Abstract
ZnO–SnO2 films with a thickness of up to 120 nm have been prepared on glass substrates by pyrolysis at 550 °C of three spin-coated organic precursors films. Films of four compositions were obtained on glass substrates. The prepared films were characterized by SEM, XRD, and XPS analysis. Electrophysical studies have shown that the activation energy of the temperature conductivity for all films is equal to 0.75 eV. While the gas-sensitive characteristics by CO treatment in low concentrations at a temperature of 200–300 °C was studied, their rapid degradation was found. Studies using the XPS method have shown that ZnO–SnO2 films contain sodium, which is diffused from the soda-lime glass substrate during the film formation. Studies of XPS spectra after CO treatment have shown that the film surface is almost 50% composed of adsorbed water molecules and OH groups. OH groups are part of the sodium, tin, and zinc hydroxides formed on the surface. In addition, zinc hydrocarbonates are formed on the surface of the films. The detected insoluble compounds lead to the degradation of gas-sensitive properties of ZnO–SnO2 films.
1. Introduction
Creating of gas-sensitive sensors for detecting environmentally hazardous gases based on nanoscale film materials is an important task for most researchers due to increased atmospheric pollution. Research work in this direction has been actively carried out over the past 20 years [1,2], and it has been established that the sensitivity of gas-sensitive sensors is affected by both the nature of the nanomaterial and its structure [3,4].
The most widespread materials for gas-sensitive-resistive sensors are semiconductor metal oxides obtained in the film form, such as SnO2 [5], ZnO [6], In2O3 [7], WO3 [8], and others [9,10] due to their special properties, such as stability to the environment, high sensitivity, etc. There are also frequent cases of using nanocomposite materials based on the abovementioned oxides, among which tin dioxide plays important role due to its high sensitivity to different hazardous gases [11]. For example, films of the composition 40SnO2:60WO3 demonstrate the greatest sensitivity to carbon dioxide at room temperature [12]. In [13], a one-step hydrothermal method was used to synthesize films based on tin dioxide, in which bismuth ions were used as doping agents. It was shown that sensors based on films of these gas-sensitive materials demonstrate high sensitivity to benzene, and the introduction of bismuth ions into the SnO2 structure leads to a narrowing of the effective band gap. Similar results were obtained in [14] where gas-sensitive film material based on a mixture of three oxides (SiO2-SnOx-CuOy) was obtained via sol–gel method.
Along with materials based on tin dioxide, materials based on zinc oxide are also of interest, both in pure form and when doped with different modifying additives. The authors [15] studied the gas-sensitive properties of pure zinc oxide films doped with aluminum ions, platinum ions, and together with aluminum and platinum ions. It was shown that the best results are achieved when doping agents are used simultaneously. The introduction of copper ions into the structure of zinc oxide made it possible to obtain structured nanomaterials in the form of columnar structures, and the resulting materials showed high sensitivity to carbon monoxide under different conditions [16].
Promising results obtained for materials based on zinc and tin oxides could propose the collaborative possibility for using these oxides in the gas-sensitive sensors creation. The ZnO–SnO2 system may be of particular interest for research, since these metals have symmetrical s-orbitals, which may explain the unique properties of composite materials based on them, such as high resistance, gas sensitivity, unique optical properties, etc. [17,18]. In addition, a number of studies indicate the simultaneous existence of composites and doped phases with high gas-sensitive properties [19,20]. Based on these data, we selected SnO2 and ZnO as the most promising materials in practical application.
When a branched system of nanorods using a one-step hydrothermal method was created, where the backbone is ZnO films and the “branches” are SnO2, a higher sensitivity to ethanol vapor was shown compared to pure zinc oxide nanofilms [21]. When hydrothermally synthesized materials based on composite material ZnO–SnO2 were compared with pure tin oxide, it was shown that ZnO nanoparticles were clearly observed on the surface of SnO2 hollow spheres; the chemisorption capacity of ZnO/SnO2 composites surface was significantly higher, which allows the authors [22] to recommend the obtained film materials as highly sensitive sensors for detecting ethanol. In addition, the authors demonstrated the stability of synthesized materials for 200 cycles.
The synthesis of ultrathin mesoporous ZnO–SnO2 nanosheets was described in detail [23]. It was shown that the materials form nanosheets and were highly sensitive to ethanol. Compared to pure ZnO and SnO2, the synthesized films are more stable, sensitive, and selective and have a faster recovery/response time. The synthesis of materials using the core–shell technology also shows promising results, e.g., materials with high sensitivity to ethanol were obtained in [24]. The hollow nanorods were synthesized in two steps—ZnO was applied to the electrochemically obtained SnO2 rods by hydrothermal method.
One of the most serious problems when using gas sensors is the deterioration of their parameters. Only a few scientific groups have studied the degradation of gas sensors based on metal oxides. These issues are studied in more detail in the works of Korotkenkov and Cho [19,25]. The authors showed that the degradation of gas sensor parameters is influenced by use of incorrectly selected surface-modified agents of oxide semiconductors or mixed oxides (contain two or more components). It was shown that the degradation is also affected by the characteristics of gas sensors metal contacts [26]. However, these studies have investigated the reasons of degradation of gas-sensitive characteristics for a sufficiently long time.
On the one hand, there are well-known processes that can be called “poisoning” (or deactivation) of gas-sensitive material. These are usually the processes that lead to a rapid deterioration of gas-sensitive properties. On the other hand, no relevant articles were found except [27,28,29,30] on the deactivation of gas-sensitive properties of zinc/tin films. In this regard, we consider it necessary to publish this material as original and relevant, which may be useful for other researchers working in this field.
The reasons for the deactivation of gas-sensitive sensors can be different, but in general, they can be bundled into three large groups—mechanical, chemical, and thermal reasons [27]. The most problematic group is chemical processes of deactivation, i.e., poisoning of sensors as an active sorption result or interaction with volatile substances. As a rule, during this type of deactivation, chemosorption occurs on the active center of the catalyst surface, which leads to sensor poisoning; when the catalyst surface is treated by any gas or volatile substances, compounds are formed that block the active surface. In the case of CO sensor treatment, blocking of the active catalyst centers may occur, but more often, it is possible that oxygen is oxidized to carbon dioxide and carbonates or hydrocarbonates are formed on the sensor surface.
There are several possible mechanisms for this process [27,28,29]. Whatever the reason for the degradation of gas sensor parameters, it is based on the processes occurring on the gas-sensitive materials surface [30].
The purpose of this work is to study the surface processes that lead to degradation of gas-sensitive properties of thin (up to 130 nm) ZnO–SnO2 films deposited on glass (soda-lime) substrates when they are treated to a controlled concentration of carbon monoxide in a mixture with moist air.
2. Materials and Methods
2.1. Deposition of ZnO–SnO2 Film on Substrates
All chemicals used were of analytical grade or of the highest purity available and were purchased from “ECROS,” Russia. Binary ZnO–SnO2 (ZTO) thin films with varying SnO2 concentrations were grown on soda-lime glass substrate. ZTO transparent films were obtained from organic compounds of zinc (II) and tin (IV) by pyrolysis.
The organic intermediates of zinc and tin (IV) were obtained through the reaction between abietic acid and acetate crystallohydrates of zinc and chloride crystallohydrates of tin (IV). Then, the solutions of organic compounds of zinc and tin (IV) were prepared with the molar ratio of Zn:Sn = 99:1 (material 1) and 95:5 (material 2). Chemical cleaning of the soda-lime glass substrates was done by treatment with a hot mixture of potassium dichromate and concentrated nitric acid for 10 min, then washing three times with distilled water, and finally, treating with alcohol. To remove the residual alcohol, the substrates were washed twice in distilled water and dried. The precursor organic composition was applied onto the glass substrates by spin-coating. After that, it was dried for 20 min at a temperature of 120 °C. If necessary, this operation was repeated several times. ZTO films were prepared on a substrate by firing the solution of organic precursors. Heat treatment of the samples was carried out in air with heating at a speed of 10 °C/min, with exposure for 1 h at a temperature of 550 °C. The films were cooled slowly to room temperature.
2.2. Characterization
Structural properties of the synthesized materials were studied by X-ray diffraction (XRD, ARL X’TRA diffractometer, CuKα1-radiation), operated at 35 kV and 30 mA. The mean crystallite size (D) was evaluated according to broadening of the highest intensity peak diffraction plane using the Scherrer equation D = kλ/βcosΘ, where k is the shape factor (k = 0.9), λ is the X-ray wavelength (λ = 0.1540562 nm), β is the full width at the half maximum of the diffraction line, and Θ is the diffraction angle. The values of β and Θ are taken for crystal plane (101) of the ZnO wurtzite phase [31].
The surface morphology was characterized by scanning electron microscope (SEM, Nova NanoLab 600).
To study the elemental composition and chemical state of the gas-sensitive films materials, the most informative surface analysis method, i.e., X-ray photoelectron spectroscopy (XPS), was used [2,32,33,34,35,36]. In our case, the XPS analysis was carried out in an ultra-high vacuum (1,8∙10−9 mbar) using K-Alpha Thermo Scientific spectrometer with monochromatic Al-Kα X-rays source (1486.6 eV).
