Carbon Nanostructures as a Multi-Functional Platform for Sensing Applications
Abstract
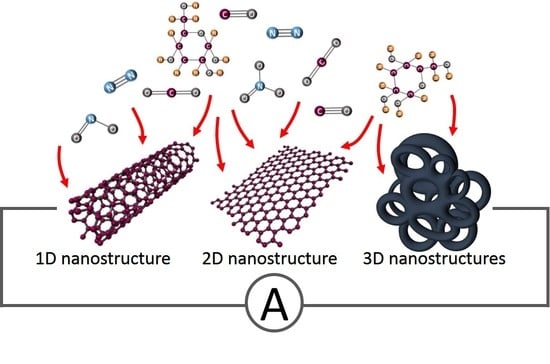
1. Introduction
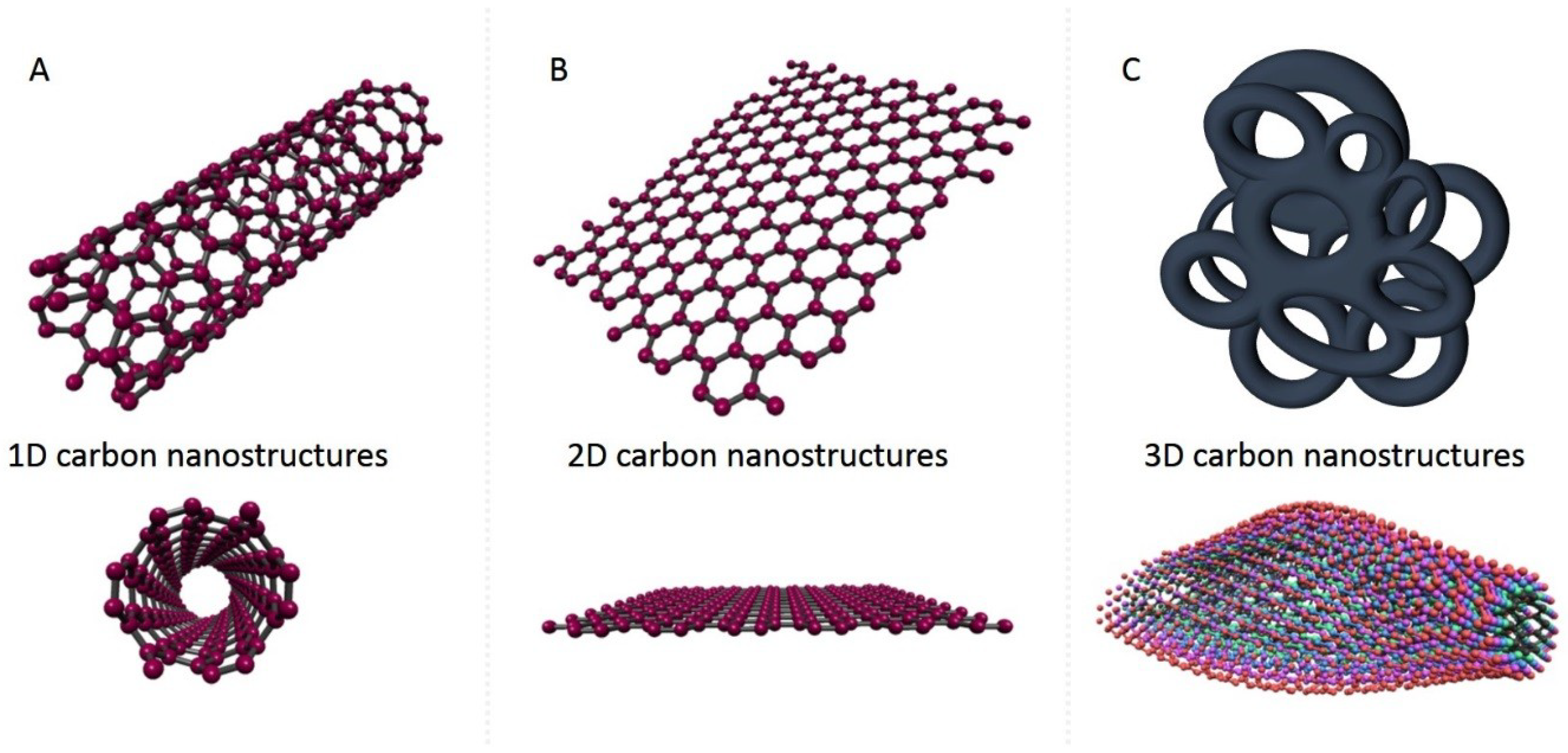
2. 1D Carbon Nanostructures
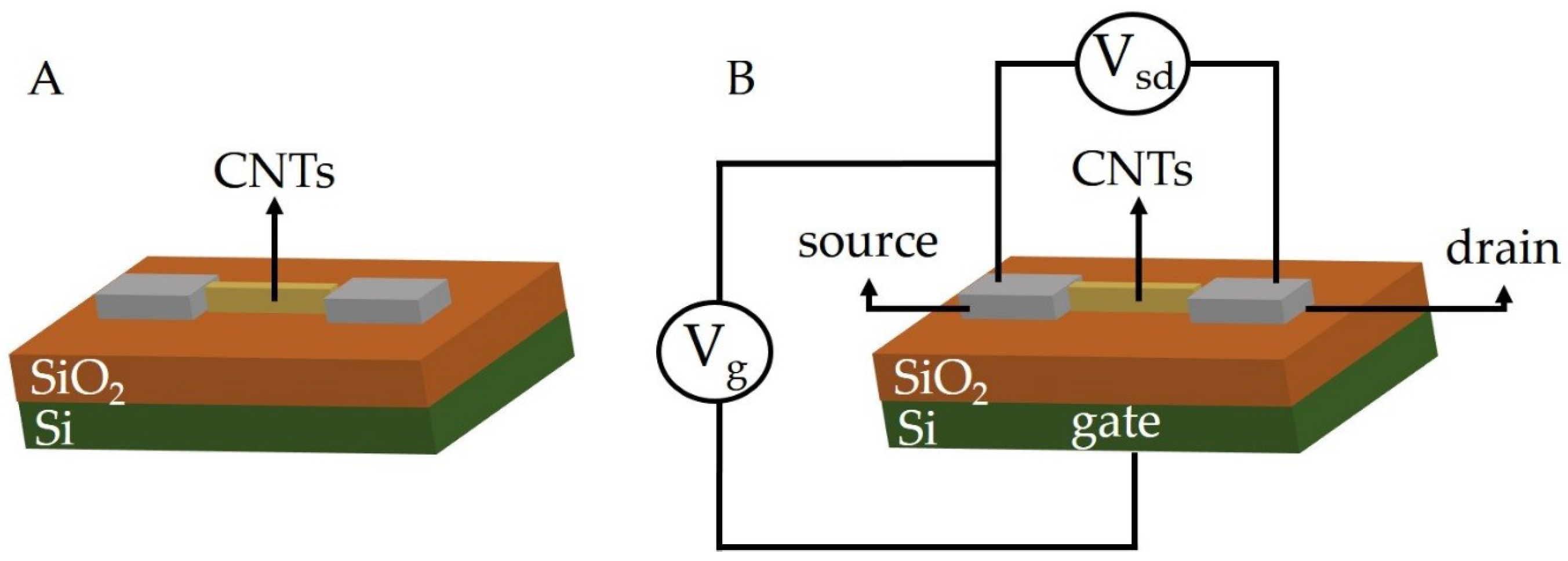
2.1. Carbon Nanotubes as Chemiresistors and Chemical Field-Effect Transistor
2.2. Carbon Nanotubes as Biosensors
3. 2D Carbon Nanostructures
3.1. 2D Nanomaterials as Chemiresistors and ChemFET
3.2. 2D Carbon Nanomaterials as Biosensors
4. 3D Carbon Nanostructures
4.1. 3D Carbon Nanostructures as Chemical and Electrochemical Sensors
4.2. 3D Carbon Nanostructures Biosensors
4.3. Other Sensors Based on 3D Carbon Nanostructures
5. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Segawa, Y.; Ito, H.; Itami, K. Structurally uniform and atomically precise carbon nanostructures. Nat. Rev. Mater. 2016, 1, 15002. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Praveen, V.K.; Das, G.; Ajayaghosh, A. Hybrid materials of 1D and 2D carbon allotropes and synthetic π-systems. NPG Asia Mater. 2018, 10, 107–126. [Google Scholar] [CrossRef]
- Nasir, S. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.G.; Bachmatiuk, A.; Büchner, B.; Cuniberti, G.; Rümmeli, M.H. Carbon nanostructures as multi-functional drug delivery platforms. J. Mater. Chem. B 2013, 1, 401–428. [Google Scholar] [CrossRef]
- Dinadayalane, T.C.; Leszczynski, J. Fundamental Structural, Electronic, and Chemical Properties of Carbon Nanostructures: Graphene, Fullerenes, Carbon Nanotubes, and Their Derivatives; Leszczynski, J., Ed.; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Eletskii, A.V. Mechanical properties of carbon nanostructures and related materials Mechanical properties of carbon nanostructures and related materials. Phys. Uspekhi 2007, 50, 225–265. [Google Scholar] [CrossRef]
- Meunier, V. Physical properties of low-dimensional sp2-based carbon nanostructures. Rev. Mod. Phys. 2016, 88, 1–50. [Google Scholar] [CrossRef]
- Lahiri, I.; Das, S.; Kang, C.; Choi, W. Application of carbon nanostructures—Energy to electronics. J. Miner. 2011, 63, 70–76. [Google Scholar] [CrossRef]
- Zanolli, Z.; Leghrib, R.; Felten, A.; Pireaux, J.; Llobet, E. Gas Sensing with Au-Decorated Carbon Nanotubes. ACS Nano 2011, 6, 4592–4599. [Google Scholar] [CrossRef]
- Rajavel, K.; Lalitha, M.; Radhakrishnan, J.K.; Senthilkumar, L.; Thangavelu, R.; Kumar, R. Multiwalled Carbon Nanotube Oxygen Sensor: Enhanced Oxygen Sensitivity at Room Temperature and Mechanism of Sensing. ACS Appl. Mater. Interfaces 2015, 7, 23857–23865. [Google Scholar] [CrossRef]
- Doroodmand, M.M.; Nasresfahani, S.; Sheikhi, M.H. Fabrication of ozone gas sensor based on FeOOH/single walled carbon nanotube-modified field effect transistor. Int. J. Environ. Anal. Chem. 2013, 93, 946–958. [Google Scholar] [CrossRef]
- Debataraja, A.; Muchtar, A.R.; Septiani, N.L.W.; Yuliarto, B.; Nugraha; Sunendar, B. High Performance Carbon Monoxide Sensor Based on Nano Composite of SnO2-Graphene. IEEE Sens. J. 2017, 17, 8297–8305. [Google Scholar] [CrossRef]
- Zhang, G.; Tai, H.; Xie, G.; Jiang, Y.; Zhou, Y. A carbon monoxide sensor based on single-walled carbon nanotubes doped with copper chloride. Sci. China Technol. Sci. 2013, 56, 2576–2580. [Google Scholar] [CrossRef]
- Mendoza, F.; Hernández, D.M.; Makarov, V.; Febus, E.; Weiner, B.R.; Morell, G. Sensors and Actuators B: Chemical Room temperature gas sensor based on tin dioxide-carbon nanotubes composite films. Sens. Actuators B Chem. 2014, 190, 227–233. [Google Scholar] [CrossRef]
- Bannov, A.G.; Jašek, O.; Manakhov, A.; Márik, M.; Nečas, D.; Zajíčková, L. High-Performance Ammonia Gas Sensors Based on Plasma Treated Carbon Nanostructures. IEEE Trans. Plasma Sci. 2017, 17, 1964–1970. [Google Scholar] [CrossRef]
- Kumar, M.K.; Ramaprabhu, S. Nanostructured Pt Functionlized Multiwalled Carbon Nanotube Based Hydrogen Sensor. J. Phys. Chem. B 2006, 110, 11291–11298. [Google Scholar] [CrossRef]
- Sayago, I.; Terrado, E.; Lafuente, E.; Horrillo, M.C.; Maser, W.K.; Benito, A.M.; Navarro, R.; Urriolabeitia, E.P.; Martinez, M.T.; Gutierrez, J. Hydrogen sensors based on carbon nanotubes thin films. Synth. Met. 2005, 148, 15–19. [Google Scholar] [CrossRef]
- Someya, T.; Small, J.; Kim, P.; Nuckolls, C.; Yardley, J.T. Alcohol Vapor Sensors Based on Single-Walled Carbon Nanotube Field Effect Transistors. Nano Lett. 2003, 3, 877–881. [Google Scholar] [CrossRef]
- Chobsilp, T.; Muangrat, W.; Issro, C.; Chaiwat, W.; Eiad-ua, A.; Suttiponparnit, K.; Wongwiriyapan, W.; Charinpanitkul, T. Sensitivity Enhancement of Benzene Sensor Using Ethyl Cellulose- Coated Surface-Functionalized Carbon Nanotubes. J. Sens. 2018, 2018, 6956973. [Google Scholar] [CrossRef]
- Ngo, Y.H.; Brothers, M.; Martin, J.A.; Grigsby, C.C.; Fullerton, K.; Naik, R.R.; Kim, S.S. Chemically Enhanced Polymer-Coated Carbon Nanotube Electronic Gas Sensor for Isopropyl Alcohol Detection. ACS Omega 2018, 3, 6230–6236. [Google Scholar] [CrossRef]
- Szkudlarek, A.; Kollbek, K.; Klejna, S.; Rydosz, A. Electronic sensitization of CuO thin films by Cr-doping for enhanced gas sensor response at low detection limit. Mater. Res. Express 2018, 5, 126406. [Google Scholar] [CrossRef]
- Rydosz, A.; Szkudlarek, A.; Ziabka, M.; Domanski, K.; Maziarz, W.; Pisarkiewicz, T. Performance of Si-Doped WO3 Thin Films for Acetone Sensing Prepared by Glancing Angle DC Magnetron Sputtering. IEEE Sens. J. 2016, 16, 1004–1012. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Q.; Zhang, Y.; Zhu, Z.; Liu, Q. Methanol Gas-Sensing Properties of SWCNT-MIP Composites. Nanoscale Res. Lett. 2016, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Cash, K.J.; Clark, H.A. Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol. Med. 2010, 16, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Rahim, A.; Rehman, Z.U.; Mir, S.; Muhammad, N.; Rehman, F.; Nawaz, M.H.; Yaqub, M.; Siddiqi, S.A.; Chaudhry, A.A. A non-enzymatic glucose sensor based on CuO-nanostructure modified carbon ceramic electrode. J. Mol. Liq. 2017, 248, 425–431. [Google Scholar] [CrossRef]
- Sainio, S.; Palomäki, T.; Tujunen, N.; Protopopova, V.; Koehne, J.; Kordas, K.; Koskinen, J.; Meyyappan, M.; Laurila, T. Integrated Carbon Nanostructures for Detection of Neurotransmitters. Mol. Neurobiol. 2015, 52, 859–866. [Google Scholar] [CrossRef]
- Pardakhty, A.; Ahmadzadeh, S.; Avazpour, S.; Kumar, V. Highly sensitive and ef fi cient voltammetric determination of ascorbic acid in food and pharmaceutical samples from aqueous solutions based on nanostructure carbon paste electrode as a sensor. J. Mol. Liq. 2016, 216, 387–391. [Google Scholar] [CrossRef]
- Tahira, A.; Nafady, A.; Baloach, Q.; Sirajuddin; Sherazi, S.T.H.; Shaikh, T.; Arain, M.; Willander, M.; Hussain, Z. Ascorbic Acid Assisted Synthesis of Cobalt Oxide Nanostructures, Their Electrochemical Sensing Application for the Sensitive Determination of Hydrazine. J. Electron. Mater. 2016, 45, 3695–3701. [Google Scholar] [CrossRef]
- Wu, W.; Min, H.; Wu, H.; Ding, Y.; Yang, S. Electrochemical Determination of Uric Acid Using a Multiwalled Carbon Nanotube Platinum–Nickel Alloy Glassy Carbon Electrode. Electrochemistry 2017, 50, 91–104. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Cai, D.; Zhang, S.; Zhang, B.; Tang, J.; Wu, Z. Talanta A hydrogen peroxide sensor based on Ag nanoparticles electrodeposited on natural nano-structure attapulgite modified glassy carbon electrode. Talanta 2011, 86, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhang, J.; Li, L.; Wang, Z.; Estrela, P. Carbon Nanostructure-Based Field-Effect Transistors for Label-Free Chemical/Biological Sensors. Sensors 2010, 10, 5133–5159. [Google Scholar] [CrossRef]
