Polyvinyl Acetate Film-Based Quartz Crystal Microbalance for the Detection of Benzene, Toluene, and Xylene Vapors in Air
Abstract
1. Introduction
2. Materials and Methods
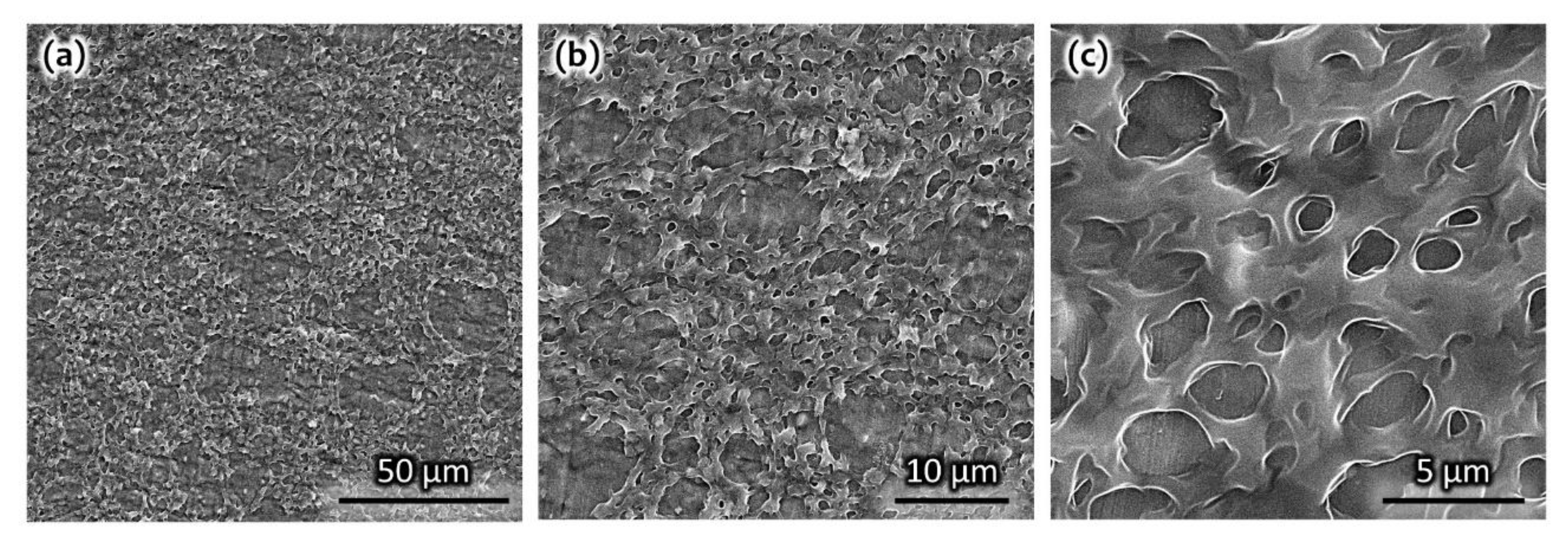
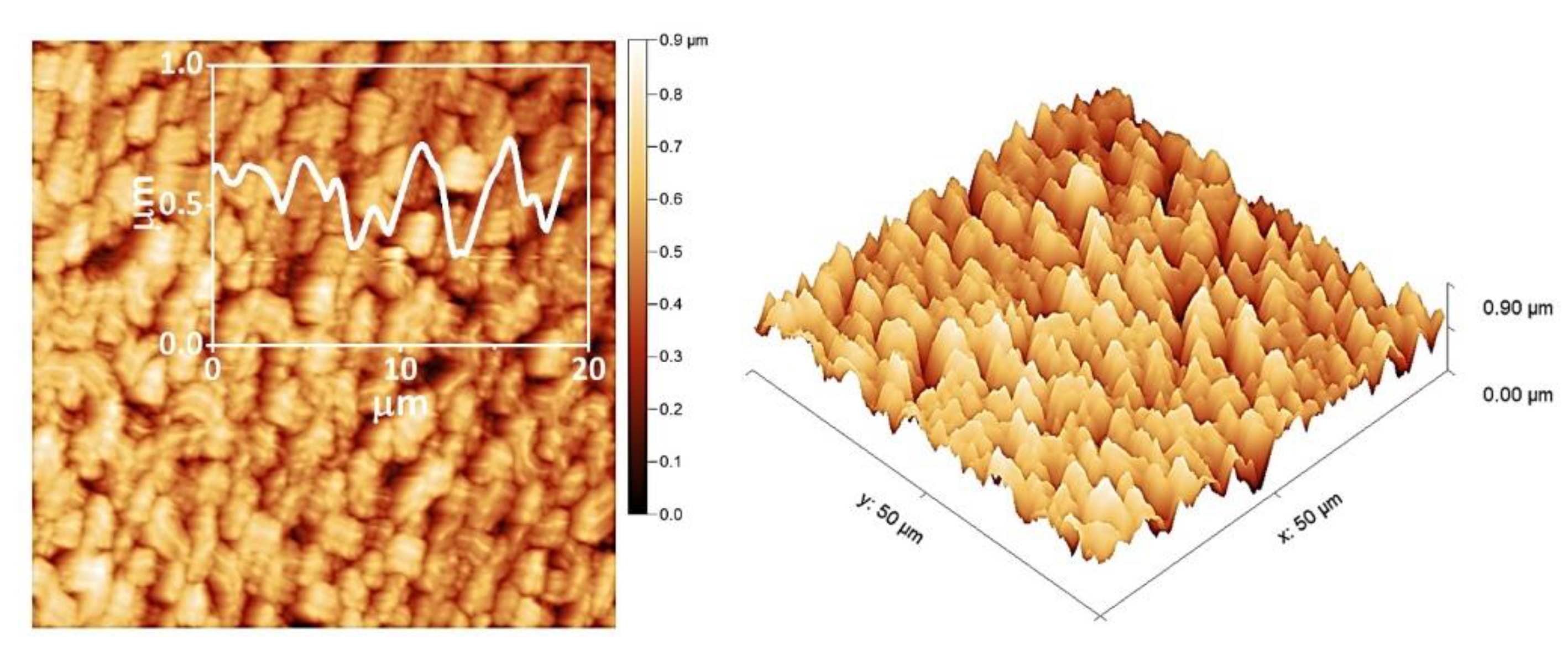
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Brunet, J.; Varenne, C.; Ndiaye, A.; Pauly, A.; Penza, M.; Alvisi, M. Tetra-tert-butyl copper phthalocyanine-based QCM sensor for toluene detection in air at room temperature. Sens. Actuators B Chem. 2015, 210, 398–407. [Google Scholar] [CrossRef]
- Ng, T.P.; Foo, S.C.; Yoong, T. Risk of spontaneous abortion in workers exposed to toluene. Occup. Environ. Med. 1992, 49, 804–808. [Google Scholar] [CrossRef][Green Version]
- Sui, L.; Zhang, X.; Cheng, X.; Wang, P.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. Au-loaded hierarchical MoO3 Hollow spheres with enhanced gas-sensing performance for the detection of BTX (Benzene, Toluene, And Xylene) and the sensing mechanism. ACS Appl. Mater. Interfaces 2017, 9, 1661–1670. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, X.; Ma, X.; Mi, R.; Chen, Y.; Ruan, S. The significant improvement for BTX (benzene, toluene and xylene) sensing performance based on Au-decorated hierarchical ZnO porous rose-like architectures. Sens. Actuators B Chem. 2018, 262, 86–94. [Google Scholar] [CrossRef]
- Acharyya, D.; Bhattacharyya, P. An efficient BTX sensor based on ZnO nanoflowers grown by CBD method. Solid. State. Electron. 2015, 106, 18–26. [Google Scholar] [CrossRef]
- Young, C.R.; Menegazzo, N.; Riley, A.E.; Brons, C.H.; Disanzo, F.P.; Givens, J.L.; Martin, J.L.; Disko, M.M.; Mizaikoff, B. Infrared hollow waveguide sensors for simultaneous gas phase detection of benzene, toluene, and xylenes in field environments. Anal. Chem. 2011, 83, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Girschikofsky, M.; Rosenberger, M.; Belle, S.; Brutschy, M.; Waldvogel, S.R.; Hellmann, R. Optical planar Bragg grating sensor for real-time detection of benzene, toluene and xylene in solvent vapour. Sens. Actuators B Chem. 2012, 171–172, 338–342. [Google Scholar] [CrossRef]
- Dubrawski, S.; Levine, M.; DiScenza, D.; Intravaia, L.; Healy, A. Fluorescence-Based Detection of Benzene, Toluene, Ethylbenzene, Xylene, and Cumene (BTEXC) Compounds in Fuel-Contaminated Snow Environments. Chemosensors 2019, 7, 5. [Google Scholar] [CrossRef]
- Bender, S.; Dickert, F.L.; Mokwa, W.; Pachatz, P. Investigations on temperature controlled monolithic integrated surface acoustic wave (SAW) gas sensors. Sens. Actuators B Chem. 2003, 93, 164–168. [Google Scholar] [CrossRef]
- Hidayat, S.N.; Julian, T.; Rianjanu, A.; Kusumaatmadja, A.; Triyana, K.; Roto, R. Quartz crystal microbalance coated by PAN nanofibers and PEDOT:PSS for humidity sensor. In Proceedings of the 2017 International Seminar on Sensors, Instrumentation, Measurement and Metrology (ISSIMM), Surabaya, Indonesia, 25–26 August 2017; pp. 119–123. [Google Scholar]
- Chen, J.Y.; Penn, L.S.; Xi, J. Quartz crystal microbalance: Sensing cell-substrate adhesion and beyond. Biosens. Bioelectron. 2018, 99, 593–602. [Google Scholar] [CrossRef]
- Schirhagl, R.; Bajwa, S.; Afzal, A.; Latif, U.; Feroz, S.; Mujahid, A. Gravimetric Viral Diagnostics: QCM Based Biosensors for Early Detection of Viruses. Chemosensors 2017, 5, 7. [Google Scholar] [CrossRef]
- Çiçek, Ç.; Yılmaz, F.; Özgür, E.; Yavuz, H.; Denizli, A. Molecularly Imprinted Quartz Crystal Microbalance Sensor (QCM) for Bilirubin Detection. Chemosensors 2016, 4, 21. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Zeitschrift für Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Rianjanu, A.; Julian, T.; Hidayat, S.N.; Suyono, E.A.; Kusumaatmaja, A.; Triyana, K. Polyacrylonitrile nanofiber as polar solvent N,N-dimethyl formamide sensor based on quartz crystal microbalance technique. J. Phys. Conf. Ser. 2018, 1011, 012067. [Google Scholar] [CrossRef]
- Bearzotti, A.; Macagnano, A.; Papa, P.; Venditti, I.; Zampetti, E. A study of a QCM sensor based on pentacene for the detection of BTX vapors in air. Sens. Actuators B Chem. 2017, 240, 1160–1164. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Cha, X.; Wu, Y.; Xu, J.; Cheng, Z.; Xiang, Q. Superhydrophobic Polymerized n-Octadecylsilane Surface for BTEX Sensing and Stable Toluene/Water Selective Detection Based on QCM Sensor. ACS Omega 2018, 3, 2437–2443. [Google Scholar] [CrossRef]
- Fan, X.; Du, B. Selective detection of trace p-xylene by polymer-coated QCM sensors. Sens. Actuators B Chem. 2012, 166–167, 753–760. [Google Scholar] [CrossRef]
- El Sabahy, J.; Berthier, J.; Ricoul, F.; Jousseaume, V. Toward optimized SiOCH films for BTEX detection: Impact of chemical composition on toluene adsorption. Sens. Actuators B Chem. 2018, 258, 628–636. [Google Scholar] [CrossRef]
- Rianjanu, A.; Roto, R.; Julian, T.; Hidayat, S.N.; Kusumaatmaja, A.; Suyono, E.A.; Triyana, K. Polyacrylonitrile Nanofiber-Based Quartz Crystal Microbalance for Sensitive Detection of Safrole. Sensors 2018, 18, 1150. [Google Scholar] [CrossRef]
- Triyana, K.; Sembiring, A.; Rianjanu, A.; Hidayat, S.; Riowirawan, R.; Julian, T.; Kusumaatmaja, A.; Santoso, I.; Roto, R. Chitosan-Based Quartz Crystal Microbalance for Alcohol Sensing. Electronics 2018, 7, 181. [Google Scholar] [CrossRef]
- Wang, X.; Cui, F.; Lin, J.; Ding, B.; Yu, J.; Al-Deyab, S.S. Functionalized nanoporous TiO2 fibers on quartz crystal microbalance platform for formaldehyde sensor. Sens. Actuators B Chem. 2012, 171–172, 658–665. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Jia, Y.; Li, X.; Zhu, Z.; Li, Y.; Si, Y.; Ding, B.; Wang, X.; Yu, J. Highly sensitive formaldehyde sensors based on polyvinylamine modified polyacrylonitrile nanofibers. RSC Adv. 2013, 3, 22994. [Google Scholar] [CrossRef]
- Rianjanu, A.; Hidayat, S.N.; Julian, T.; Suyono, E.A.; Kusumaatmaja, A.; Triyana, K. Swelling Behavior in Solvent Vapor Sensing based on Quartz Crystal Microbalance (QCM) Coated Polyacrylonitrile (PAN) Nanofiber. IOP Conf. Ser. Mater. Sci. Eng. 2018, 367, 012020. [Google Scholar] [CrossRef]
- Rianjanu, A.; Kusumaatmaja, A.; Suyono, E.A.; Triyana, K. Solvent vapor treatment improves mechanical strength of electrospun polyvinyl alcohol nanofibers. Heliyon 2018, 4, e00592. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Speight, J.G. Lange’s handbook of chemistry, 16th ed.; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Fraden, J. Handbook of Modern Sensors; Springer: Berlin, Germany, 2013. [Google Scholar]
- Bayram, A.; Özbek, C.; Şenel, M.; Okur, S. CO gas sorption properties of ferrocene branched chitosan derivatives. Sens. Actuators B Chem. 2017, 241, 308–313. [Google Scholar] [CrossRef]
- Horzum, N.; Tascioglu, D.; Ozbek, C.; Okur, S.; Demir, M.M. VOC sensors based on a metal oxide nanofibrous membrane/QCM system prepared by electrospinning. New J. Chem. 2014, 38, 5761–5768. [Google Scholar] [CrossRef]
- Taylor, J.R. An Introduction to Error Analysis: The Study of Uncertainties in Physical Measurements; Universities Science Books: Sausalito, CA, USA, 1982; ISBN 0-935702-75-X. [Google Scholar]
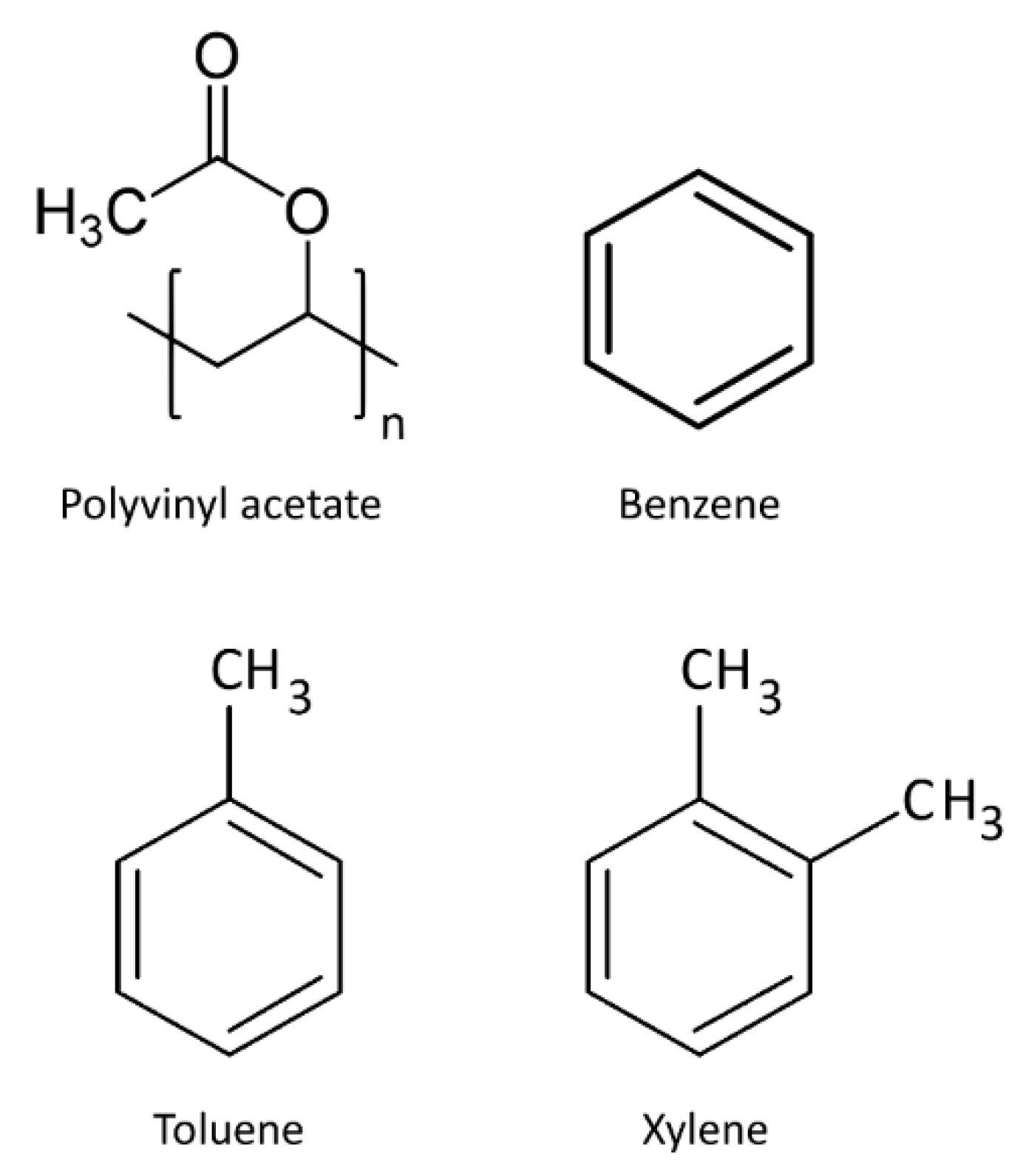
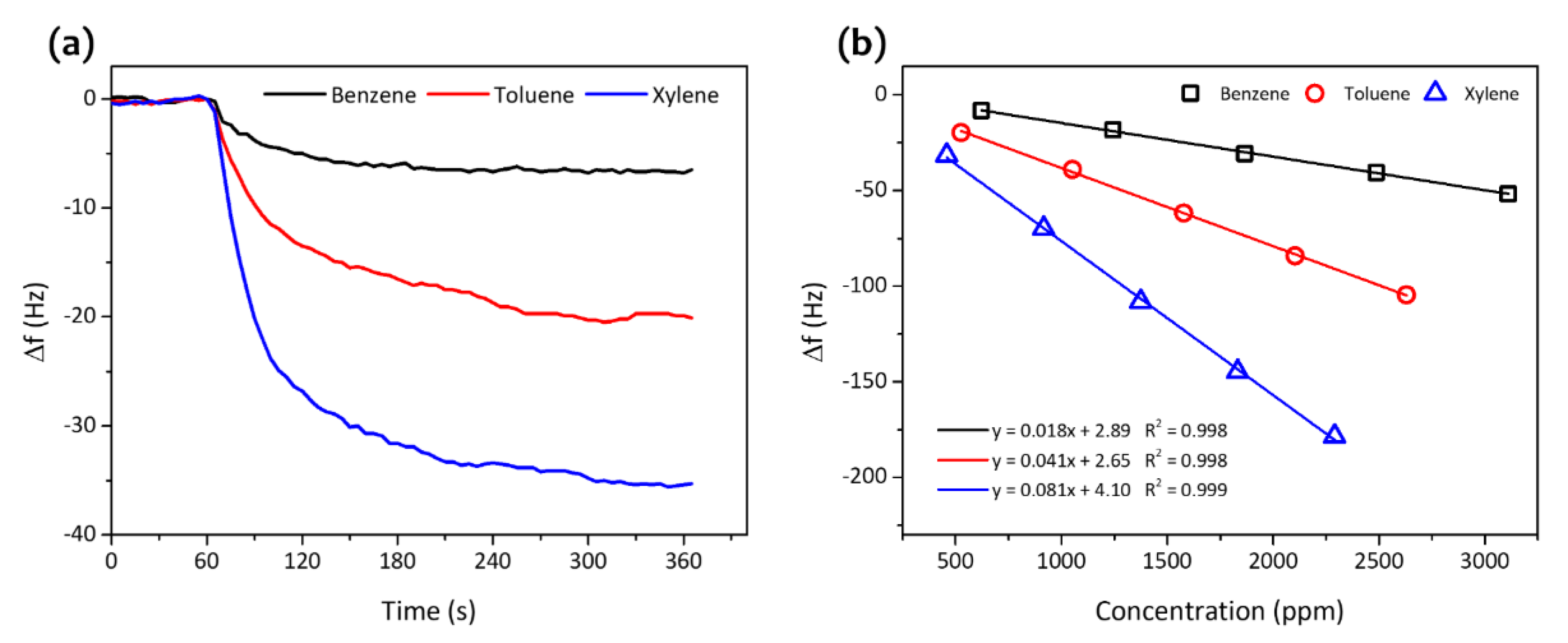
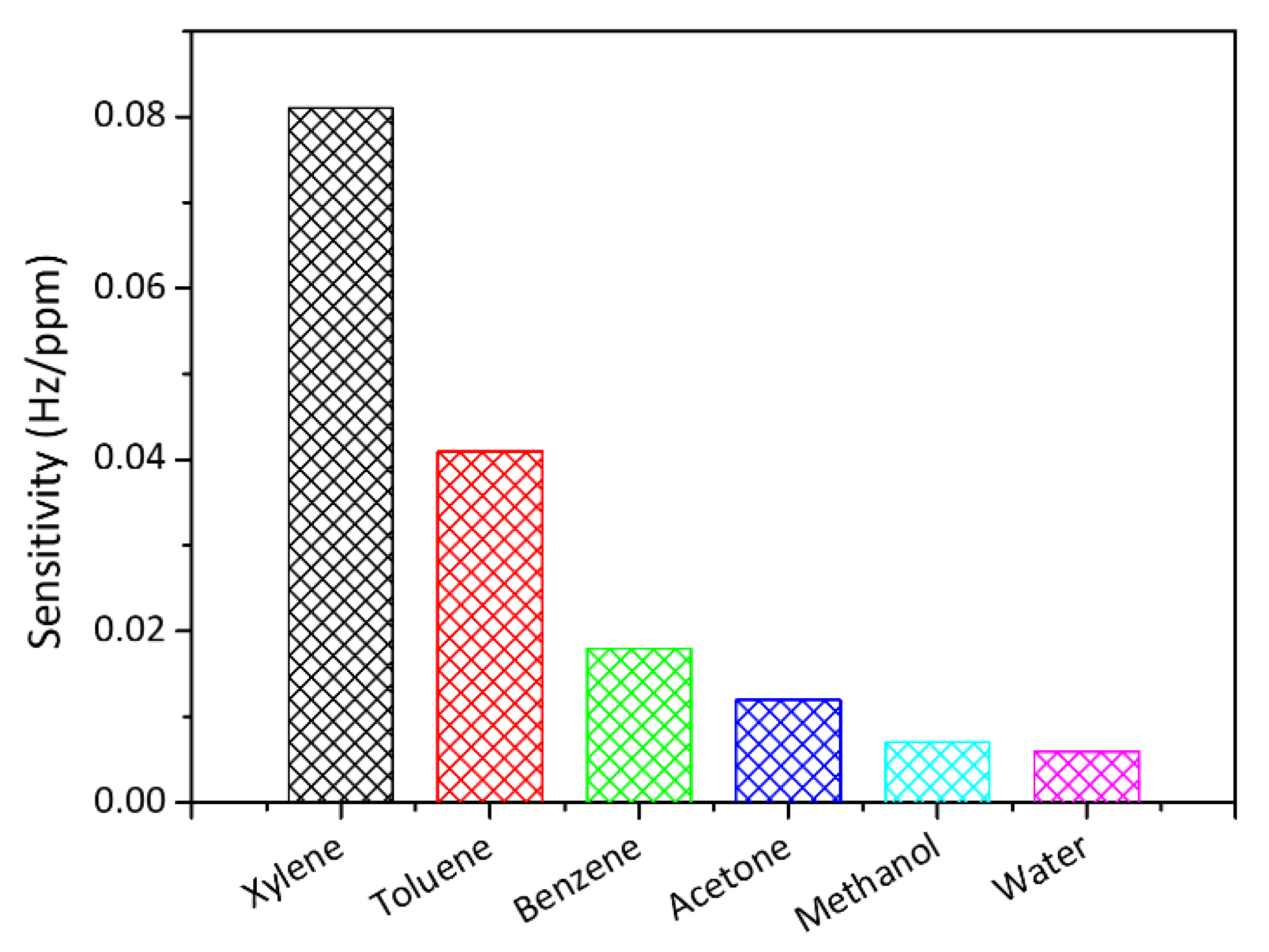
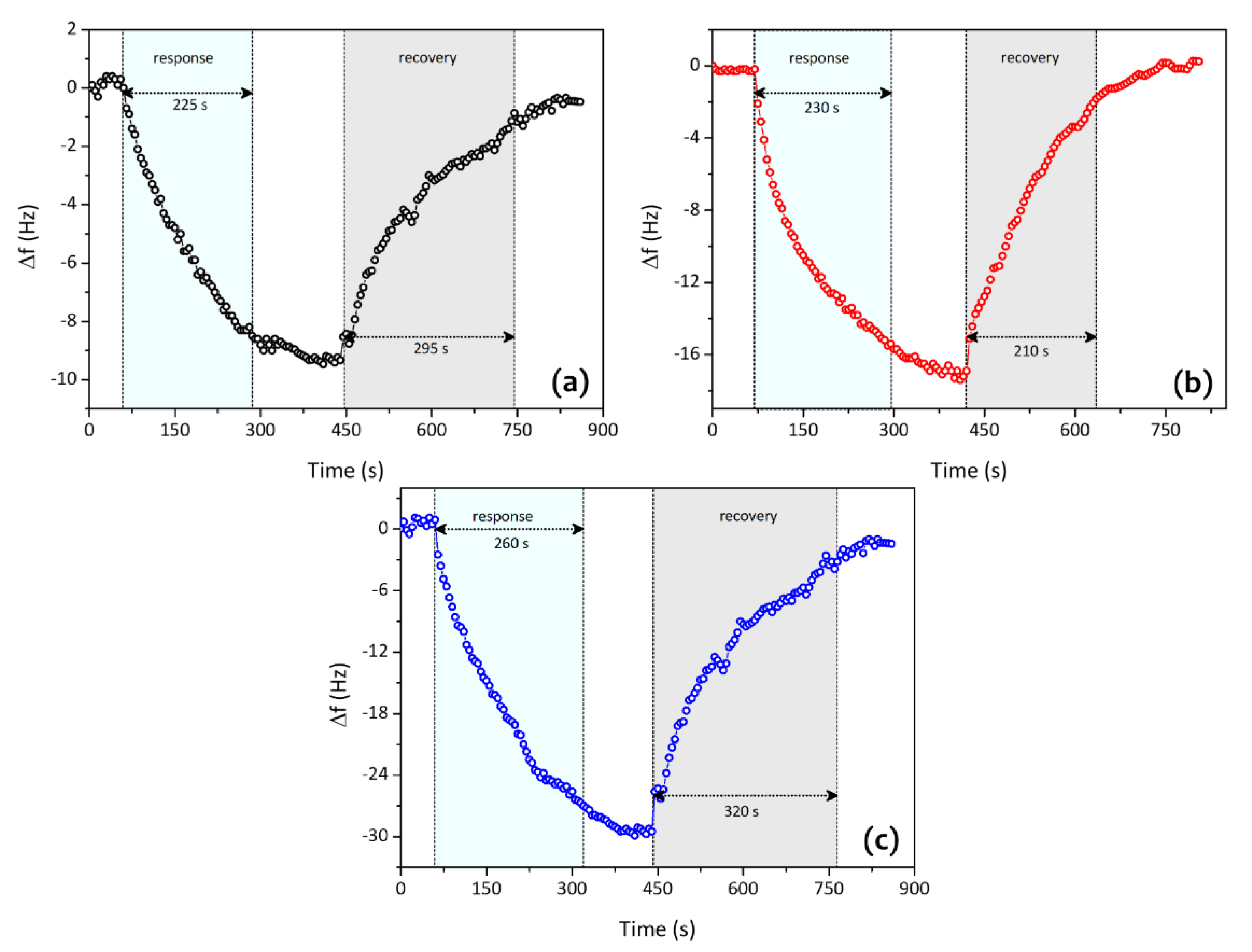
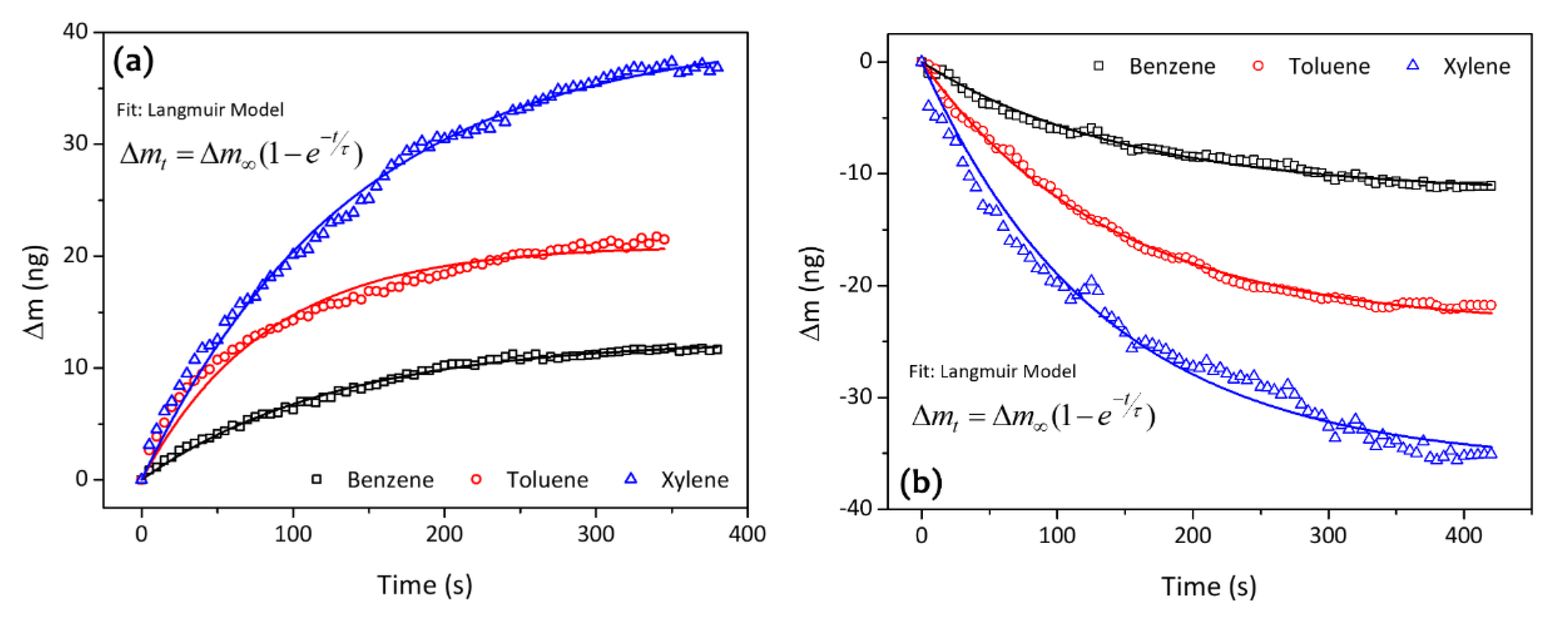
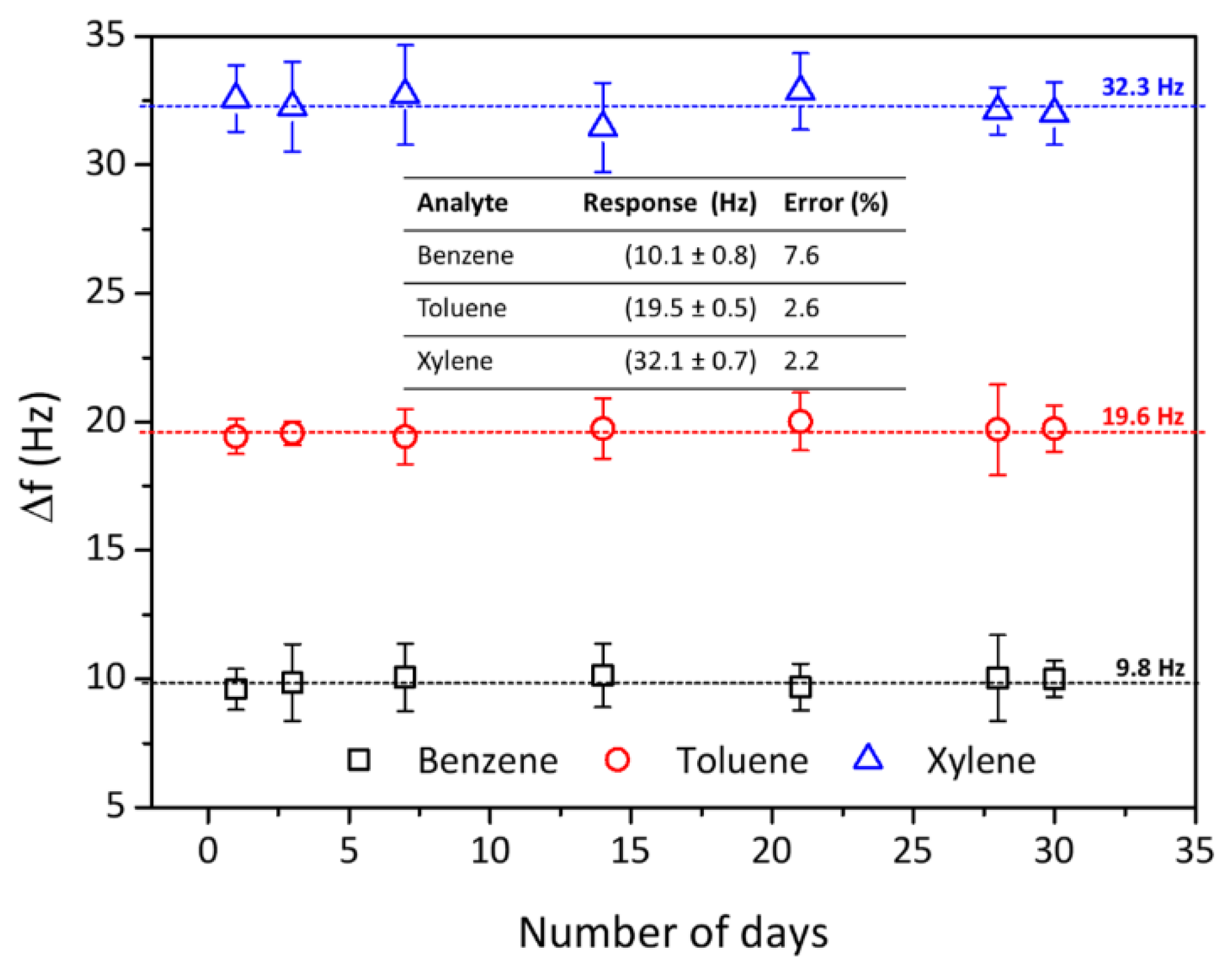
| Analytes | Sensitivity (Hz/ppm) | Relative Energy Density (RED) | Vapor Pressure (mmHg) | Boiling Temperature (K) |
|---|---|---|---|---|
| Xylene | 0.081 | 1.011 | 8.8 | 407.4 |
| Toluene | 0.041 | 1.005 | 28.4 | 383.6 |
| Benzene | 0.018 | 1.058 | 94.8 | 353.1 |
| Acetone | 0.012 | 0.813 | 231.0 | 329.0 |
| Methanol | 0.007 | 1.297 | 127.0 | 337.7 |
| Water | 0.006 | 2.532 | 23.8 | 373.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rianjanu, A.; Hasanah, S.A.; Nugroho, D.B.; Kusumaatmaja, A.; Roto, R.; Triyana, K. Polyvinyl Acetate Film-Based Quartz Crystal Microbalance for the Detection of Benzene, Toluene, and Xylene Vapors in Air. Chemosensors 2019, 7, 20. https://doi.org/10.3390/chemosensors7020020
Rianjanu A, Hasanah SA, Nugroho DB, Kusumaatmaja A, Roto R, Triyana K. Polyvinyl Acetate Film-Based Quartz Crystal Microbalance for the Detection of Benzene, Toluene, and Xylene Vapors in Air. Chemosensors. 2019; 7(2):20. https://doi.org/10.3390/chemosensors7020020
Chicago/Turabian StyleRianjanu, Aditya, Siti A. Hasanah, Doni B. Nugroho, Ahmad Kusumaatmaja, Roto Roto, and Kuwat Triyana. 2019. "Polyvinyl Acetate Film-Based Quartz Crystal Microbalance for the Detection of Benzene, Toluene, and Xylene Vapors in Air" Chemosensors 7, no. 2: 20. https://doi.org/10.3390/chemosensors7020020
APA StyleRianjanu, A., Hasanah, S. A., Nugroho, D. B., Kusumaatmaja, A., Roto, R., & Triyana, K. (2019). Polyvinyl Acetate Film-Based Quartz Crystal Microbalance for the Detection of Benzene, Toluene, and Xylene Vapors in Air. Chemosensors, 7(2), 20. https://doi.org/10.3390/chemosensors7020020