Before conducting the research, reference photoelectronic lines values of the reference samples, i.e., Au 4f = 84, Ag 3d = 368.2, and Cu 2p = 932.6 eV were obtained. To neutralize the surface charge, a calibrated (C 1s = 284.8 eV) compensating electron flood gun was used. The basic element composition of the surface is determined from the survey spectra obtained in the mode of constant pass energy of 200 eV at a spectral resolution of 1 eV. The signal intensity values were averaged statistically over 10 measurements. To determine the chemical state, high-resolution spectra with an X-ray beam diameter of 400 µ were recorded in the energy pass 20 eV at a spectral resolution of 0.1 eV and a statistical accumulation value of N = 20. Component analysis is performed by constructing curves using joint Shirley and Tougaard functions to determine the peaks background; the line shape of the plotted curves was obtained at a 30% ratio of Lorenz/Gauss mixture and the ratio of doublet peaks intensities is determined according to the quantum number selection rule, which corresponds to 1/2 for p-electrons and 2/3 for d-electrons. The calculation of the element atoms concentrations is carried out by determining the curve areas of the corresponding components and their normalization as follows:
where Nelement—x-element concentration; Snorm—normalized area under the peak of the x-element; Fx—sensitivity factor of the x-element, which depends on the orbital; Tx—transmission function (depends on the sensitivity factor); and Cx—attenuation length, correction for the free path length of nonelastically scattered electrons. The values Fx, Tx, and Cx are defined from the Scofield libraries.
ZTO was deposited as thin films using pyrolysis on glass substrates. To study the electrophysical and gas-sensitive properties, V-Ni metal contacts with a thickness of 0.2–0.3 µ were formed on top of ZTO by vacuum thermal deposition, the distance between the contacts was 1 mm. It was measured using an automated installation for determining the parameters of gas sensors at the Center for Collective Use “Microsystem Technics and Integral Sensors” [37].
3. Results and Discussion
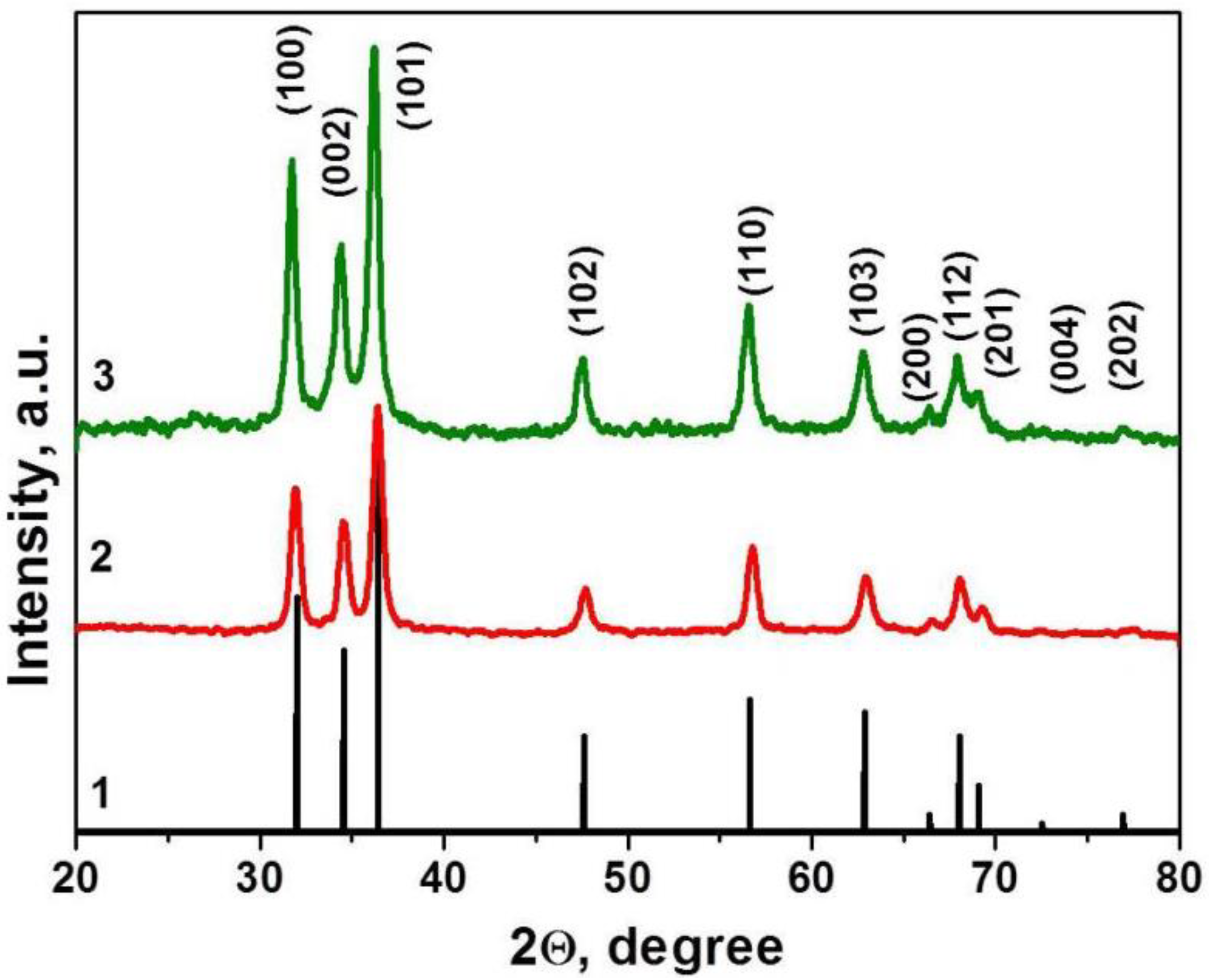
The crystal structure and the purity of the as-deposited thin film were investigated by XRD. The results are presented in Figure 1. No additional diffraction peaks were detected in the XRD patterns, indicating that thin films yield pure phase. It was shown that obtained materials contain single-phase, hexagonal wurtzite structured ZnO, according to Crystallography Open Database, COD ID 2300113 [38] (Figure 1). The presence of diffraction peaks along different planes indicates that the film’s nanocrystalline structure is growing in different directions and is isotropic. It was found that signals on the XRD pattern are expanded, which is typical for nanocrystalline materials. According to Scherrer’s equation, the crystalline size was about 10–17 nm.
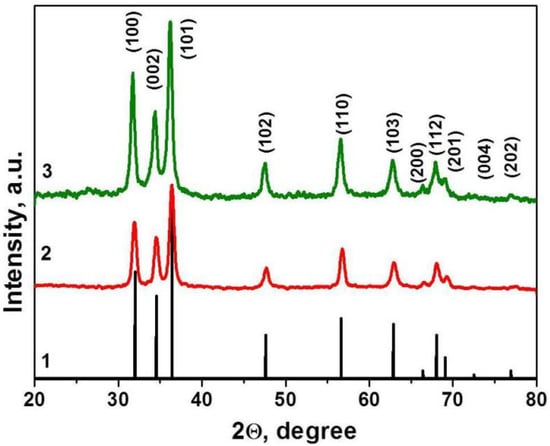
Figure 1.
XRD patterns of ZnO–SnO2 (ZTO) films. ZnO phase (curve 1), Crystallography Open Database (COD) ID 2300113, and material 1 (curve 2), 2 (curve 3).