- Stetter, J.R.; Penrose, W.R.; Yao, S. Sensors, Chemical Sensors, Electrochemical Sensors, and ECS. J. Electrochem. Soc. 2003, 150, S11–S16. [Google Scholar] [CrossRef]
- Jiménez-Cadena, G.; Riu, J.; Rius, F.X. Gas sensors based on nanostructured materials. Analyst 2007, 132, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Septiani, N.L.W.; Yuliarto, B. Review—The Development of Gas Sensor Based on Carbon Nanotubes. J. Electrochem. Soc. 2016, 163, B97–B106. [Google Scholar] [CrossRef]
- Chavan, R.; Desai, U.; Mhatre, P.; Chinchole, R. A review: Carbon nanotubes. Int. J. Pharm. Sci. Rev. Res. 2012, 13, 124–134. [Google Scholar] [CrossRef]
- Baptista, F.R.; Belhout, S.A.; Giordani, S.; Quinn, S.J. Recent developments in carbon nanomaterial sensors. Chem. Soc. Rev. 2015, 44, 4433–4453. [Google Scholar] [CrossRef]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Yan, M.; Yu, J. Carbon nanostructures in biology and medicine. J. Mater. Chem. B 2017, 5, 6437–6450. [Google Scholar] [CrossRef]
- Idowu, A.; Boesl, B.; Agarwal, A. 3D graphene foam-reinforced polymer composites—A review. Carbon N. Y. 2018, 135, 52–71. [Google Scholar] [CrossRef]
- Sha, J.; Gao, C.; Lee, S.K.; Li, Y.; Zhao, N.; Tour, J.M. Preparation of three-dimensional graphene foams using powder metallurgy templates. ACS Nano 2016, 10, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhao, Y. Three-Dimensional Porous Graphene Networks and Hybrids for Lithium-Ion Batteries and Supercapacitors. Chem 2017, 2, 171–200. [Google Scholar] [CrossRef]
- Olszowska, K.; Pang, J.; Wrobel, P.S.; Zhao, L.; Ta, H.Q.; Liu, Z.; Trzebicka, B.; Bachmatiuk, A.; Rummeli, M.H. Three-dimensional nanostructured graphene: Synthesis and energy, environmental and biomedical applications. Synth. Met. 2017, 234, 53–85. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Avouris, P. Carbon Nanotubes: Synthesis, Properties, and Applications; Springer: Berlin, Germany, 2001; ISBN 3540410864. [Google Scholar]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Ajayan, P.M. Nanotubes from Carbon. Chem. Rev. 1999, 7, 1787–1800. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Z.; Yang, Z.; Chen, X.; Xu, D. Gas sensors based on deposited single-walled carbon nanotube networks for DMMP detection. Nanotechnology 2009. [Google Scholar] [CrossRef] [PubMed]
- Valentini, L.; Armentano, I.; Kenny, J.M.; Cantalini, C.; Lozzi, L.; Santucci, S. Sensors for sub-ppm NO2 gas detection based on carbon nanotube thin films. Appl. Phys. Lett. 2003, 82, 961–963. [Google Scholar] [CrossRef]
- Ueda, T.; Bhuiyan, M.M.H.; Norimatsu, H.; Katsuki, S.; Ikegami, T.; Mitsugi, F. Development of carbon nanotube-based gas sensors for NOx gas detection working at low temperature. Phys. E Low Dimens. Syst. Nanostruct. 2008, 40, 2272–2277. [Google Scholar] [CrossRef]
- Higginbotham, A.L.; Kosynkin, D.V.; Sinitskii, A.; Sun, Z.; Tour, J.M. Lower-Defect Graphene Oxide Nanoribbons from Multiwalled Carbon Nanotubes. ACS Nano 2010, 4, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Dutko, O.; Plachá, D.; Mikeska, M.; Martynková, G.S.; Wróbel, P.; Bachmatiuk, A.; Rümmeli, M.H. Comparison of Selected Oxidative Methods for Carbon Nanotubes: Structure and Functionalization Study. J. Nanosci. Nanotechnol. 2016, 16, 7822–7825. [Google Scholar] [CrossRef]
- Scheibe, B.; Borowiak-Palen, E.; Kalenczuk, R.J. Oxidation and reduction of multiwalled carbon nanotubes—Preparation and characterization. Mater. Charact. 2010, 61, 185–191. [Google Scholar] [CrossRef]
- Čech, J.; Curran, S.A.; Zhang, D.; Dewald, J.L.; Avadhanula, A.; Kandadai, M.; Roth, S. Functionalization of multi-walled carbon nanotubes: Direct proof of sidewall thiolation. Phys. Status Solidi B 2006, 3225, 3221–3225. [Google Scholar] [CrossRef]
- Lim, J.K.; Yun, W.S.; Yoon, M.; Lee, S.K.; Kim, C.H.; Kim, K.; Kim, S.K. Selective thiolation of single-walled carbon nanotubes. Synth. Met. 2003, 139, 521–527. [Google Scholar] [CrossRef]
- Curran, S.A.; Cech, J.; Zhang, D.; Dewald, J.L.; Avadhanula, A.; Kandadai, M.; Roth, S. Thiolation of carbon nanotubes and sidewall functionalization. J. Mater. Res. 2006, 21, 1012–1018. [Google Scholar] [CrossRef]
- Hines, D.; Rümmeli, M.H.; Adebimpe, D.; Akins, D.L. High-yield photolytic generation of brominated single-walled carbon nanotubes and their application for gas sensing. Chem. Commun. 2014, 50, 11568–11571. [Google Scholar] [CrossRef] [PubMed]
- Sayogo, I.; Santos, H.; Horrilo, M.; Aleixandre, M.; Fernandez, M.; Terrado, E.; Tacchini, I.; Aroz, R.; Maser, W.; Benito, A.; et al. Carbon nanotube networks as gas sensors for NO2 detection. Talanta 2008, 77, 758–764. [Google Scholar] [CrossRef]
- Guo, M.A.; Xu, M.E.; Pan, M.; Chen, Y.Q. Effect of thiol on formaldehyde sensors based on CNTs. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; Volume 1–11, pp. 3793–3795. [Google Scholar]
- Xie, H.; Sheng, C.; Chen, X.; Wang, X.; Li, Z.; Zhou, J. Multi-wall carbon nanotube gas sensors modified with amino-group to detect low concentration of formaldehyde. Sens. Actuators B Chem. 2012, 168, 34–38. [Google Scholar] [CrossRef]
- Zhang, T.; Mubeen, S.; Myung, N.V.; Deshusses, M.A. Recent progress in carbon nanotube-based gas sensors. Nanotechnology 2008, 19, 332001. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H.; Chongwu, Z.; Chapline, M.G.; Peng, S.; et al. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Shimizu, Y.; Egashira, M. Basic aspects and challenges of semiconductor gas sensors. J. MRS Bull. 1999, 24, 18–24. [Google Scholar] [CrossRef]
- Star, A.; Joshi, V.; Skarupo, S.; Thomas, D.; Gabriel, J.P.; Emery, V. Gas Sensor Array Based on Metal-Decorated Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 21014–21020. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.P.; Snow, E.S.; Houser, E.J.; Park, D.; Stepnowski, J.L.; McGill, R.A. Nerve agent detection using networks of single-walled carbon nanotubes. Appl. Phys. Lett. 2003, 83, 4026–4028. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, F.; Tu, Y.; Ren, Z. Glucose Biosensors Based on Carbon Nanotube Nanoelectrode Ensembles. Nano Lett. 2004, 4, 191–195. [Google Scholar] [CrossRef]
- Liu, X.; Shi, L.; Niu, W.; Li, H.; Xu, G. Amperometric glucose biosensor based on single-walled carbon nanohorns. Biosens. Bioelectron. 2008, 23, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.L.; Shiu, K.K. A mediator-free bienzyme amperometric biosensor based on horseradish peroxidase and glucose oxidase immobilized on carbon nanotube modified electrode. Electroanalysis 2008, 20, 2090–2095. [Google Scholar] [CrossRef]
- Zhao, K.; Zhuang, S.; Chang, Z.; Songm, H.; Dai, L.; He, P.; Fang, Y. Amperometric Glucose Biosensor Based on Platinum Nanoparticles Combined Aligned Carbon Nanotubes Electrode. Electroanalysis 2007, 19, 1069–1074. [Google Scholar] [CrossRef]
- Wang, J.; Tangkuaram, T.; Loyprasert, S.; Vazquez-Alvarez, T.; Veerasai, W.; Kanatharana, P.; Thavarungkul, P. Electrocatalytic detection of insulin at RuOx/carbon nanotube-modified carbon electrodes. Anal. Chim. Acta 2007, 581, 1–6. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Revenga-Parra, M.; Gennari, M.; Pariente, F.; Mas-Ballesté, R.; Zamora, F.; Lorenzo, E. Insulin sensor based on nanoparticle-decorated multiwalled carbon nanotubes modified electrodes. Sens. Actuators B Chem. 2016, 222, 331–338. [Google Scholar] [CrossRef]
- Swamy, B.E.K.; Venton, B.J. Carbon nanotube-modified microelectrodes for simultaneous detection of dopamine and serotonin in vivo. Analyst 2007, 132, 876–884. [Google Scholar] [CrossRef]
- Sun, Y.; Fei, J.; Hou, J.; Zhang, Q. Simultaneous determination of dopamine and serotonin using a carbon nanotubes-ionic liquid gel modified glassy carbon electrode. Microchim. Acta 2009, 165, 373–379. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon N. Y. 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of Large-Area Graphene Films for High-Performance Transparent Conductive Electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef]
- Ko, G.; Kim, H.-Y.; Ahn, J.; Park, Y.-M.; Lee, K.-Y.; Kim, J. Graphene-based nitrogen dioxide gas sensors. Curr. Appl. Phys. 2010, 10, 1002–1004. [Google Scholar] [CrossRef]
- Pearce, R.; Iakimov, T.; Andersson, M.; Hultman, L.; Spetz, A.L.; Yakimova, R. Epitaxially grown graphene based gas sensors for ultra sensitive NO2 detection. Sens. Actuators B Chem. 2011, 155, 451–455. [Google Scholar] [CrossRef]
- Yavari, F.; Castillo, E.; Gullapalli, H.; Ajayan, P.M.; Koratkar, N. High sensitivity detection of NO2 and NH3 in air using chemical vapor deposition grown graphene. Appl. Phys. Lett. 2012, 100, 203120. [Google Scholar] [CrossRef]
- Miasik, J.J.; Hooper, A.; Tofield, B.C. Conducting polymer gas sensors. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1986, 82, 1117–1126. [Google Scholar] [CrossRef]
- Choi, H.; Jeong, H.Y.; Lee, D.; Choi, C.-G.; Choi, S. Flexible NO2 gas sensor using multilayer graphene films by chemical vapor deposition. Carbon Lett. 2013, 14, 186–189. [Google Scholar] [CrossRef]
- Yoon, H.J.; Jun, D.H.; Yang, J.H.; Zhou, Z.; Yang, S.S.; Cheng, M.M.-C. Carbon dioxide gas sensor using a graphene sheet. Sens. Actuators B Chem. 2011, 157, 310–313. [Google Scholar] [CrossRef]
- Nemade, K.R.; Waghuley, S.A. Chemiresistive Gas Sensing by Few-Layered Graphene. J. Electron. Mater. 2013, 42, 2857–2866. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Jayatissa, A.H. Graphene based field effect transistor for the detection of ammonia. J. Appl. Phys. 2012, 112, 064304. [Google Scholar] [CrossRef]
- Prezioso, S.; Perrozzi, F.; Giancaterini, L.; Cantalini, C.; Treossi, E.; Palermo, V.; Nardone, M.; Santucci, S.; Ottaviano, L. Graphene Oxide as a Practical Solution to High Sensitivity Gas Sensing. J. Phys. Chem. 2013, 117, 10683–10690. [Google Scholar] [CrossRef]
- Taylor, A.P.; Velásquez-garcía, L.F. Electrospray-printed nanostructured graphene oxide gas sensors. Nanotechnology 2015, 26, 505301. [Google Scholar] [CrossRef]
- Wróbel, P.; Wlodarski, M.; Jedrzejewska, A.; Placek, K.; Szukiewicz, R.; Kotowicz, S.; Tokarska, K.; Ta, H.Q.; Mendes, R.G.; Liu, Z.; et al. A comparative study on simple and practical chemical gas sensors from chemically modified graphene films. Mater. Res. Express 2018, 6, 1. [Google Scholar] [CrossRef]
- Fowler, J.D.; Allen, Ќ.M.J.; Tung, Ќ.V.C.; Yang, Y.; Kaner, R.B.; Weiller, B.H. Practical Chemical Sensors from Chemically Derived Graphene. ACS Nano 2009, 3, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ocola, L.E.; Chen, J. Gas detection using low-temperature reduced graphene oxide sheets. Appl. Phys. Lett. 2009, 94, 083111. [Google Scholar] [CrossRef]
