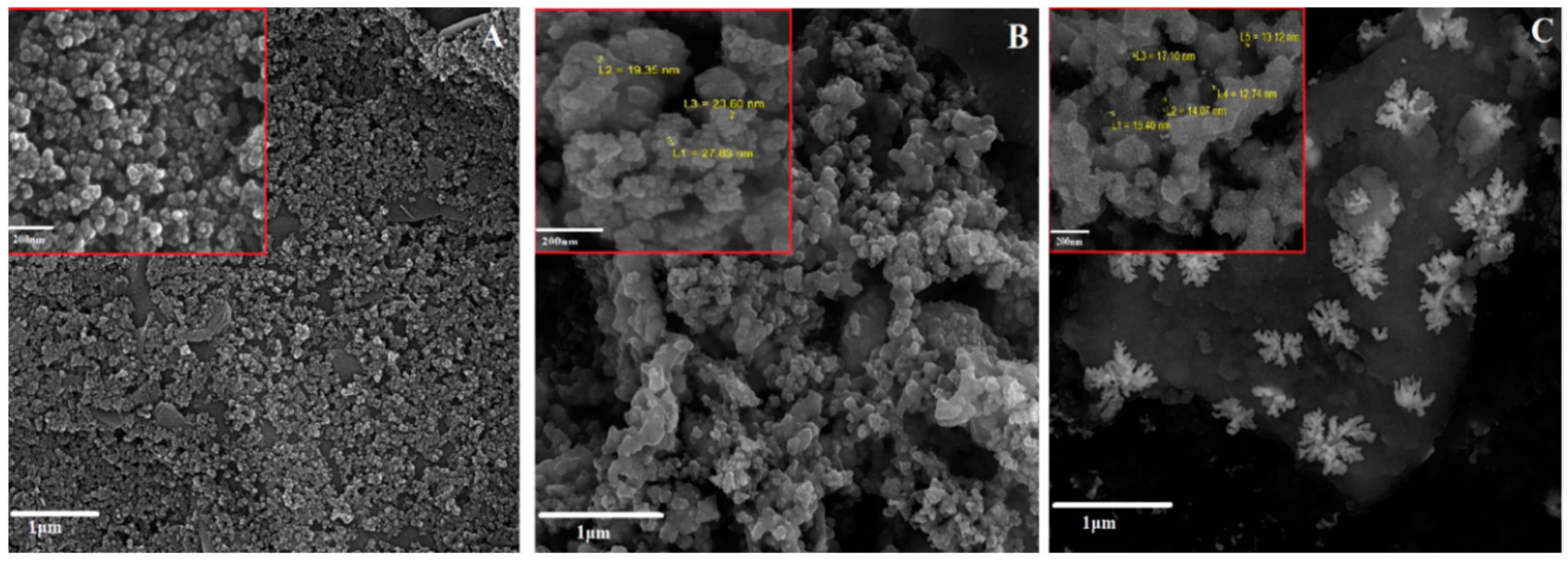
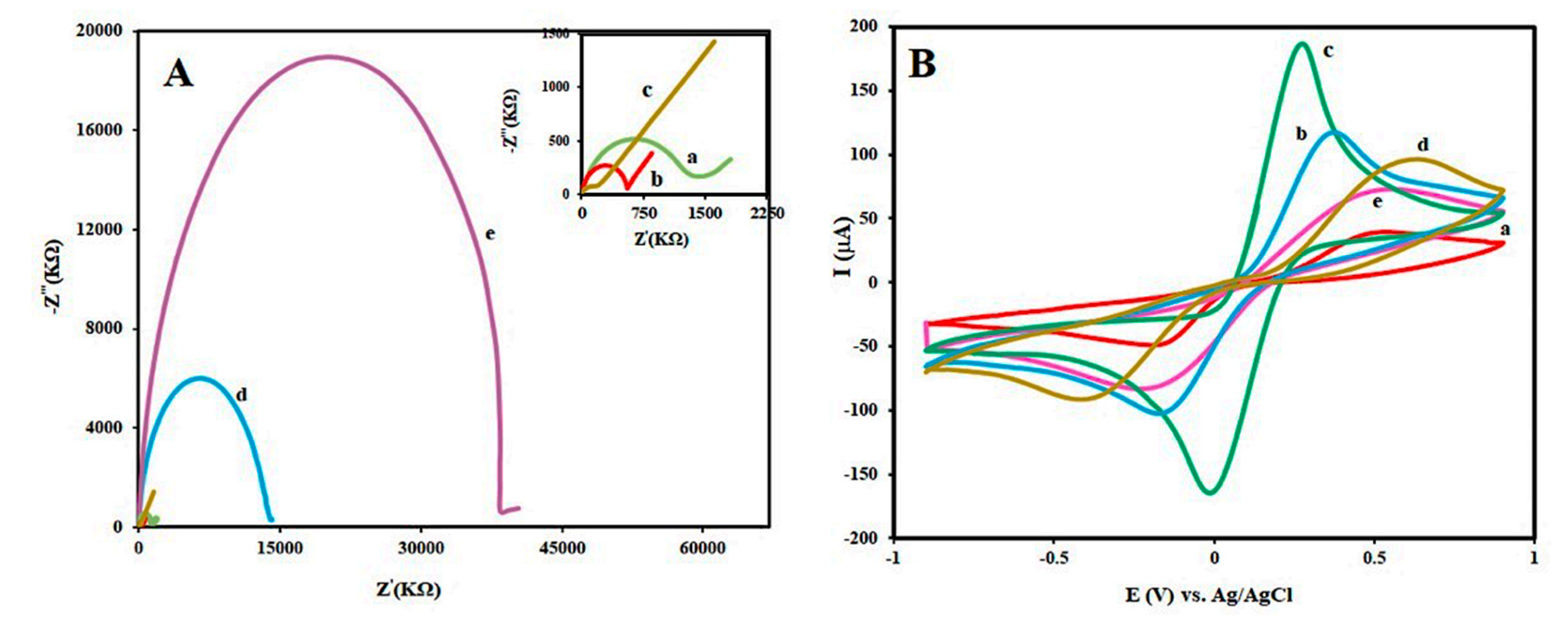
3.1. Characterization of Sensing Interface
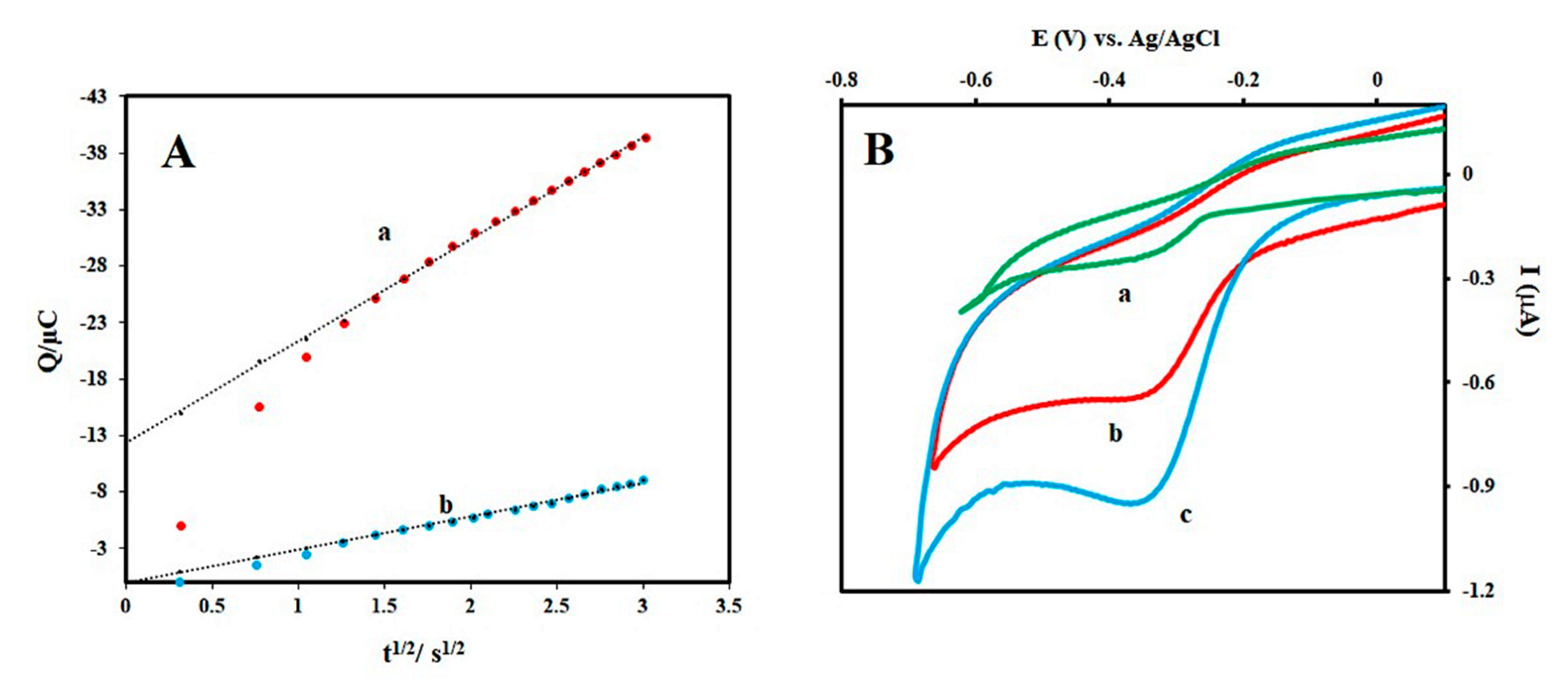
3.2. Aptamer Surface Density and Detection Feasibility of Trp Aptasensor
3.3. Optimization of the Proposed Aptasensor
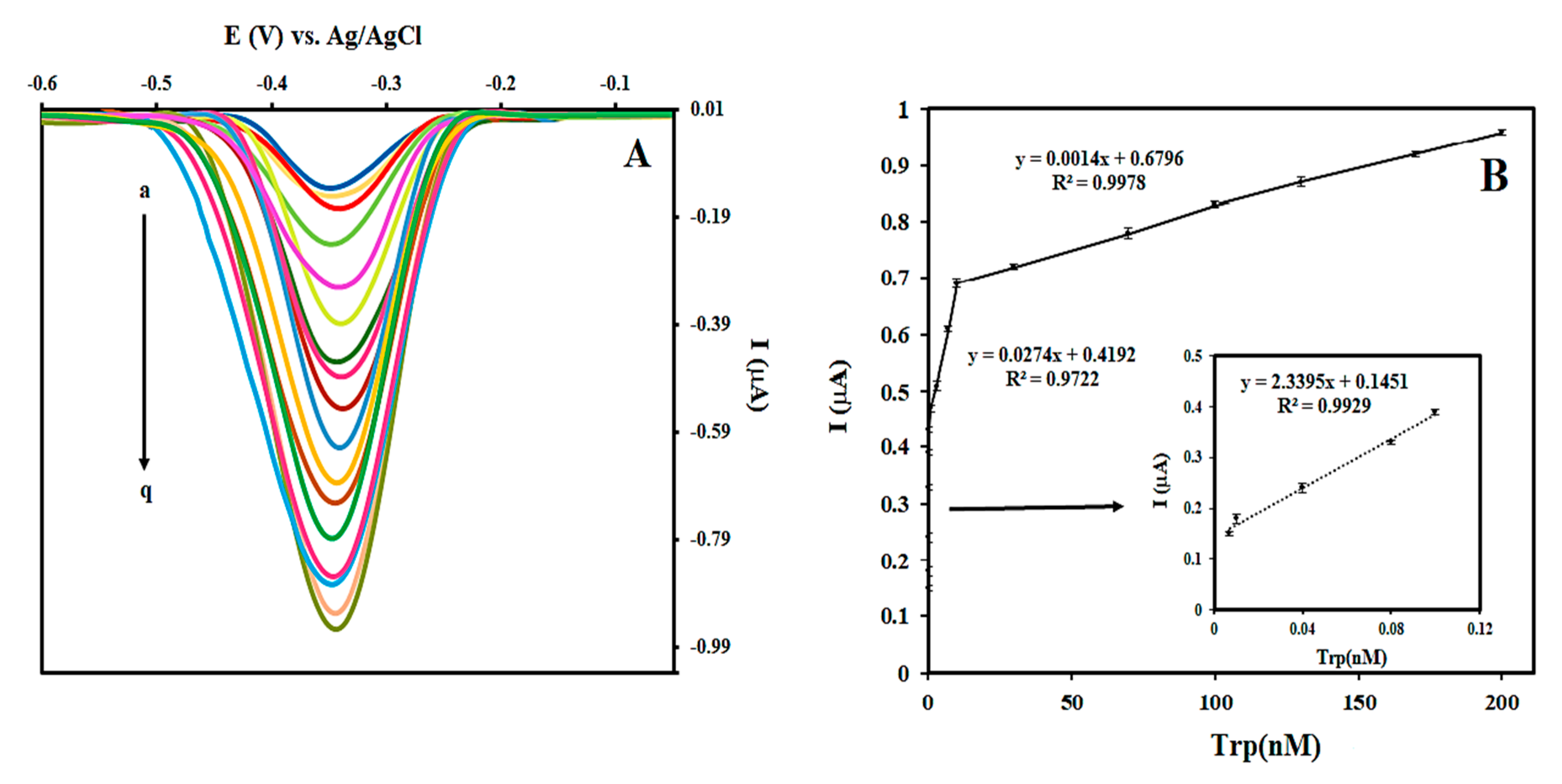
3.4. Sensitivity of the Prepared Aptasensor
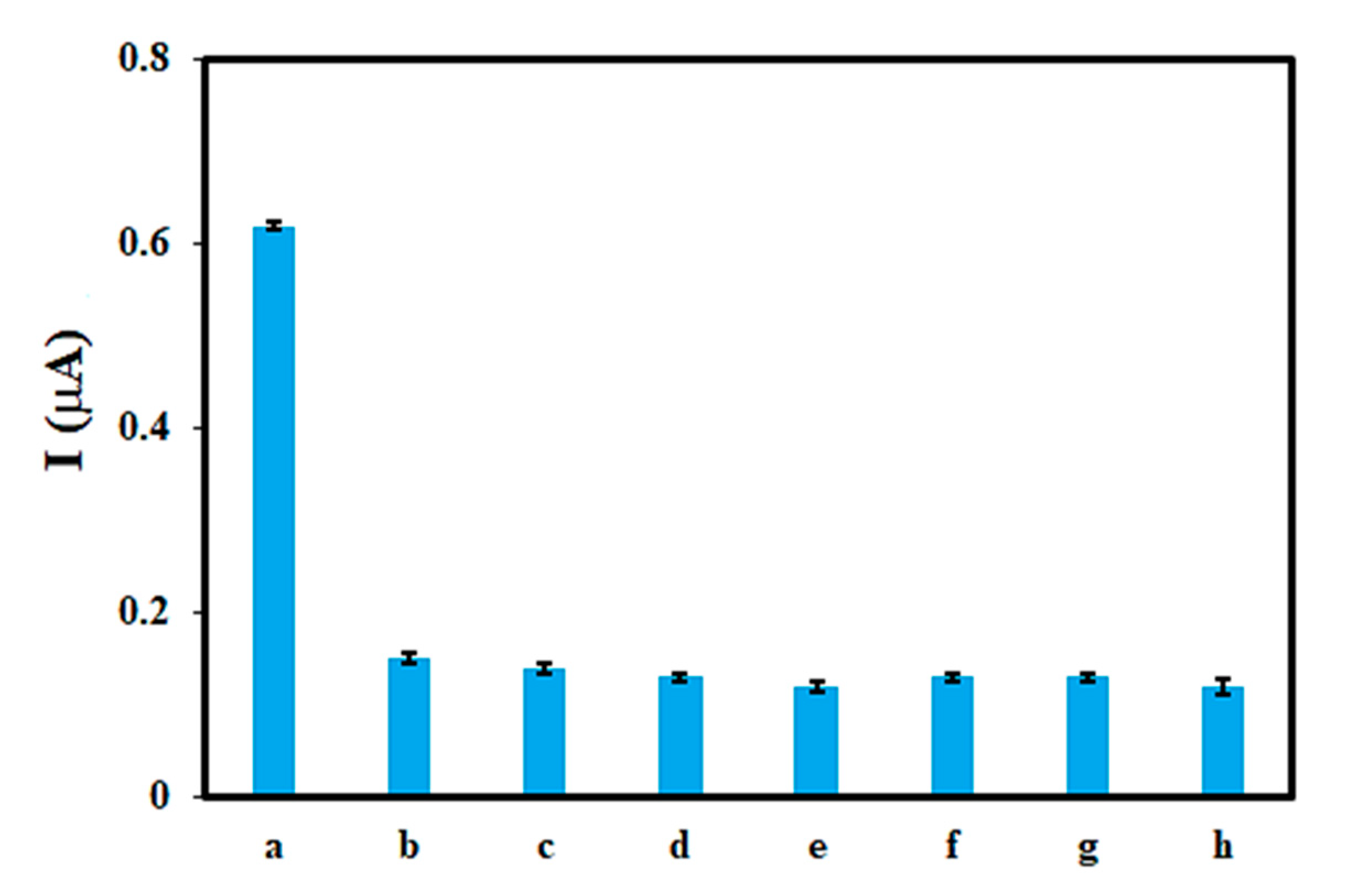
3.5. Selectivity, Real Sample, Reproducibility, and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Tryptophan Res. 2018, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J. Amino Acids 2016, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Sánchez-López, E.; Wang, Q.; Jiang, Z.; Marina, M.L. Determination of L-Norvaline and L-Tryptophan in Dietary Supplements by Nano-LC Using an O-[2-(Methacryloyloxy)-Ethylcarbamoyl]-10,11-Dihydroquinidine-Silica Hybrid Monolithic Column. J. Pharm. Anal. 2019, 10, 70–77. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.; Jiménez, S.; Morales, F.J. Tryptophan determination in milk-based ingredients and dried sport supplements by liquid chromatography with fluorescence detection. Food Chem. 2006, 98, 580–585. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Zhao, M.; Wang, J.; Cui, C.; Yang, B. Spectrophotometric Method for Determination of Tryptophan in Protein Hydrolysates. J. Food Tech. Biotech. 2007, 45, 360–366. [Google Scholar]
- Lin, Z.; Chen, X.; Cai, Z.; Li, P.; Chen, X.; Wang, X. Chemiluminescence of Tryptophan and Histidine in Ru(Bpy)32+-KMnO4 Aqueous Solution. Talanta 2008, 75, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Eser, B.; Özkan, Y.; Dinçel, A.S. Determination of Tryptophan and Kynurenine by LC-MS/MS by Using Amlodipine as an Internal Standard. J. Am. Soc. Mass Spectrom. 2020, 31, 379–385. [Google Scholar] [CrossRef]
- Hazra, C.; Samanta, T.; Mahalingam, V. A resonance energy transfer approach for the selective detection of aromatic amino acids. J. Mater. Chem. C 2014, 2, 10157–10163. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Ding, Z.; Li, G.; Liu, J.; Zuberi, Z.; He, Q. Rapid recognition and determination of tryptophan by carbon nanotubes and molecularly imprinted polymer-modified glassy carbon electrode. Bioelectrochemistry 2020, 131, 107393. [Google Scholar] [CrossRef]
- Idili, A.; Gerson, J.; Parolo, C.; Kippin, T.; Plaxco, K.W. An electrochemical aptamer-based sensor for the rapid and convenient measurement of l-tryptophan. Anal. Bioanal. Chem. 2019, 411, 4629–4635. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Feng, J.; Wu, Y.; Tian, Y.; Li, G.; Chen, D. Sensitive Voltammetric Sensor for Tryptophan Detection by Using Polyvinylpyrrolidone Functionalized Graphene/GCE. Nanomaterials 2020, 10, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashkavayi, A.B.; Raoof, J.-B.; Ojani, R. Construction of a highly sensitive signal-on aptasensor based on gold nanoparticles/functionalized silica nanoparticles for selective detection of tryptophan. Anal. Bioanal. Chem. 2017, 409, 6429–6438. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Raoof, J.-B. Ultrasensitive and reusable electrochemical aptasensor for detection of tryptophan using of [Fe(bpy)3](p-CH3C6H4SO2)2 as an electroactive indicator. J. Pharm. Biomed. Anal. 2019, 163, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Hashkavayi, A.B.; Raoof, J.-B.; Ojani, R.; Asl, E.H. Label-Free Electrochemical Aptasensor for Determination of Chloramphenicol Based on Gold Nanocubes-Modified Screen-Printed Gold Electrode. Electroanalysis 2015, 27, 1449–1456. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Raoof, J.B.; Azimi, R.; Ojani, R. Label-free and sensitive aptasensor based on dendritic gold nanostructures on functionalized SBA-15 for determination of chloramphenicol. Anal. Bioanal. Chem. 2016, 408, 2557–2565. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Raoof, J.-B.; Ojani, R.; Kavoosian, S. Ultrasensitive electrochemical aptasensor based on sandwich architecture for selective label-free detection of colorectal cancer (CT26) cells. Biosens. Bioelectron. 2017, 92, 630–637. [Google Scholar] [CrossRef]
- Park, K.S. Nucleic acid aptamer-based methods for diagnosis of infections. Biosens. Bioelectron. 2018, 102, 179–188. [Google Scholar] [CrossRef]
- Park, K.S.; Lee, C.Y.; Kang, K.S.; Park, H.G. Aptamer-mediated universal enzyme assay based on target-triggered DNA polymerase activity. Biosens. Bioelectron. 2017, 88, 48–54. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Raoof, J.-B.; Ojani, R. Preparation of Epirubicin Aptasensor Using Curcumin as Hybridization Indicator: Competitive Binding Assay between Complementary Strand of Aptamer and Epirubicin. Electroanalysis 2018, 30, 378–385. [Google Scholar] [CrossRef]
- Yu, X.; Lin, Y.; Wang, X.; Xu, L.; Wang, Z.; Fu, F. Exonuclease-assisted multicolor aptasensor for visual detection of ochratoxin A based on G-quadruplex-hemin DNAzyme-mediated etching of gold nanorod. J. Microchim. Acta 2018, 185, 259. [Google Scholar] [CrossRef]
- Golub, E.; Albada, H.B.; Liao, W.-C.; Biniuri, Y.; Willner, I.; Albada, B. Nucleoapzymes: Hemin/G-Quadruplex DNAzyme—Aptamer Binding Site Conjugates with Superior Enzyme-like Catalytic Functions. J. Am. Chem. Soc. 2016, 138. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yu, Y.; Chen, Z.; Zhang, L.; He, S.; Shi, Q.; Yang, H. A label-free hemin/G-quadruplex DNAzyme biosensor developed on electrochemically modified electrodes for detection of a HBV DNA segment. RSC Adv. 2015, 5, 11541–11548. [Google Scholar] [CrossRef]
- Wu, S.-H.; Tang, Y.; Chen, L.; Ma, X.-G.; Tian, S.-M.; Sun, J.-J. Amplified electrochemical hydrogen peroxide reduction based on hemin/G-quadruplex DNAzyme as electrocatalyst at gold particles modified heated copper disk electrode. Biosens. Bioelectron. 2015, 73, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Wang, Q.; Zhu, D.; Luo, J.; Cheng, G.; He, P.; Fang, Y. G-Quadruplex-Based DNAzymes Aptasensor for the Amplified Electrochemical Detection of Thrombin. Electroanalysis 2010, 22, 2985–2990. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Hashemnia, S.; Osfouri, S.; Zarei, S. Electrochemical Study of Antioxidant Capacity of Gracilaria Pygmaea Macro-Algae Based on the Green Synthesis of Gold Nanoparticles: Assessment of Its Cytotoxic Effect on Four Cancer Cell Lines. J. Electrochem. Soc. 2019, 166, B969–B977. [Google Scholar] [CrossRef]
- Raoof, J.-B.; Bagheryan, Z.; Hashkavayi, A.B. Development of a DNA biosensor based on MCM41 modified screen-printed graphite electrode for the study of the short sequence of the p53 tumor suppressor gene in hybridization and its interaction with the flutamide drug using hemin as the electrochemical label. New J. Chem. 2020, 44, 2016–2021. [Google Scholar]
- Rahimi-Mohseni, M.; Raoof, J.B.; Ojani, R.; Aghajanzadeh, T.A.; Hashkavayi, A.B. Development of a new paper based nano-biosensor using the co-catalytic effect of tyrosinase from banana peel tissue (Musa Cavendish) and functionalized silica nanoparticles for voltammetric determination of l-tyrosine. Int. J. Biol. Macromol. 2018, 113, 648–654. [Google Scholar] [CrossRef]
- Li, F.; Huang, Y.; Huang, K.; Lin, J.; Huang, P. Functional Magnetic Graphene Composites for Biosensing. Int. J. Mol. Sci. 2020, 21, 390. [Google Scholar] [CrossRef] [Green Version]
- Doaga, R.; McCormac, T.; Dempsey, E. Functionalized magnetic nanomaterials for electrochemical biosensing of cholesterol and cholesteryl palmitate. J. Microchim. Acta 2020, 187, 1–10. [Google Scholar] [CrossRef]
- Rajeev, G.; Cowin, A.J.; Voelcker, N.H.; Simon, B.P. Magnetic Nanoparticles Enhance Pore Blockage-Based Electrochemical Detection of a Wound Biomarker. Front. Chem. 2019, 7, 438. [Google Scholar] [CrossRef] [Green Version]
- Cho, I.-H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. J. Biomater. Res. 2020, 24, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.M.; Tavallaie, R.; Sandiford, L.; Tilley, R.D.; Gooding, J.J. Gold Coated Magnetic Nanoparticles: From Preparation to Surface Modification for Analytical and Biomedical Applications. Chemcomm 2016, 47, 7528–7540. [Google Scholar]
- Phama, X.-H.; Hahm, E.; Kim, H.-M.; Son, B.S.; Jo, A.; An, J.; Thi, T.A.T.; Nguyen, D.Q.; Jun, B.-H. Silica-Coated Magnetic Iron Oxide Nanoparticles Grafted onto Graphene Oxide for Protein Isolation. Nanomaterials 2020, 10, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaria, A.; Soulé, S.; Hallali, N.; Ojo, W.-S.; Mirjolet, M.; Fuks, G.; Cornejo, A.; Allouche, J.; Dupin, J.C.; Martinez, H.; et al. Silica coated iron nanoparticles: Synthesis, interface control, magnetic and hyperthermia properties. RSC Adv. 2018, 8, 32146–32156. [Google Scholar] [CrossRef] [Green Version]
- Sharafi, Z.; Bakhshi, B.; Javidi, J.; Adrangi, S. Synthesis of Silica-coated Iron Oxide Nanoparticles: Preventing Aggregation without Using Additives or Seed Pretreatment. Iran. J. Pharm. Res. IJPR 2018, 17, 386–395. [Google Scholar]
- Hashkavayi, A.B.; Raoof, J.B. Design an Aptasensor Based on Structure-Switching Aptamer on Dendritic Gold Nanostructures/Fe3O4@ SiO2/DabCO Modified Screen Printed Electrode for Highly Selective Detection of Epirubicin. Biosens. Bioelectron. 2017, 91, 650–657. [Google Scholar] [CrossRef]
- Pajkossy, T. Voltammetry Coupled with Impedance Spectroscopy. J. Solid State Electrochem. 2020, 24, 2157–2159. [Google Scholar] [CrossRef]
- Steel, A.B.; Herne, T.M.; Tarlov, M.J. Electrochemical Quantitation of DNA Immobilized on Gold. J. Anal. Chem. 1998, 70, 4670–4677. [Google Scholar] [CrossRef]
- Yang, X.; Han, Q.; Zhang, Y.; Wu, J.; Tang, X.; Dong, C.; Liu, W. Determination of free tryptophan in serum with aptamer—Comparison of two aptasensors. Talanta 2015, 131, 672–677. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, J.; Harvey, S.E.; Hu, X.; Cheng, C. RNA G-quadruplex secondary structure promotes alternative splicing via the RNA-binding protein hnRNPF. Genes Dev. Chang. 2017, 31, 2296–2309. [Google Scholar] [CrossRef]
- Kikin, O.; D’Antonio, L.; Bagga, P.S. QGRS Mapper: A web-based server for predicting G-quadruplexes in nucleotide sequences. Nucleic Acids Res. 2006, 34, W676–W682. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, G.; Julián, E.P.; Agüí, L.; Cabré, N.; Joven, J.; Yáñez-Sedeño, P.; Pingarrón, J.M. An Electrochemical Enzyme Biosensor for 3-Hydroxybutyrate Detection Using Screen-Printed Electrodes Modified by Reduced Graphene Oxide and Thionine. Biosensors 2017, 7, 50. [Google Scholar] [CrossRef] [Green Version]
- Dermiş, S.; Cay, H.Y. Electrochemical behaviour of sertraline hydrochloride at a glassy carbon electrode and its determination in pharmaceutical products using osteryoung square wave voltammetry. Pharmazie 2010, 65, 182–187. [Google Scholar] [PubMed]
- Safavi, A.; Momeni, S. Electrocatalytic Oxidation of Tryptophan at Gold Nanoparticle-Modified Carbon Ionic Liquid Electrode. Electroanalysis 2010, 22, 2848–2855. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, D.; Zhan, G.; Li, C. Electrochemical Investigation of Tryptophan at a Poly (P-Aminobenzene Sulfonic Acid) Film Modified Glassy Carbon Electrode. J. Bull. Korean Chem. Soc. 2008, 29, 928. [Google Scholar]
- Xu, M.; Ma, M.; Ma, Y. Electrochemical determination of tryptophan based on silicon dioxide nanopartilces modified carbon paste electrode. Russ. J. Electrochem. 2012, 48, 489–494. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L. Electrochemical Sensor for Tryptophan Determination Based on Copper-cobalt Hexacyanoferrate Film Modified Graphite Electrode. Sensors 2007, 7, 2446–2457. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Mai, G.; Liu, Y.; Yang, C.; Qua, W. Voltammetric Determination of Tryptophan at a Single-Wall Carbon Nanotubes Modified Electrode. J. Nanosci. Nanotechnol. 2004, 4, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S.; Bayat, M. Voltammetric Determination of Tryptophan and 5-Hydroxytryptophan Using Graphite Electrode Modified with a Thin Film of Graphite/Diamond Nano-Mixture and Determination of Omeprazole Using Graphite Electrode; Department of Chemistry, Sharif University of Technology: Tehran, Iran, 2010. [Google Scholar]
- Majidi, M.R.; Salimi, A.; Alipour, E. Development of Voltammetric Sensor for Determination of Tryptophan Using MWCNTs-Modified Sol-Gel Electrode. J. Chin. Chem. Soc. 2013, 60, 1473–1478. [Google Scholar] [CrossRef]
- Chen, Z.-D.; Okamura, K.; Hanaki, M.; Nagaoka, T. Selective Determination of Tryptophan by Using a Carbon Paste Electrode Modified with an Overoxidized Polypyrrole Film. Anal. Sci. 2002, 18, 417–421. [Google Scholar] [CrossRef] [Green Version]
- Sadok, I.; Tyszczuk-Rotko, K.; Mroczka, R.; Staniszewska, M. Simultaneous voltammetric analysis of tryptophan and kynurenine in culture medium from human cancer cells. Talanta 2020, 209, 120574. [Google Scholar] [CrossRef]
| Sensing Surface | Electrochemical Method | Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|
| GNP/CILE | SWV | 5 × 10−3–9 × 10−1 M | 4 × 10–3 M | [44] |
| poly(p-ABSA) film/GCE | LSV | 1 × 10−7–10−5 M | 7 × 10−8 M | [45] |
| SiO2 nanoparticles/CPE | LSV | 1 × 10–7–5 × 10–5 M | 3.6 × 10–8 M | [46] |
| CuCoHCF/graphite electrode | Amperometry | 1 × 10–5–9 × 10–4 M | 6 × 10–6 M | [47] |
| SWNT/GCE | DPV | 4 × 10−8–1 × 10−5 M | 1 ×10−8 M | [48] |
| PGE/GND | CV | 1 × 10−8–4 × 10−6 M | 3 × 10−9 M | [49] |
| MWCNTs/SGE | DPV | 2 × 10−7–15 × 10−6 M | 14 × 10−8 M | [50] |
| OPPy/CPE | ASV | 1 × 10−2–1 × 10−1 M | 1 × 10−2 M | [51] |
| MIP-MWCNTs/GCE | CV | 2 × 10−6–1 × 10−4 M | 1 × 10−9 M | [9] |
| PVP-GR/GCE | LSV | 6 × 10−8–1 × 10−4 M | 1 × 10−8 M | [11] |
| BiF/BDDE | DPV | 1 × 10−7–75 × 10−6 M | 3 × 10−8 M | [52] |
| AuNPs/FSN/SPE | DPV | 0.06–250 × 10−9 M | 1 × 10−11 M | [12] |
| DGNs/Fe3O4@SiO2-DABCO/SPE | DPV | 0.007–0.1, 0.1–10, and 10–200 × 10−9 M | 2 × 10−12 M | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashkavayi, A.B.; Raoof, J.B.; Park, K.S. Sensitive Electrochemical Detection of Tryptophan Using a Hemin/G-Quadruplex Aptasensor. Chemosensors 2020, 8, 100. https://doi.org/10.3390/chemosensors8040100
Hashkavayi AB, Raoof JB, Park KS. Sensitive Electrochemical Detection of Tryptophan Using a Hemin/G-Quadruplex Aptasensor. Chemosensors. 2020; 8(4):100. https://doi.org/10.3390/chemosensors8040100
Chicago/Turabian StyleHashkavayi, Ayemeh Bagheri, Jahan Bakhsh Raoof, and Ki Soo Park. 2020. "Sensitive Electrochemical Detection of Tryptophan Using a Hemin/G-Quadruplex Aptasensor" Chemosensors 8, no. 4: 100. https://doi.org/10.3390/chemosensors8040100
APA StyleHashkavayi, A. B., Raoof, J. B., & Park, K. S. (2020). Sensitive Electrochemical Detection of Tryptophan Using a Hemin/G-Quadruplex Aptasensor. Chemosensors, 8(4), 100. https://doi.org/10.3390/chemosensors8040100