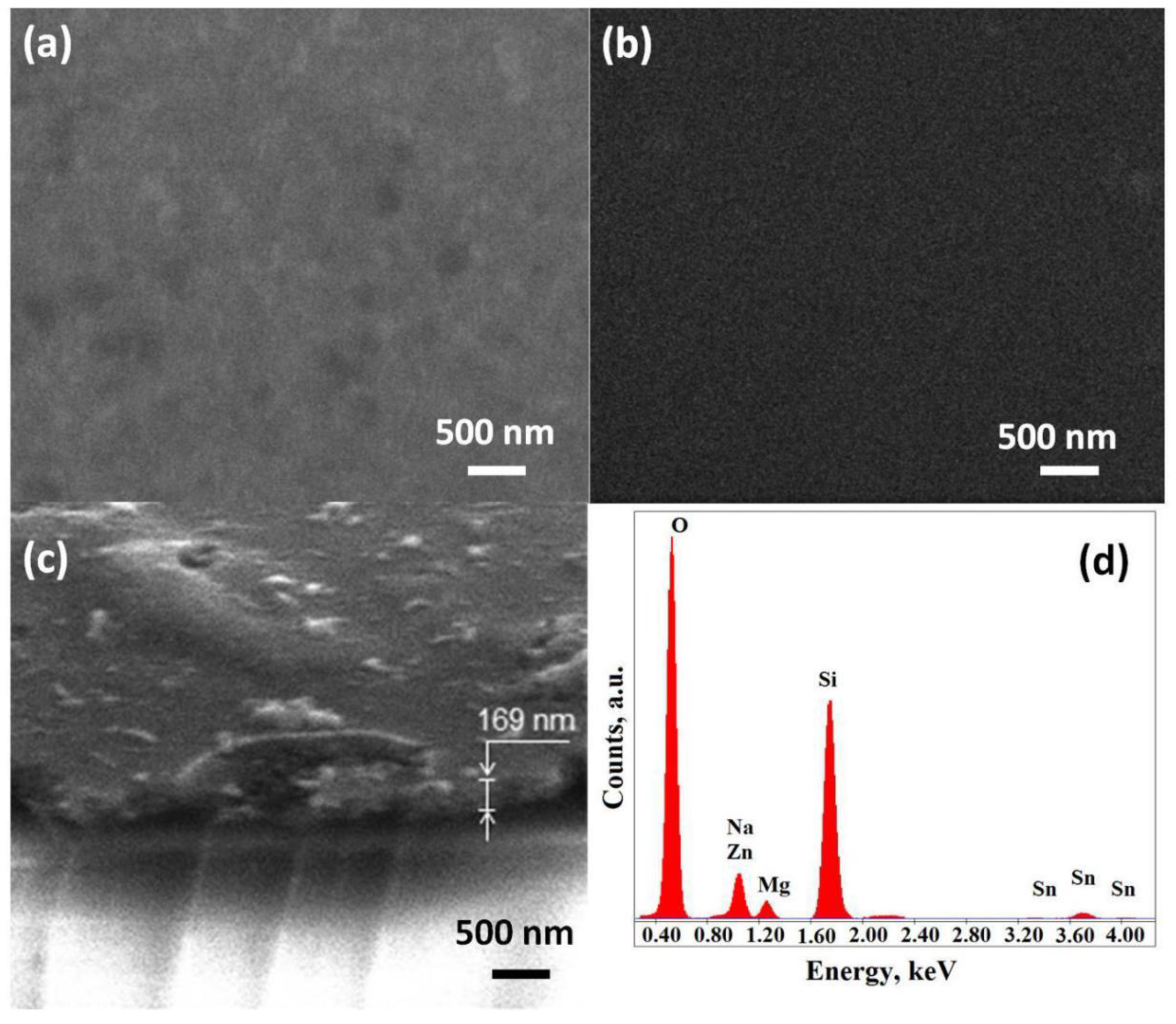
The samples were investigated by SEM analysis, and also, the EDAX analysis was performed (Figure 2). The films surface was not uniform and contained different phase inclusions of different sizes. The film thickness was estimated programmatically using the SEM, Nova NanoLab 600 device. The tilt of the film was considered during measurements. The thickness estimation based on SEM measurements showed that the film on one sample has a thickness spread in the range of 140–220 nm. Figure 2c shows a typical fragment of film sample obtained from solution with a ZnO–SnO2 ratio of 99:1.
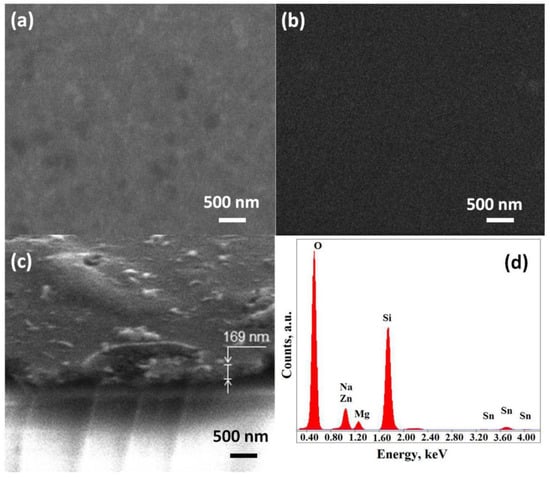
Figure 2.
SEM images of materials 1 (a) and 2 (b) and surfaces and typical fragment (c), and EDX spectra (d) of material 1 film.
The elemental composition of all the studied films was determined using energy-dispersive X-ray spectroscopy (Figure 2d). The results of EDAX analysis showed the presence of Si, Na, and Mg atoms on the spectra, which were part of the substrate. When studying samples containing zinc, the line in the vicinity of energy 1 keV was refined and divided into Na and Zn components, which allowed us to interpret the qualitatively and quantitatively presented components according to the method presented in [39]. It should be noted that the depth of electron penetration in EDAX analysis was in units of microns, and the thickness of the studied films did not exceed 220 nm. This did not allowed us to unambiguously judge the possible diffusion, e.g., of Na atoms on the studied films surface.
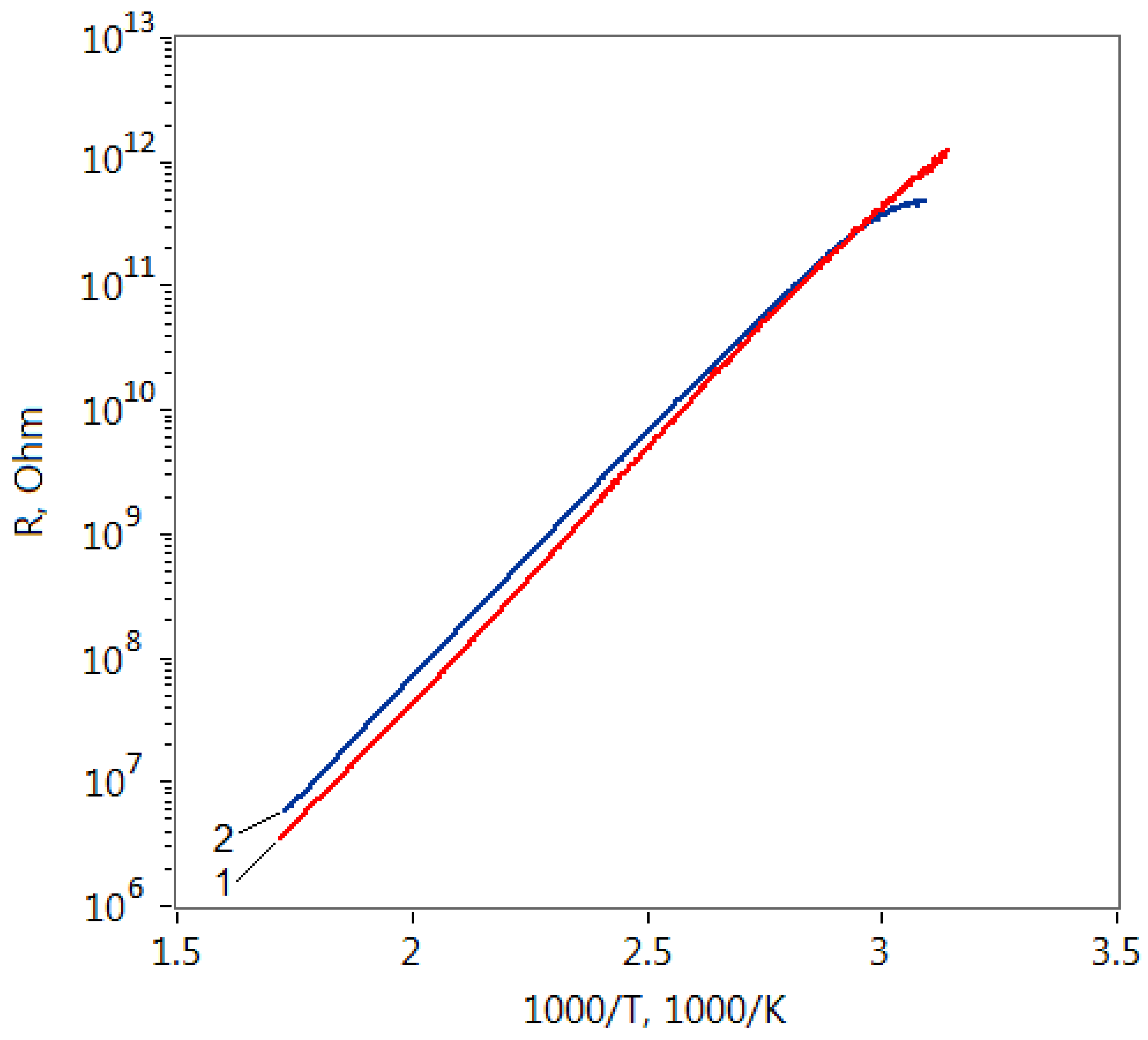
The resistance dependence of the formed structures based on ZTO film grown on the soda-lime glass substrate on the reverse temperature is shown in Figure 3. It is seen that despite the different concentrations of modifying agent, these dependencies are very close to each other for both heating and cooling curves. The observed identical nature of the resistance temperature dependence can be attributed to the presence of Na atoms in the films structure which were diffused from the substrate to the film. It is known that the activation energy of the ZnO films conductivity doped with Na can be equal to 0.8 eV [40]. In our case, this dependence was well approximated by the Arrhenius equation:
where ρ0 is preexponential factor, Ea is activation energy of conductivity, and k is Boltzmann constant. The activation energy Ea of the conductivity deduced from the slopes of the lines fitting the curves is 0.75 eV for all films.
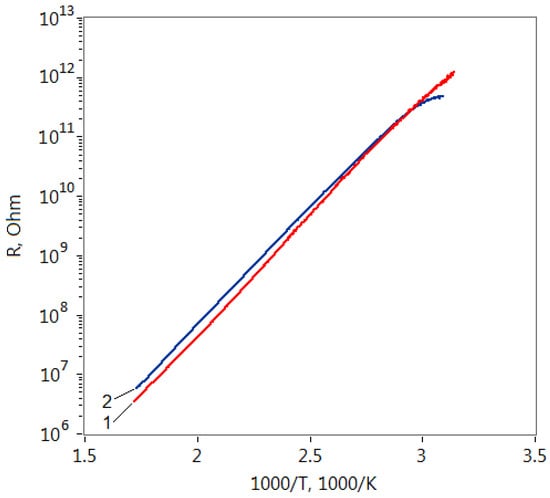
Figure 3.
Resistance (R) dependences on reverse temperature (1000/T) of ZnO–SnO2 films with the Zn:Sn ratios: 1—99:1 and 2—95:5.
To confirm this hypothesis, it could be noted that the same films, but formed on substrates of aluminum oxide (polycor), showed a completely different character of the temperature dependence of the conductivity, as shown in [18]. In addition, zinc oxide films that do not contain Na, as a rule, show a different nature of the R on T dependence when heated and cooled [41].
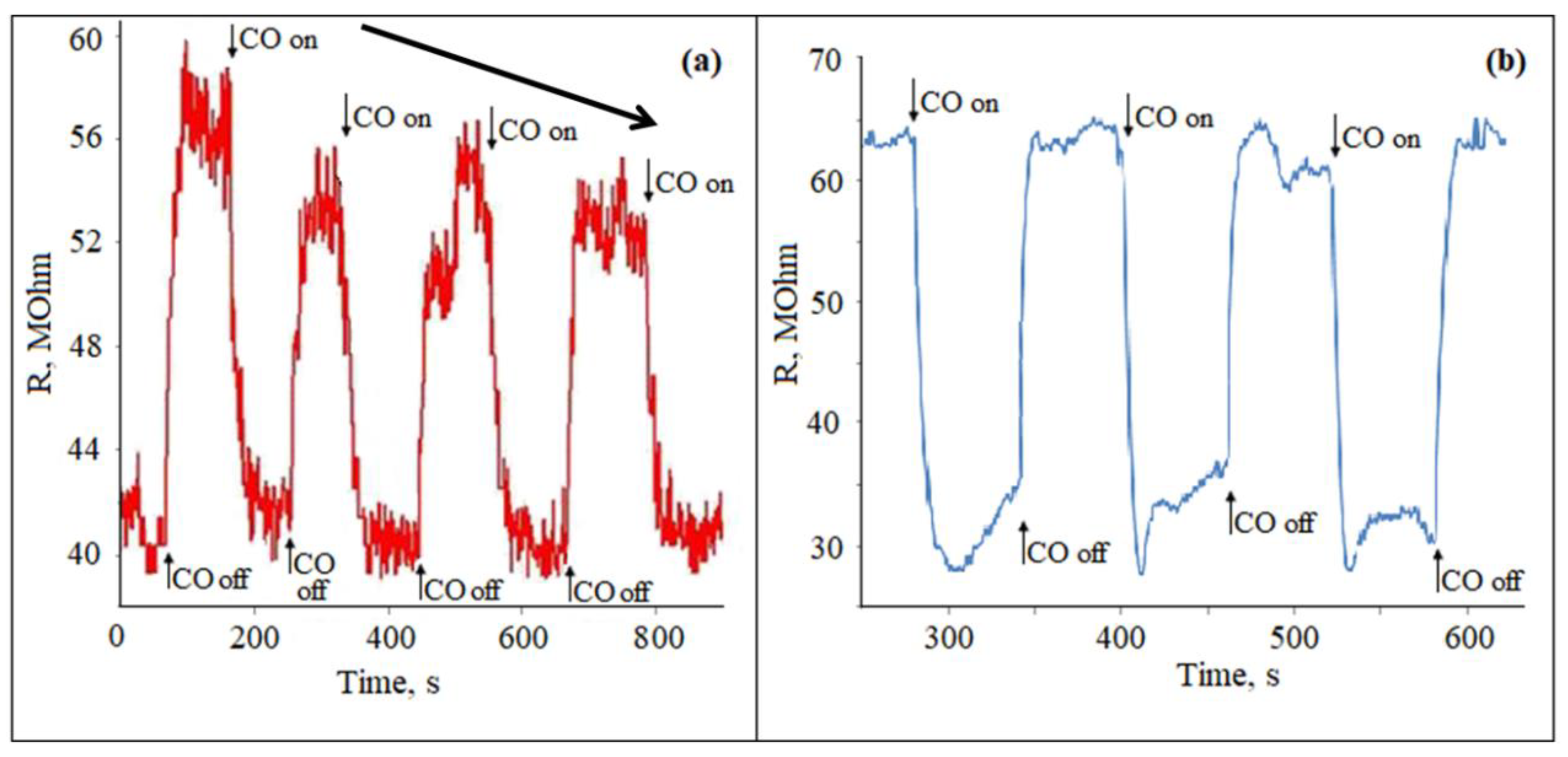
Gas sensitivity studies were carried out for all the studied films materials with the Zn:Sn ratio of 95:5 in relation to CO with a concentration of 50 ppm mixed with air (humidity was about 40–60%) at temperature range of 200–300 °C. In one cycle, the gas supply and air purge were performed three times alternately with a frequency of 120 s. Studies have shown that when exposed to CO, the studied films resistance Rg decreases. The reaction and recovery times were quite short, i.e., of about 30 and 60 s, respectively. However, already in the first cycle of CO treatment, there was a tendency to decreasing the sensor response (Figure 4a). It was also seen that the response of the gas sensor to CO treatment with a concentration of 50 ppm was lower compared to the work of other authors [26,42].
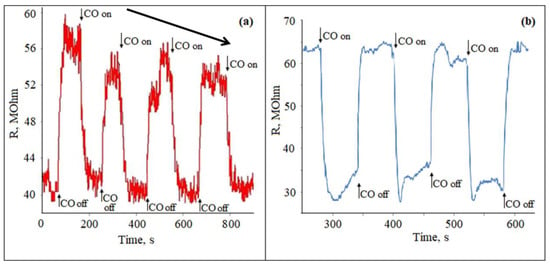
Figure 4.
Poisoning effect of the ZTO films surface with the Zn:Sn ratio of 95:5 grown on the glass (a) and polycor (b) substrates when exposed to CO with a concentration of 50 ppm at 300 °C.
After the first measurement cycle, the samples were cooled to room temperature and reheated after 1 h. The measurement cycle was performed for the second time. Starting from the second cycle, the measurements were not reproduced, and the sensitivity dropped sharply. This type of measurement was observed for all studied films. This indicated that the effect of the gas-sensitive material “poisoning” was observed.
For comparison, a similar experiment was conducted for a material with a ratio of Zn:Sn = 95:5, which was applied on the aluminum oxide (polycor) substrate (Figure 4b). The response curve showed that the sensitivity to CO was slightly higher. In addition, this sample did not have a rapid deterioration in gas-sensitive properties.
In general, the “poisoning” of the gas-sensitive material surface occurs as a result of formation of the different compounds on its surface, and this process is irreversible. The chemical composition of the film surface before and after exposure to CO was assessed using the XPS method (Figure 5, Figure 6 and Figure 7).
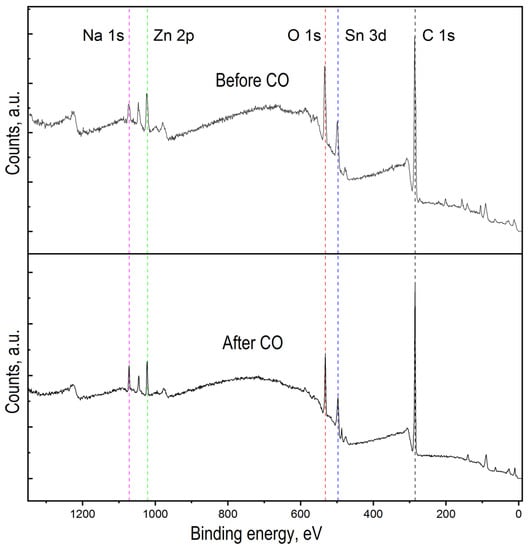
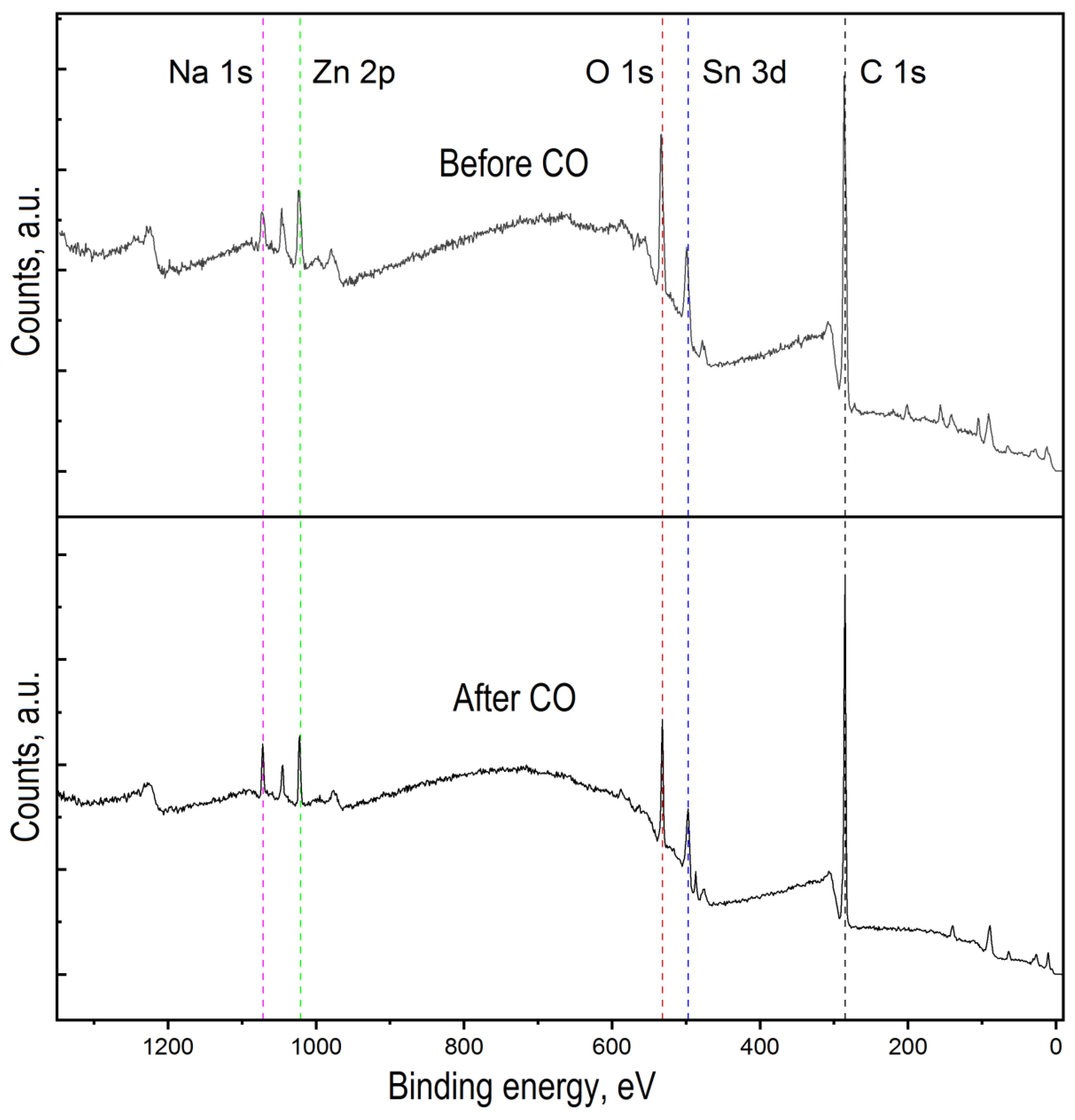
Figure 5.
Overview spectra of ZTO before and after CO treatment.
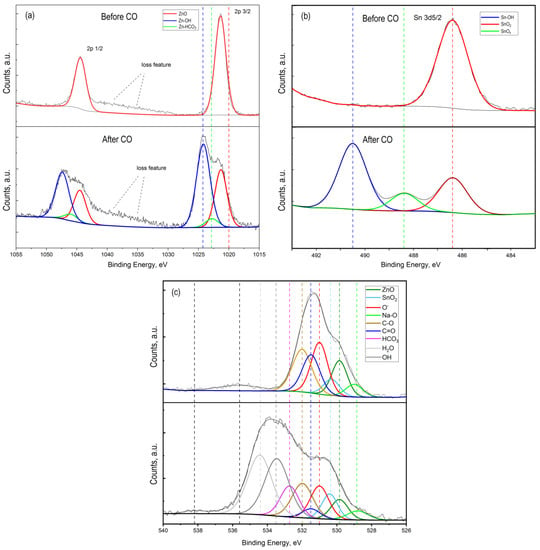
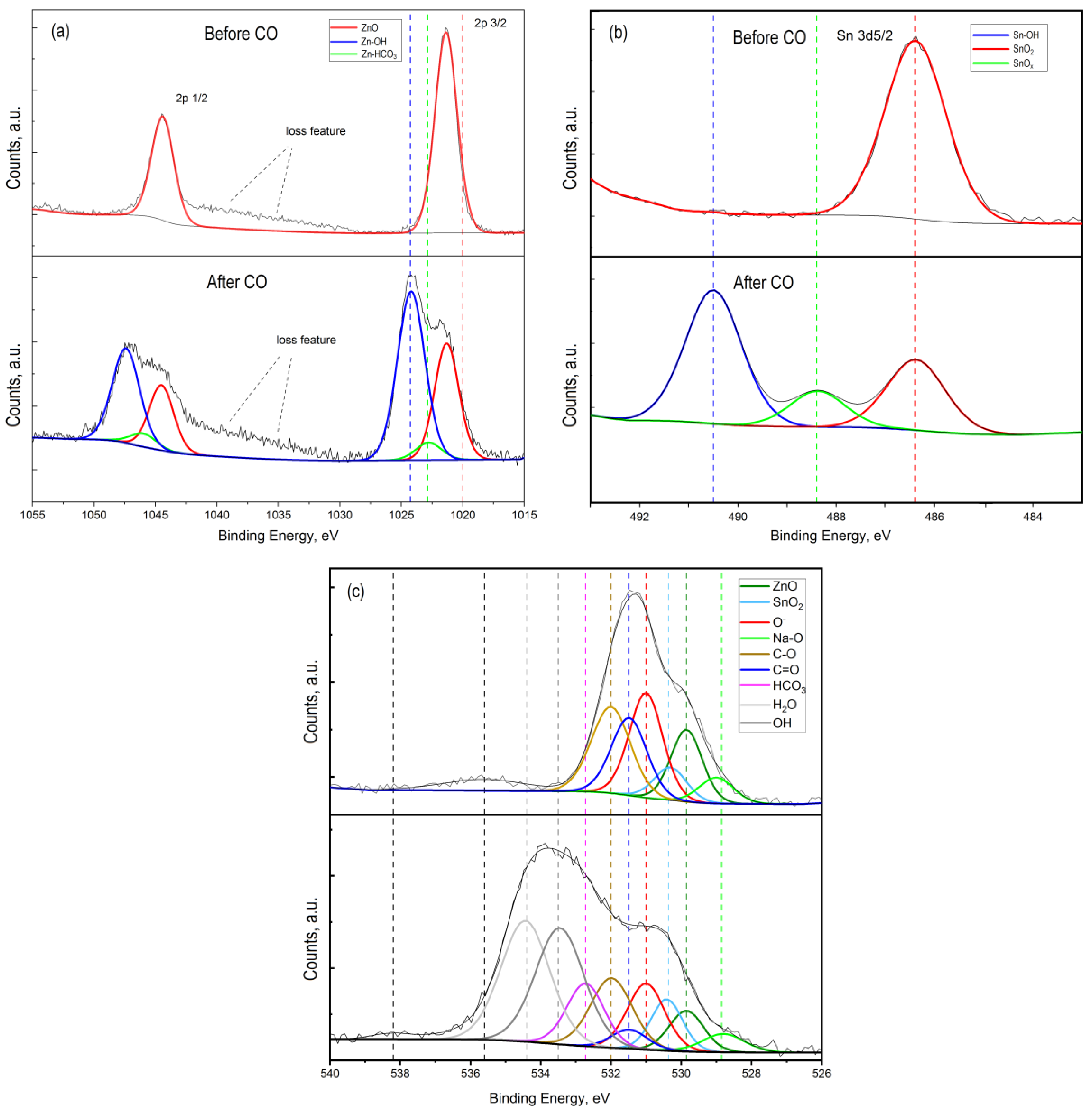
Figure 6.
High-resolution XPS spectrum of Zn 2p (a), Sn 2p (b), and O 1s (c) for ZTO film with Zn:Sn ratio 99:1 before and after CO treatment at T = 300 °C.
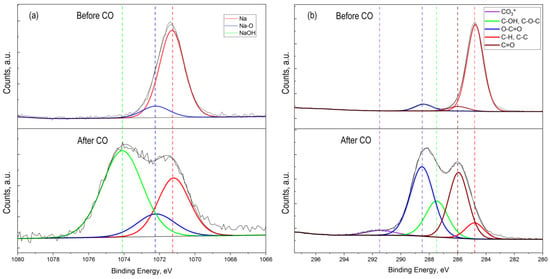
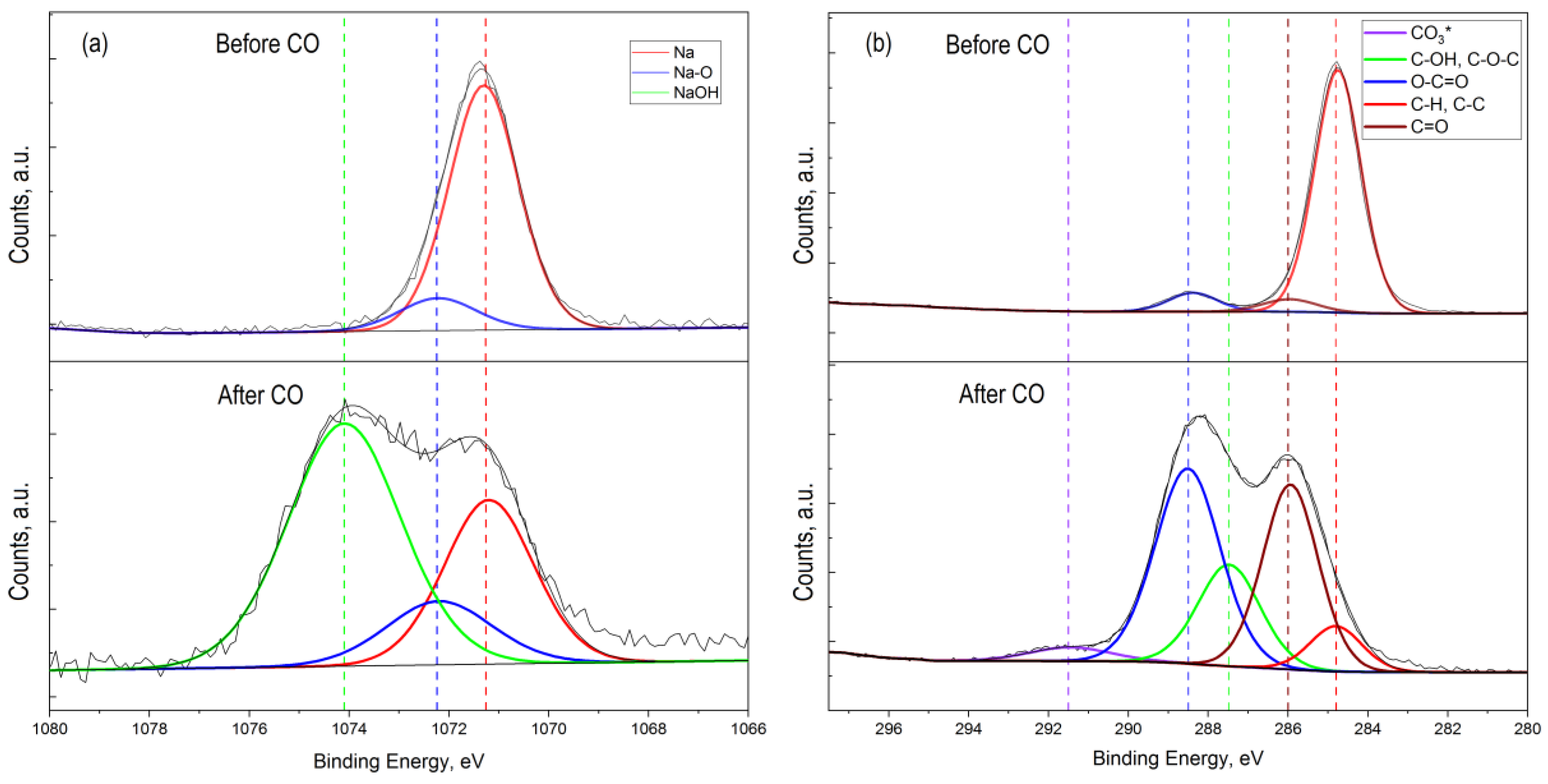
Figure 7.
High-resolution XPS spectrum of Na 1s (a) and C 1s (b) 2p for ZTO film with Zn:Sn ratio = 95:5 before and after CO treatment at T = 300 °C.
Figure 5 shows the overview spectra of ZTO film with the Zn:Sn ratio of 95:5 before and after CO treatment with a concentration of 50 ppm at a temperature of 300 °C. It can be seen that in both cases, the film surface contains the following elements: Zn, Sn, O, C, and Na. To assess the chemical composition of ZTO films through qualitative and quantitative analysis, high-resolution peaks spectra of the elements Zn 2p, Sn 2p, O 1s, Na 1s, and C 1s were studied (Figure 5 and Figure 6). Before exposure to CO, zinc and tin oxides were present on ZTO films surface (Figure 5). The presence of carbon on the ZTO film surface before CO treatment was associated with the film’s synthesis method from organic precursors, as evidenced by the high-resolution spectrum of the C 1s line, whose component composition corresponded to the C-C and C=O bonds [43,44,45,46] (Figure 6b).
High-resolution spectrum analysis of the C 1s photoelectronic line for ZnO–SnO2 after CO treatment showed a significant increase in the amount of sorbed carbon with different chemical states and new chemical bonds: C-C, C-H, C-O, C=O, and CO32− (Figure 6b). The absence of a silicon photoelectronic signal, which is part of the glass, indicated a continuous coating of the substrate by ZTO film with a thickness of at least 10 nm.
The presence of Na 1s peak in the survey spectrum for a sample that was not treated by CO (Figure 7a) indicated the initial presence of sodium in the oxide film. The presence of sodium can be explained by its diffusion from the glass into the ZnO–SnO2 film during synthesis process. In this sample, sodium was present as interstitial defects in oxides (Na* red line component at 1071.7 eV) and as Na-O bond at 1072.2 eV (Figure 7a). The presence of sodium in the film is also evidenced by the obtained energy activation of conductivity, which is close to the values reported in [40]. However, on a sample that was treated by carbon monoxide, the concentration of sodium that corresponds to interstitial defects in oxides was decreased. At the same time, a peak of Na 1s with energy of 1074.1 eV appeared, corresponding to the association of Na with the OH− group (Figure 7a, after CO treatment). Probably, Na+ cations located on the surface contributed to the sorption of water molecules from the atmosphere to form sodium hydroxide (NaOH). This was also evidenced by the high-resolution XPS spectrum for oxygen lines 1s with an energy of 533.7 eV (Figure 6c, after CO treatment).
The obtained results do not refute the known facts of water molecules dissociation on the metal oxides surface with the formation of OH− groups [47,48]. The presence of OH− groups on the ZnO–SnO2 film surface leads to the formation of Zn-OH (Zn 2P peak with an energy of 1022.8 eV (Figure 6a, after CO treatment)) and Sn-OH (Sn 3d5/2 peak with an energy of 490.5 eV (Figure 6b, after CO treatment)) bonds.
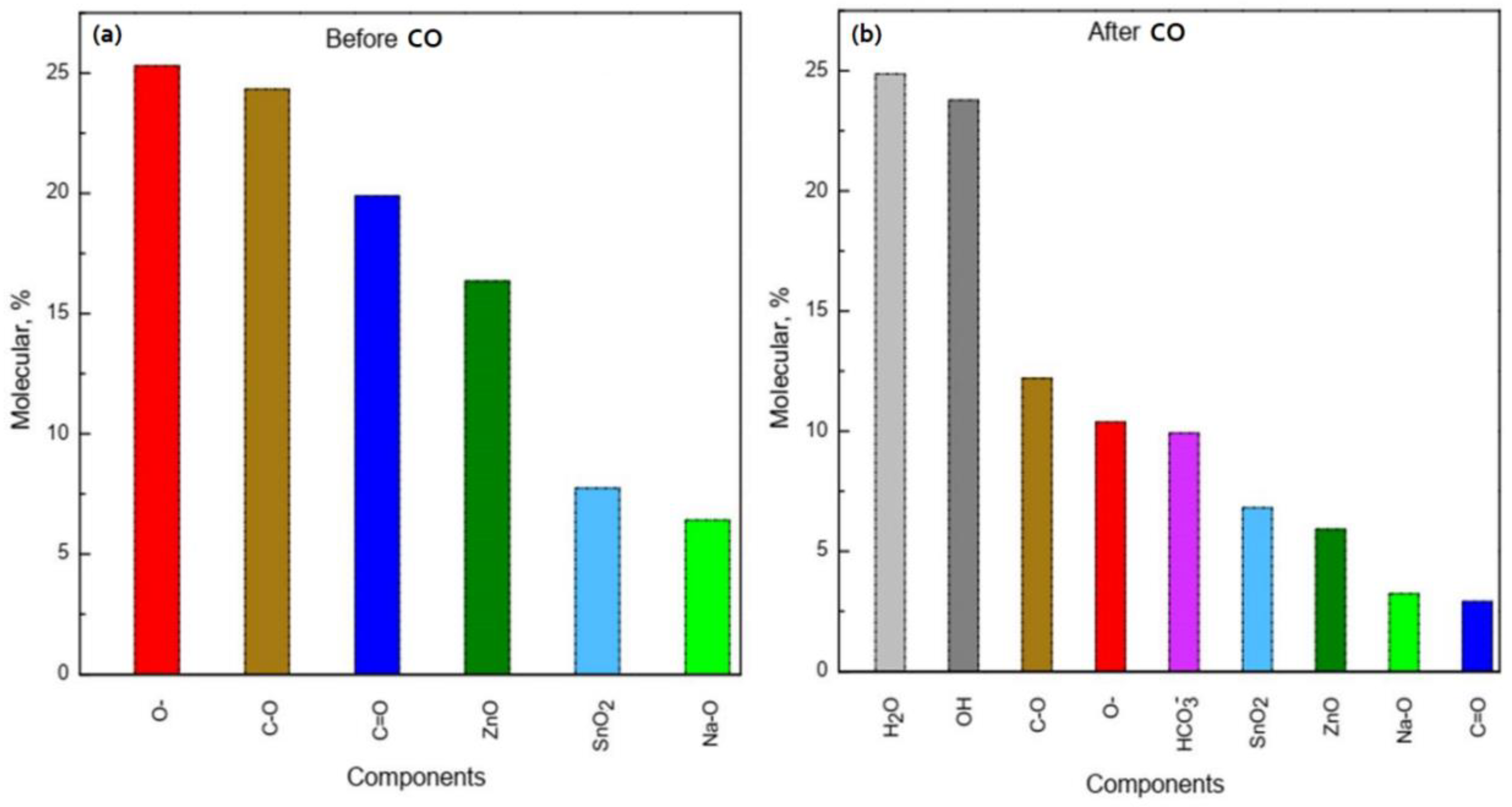
By comparing the intensity and area of the curve components in the high-resolution spectrum of oxygen O 1s (Figure 6c), we estimated the amount of sorbed water and the ratio of formed OH– groups and other components of the ZTO film surface before and after CO gas treatment (Figure 8). Their number was about 50% of the total number of the surface components (Figure 8b). It is worth noting that the components H2O and OH− groups in the high-resolution spectra of O 1s, Na 1s, Sn 3d, and Zn 2p for the ZnO–SnO2 film before CO treatment were not detected (Figure 8a). From the quantitative ratios of SnO2 and ZnO before and after CO treatment, it was seen that after CO treatment, the amount of zinc oxide relative to the amount of tin dioxide decreased. This indicated that zinc oxide interacted more actively with OH− groups and CO to form hydroxides and hydrocarbonates, respectively, than tin dioxide.
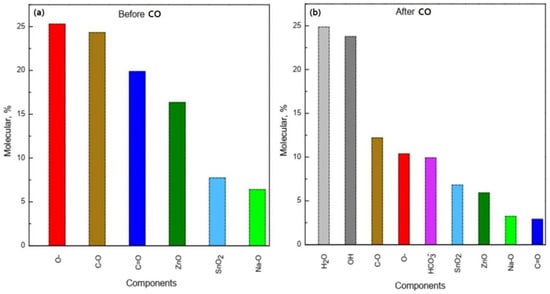
Figure 8.
Chemical composition of the ZnO–SnO2 film surface with the Zn:Sn ratio: 95:5 before (a) and after (b) CO treatment.
In addition, a detailed examination photoelectronic line C 1s of the high-resolution spectrum (Figure 7b) obtained from the ZnO–SnO2 metal oxide system after CO treatment showed a new component at a binding energy of 291.5 eV, which corresponded to carbonate compounds. This fact can be explained by the oxidation of carbon monoxide when exposed to oxygen, which is adsorbed from air on the film surface [48,49].
In the experiment, we fed CO at a concentration of 50 ppm along with atmospheric air that contains water (humidity was about 40–60%). Water molecules are adsorbed on the surface of oxides and then dissociate to form OH− groups [19,25,47,48]. As a result, molecular water and OH− groups are present on the film surface. This is clearly seen from Figure 8b, where the total number of OH- groups and water molecules are about 50% of the total amount of substance on the surface. It is known that sodium is present in the ZnO film doped with SnO2 in the internodes [16]. It is also shown that Na+ is attracted by the OH− around the nanocrystal and forms a virtual capping layer [50]. The presence of OH− ions on the zinc oxide surface and zinc ions located in the internodes leads to the appearance of Zn-OH bonds. This is indicated by a decreasing of the Na peak intensity (red curve) after CO treatment and the appearance of a peak corresponding to the Na-OH bonds (Figure 7a). The presence of Na-OH bonds with simultaneous exposure to CO leads to the formation of zinc hydroxocarbonates insoluble compounds, which block the active adsorption centers. A number of works show [51,52] that the synthesis of zinc hydroxocarbonate (Zn5(CO3)2(OH)6) occurs in the presence of NaOH or during CO treatment. These conclusions are also confirmed by the results of the XPS studies (Figure 8), i.e., the number of bonds corresponding to zinc oxide was about 17% before CO treatment, whereas after CO treatment, it became about 7%. At the same time, about 10% of hydroxocarbonates were formed. Thus, chemical processes that lead to surface poisoning can be most probably initiated by the presence of sodium on the ZnO–SnO2 films surface, which was diffused from the substrate and able to form Na-OH bonds.
Thus, as a result of the carbon monoxide treatment on the gas-sensitive ZnO–SnO2 film containing sodium, which was diffused from the soda-lime glass substrate, the formation of metal hydroxides and carbonates occurred on the film surface. As a result, the surface of the ZTO film became unable to respond further to the carbon monoxide influence.
4. Conclusions
The discovered fact of the gas-sensitive material “poisoning” can be explained by a number of consecutive chemical reactions, the activator of which is sodium diffused into the oxide film from the substrate. The first step is the adsorption of water molecules on the surface areas of the ZnO–SnO2 film containing Na, which can create Na-OH bonds. The formation of Na-OH bonds on the surface during CO treatment also leads to the formation of zinc hydroxocarbonates insoluble compounds. This fact, as well as the formation of Zn-OH and Sn-OH bonds, leads to the blocking of the surface-active adsorption centers. Due to the ionic nature of the reactions occurring on the surface, the rapid decrease of gas-sensitive properties of ZnO–SnO2 thin films grown on the glass substrate is observed.
Thus, the presence of sodium in the surface layers of the gas-sensitive ZnO–SnO2 thin films grown on the glass substrate significantly affected the operation of the sensor and led to its “poisoning.”
Author Contributions
Conceptualization, V.V.P., E.M.B., and S.A.K.; XPS, S.A.K.; SEM, electrophysical and gas sensitive properties, V.V.P. and Y.N.V.; XRD analysis, E.M.B. and M.G.V.; writing-original draft preparation, V.V.P., S.A.K., E.M.B., and M.G.V.; and writing-review and editing, V.V.P., E.M.B., and S.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Foundation for Basic Research (RFBR), grant number 20-07-00653 A.
Acknowledgments
The authors thank REC “Nanotechnology” SFedU for SEM analysis and CCU “Microsystems Engineering and Integrated Sensing Technology” SFedU for gas-sensitive properties measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mahajan, S.; Jagtap, S. Metal-oxide semiconductors for carbon monoxide (CO) gas sensing: A review. Appl. Mater. Today 2020, 18, 100483. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, W.; Liu, W.; Cao, P.; Han, S.; Zhu, D.; Lu, Y. Structural, chemical, optical, and electrical evolution of solution-processed SnO2 films and their applications in thin-film transistors. J. Phys. D Appl. Phys. 2020, 53, 175106. [Google Scholar] [CrossRef]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: State of the art and approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Lee, J.-H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuators B Chem. 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Li, N.; Fan, Y.; Shi, Y.; Xiang, Q.; Wang, X.; Xu, J. A low temperature formaldehyde gas sensor based on hierarchical SnO/SnO2 nano-flowers assembled from ultrathin nanosheets: Synthesis, sensing performance and mechanism. Sens. Actuators B Chem. 2019, 294, 106–115. [Google Scholar] [CrossRef]
- Gong, B.; Shi, T.; Zhu, W.; Liao, G.; Li, X.; Huang, J.; Zhou, T.; Tang, Z. UV irradiation-assisted ethanol detection operated by the gas sensor based on ZnO nanowires/optical fiber hybrid structure. Sens. Actuators B Chem. 2017, 245, 821–827. [Google Scholar] [CrossRef]
- Lim, S.K.; Hwang, S.-H.; Chang, D.; Kim, S. Preparation of mesoporous In2O3 nanofibers by electrospinning and their application as a CO gas sensor. Sens. Actuators B Chem. 2010, 149, 28–33. [Google Scholar] [CrossRef]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Hunter, G.W.; Xu, J.C.; Kulis, M.J.; Berger, G.M.; Ticich, T.M. Metaloxide nanostructure and gas-sensing performance. Sens. Actuators B Chem. 2009, 138, 113–119. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef]
- Marsal, A.; Cornet, A.; Morante, J.R. Study of the CO and humidity interference in La doped tin oxide CO2 gas sensor. Sens. Actuators B Chem. 2003, 94, 324–329. [Google Scholar] [CrossRef]
- Dhannasare, S.B.; Yawale, S.S.; Unhale, S.B.; Yawale, S.P. Application of nanosize polycrystalline SnO2-WO3 solid material as CO2 gas sensor. Rev. Mex. Fis. 2012, 58, 445–450. [Google Scholar]
- Guo, W.; Zhou, Q.; Zhang, J.; Fu, M.; Radacsi, N.; Li, Y. Hydrothermal synthesis of Bi-doped SnO2/rGO nanocomposites and the enhanced gas sensing performance to benzene. Sens. Actuators B Chem. 2019, 299, 126959. [Google Scholar] [CrossRef]
- Petrov, V.V.; Nazarova, T.N.; Kopilova, N.F.; Zabluda, O.V.; Kisilev, I.; Bruns, M. Study of Physical and Chemical, Electrophysical Properties and Gas Sensitive Characteristics of SiO2-SnOx-CuOy Nanocomposite Films. Nano- Microsyst. Technol. 2010, 8, 15–21. [Google Scholar]
- Koo, A.; Yoo, R.; Woo, S.P.; Lee, H.; Lee, W. Enhanced acetone-sensing properties of Pt-decorated Al-doped ZnO nanoparticles. Sens. Actuators B Chem. 2019, 280, 109–119. [Google Scholar] [CrossRef]
- Gong, H.; Hu, J.Q.; Wang, J.H.; Ong, C.H.; Zhu, F.R. Nano-crystalline Cu-doped ZnO thin film gas sensor for CO. Sens. Actuators B Chem. 2006, 115, 247–251. [Google Scholar] [CrossRef]
- Lee, S.; Hwang, C.; Pi, J.; Ryu, M.; Oh, H.; Cho, S.H.; Yang, J.; Ko, S.; Hye, P.; Chu, Y. Characterization of ZnO–SnO2 nanocomposite thin films deposited by pulsed laser ablation and their field effect electronic properties. Mater. Lett. 2014, 122, 94–97. [Google Scholar] [CrossRef]
- Petrov, V.V.; Varzarev, Y.N.; Bayan, E.M.; Storozhenko, V.Y.; Rozhko, A.A. Study of the Electrophysical Properties of Thin Films of Mixed Zinc and Tin Oxides. In Proceedings of the 2019 IEEE International Conference on Electrical Engineering and Photonics, St. Petersburg, Russia, 17–18 October 2019; pp. 242–243. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Sergiienko, S.A.; Kukla, O.L.; Yaremov, P.S.; Solomakha, V.N.; Shvets, O.V. The influence of preparation conditions and doping on the physicochemical and sensor properties of mesoporous tin oxide. Sens. Actuators B 2013, 177, 643–653. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Yu, Q.; Zhao, L.; Sun, P.; Wang, T.; Liu, F.; Yan, X.; Gao, Y.; Liang, X.; et al. One step synthesis of branched SnO2/ZnO heterostructures and their enhanced gas-sensing properties. Sens. Actuators B Chem. 2019, 281, 415–423. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Wang, B.; Sun, P.; Yang, Q.; Liang, X.; Song, H.; Lu, G. Highly sensitive and low detection limit of ethanol gas sensor based on hollow ZnO/SnO2 spheres composite material. Sens. Actuators B Chem. 2017, 245, 551–559. [Google Scholar] [CrossRef]
- Qin, S.; Tang, P.; Feng, Y.; Li, D. Novel ultrathin mesoporous ZnO-SnO2 n-n heterojunction nanosheets with high sensitivity to ethanol. Sens. Actuators B Chem. 2020, 309, 127801. [Google Scholar] [CrossRef]
- Li, W.; Ma, S.; Li, Y.; Yang, G.; Mao, Y.; Luo, J.; Gengzang, D.; Xu, X.; Yan, S. Enhanced ethanol sensing performance of hollow ZnO–SnO2 core–shell nanofibers. Sens. Actuators B Chem. 2015, 211, 392–402. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Pronin, I.A.; Ham, M.H.; Cho, B.K. Metal Oxides for Application in Conductometric Gas Sensors: How to Choose? Solid State Phenom. 2017, 266, 187–195. [Google Scholar] [CrossRef]
- Dharmalingam, G.; Sivasubramaniam, R.; Parthiban, S. Quantification of Ethanol by Metal-Oxide-Based Resistive Sensors: A Review. J. Electron. Mater. 2020, 49, 3009–3024. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A 2012, 212, 17–60. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Fu, Y.; Luo, W.; Tian, Y. Deactivation mechanism and anti-deactivation modification of SnO2-based catalysts for methane gas sensors. Sens. Actuators B Chem. 2019, 299, 126939. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, B.; Jo, Y.K.; Dai, Z.; Lee, J. Chemiresistive trimethylamine sensor using monolayer SnO2 inverse opals decorated with Cr2O3 nanoclusters. Sens. Actuators B Chem. 2020, 309, 127805. [Google Scholar] [CrossRef]
- Hosseini, Z.S.; Mortezaali, A.; Iraji zad, A.; Fardindoost, S. Sensitive and selective room temperature H2S gas sensor based on Au sensitized vertical ZnO nanorods with flowerlike structures. J. Alloys Compd. 2015, 628, 222–229. [Google Scholar] [CrossRef]
- Maniv, S.; Zangvil, A. Controlled texture of reactively rf-sputtered ZnO thin films. J. Appl. Phys. 1978, 47, 2787–2792. [Google Scholar] [CrossRef]
- Siegbahn, K. Electron Spectroscopy for Atoms, Molecules and Condensed Matter. Rev. Mod. Phys. 1982, 54, 709. [Google Scholar] [CrossRef]
- Shmatko, V.A.; Yalovega, G.E.; Myasoedova, T.N.; Brzhezinskaya, M.M.; Shtekhin, I.E.; Petrov, V.V. Influence of the surface morphology and structure on the gas-sorption properties of SiO2CuOx nanocomposite materials: X-ray spectroscopy investigations. Phys. Solid State 2015, 57, 399–406. [Google Scholar] [CrossRef]
- Myasoedova, T.N.; Yalovega, G.E.; Shmatko, V.A.; Funik, A.O.; Petrov, V.V. SiO2CuOx films for nitrogen dioxide detection: Correlation between technological conditions and properties. Sens. Actuators B Chem. 2016, 230, 167–175. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J.; Rumble, J.R., Jr. NIST Standard Reference Database 20, Version 3.4 (Web Version); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2003. [CrossRef]
- Petrov, V.V.; Starnikova, A.P.; Varzarev, Y.N.; Abdullin, K.A.; Makarenko, D.P. Gas sensitive properties of ZnO nanorods formed on silicon and glass substrates. IOP Conf. Ser. Mater. Sci. Eng. 2019, 703, 012038. [Google Scholar] [CrossRef]
- Heidrun, S.; Ahsbahs, H. High-pressure X-ray investigation of zincite ZnO single crystals using diamond anvils with an improved shape. J. Appl. Crystallogr. 2006, 39, 169–175. [Google Scholar] [CrossRef]
- Huang, L.; Wright, S.; Yang, S.; Shen, D.; Gu, B.; Du, Y. ZnO well-faceted fibers with periodic junctions. J. Phys. Chem. B 2004, 108, 19901–19903. [Google Scholar] [CrossRef]
- Wahl, U.; Correia, J.G.; Amorim, L.; Decoster, S.; da Silva, M.R.; Pereira, L.M.C. Lattice sites of Na dopants in ZnO. Semicond. Sci. Technol. 2016, 31, 095005. [Google Scholar] [CrossRef]
- Rambua, A.P.; Iftimieb, N.; Rusu, G.I. Influence of the substrate nature on the properties of ZnO thin films. Mater. Sci. Eng. B 2012, 177, 157–163. [Google Scholar] [CrossRef]
- Pathan, I.G.; Suryawanshi, D.N.; Bari, A.R.; Rane, D.S.; Patil, L.A. Preparation and Gas Sensing Properties of Nanostructured ZnSnO3 Thin Films. Adv. Nanomater. Nanotechnol. 2013, 143–157. [Google Scholar] [CrossRef]
- Barr, T.L.; Seal, S. Nature of the use of adventitious carbon as a binding energy standard. J. Vac. Sci. Technol. A 1995, 13, 1239–1246. [Google Scholar] [CrossRef]
- Swift, P. Adventitious carbon—The panacea for energy referencing? Surf. Interface Anal. 1982, 4, 47–51. [Google Scholar] [CrossRef]
- Miller, D.J.; Biesinger, M.C.; McIntyre, N.S. Interactions of CO2 and CO at fractional atmosphere pressures with iron and iron oxide surfaces: One possible mechanism for surface contamination? Surf. Interface Anal. 2002, 33, 299–305. [Google Scholar] [CrossRef]
- Piao, H.; McIntyre, N.S. Adventitious carbon growth on aluminium and gold–aluminium alloy surfaces. Surf. Interface Anal. 2002, 33, 591–594. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2 sensors in the presence of humidity. J. Phys. Condens. Matter. 2003, 15, 813–839. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Blinov, I.; Brinzari, V.; Stetter, J.R. Effect of air humidity on gas response of SnO2 thin film ozone sensors. Sens. Actuators B Chem. 2007, 122, 519–526. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Saeidi, N. Sn-embedded graphene: An active catalyst for CO oxidation to CO2? Phys. E Low-Dimens. Syst. Nanostruct. 2015, 74, 382–387. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-Dimensional ZnO Nanostructures: Solution Growth and Functional Properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef]
- Nikolaeva, N.S.; Ivanov, V.V.; Shubin, A.A. Synthesis of highly dispersed forms of zinc oxide: Chemical deposition and thermolysis. J. Sib. Fed. Univ. Chem. 2010, 2, 153–173. [Google Scholar]
- Wang, G.Y.; Zhang, W.X.; Lian, H.L.; Jiang, D.Z.; Wu, T.H. Effect of calcination temperatures and precipitant on the catalytic performance of Au/ZnO catalysts for CO oxidation at ambient temperature and in humid circumstances. Appl. Catal. A Gen. 2003, 239, 1–10. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).