- Lu, G.; Park, S.; Yu, K.; Ruoff, R.S.; Ocola, L.E.; Rosenmann, D.; Chen, J. Toward practical gas sensing with highly reduced graphene oxide: A new signal processing method to circumvent run-to-run and device-to-device variations. ACS Nano 2011, 5, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Dua, V.; Surwade, S.P.; Ammu, S.; Agnihotra, S.R.; Jain, S.; Roberts, K.E.; Park, S.; Ruoff, R.S.; Manohar, S.K. All-Organic Vapor Sensor Using Inkjet-Printed Reduced Graphene Oxide. Angew. Chem. Int. Ed. 2010, 49, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, W.-J.; Lee, H.-K.; Lee, D.-S.; Shin, J.-H.; Jun, Y.; Yun, Y.J. Highly flexible, mechanically stable, and sensitive NO2 gas sensors based on reduced graphene oxide nanofibrous mesh fabric for flexible electronics. Sens. Actuators B Chem. 2018, 257, 846–852. [Google Scholar] [CrossRef]
- Lipatov, A.; Varezhnikov, A.; Wilson, P.; Sysoev, V.; Kolmakov, A.; Sinitskii, A. Highly selective gas sensor arrays based on thermally reduced graphene oxide. Nanoscale 2013, 5, 5426–5434. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Liu, A.; Huang, L.; Li, C.; Shi, G. High-Performance NO2 Sensors Based on Chemically Modified Graphene. Adv. Mater. 2013, 25, 766–771. [Google Scholar] [CrossRef]
- Zöpfl, A.; Lemberger, M.; König, M.; Ruhl, G.; Matysik, F.; Hirsch, T. Reduced graphene oxide and graphene composite materials for improved gas sensing at low temperature. Faraday Discuss. 2014, 173, 403–414. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Song, J.; Han, D.; Ivaska, A.; Niu, L. Direct Electrochemistry of Glucose Oxidase and Biosensing for Glucose Based on Graphene. Anal. Chem. 2009, 81, 2378–2382. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Kang, X.; Wang, C.; Wang, D.; Liu, J.; Aksay, I.A.; Lin, Y. Talanta Glucose biosensor based on immobilization of glucose oxidase in platinum nanoparticles/graphene/chitosan nanocomposite film. Talanta 2009, 80, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Kima, W.; Jun, J.; Lee, J.S.; Kim, J.H.; Jang, J. Highly selective FET-type glucose sensor based on shape-controlled palladium nanoflower-decorated graphene. Sens. Actuators B Chem. 2018, 264, 216–223. [Google Scholar] [CrossRef]
- Meng, L.; Jin, J.; Yang, G.; Lu, T.; Zhang, H.; Cai, C. Nonenzymatic Electrochemical Detection of Glucose Based on Palladium−Single-Walled Carbon Nanotube Hybrid Nanostructures. Anal. Chem. 2009, 81, 7271–7280. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Feng, W.; Miao, X.; Zhang, X.; Chen, S.; Chen, Y.; Wang, W.; Zhang, Y. A flexible and highly sensitive nonenzymatic glucose sensor based on DVD-laser scribed graphene substrate. Biosens. Bioelectron. 2018, 110, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Yoon, H.S.; Park, J.Y. A wearable electrochemical glucose sensor based on simple and low-cost fabrication supported micro-patterned reduced graphene oxide nanocomposite electrode on flexible substrate. Biosens. Bioelectron. 2018, 109, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Jarjes, Z.A.; Desprez, V.; Kilmartin, P.A.; Travas-Sejdic, J. Sensitive, selective, disposable electrochemical dopamine sensor based on PEDOT-modified laser scribed graphene. Biosens. Bioelectron. 2018, 107, 184–191. [Google Scholar] [CrossRef]
- Pandey, A.; Gurbuz, Y.; Ozguz, V.; Niazi, J.H.; Qureshi, A. Graphene-interfaced electrical biosensor for label-free and sensitive detection of foodborne pathogenic E. coli O157:H7. Biosens. Bioelectron. 2017, 91, 225–231. [Google Scholar] [CrossRef]
- Thakur, B.; Zhou, G.; Chang, J.; Pu, H.; Jin, B.; Sui, X.; Yuan, X.; Yang, C.-H.; Magruder, M.; Chen, J. Rapid detection of single E. coli bacteria using a graphene-based field-effect transistor device. Biosens. Bioelectron. 2018, 110, 16–22. [Google Scholar] [CrossRef]
- Singh, R.; Hong, S.; Jang, J. Label-free Detection of Influenza Viruses using a Reduced Graphene Oxide-based Electrochemical Immunosensor Integrated with a Microfluidic Platform. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2014, 1530, 1530–1534. [Google Scholar] [CrossRef]
- Georgios, D.K.; Emmanuel, T.; George, F.E.; Maroulis, G.; Simos, T.E. Pillared Graphene: A New 3-D Innovative Network Nanostructure Augments Hydrogen Storage. AIP Conf. Proc. 2009, 1148, 388–391. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Worsley, M.A.; Kucheyev, S.O.; Mason, H.E.; Merrill, M.D.; Mayer, B.P.; Lewicki, J.; Valdez, C.A.; Suss, M.E.; Stadermann, M.; Pauzauskie, P.J.; et al. Mechanically robust 3D graphene macroassembly with high surface area. Chem. Commun. 2012, 48, 8428–8430. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.Y.; Wei, D.; Anitowska, E.; Bellani, V.; Ortolani, L.; Morandi, V.; Gazzano, M.; Zanelli, A.; Borini, S.; Palermo, V. Electrochemically exfoliated graphene oxide/iron oxide composite foams for lithium storage, produced by simultaneous graphene reduction and Fe(OH)3 condensation. Carbon N. Y. 2015, 84, 254–262. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, J.Z.; Chang, S.L.Y.; Wu, Y.; Li, D. Biomimetic superelastic graphene-based cellular monoliths. Nat. Commun. 2012, 3, 1241. [Google Scholar] [CrossRef] [PubMed]
- Hydrogel, S.G. Self-Assembled Graphene Hydrogel. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pham, T.T.; Yan, A.; Shin, S.J.; Lee, J.R.I.; Bagge-Hansen, M.; Mickelson, W.; Zettl, A. Synthesis and characterization of highly crystalline graphene aerogels. ACS Nano 2014, 8, 11013–11022. [Google Scholar] [CrossRef]
- Wu, C.; Fang, L.; Huang, X.; Jiang, P. Three-dimensional highly conductive graphene-silver nanowire hybrid foams for flexible and stretchable conductors. ACS Appl. Mater. Interfaces 2014, 6, 21026–21034. [Google Scholar] [CrossRef]
- Yao, H.B.; Ge, J.; Wang, C.F.; Wang, X.; Hu, W.; Zheng, Z.J.; Ni, Y.; Yu, S.H. A flexible and highly pressure-sensitive graphene-polyurethane sponge based on fractured microstructure design. Adv. Mater. 2013, 25, 6692–6698. [Google Scholar] [CrossRef]
- Samad, Y.A.; Li, Y.; Alhassan, S.M.; Liao, K. Novel graphene foam composite with adjustable sensitivity for sensor applications. ACS Appl. Mater. Interfaces 2015, 7, 9196–9202. [Google Scholar] [CrossRef]
- Yavari, F.; Chen, Z.; Thomas, A.V.; Ren, W.; Cheng, H.M.; Koratkar, N. High sensitivity gas detection using a macroscopic three-dimensional graphene foam network. Sci. Rep. 2011, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Xie, X.; Sun, J.; Qin, G.; Tang, C.; Zhang, Z.; Ding, Z.; Yue, W. Graphene foam chemical sensor system based on principal component analysis and backpropagation neural network. Adv. Condens. Matter Phys. 2018, 2018, 2361571. [Google Scholar] [CrossRef]
- Mei, W.; Huang, J.; Zhu, L.; Ye, Z.; Mai, Y.; Tu, J. Synthesis of porous rhombus-shaped Co3O4 nanorod arrays grown directly on a nickel substrate with high electrochemical performance. J. Mater. Chem. 2012, 22, 9315–9321. [Google Scholar] [CrossRef]
- Cao, A.M.; Hu, J.S.; Liang, H.P.; Song, W.G.; Wan, L.J.; He, X.L.; Gao, X.G.; Xia, S.H. Hierarchically structured cobalt oxide (Co3O4): The morphology control and its potential in sensors. J. Phys. Chem. B 2006, 110, 15858–15863. [Google Scholar] [CrossRef] [PubMed]
- Vladimirova, S.; Krivetskiy, V.; Rumyantseva, M.; Gaskov, A.; Mordvinova, N.; Lebedev, O.; Martyshov, M.; Forsh, P. Co3O4 as p-type material for CO sensing in humid air. Sensors 2017, 17, 2216. [Google Scholar] [CrossRef]
- Patil, D.; Patil, P.; Subramanian, V.; Joy, P.A.; Potdar, H.S. Highly sensitive and fast responding CO sensor based on Co3O4 nanorods. Talanta 2010, 81, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, M.; He, S.; Chen, W. Freestanding 3D mesoporous Co3O4@carbon foam nanostructures for ethanol gas sensing. Anal. Chem. 2014, 86, 7996–8002. [Google Scholar] [CrossRef] [PubMed]
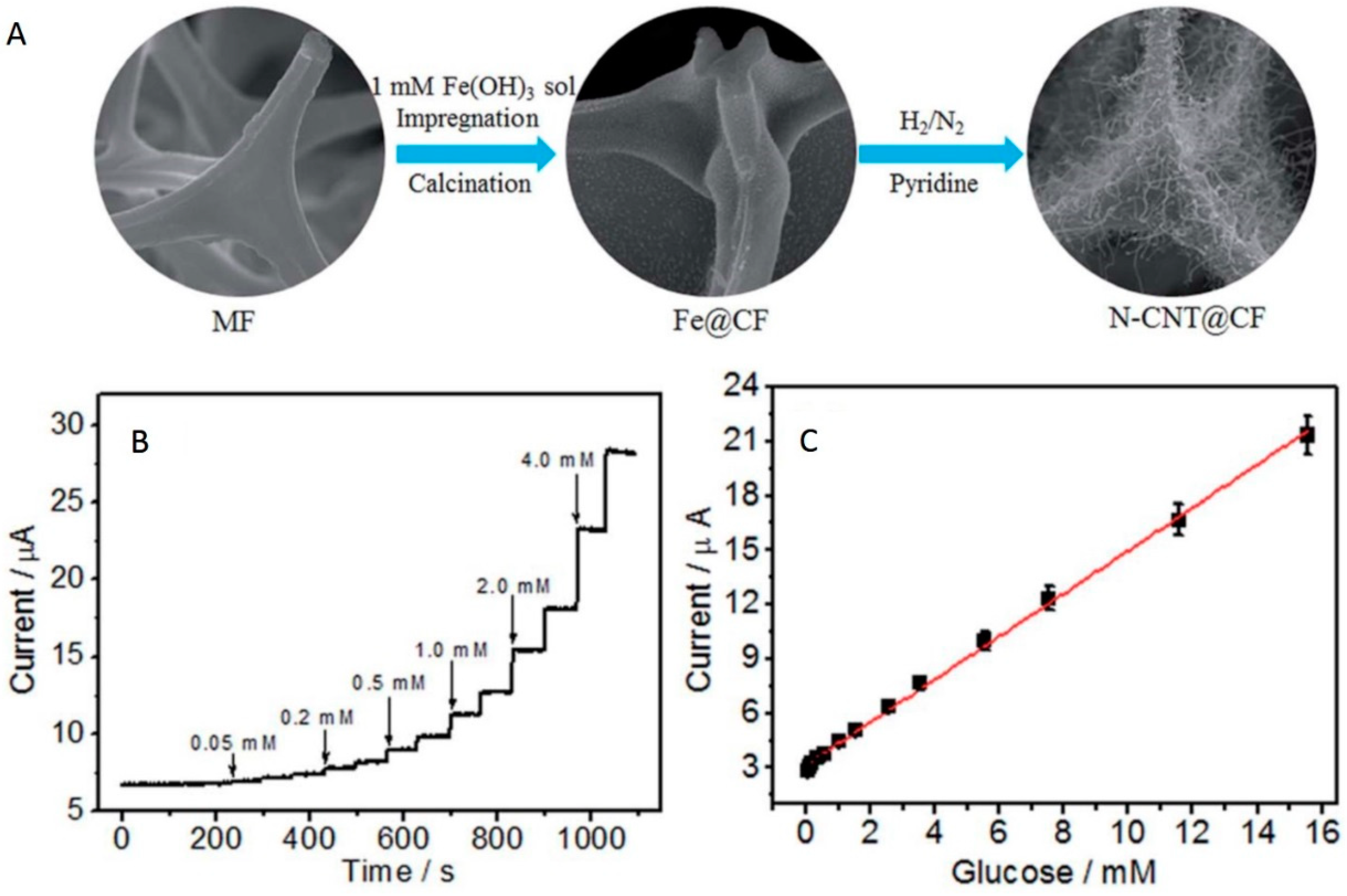
- Ma, Y.; Song, X.; Ge, X.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. In situ growth of α-Fe2O3 nanorod arrays on 3D carbon foam as an efficient binder-free electrode for highly sensitive and specific determination of nitrite. J. Mater. Chem. A 2017, 5, 4726–4736. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Chen, S.; Xu, F.; Chen, S.; Jia, J.; Tan, H.; Hou, H.; Song, Y. Electrochemical sensing and biosensing platform based on biomass-derived macroporous carbon materials. Anal. Chem. 2014, 86, 1414–1421. [Google Scholar] [CrossRef]
- Lin, D.; Wu, J.; Ju, H.; Yan, F. Nanogold/mesoporous carbon foam-mediated silver enhancement for graphene-enhanced electrochemical immunosensing of carcinoembryonic antigen. Biosens. Bioelectron. 2014, 52, 153–158. [Google Scholar] [CrossRef]
- Dong, X.C.; Xu, H.; Wang, X.W.; Huang, Y.X.; Chan-Park, M.B.; Zhang, H.; Wang, L.H.; Huang, W.; Chen, P. 3D graphene-cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Liu, L.; Guo, Q.; Wang, J.; Yang, J.; Guan, X.; Chen, S.; Hou, H. Three-dimensional N-doped carbon nanotube@carbon foam hybrid: An effective carrier of enzymes for glucose biosensors. RSC Adv. 2017, 7, 26574–26582. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Yu, J.; He, J.; Yang, H.; Ye, Y.; Song, Y. A green and simple strategy to prepare graphene foam-like three-dimensional porous carbon/Ni nanoparticles for glucose sensing. Sens. Actuators B Chem. 2017, 239, 172–179. [Google Scholar] [CrossRef]
- Si, P.; Dong, X.C.; Chen, P.; Kim, D.H. A hierarchically structured composite of Mn3O4/3D graphene foam for flexible nonenzymatic biosensors. J. Mater. Chem. B 2013, 1, 110–115. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Wang, J.; Wang, E. Mussel-inspired biopolymer modified 3D graphene foam for enzyme immobilization and high performance biosensor. Electrochim. Acta 2015, 161, 17–22. [Google Scholar] [CrossRef]
- Kung, C.C.; Lin, P.Y.; Buse, F.J.; Xue, Y.; Yu, X.; Dai, L.; Liu, C.C. Preparation and characterization of three dimensional graphene foam supported platinum-ruthenium bimetallic nanocatalysts for hydrogen peroxide based electrochemical biosensors. Biosens. Bioelectron. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Hsu, T.K.; Sun, C.L.; Nien, Y.T.; Pu, N.W.; Ger, M. Der Synthesis of CuO/graphene nanocomposites for nonenzymatic electrochemical glucose biosensor applications. Electrochim. Acta 2012, 82, 152–157. [Google Scholar] [CrossRef]
- Jindal, K.; Tomar, M.; Gupta, V. CuO thin film based uric acid biosensor with enhanced response characteristics. Biosens. Bioelectron. 2012, 38, 11–18. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, M.; Cai, B.; Wang, W.; Ye, Z.; Huang, J. 3D graphene foams decorated by CuO nanoflowers for ultrasensitive ascorbic acid detection. Biosens. Bioelectron. 2014, 59, 384–388. [Google Scholar] [CrossRef]
- Dong, X.; Wang, X.; Wang, L.; Song, H.; Zhang, H.; Huang, W.; Chen, P. 3D graphene foam as a monolithic and macroporous carbon electrode for electrochemical sensing. ACS Appl. Mater. Interfaces 2012, 4, 3129–3133. [Google Scholar] [CrossRef]
- Yue, H.Y.; Huang, S.; Chang, J.; Heo, C.; Yao, F.; Adhikari, S.; Gunes, F.; Liu, L.C.; Lee, T.H.; Oh, E.S.; et al. ZnO nanowire arrays on 3D hierachical graphene foam: Biomarker detection of parkinson’s disease. ACS Nano 2014, 8, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
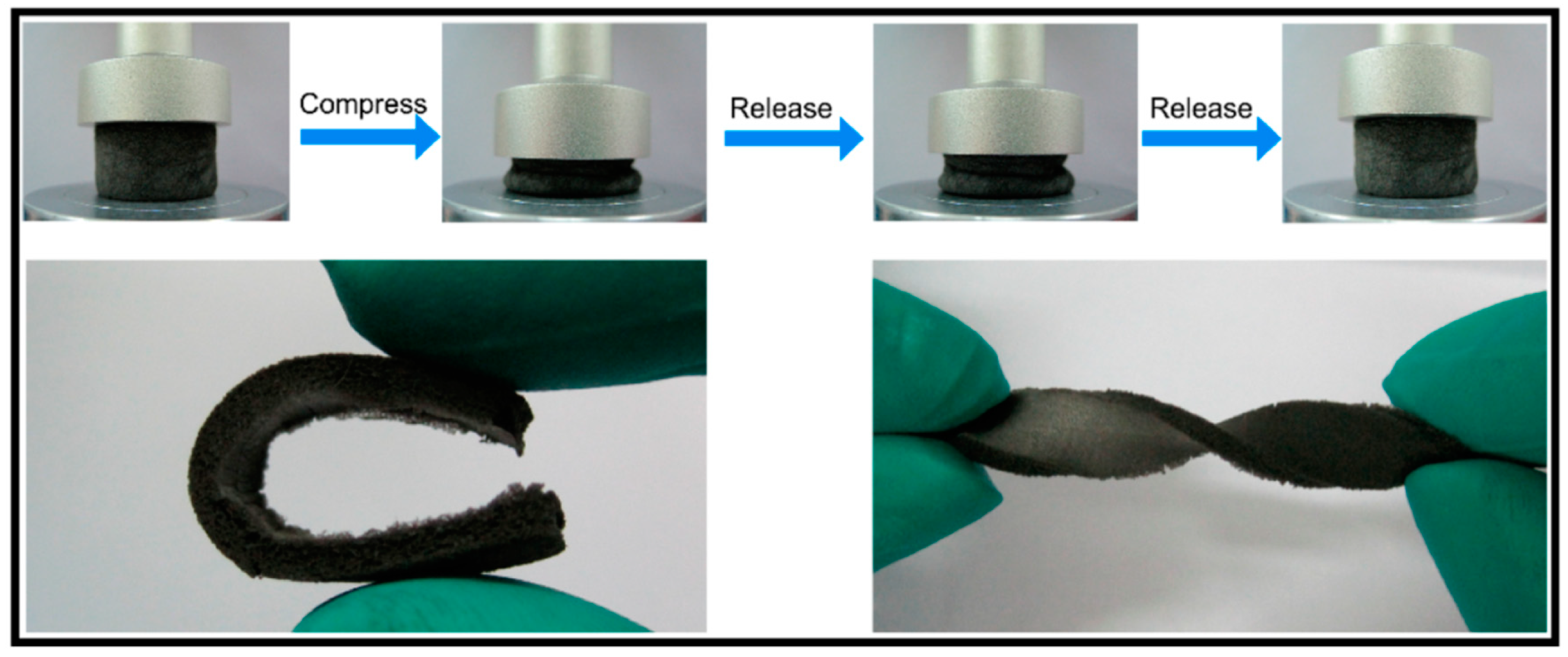
- Xu, R.; Lu, Y.; Jiang, C.; Chen, J.; Mao, P.; Gao, G.; Zhang, L.; Wu, S. Facile fabrication of three-dimensional graphene foam/poly(dimethylsiloxane) composites and their potential application as strain sensor. ACS Appl. Mater. Interfaces 2014, 6, 13455–13460. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Peng, Q.; Ding, Y.; Lin, Z.; Wang, C.; Li, Y.; