Hydrogen Sensing Properties of Co-Doped ZnO Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Co-Doped ZnO
2.2. Characterization
2.3. Gas Sensing Measurements
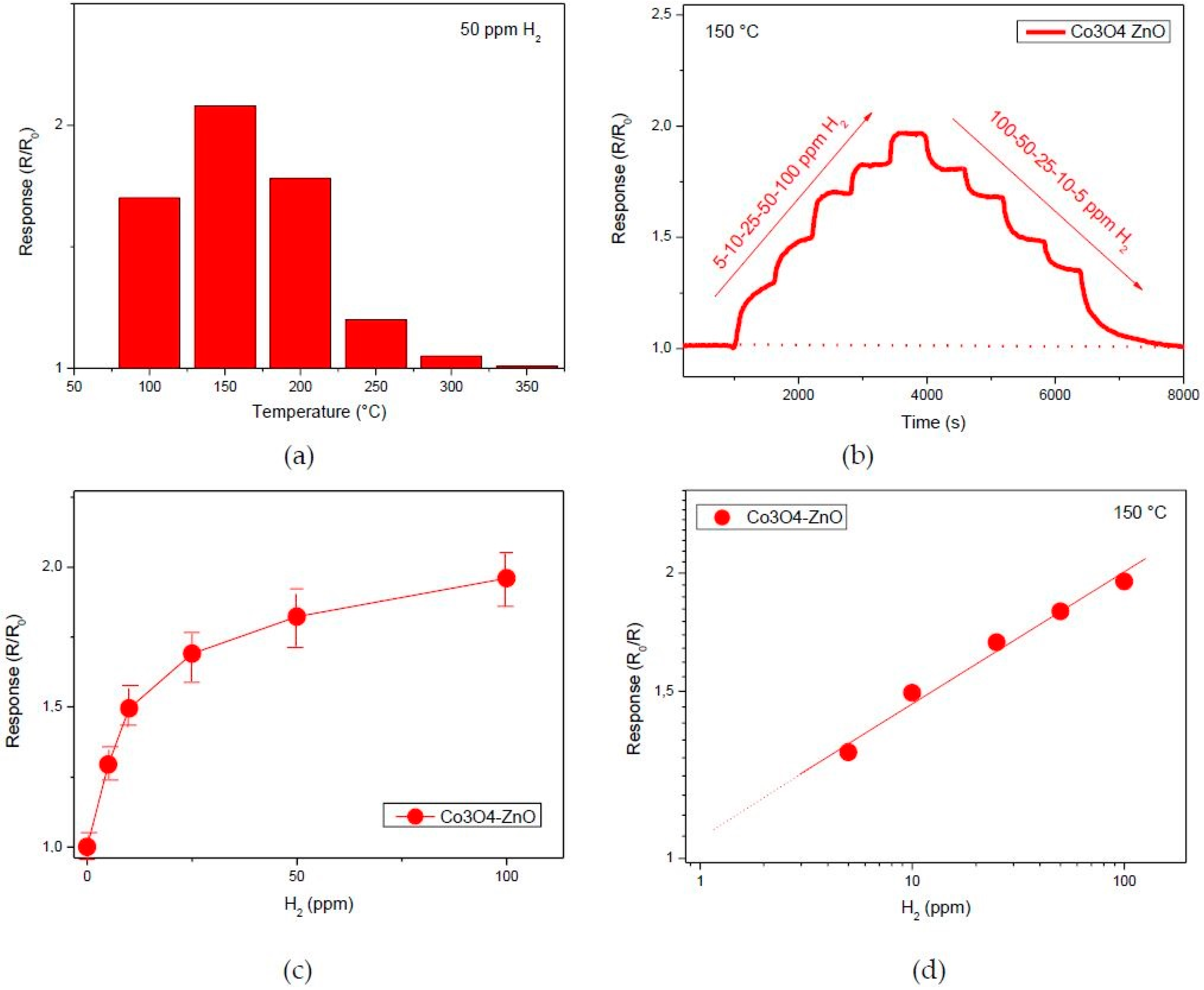
3. Results
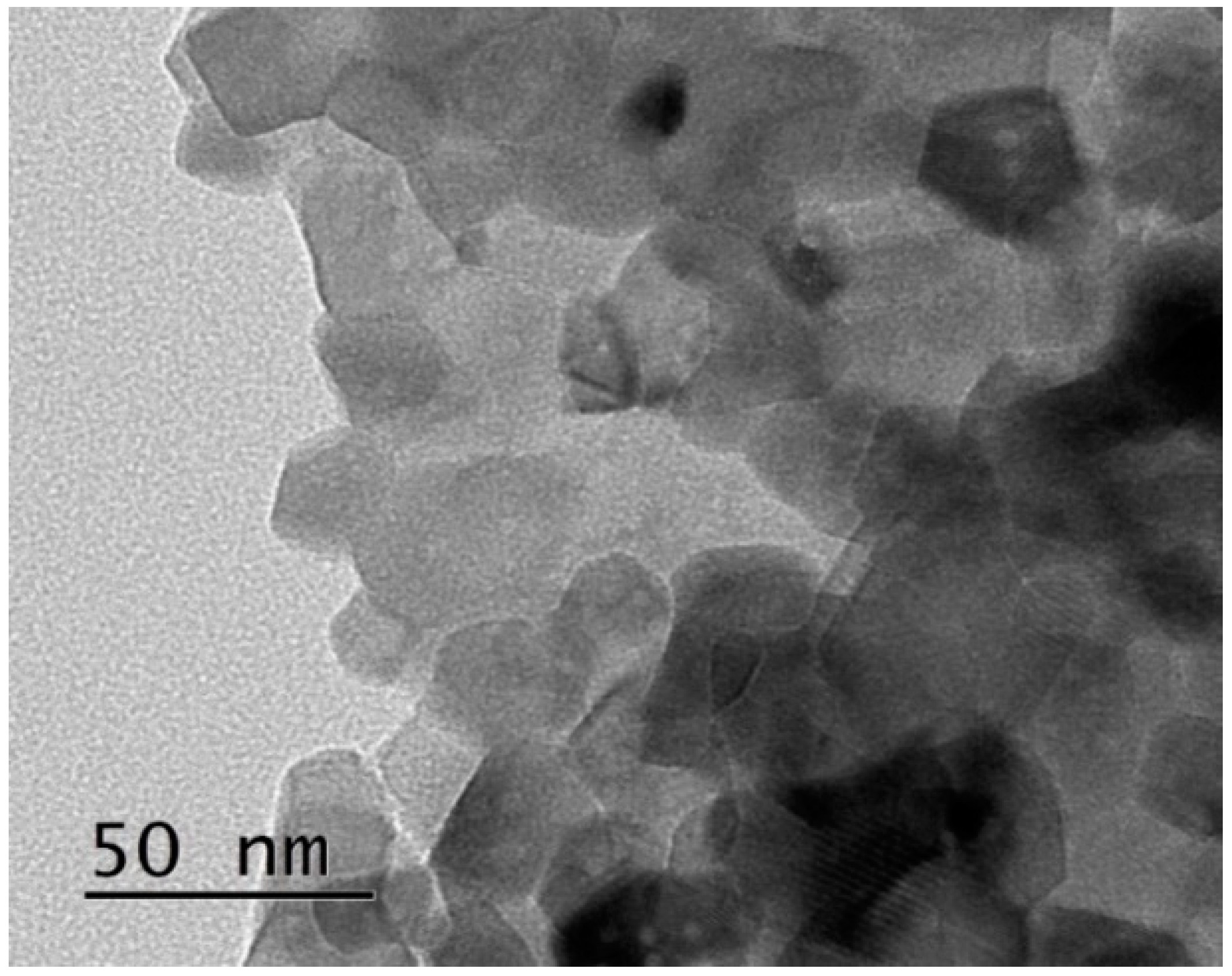
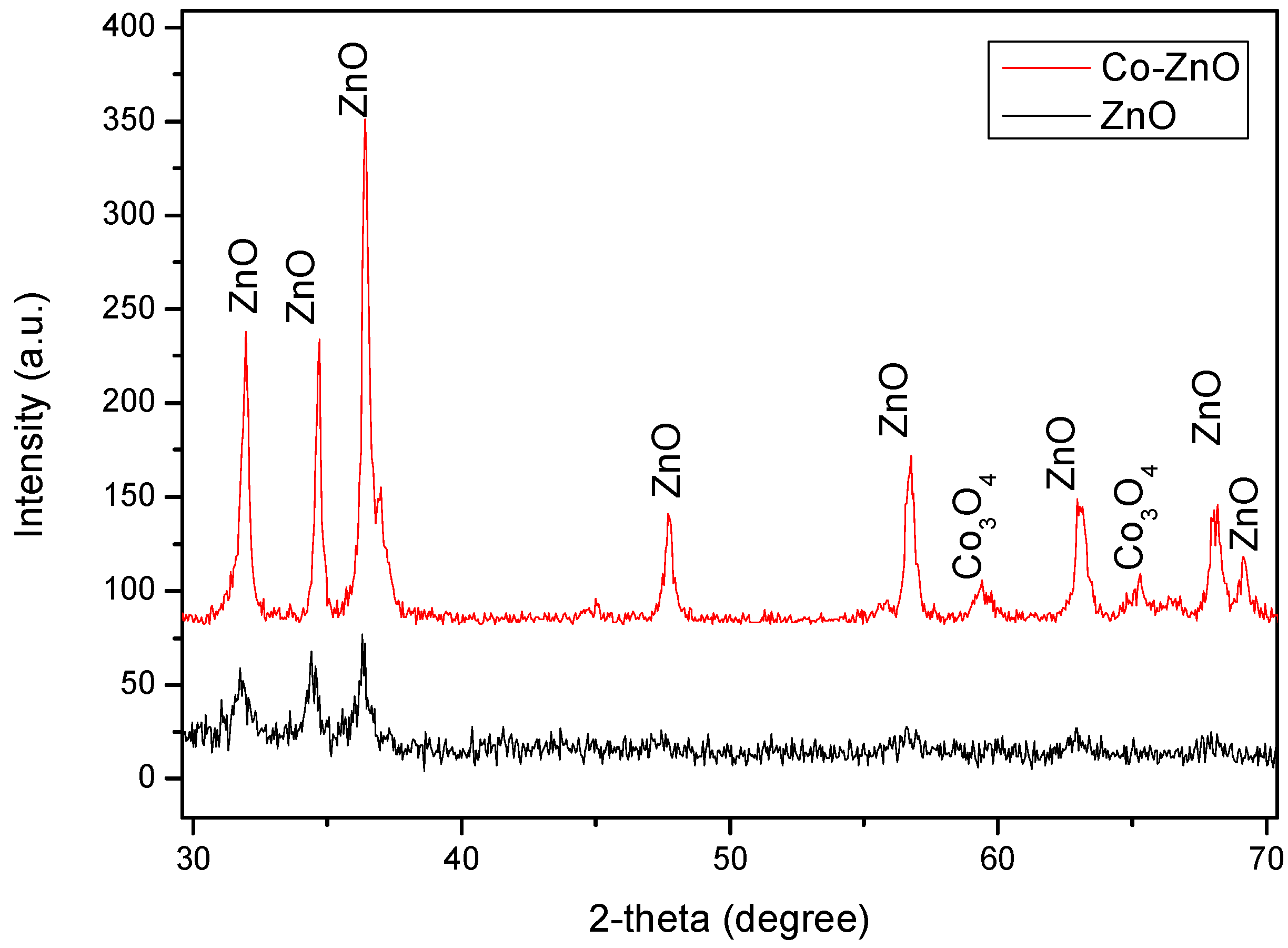
3.1. Morphological and Microstructural Characterization
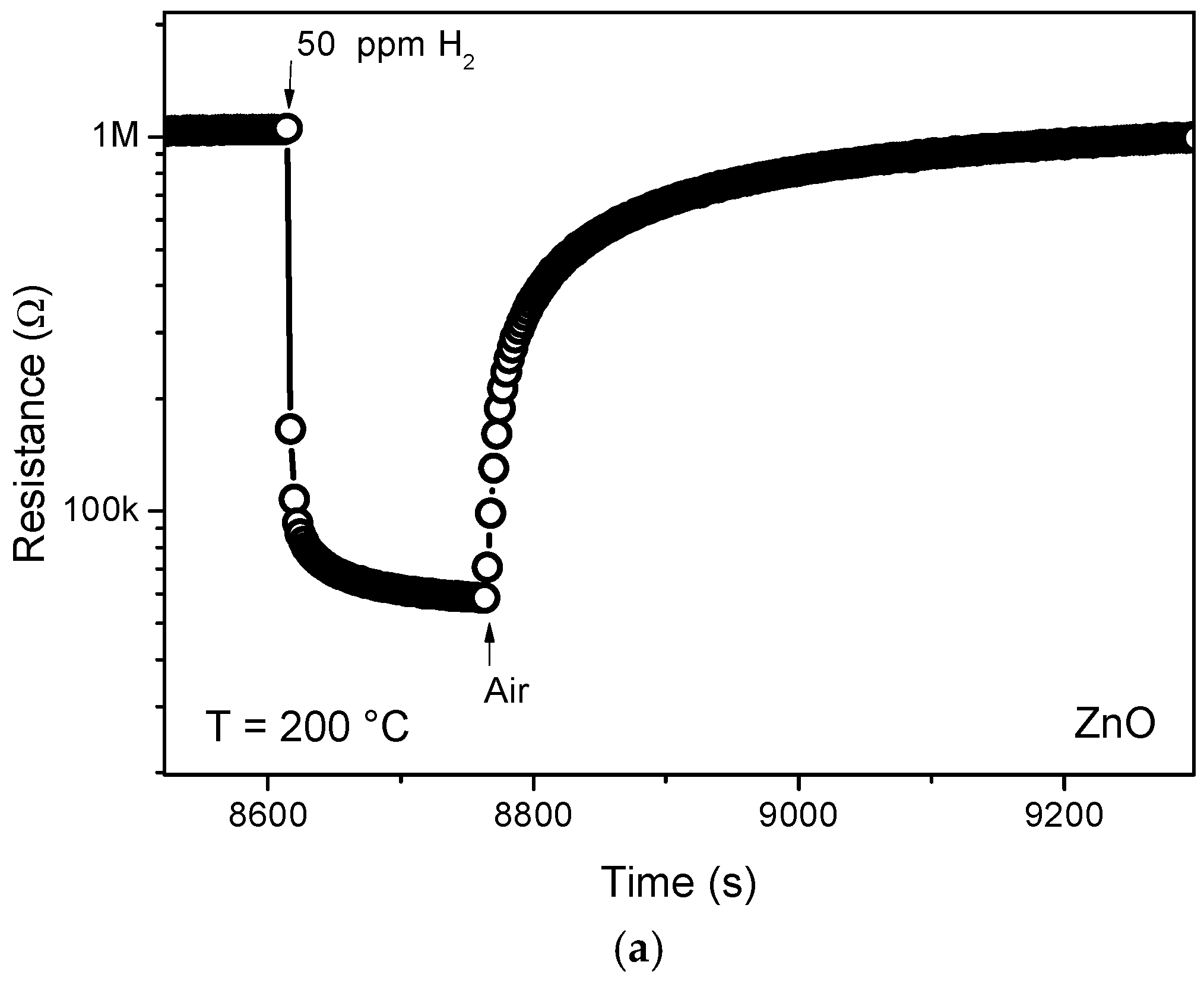
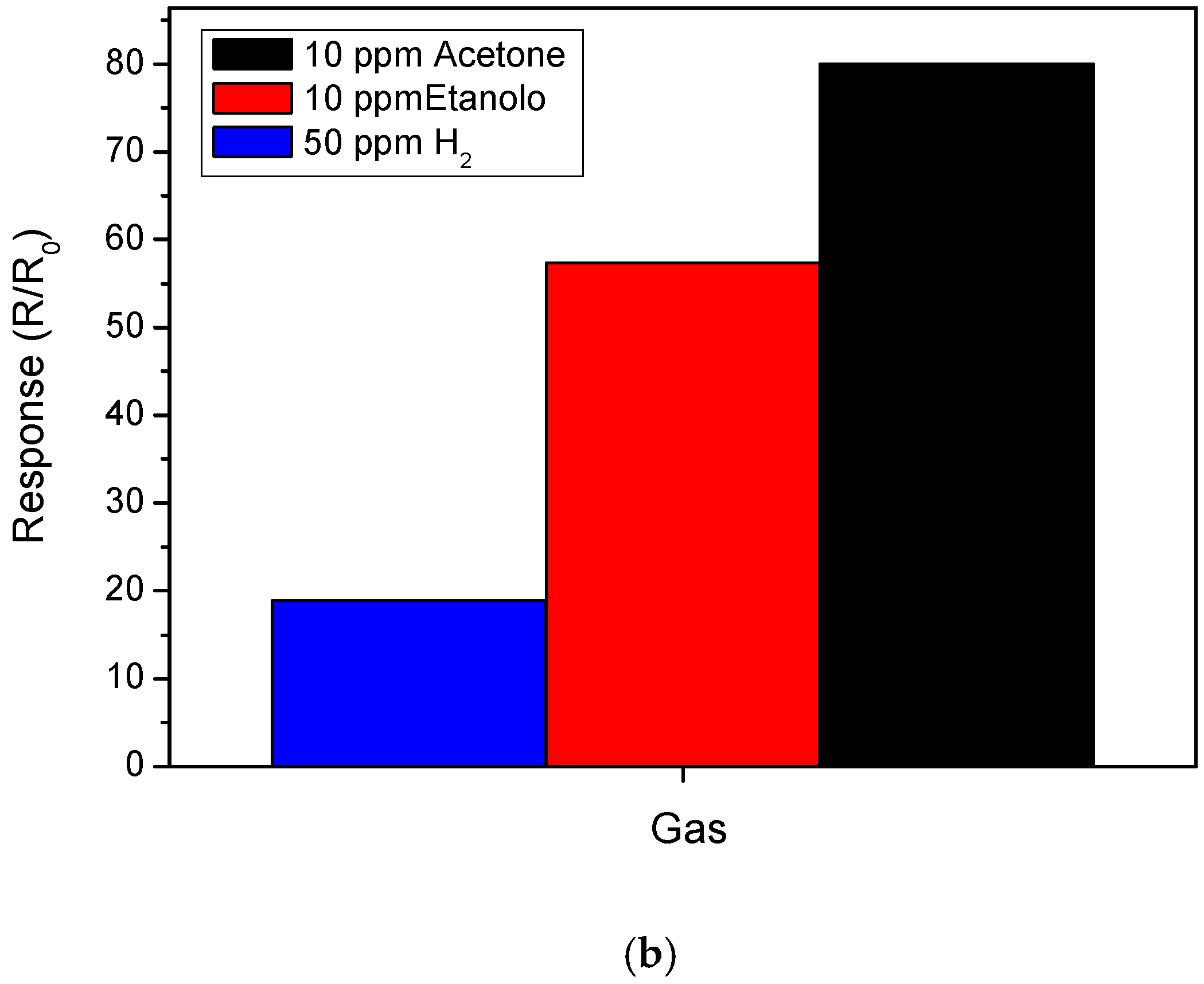
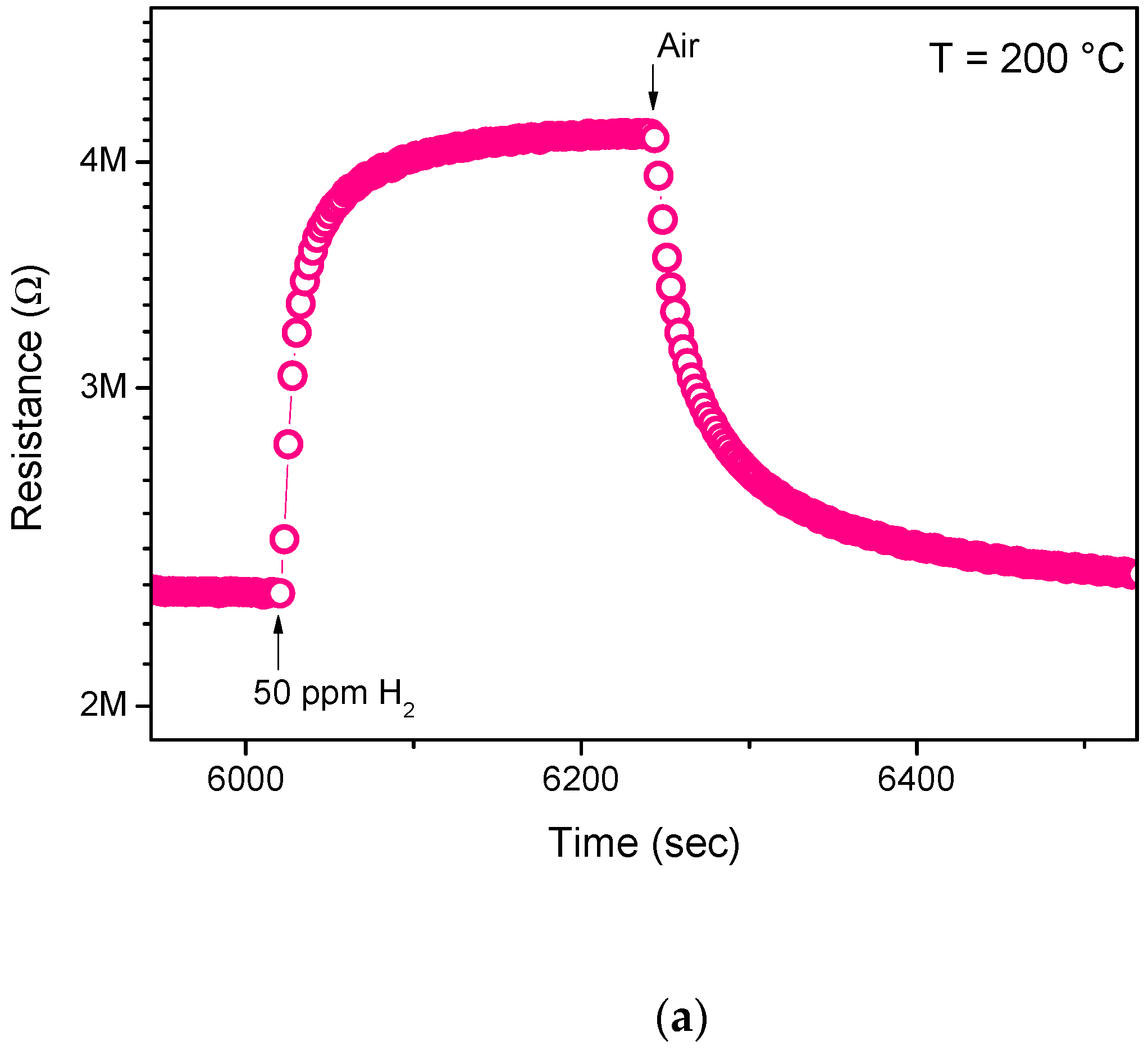
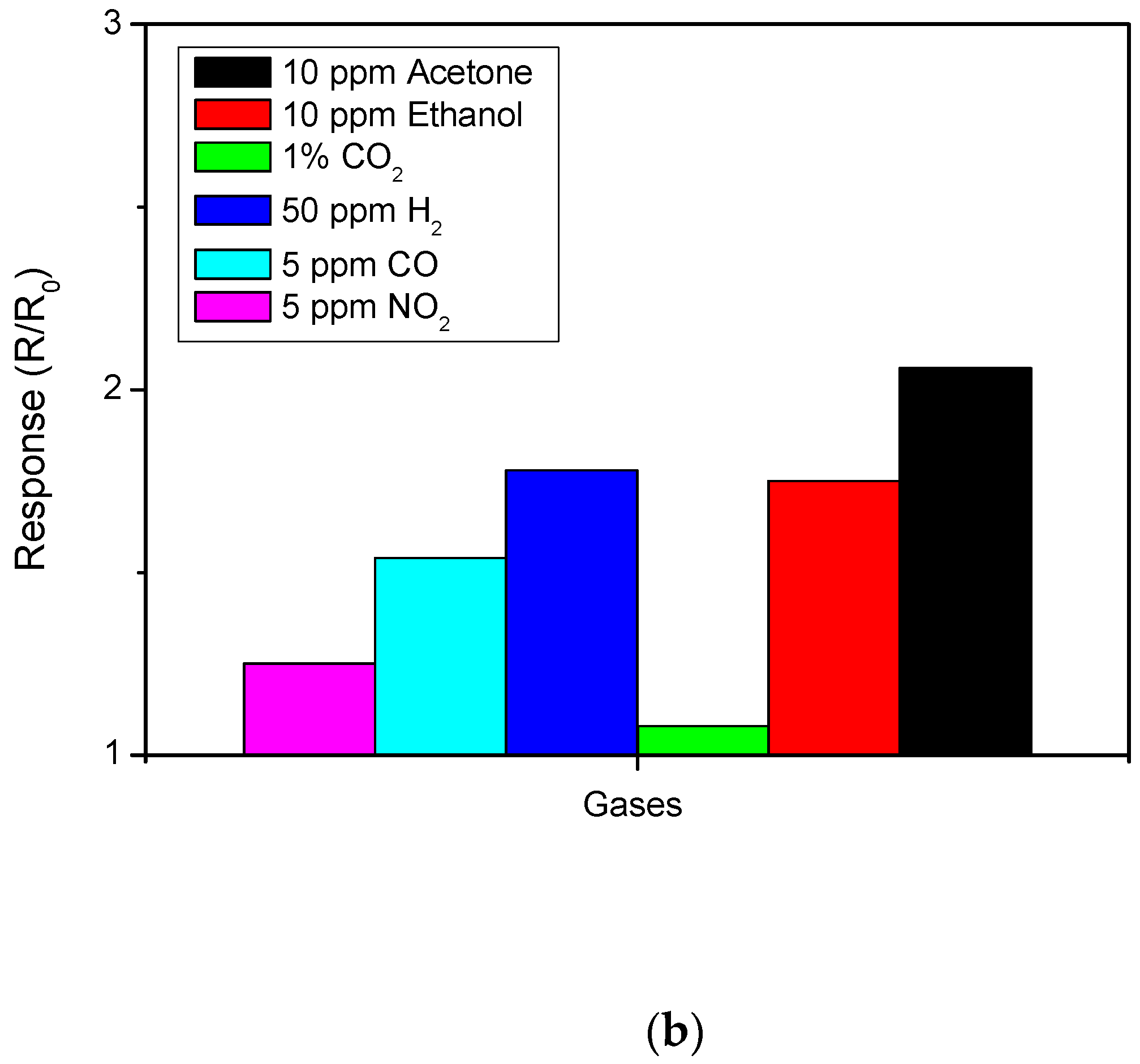
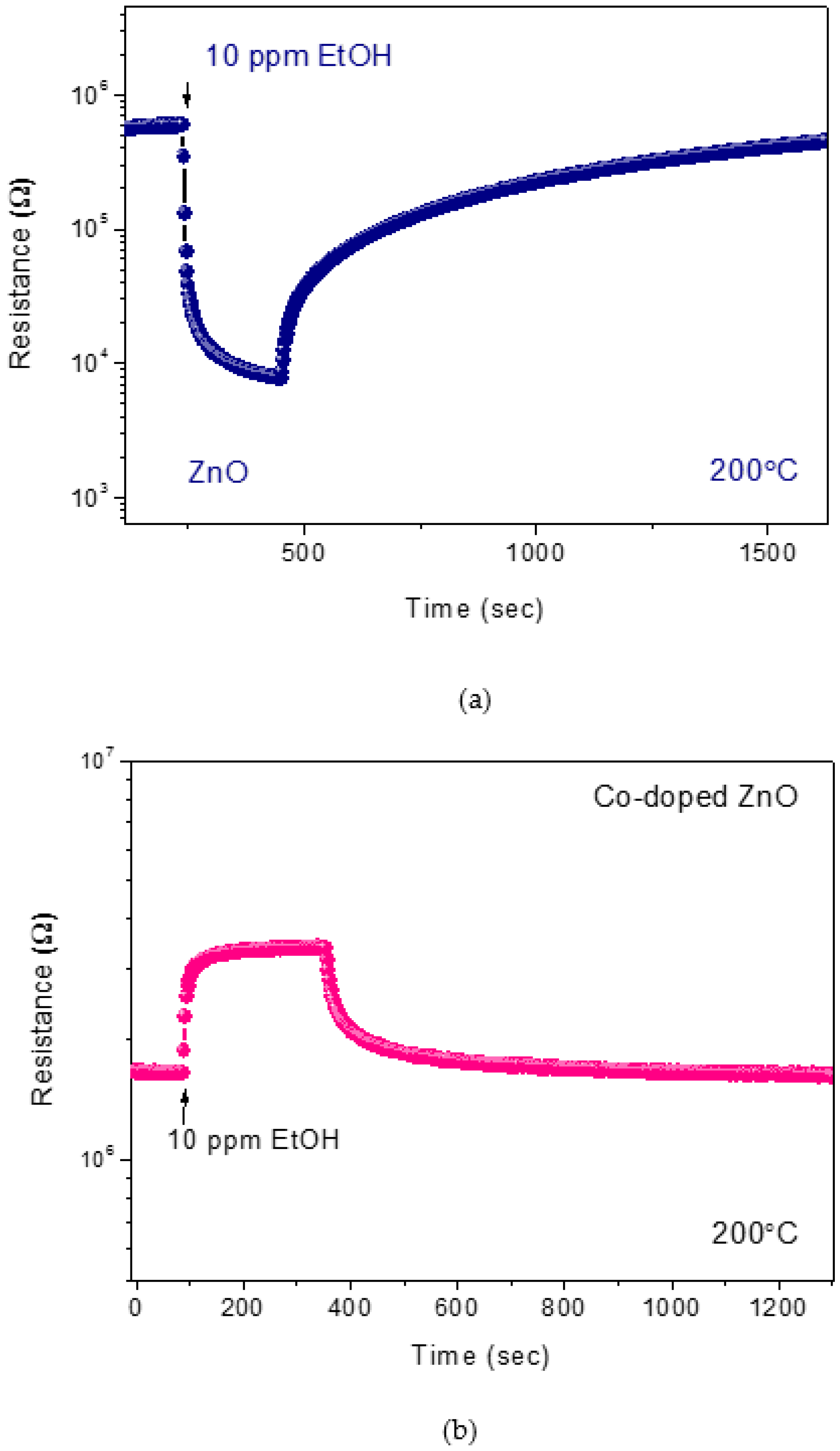
3.2. Sensing Tests
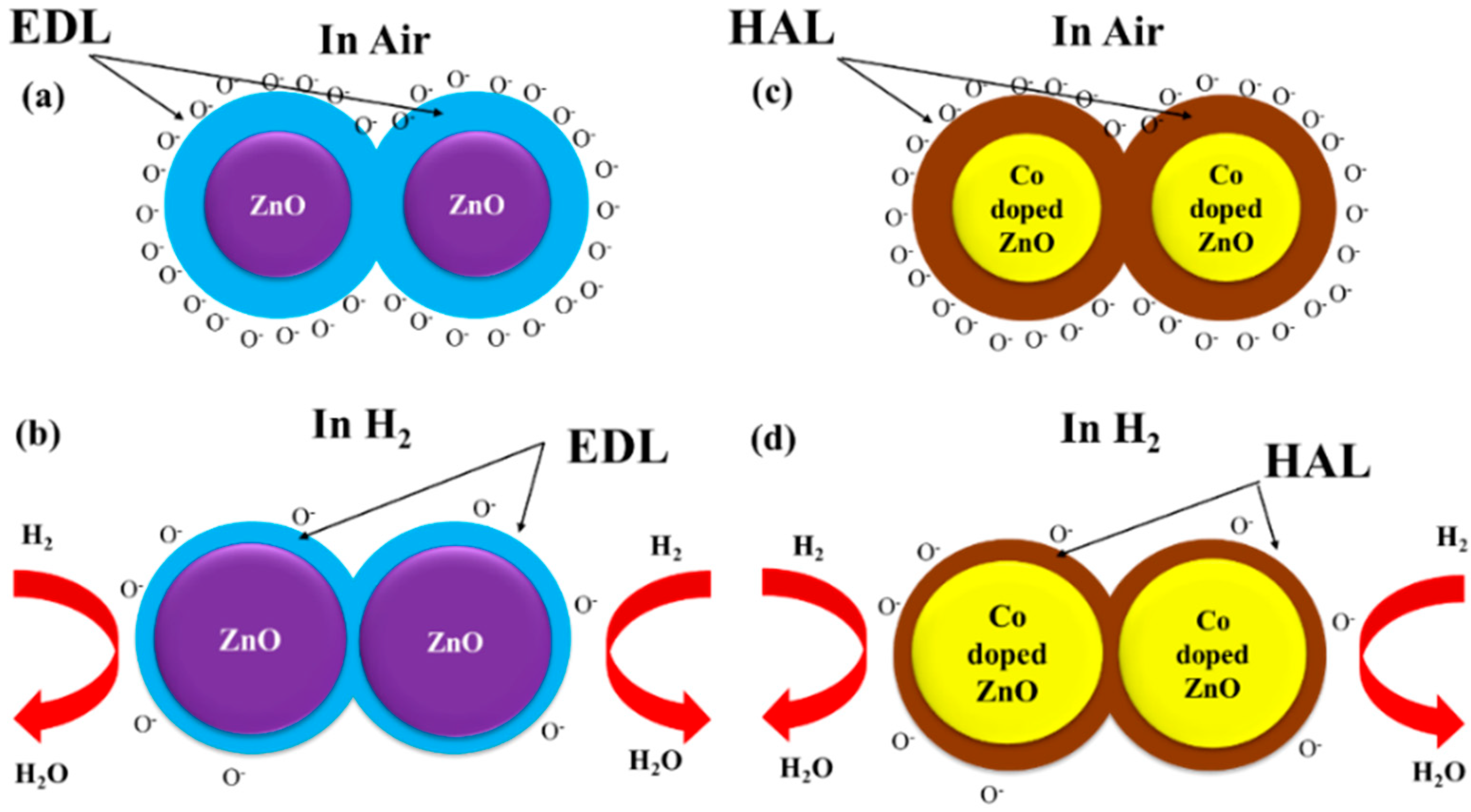
3.3. Hydrogen Sensor Performances
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Lokhande, C.D. Chemical route to synthesis of mesoporous ZnO thin films and their liquefied petroleum gas sensor performance. J. Alloys. Compd. 2011, 509, 10092–10097. [Google Scholar] [CrossRef]
- Qi, Q.; Zeng, T.; Zeng, Y.; Yang, H. Humidity sensing properties of KCl-doped Cu–Zn/CuO–ZnO nanoparticles. Sens. Actuators B Chem. 2009, 137, 21–26. [Google Scholar] [CrossRef]
- Lee, J.H.; Ko, K.H.; Park, B.O. Electrical and optical properties of ZnO transparent conducting films by the sol-gel method. J. Cryst. Growth 2003, 247, 119. [Google Scholar] [CrossRef]
- Olson, D.C.; Piris, J.; Collins, R.T.; Shaheen, S.E.; Ginley, D.S. Hybrid photovoltaic devices of polymer and ZnO nanofiber composites. Thin Solid Films 2006, 496, 26. [Google Scholar] [CrossRef]
- Powell, D.A.; Kalantar-zade, K.; Wlodarski, W. Numerical calculation of SAW sensitivity: Application to ZnO/LiTaO3 transducers. Sens. Actuators A Phys. 2004, 115, 456–461. [Google Scholar] [CrossRef]
- Hu, Z.J.Y.; Xu, C.; Mei, T.; Guo, J.; White, T. Monodisperse ZnO nanodots: Synthesis, charaterization, and optoelectronic properties. J. Phys. Chem. C 2007, 111, 9757–9760. [Google Scholar] [CrossRef]
- Chen, Y.L.H.; Xie, C.; Wu, J.; Zeng, D.; Liao, Y. A comparative study on UV light activated porous TiO2 and ZnO film sensors for gas sensing at room temperature. Ceram. Int. 2012, 38, 503–509. [Google Scholar] [CrossRef]
- Mirzaei, A.; Park, S.; Kheel, H.; Sun, G.-J.; Lee, S.; Lee, C. ZnO-capped nanorod gas sensors. Ceram. Int. 2016, 42, 6187–6197. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kang, S.Y.; Mirzaei, A.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Enhancement of gas sensing properties by the functionalization of ZnO-branched SnO2 nanowires with Cr2O3 nanoparticles. Sens. Actuators B Chem. 2017, 249, 656–666. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. How shell thickness can affect the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B Chem. 2018, 258, 270–294. [Google Scholar] [CrossRef]
- Leonardi, S.G. Two-dimensional zinc oxide nanostructures for gas sensor applications. Chemosensors 2017, 5, 17. [Google Scholar] [CrossRef]
- Chu, X.; Chen, T.; Zhang, W.; Zheng, B.; Shui, H. Investigation on formaldehyde gas sensor with ZnO thick film prepared through microwave heating method. Sens. Actuators B Chem. 2009, 142, 49–54. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, T.; Yuan, M.; Kang, M.; Lu, G.; Wang, R.; Fan, H.; He, Y.; Yang, H. Growth and selective acetone detection based on ZnO nanorod arrays. Sens. Actuators B Chem. 2009, 143, 93–98. [Google Scholar] [CrossRef]
- Dong, L.F.; Cui, Z.L.; Zhang, Z.K. Gas sensing properties of nano-ZnO prepared by arc plasma method. Nanostruct. Mater. 1997, 8, 815–823. [Google Scholar] [CrossRef]
- Oh, E.; Choi, H.Y.; Jung, S.H.; Cho, S.; Kim, J.C.; Lee, K.H.; Kang, S.W.; Kim, J.; Yund, J.Y.; Jeong, S.H. High-performance NO2 gas sensor based on ZnO nanorod grown by ultrasonic irradiation. Sens. Actuators B Chem. 2009, 141, 239–243. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Zhu, Z.; Zhu, J. Effect of Pd2+ doping on ZnO nanotetrapods ammonia sensor. Colloids Surf. A 2006, 276, 59–64. [Google Scholar] [CrossRef]
- Aygün, S.; Cann, D. Hydrogen sensitivity of doped CuO/ZnO heterocontact sensors. Sens. Actuators B Chem. 2005, 106, 837–842. [Google Scholar]
- Wei, A.; Pan, L.; Huang, W. Recent Progress in the ZnO Nanostructure-Based Sensors. Mater. Sci. Eng. B 2011, 176, 1409–1421. [Google Scholar] [CrossRef]
- Rout, C.S.; Raju, A.R.; Govindaraj, A.; Rao, C.N.R. Hydrogen sensors based on ZnO nanoparticles. Solid State Commun. 2006, 138, 136–138. [Google Scholar] [CrossRef]
- Hoppe, M.; Lupan, O.; Postica, V.; Wolff, N.; Duppel, V.; Kienle, L.; Tiginyanu, I.; Adelung, R. ZnAl2O4-Functionalized zinc oxide microstructures for highly selective hydrogen gas sensing applications. Phys. Stat. Solidi 2018, 215, 1700772. [Google Scholar]
- Sett, D.; Basak, D. Highly enhanced H2 gas sensing characteristics of Co: ZnO nanorods and its mechanism. Sens. Actuators B Chem. 2018, 243, 475–483. [Google Scholar] [CrossRef]
- Reddy, P.; Reddy, K.S.; Reddy, B.; Manasa, M.V.; Devi, G.S.; Rao, G.N. Gas Sensing Characteristics of ZnO: Nb2O5 nanocomposite towards hydrogen gas. J. Adv. Phys. 2017, 6, 418–421. [Google Scholar] [CrossRef]
- Jaaniso, R.; Kiang Tan, O. Semiconductor Gas Sensors; Woodhead Publishing Group: Sawston, UK, 2013. [Google Scholar]
- Wang, H.T.; Kang, B.S.; Ren, F.; Tien, L.C.; Sadik, P.W.; Norton, D.P.; Pearton, S.J. Hydrogen-selective sensing at room temperature with ZnO nanorods. J. Appl. Phys. Lett. 2005, 86, 243503. [Google Scholar] [CrossRef]
- Bekermann, D.; Gasparotto, A.; Barreca, D.; Maccato, C.; Comini, E.; Sada, C.; Sberveglieri, G.; Devi, A.; Fischer, R.A. Co3O4/ZnO nanocomposites: From plasma synthesis to gas sensing applications. ACS Appl. Mater. Interfaces 2012, 4, 928–934. [Google Scholar] [CrossRef]
- Shin, W.; Goto, T.; Nagai, D.; Itoh, T.; Tsuruta, A.; Akamatsu, T.; Sato, K. Thermoelectric array sensors with selective combustion catalysts for breath gas monitoring. Sensors 2018, 18, 1579. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. Highly stable and selective ethanol sensor based on α-Fe2O3 nanoparticles prepared by Pechini sol-gel method. Ceram. Int. 2016, 42, 6136–6144. [Google Scholar] [CrossRef]
- Anderson, P.; Baumberg, B. Alcohol in Europe, A Public Health Perspective; A Report for the European Commission; European Commission: Brussels, Belgium, 2006. [Google Scholar]
- Karmaoui, M.; Leonardi, S.G.; Latino, M.; Tobaldi, D.M.; Donato, N.; Pullar, R.C.; Neri, G. Pt-decorated In2O3 nanoparticles and their ability as a highly sensitive (< 10 ppb) acetone sensor for biomedical applications. Sens. Actuators B Chem. 2016, 230, 697–705. [Google Scholar] [CrossRef]
- Ghosh, A.; Bannerjee, R.; Basu Majumder, S. Selective hydrogen sensing by cobalt doped ZnO thin films: A study on carrier reversal conductivity. In Proceedings of the IEEE Sensors, Busan, South Korea, 1–4 November 2015. [Google Scholar]
- Falsafi, F.; Hashemi, B.; Mirzaei, A.; Fazio, E.; Neri, F.; Donato, N.; Leonardi, S.G.; Neri, G. Sm-doped cobalt ferrite nanoparticles: A novel sensing material for conductometric hydrogen leak sensor. Ceram. Int. 2017, 43, 1029–1037. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Optimization and gas sensing mechanism of n-SnO2-p-Co3O4 composite nanofibers. Sens. Actuators B Chem. 2017, 248, 500–511. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moosavi, F.; Bahrololoom, M.E.; Kamjou, R.; Mirzaei, A.; Leonardi, S.G.; Neri, G. Hydrogen Sensing Properties of Co-Doped ZnO Nanoparticles. Chemosensors 2018, 6, 61. https://doi.org/10.3390/chemosensors6040061
Moosavi F, Bahrololoom ME, Kamjou R, Mirzaei A, Leonardi SG, Neri G. Hydrogen Sensing Properties of Co-Doped ZnO Nanoparticles. Chemosensors. 2018; 6(4):61. https://doi.org/10.3390/chemosensors6040061
Chicago/Turabian StyleMoosavi, Fatemeh, Mohammad Ebrahim Bahrololoom, Ramin Kamjou, Ali Mirzaei, Salvatore Gianluca Leonardi, and Giovanni Neri. 2018. "Hydrogen Sensing Properties of Co-Doped ZnO Nanoparticles" Chemosensors 6, no. 4: 61. https://doi.org/10.3390/chemosensors6040061
APA StyleMoosavi, F., Bahrololoom, M. E., Kamjou, R., Mirzaei, A., Leonardi, S. G., & Neri, G. (2018). Hydrogen Sensing Properties of Co-Doped ZnO Nanoparticles. Chemosensors, 6(4), 61. https://doi.org/10.3390/chemosensors6040061






