Development of Novel and Highly Specific ssDNA-Aptamer-Based Electrochemical Biosensor for Rapid Detection of Mercury (II) and Lead (II) Ions in Water
Abstract
1. Introduction
2. Experimental Methodology
2.1. Aptamers and Other Chemicals
2.2. Immobilization of Aptamers
2.3. Electrochemical Measurements
2.4. ICP-MS Measurements
3. Results and Discussion
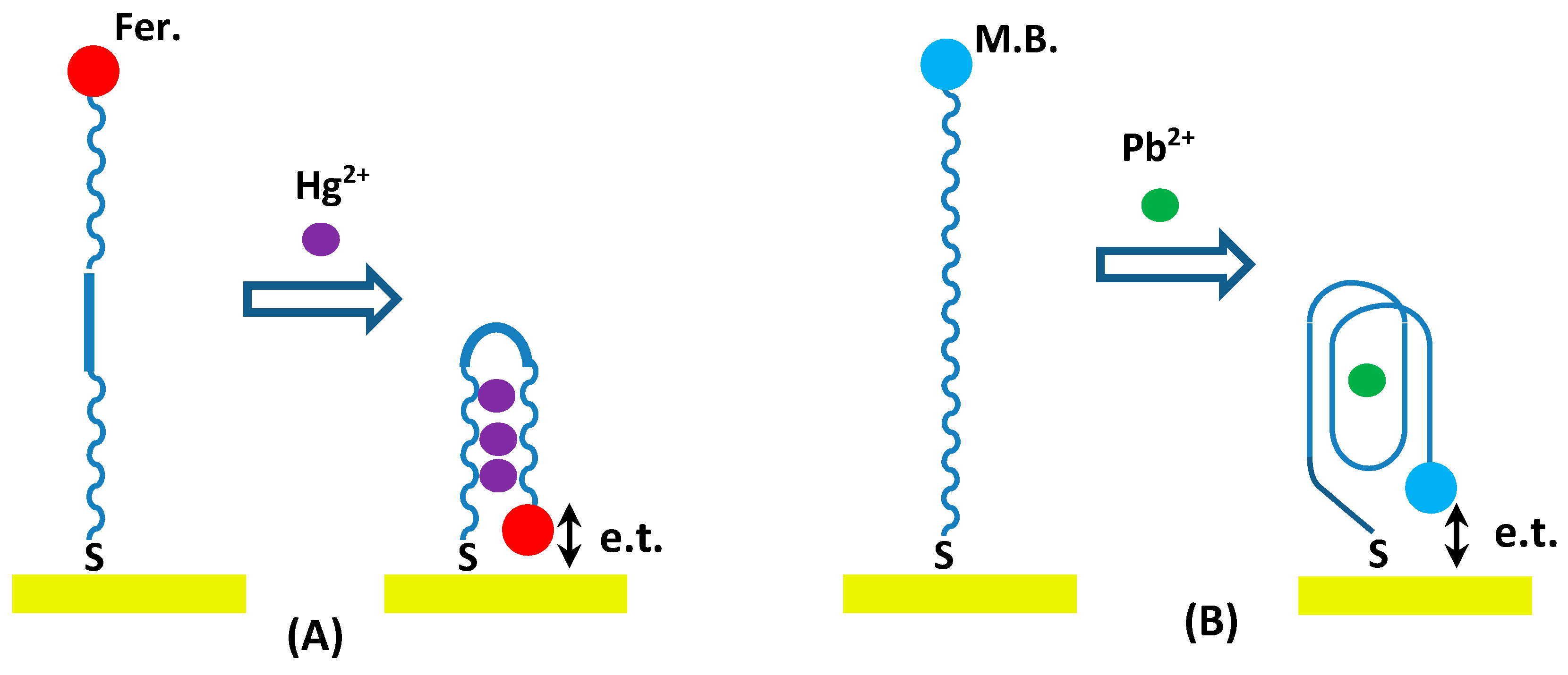
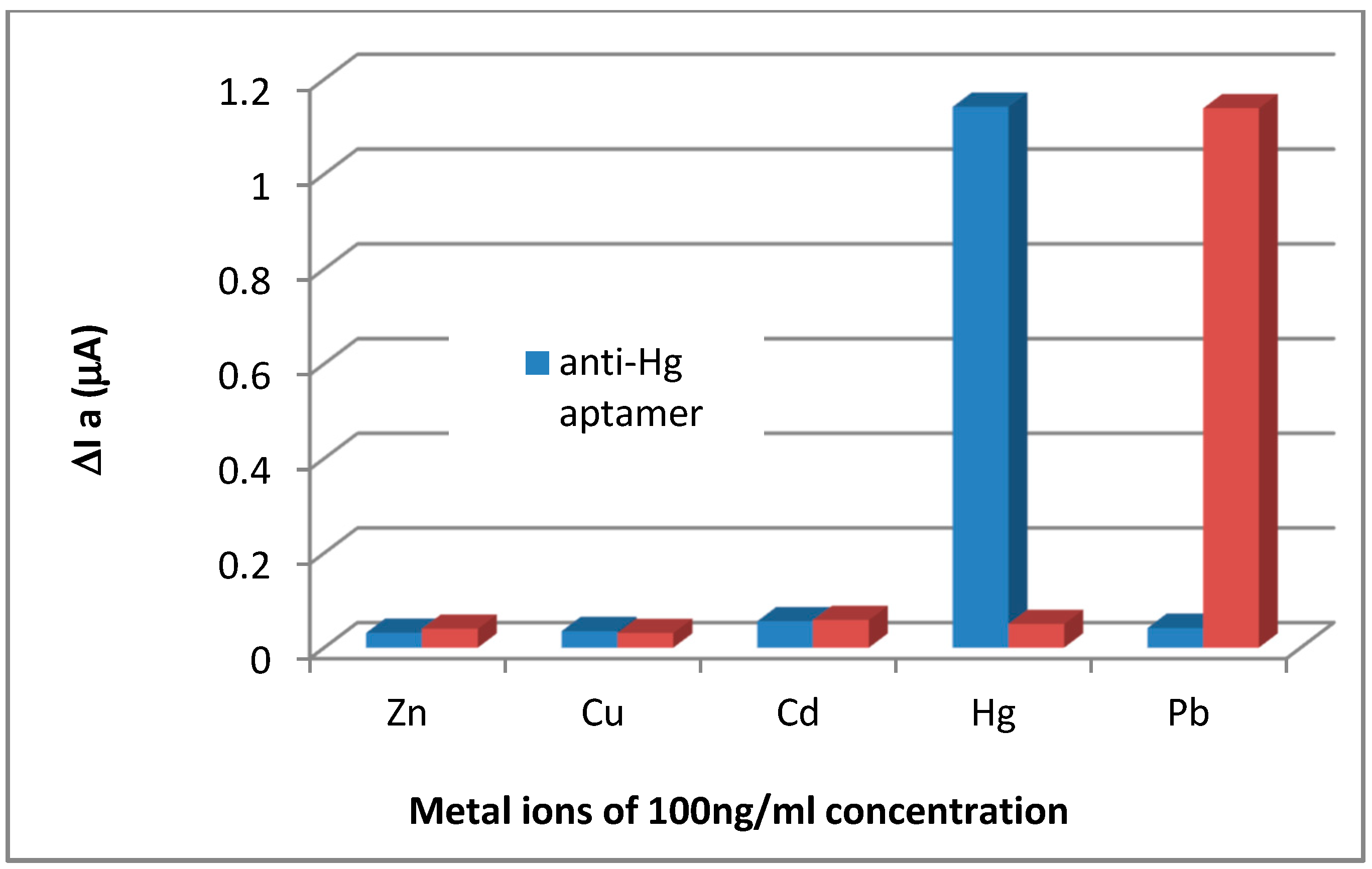
3.1. Design Strategy of the Aptasensor
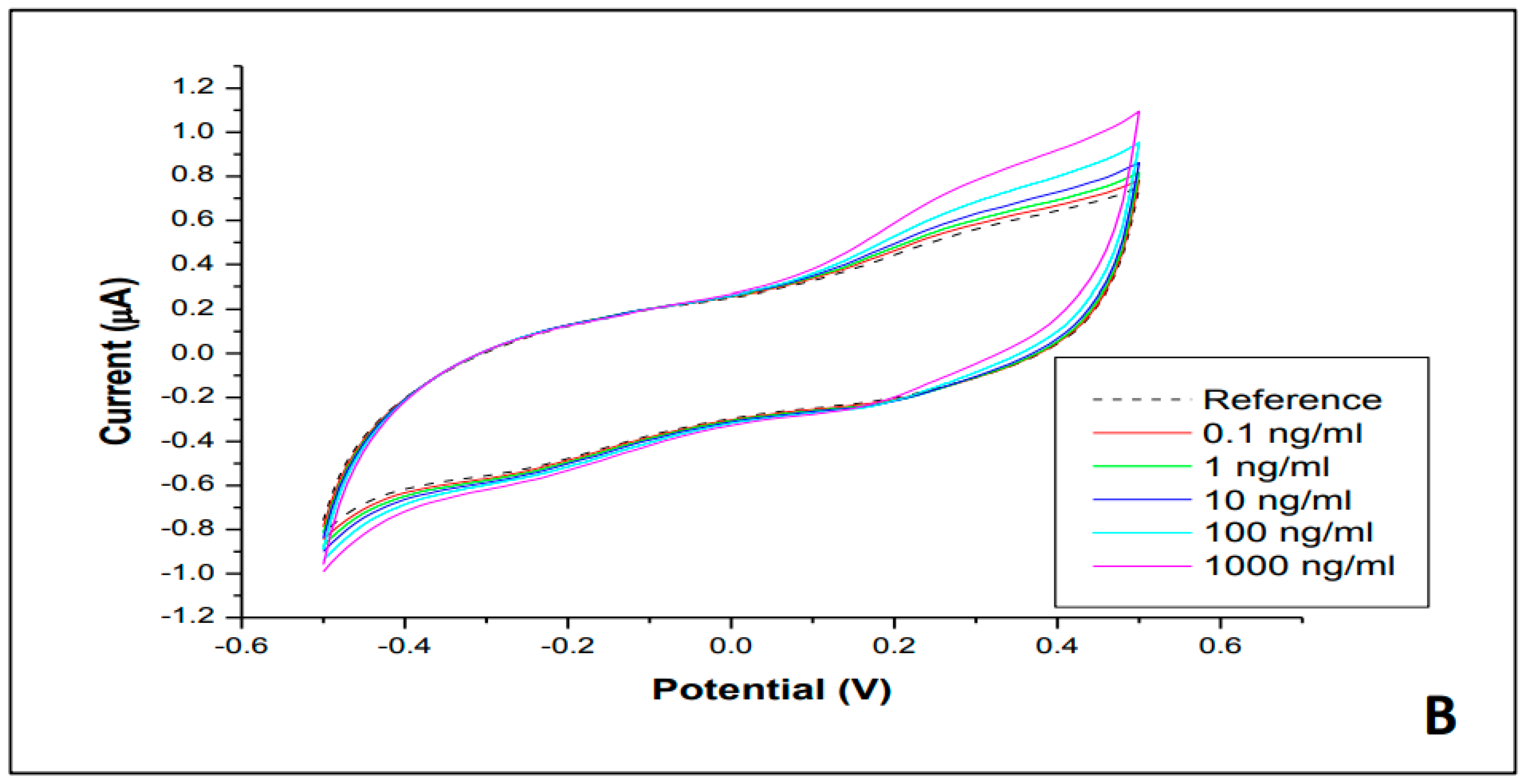
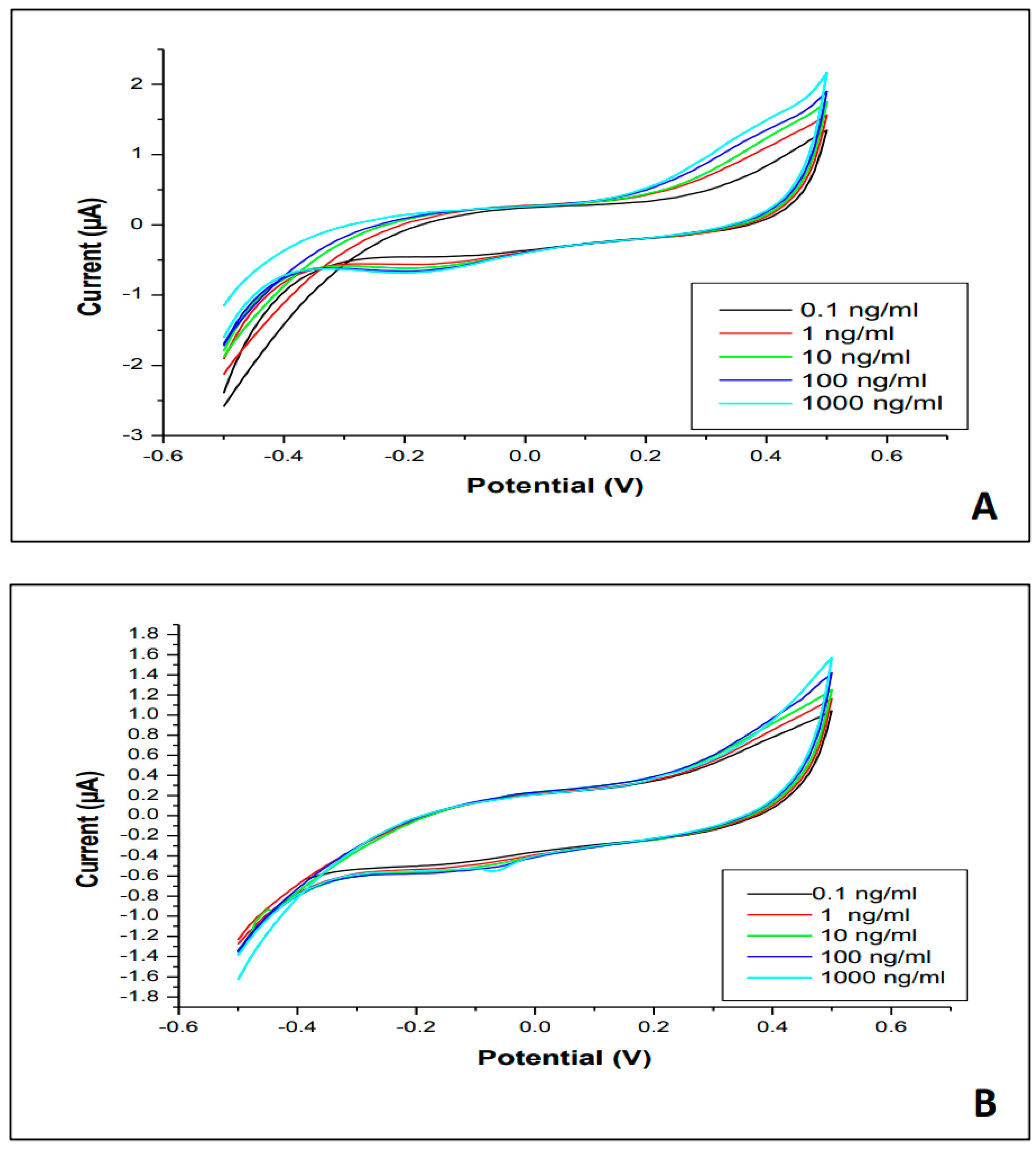
3.2. Cyclic Electrochemical Measurements
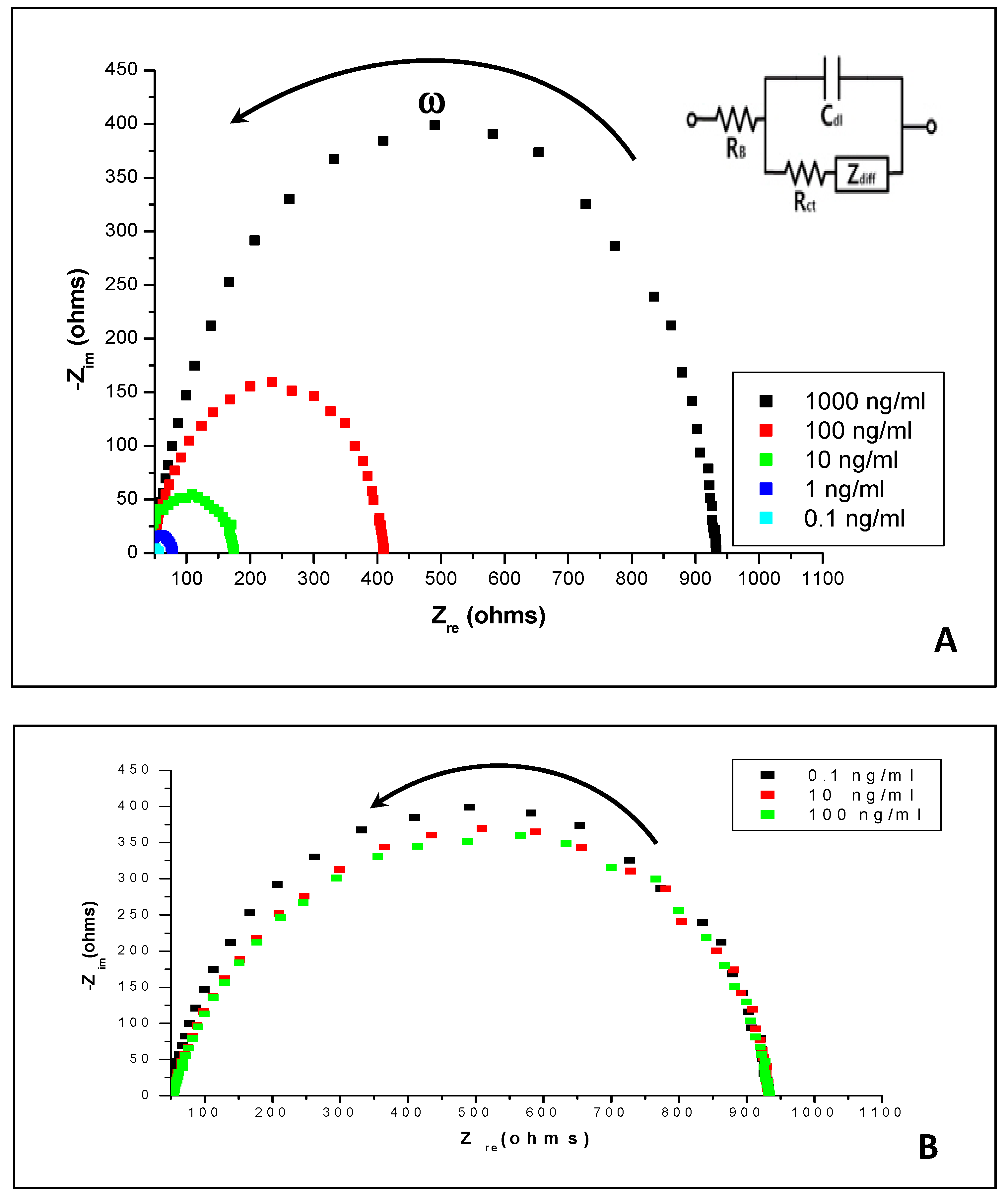
3.3. Impedance Spectroscopy Measurements
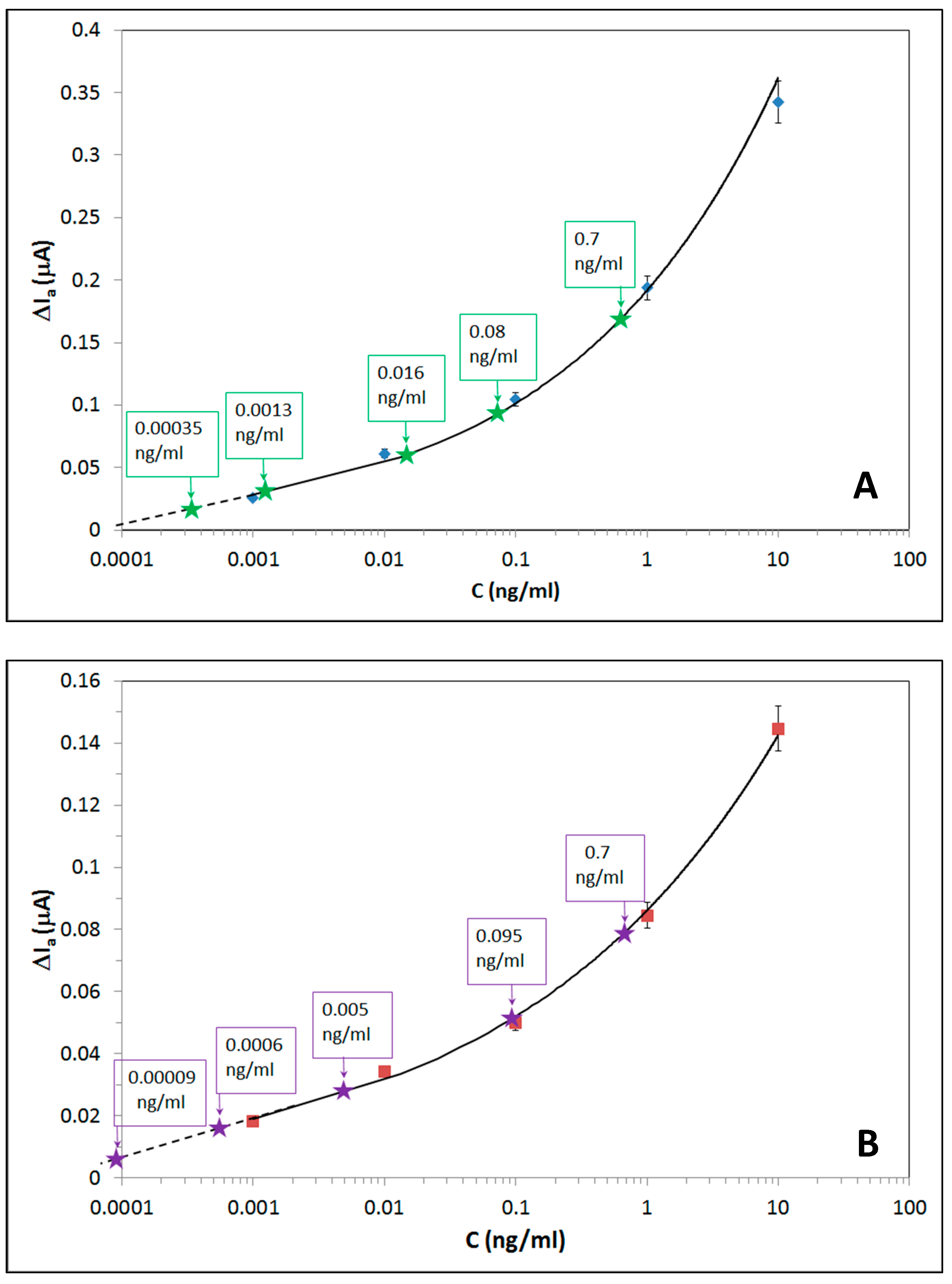
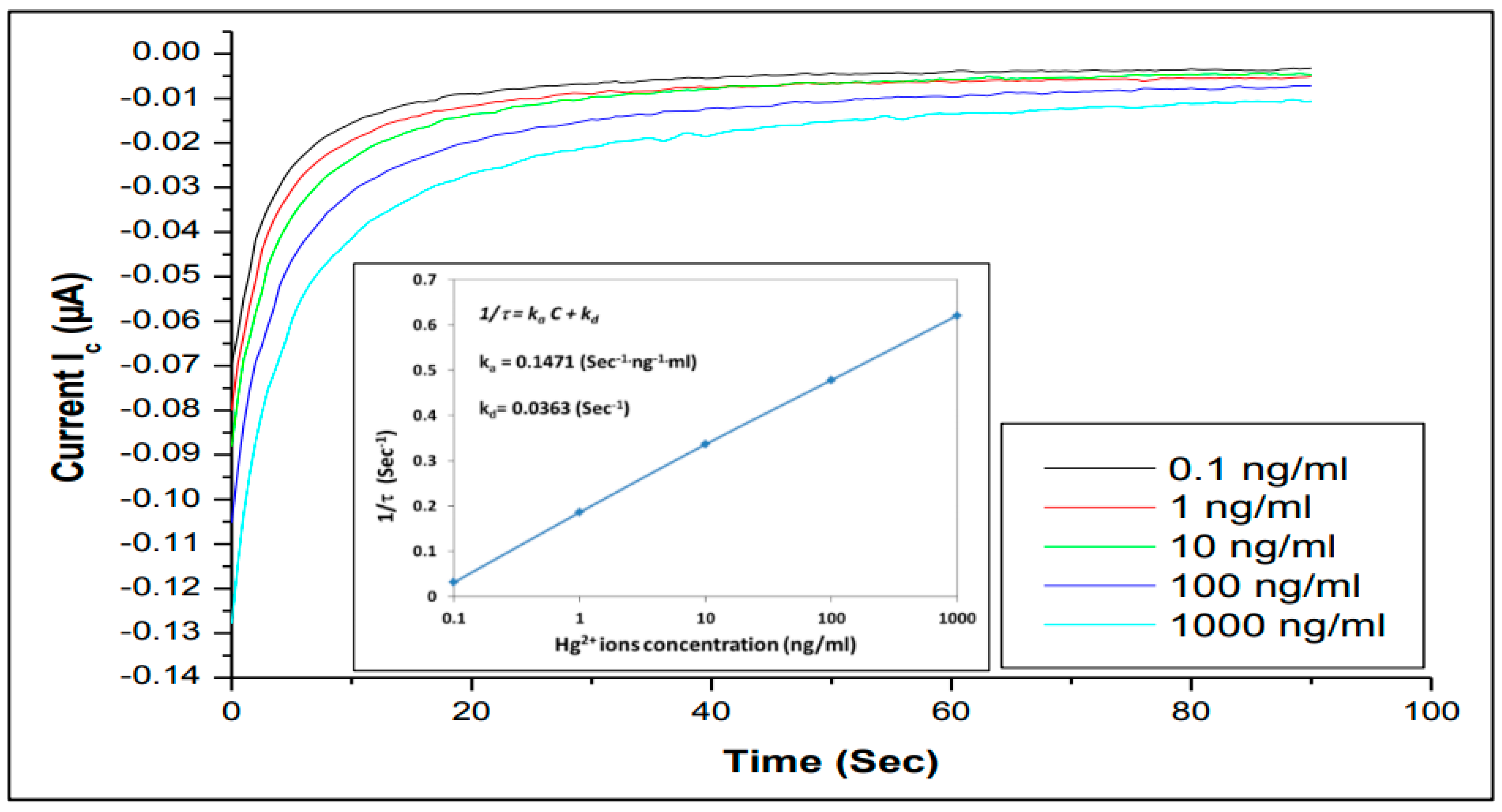
3.4. The Kinetics of Aptamers (Hg2+ and Pb2+) Binding
4. Conclusions and Future Work
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Förstner, U.; Wittmann, G.T. Metal Pollution in the Aquatic Environment; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Abu-Ali, H.; Nabok, A.; Smith, T.; Al-Shanawa, M. Inhibition Biosensor Based on DC and AC Electrical Measurements of Bacteria Samples. Proc. Technol. 2017, 27, 129–130. [Google Scholar] [CrossRef]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The toxicology of mercury current exposures and clinical manifestations. N. Eng. J. Med. 2003, 349, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Saidur, M.R.; Aziz, A.A.; Basirun, W.J. Recent advances in DNA-based electrochemical biosensors for heavy metal ion detection: A review. Biosens. Bioelectron. 2017, 90, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Galli, C.; Neri, A.; Toffoli, A.; Calciolari, E.; Manfredi, E.; Macaluso, C. Aptamers improve the bioactivity of biomaterials. Aptamers 2017, 1, 3–12. [Google Scholar]
- Wang, J.; Wu, C.; Hu, N.; Zhou, J.; Du, L.; Wang, P. Micro-fabricated electrochemical cell-based biosensors for analysis of living cells in vitro. Biosensors 2012, 2, 127–170. [Google Scholar] [CrossRef]
- Tan, F.; Cong, L.; Saucedo, N.M.; Gao, J.; Li, X.; Mulchandani, A. An electrochemically reduced graphene oxide chemiresistive sensor for sensitive detection of Hg2+ ion in water samples. J. Hazard. Mater. 2016, 320, 226–233. [Google Scholar] [CrossRef]
- Abu-Ali, H.; Nabok, A.; Smith, T.; Al-Shanawa, M. Development of electrochemical inhibition biosensor based on bacteria for detection of environmental pollutants. Sens. Bio-Sens. Res. 2017, 13, 109–114. [Google Scholar] [CrossRef]
- Ben-Yoav, H.; Almog, R.O.; Sverdlov, Y.; Sternheim, M.; Belkin, S.; Freeman, A.; Shacham-Diamand, Y. Modified working electrodes for electrochemical whole-cell microchips. Electrochim. Acta 2012, 82, 109–114. [Google Scholar] [CrossRef]
- Belkin, S.; Gu, M.B. Whole Cell Sensing Systems I; Reporter Cells and Devices; Springer: Berlin/Heidelberg, Germany, 2010; p. 220. [Google Scholar]
- Citartan, M.; Gopinath, S.C.; Tominaga, J.; Tan, S.C.; Tang, T.H. Assays for aptamer-based platforms. Biosens. Bioelectron. 2012, 34, 1–11. [Google Scholar] [CrossRef]
- Ferapontova, E.E.; Olsen, E.M.; Gothelf, K.V. An RNA aptamer-based electrochemical biosensor for detection of theophylline in serum. J. Am. Chem. Soc. 2008, 130, 4256–4258. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Y.; Fan, M.; Liu, X. Isolation and identification of the DNA aptamer target to acetamiprid. J. Agric. Food Chem. 2011, 59, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Al Rubaye, A.; Nabok, A.; Catanante, G.; Marty, J.L.; Takacs, E.; Szekacs, A. Detection of ochratoxin A in aptamer assay using total internal reflection ellipsometry. Sens. Actuators B Chem. 2018, 263, 248–251. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.; Guo, Z.; Lin, J. Practical application of aptamer-based biosensors in detection of low molecular weight pollutants in water sources. Molecules 2018, 23, 344. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Optical detection systems using immobilized aptamers. Biosens. Bioelectron 2011, 26, 3725–3736. [Google Scholar] [CrossRef] [PubMed]
- Hamula, C.L.; Guthrie, J.W.; Zhang, H.; Li, X.F.; Le, X.C. Selection and analytical applications of aptamers. Trends Anal. Chem. 2006, 25, 681–691. [Google Scholar] [CrossRef]
- Chen, L.; Cai, Q.; Luo, F.; Chen, X.; Zhu, X.; Qiu, B.; Chen, G. A sensitive aptasensor for adenosine based on the quenching of Ru (bpy)32+-doped silica nanoparticle ECL by ferrocene. Chem. Commun. 2010, 46, 7751–7753. [Google Scholar] [CrossRef]
- Yang, L.; Fung, C.W.; Cho, E.J.; Ellington, A.D. Real-time rolling circle amplification for protein detection. Anal. Chem. 2007, 79, 3320–3329. [Google Scholar] [CrossRef]
- Patolsky, F.; Weizmann, Y.; Willner, I. Redox-active nucleic-acid replica for the amplified bioelectrocatalytic detection of viral DNA. J. Am. Chem. Soc. 2002, 124, 770–772. [Google Scholar] [CrossRef]
- Deng, C.; Chen, J.; Nie, L.; Nie, Z.; Yao, S. Sensitive bifunctional aptamer-based electrochemical biosensor for small molecules and protein. Anal. Chem. 2009, 81, 9972–9978. [Google Scholar] [CrossRef]
- Li, T.; Dong, S.; Wang, E. Label-free colorimetric detection of aqueous mercury ion using Hg2+-modulated G-quadruplex-based DNAzymes. Anal. Chem. 2009, 81, 2144–2149. [Google Scholar] [CrossRef] [PubMed]
- Barthelmebs, L.; Hayat, A.; Limiadi, A.W.; Marty, J.L.; Noguer, T. Electrochemical DNA aptamer-based biosensor for OTA detection, using superparamagnetic nanoparticles. Sens. Actuators B Chem. 2011, 156, 932–937. [Google Scholar] [CrossRef]
- Zeng, G.; Zhang, C.; Huang, D.; Lai, C.; Tang, L.; Zhou, Y.; Xu, P.; Wang, H.; Qin, L.; Cheng, M. Practical and re-generable electrochemical aptasensor based on nanoporous gold and thymine-Hg2+-thymine base pairs for Hg2+ detection. Biosens. Bioelectron. 2017, 90, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, Q.; Chen, T.; Zhang, X.; Chen, Y.; Tan, W. DNA-capped mesoporous silica nanoparticles as an ion-responsive release system to determine the presence of mercury in aqueous solutions. Anal. Chem. 2012, 84, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Park, S.J.; Kwon, O.S.; Bae, J.; Jang, J. High-performance flexible graphene aptasensor for mercury detection in mussels. ACS Nano 2013, 7, 10563–10571. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Gao, C.; He, S.; Wang, Q.; Wu, A. Label-free electrochemical lead (II) aptasensor using thionine as the signalling molecule and graphene as signal-enhancing platform. Biosens. Bioelectron. 2016, 81, 15–22. [Google Scholar] [CrossRef]
- Dolati, S.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Recent nucleic acid based biosensors for Pb2+ detection. Sens. Actuators B Chem. 2017, 246, 864–878. [Google Scholar] [CrossRef]
- Wang, S.E.; Si, S. Aptamer biosensing platform based on carbon nanotube long-range energy transfer for sensitive, selective and multicolour fluorescent heavy metal ion analysis. Anal. Methods 2013, 5, 2947–2953. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Ju, H. Label-free signal-on aptasensor for sensitive electrochemical detection of arsenite. Biosens. Bioelectron. 2016, 79, 861–865. [Google Scholar] [CrossRef]
- Rhouati, A.; Yang, C.; Hayat, A.; Marty, J.-L. Aptamers: A promising tool for ochratoxin A detection in food analysis. Toxins 2013, 5, 1988–2008. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.R. Impedance spectroscopy. Ann. Biomed. Eng. 1992, 20, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, R.; Michaelsson, A.; Mattsson, L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J. Immunol. Methods 1991, 145, 229–240. [Google Scholar] [CrossRef]
- Battaglioli, G.; Liu, H.; Martin, D.L. Kinetic differences between the isoforms of glutamate decarboxylase: Implications for the regulation of GABA synthesis. J. Neurochem. 2003, 86, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Nabok, A.; Tsargorodskaya, A.; Mustafa, M.K.; Szekacs, I.; Starodub, N.F.; Szekacs, A. Detection of low molecular weight toxins using an optical phase method of ellipsometry. Sens. Actuators B Chem. 2011, 154, 232–237. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Yang, F.; Yang, X. Dual polarisation interferometry for real-time, label-free detection of interaction of mercury (II) with mercury-specific oligonucleotides. Chem. Commun. 2012, 48, 2873–2875. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]

| Sample Number | Hg2+ Ions (ng/mL) | Pb2+ Ions (ng/mL) | ||
|---|---|---|---|---|
| CV Results | ICP-MS Result | CV Results | ICP-MS Result | |
| Sample 1 | 0.00035 | 0.0017 | 0.00009 | 0.0001 |
| Sample 2 | 0.0013 | 0.0106 | 0.0006 | 0.0088 |
| Sample 3 | 0.016 | 0.078 | 0.005 | 0.034 |
| Sample 4 | 0.08 | 0.7 | 0.095 | 0.96 |
| Sample 5 | 0.7 | 0.9 | 0.7 | 1.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Ali, H.; Nabok, A.; Smith, T.J. Development of Novel and Highly Specific ssDNA-Aptamer-Based Electrochemical Biosensor for Rapid Detection of Mercury (II) and Lead (II) Ions in Water. Chemosensors 2019, 7, 27. https://doi.org/10.3390/chemosensors7020027
Abu-Ali H, Nabok A, Smith TJ. Development of Novel and Highly Specific ssDNA-Aptamer-Based Electrochemical Biosensor for Rapid Detection of Mercury (II) and Lead (II) Ions in Water. Chemosensors. 2019; 7(2):27. https://doi.org/10.3390/chemosensors7020027
Chicago/Turabian StyleAbu-Ali, Hisham, Alexei Nabok, and Thomas J. Smith. 2019. "Development of Novel and Highly Specific ssDNA-Aptamer-Based Electrochemical Biosensor for Rapid Detection of Mercury (II) and Lead (II) Ions in Water" Chemosensors 7, no. 2: 27. https://doi.org/10.3390/chemosensors7020027
APA StyleAbu-Ali, H., Nabok, A., & Smith, T. J. (2019). Development of Novel and Highly Specific ssDNA-Aptamer-Based Electrochemical Biosensor for Rapid Detection of Mercury (II) and Lead (II) Ions in Water. Chemosensors, 7(2), 27. https://doi.org/10.3390/chemosensors7020027






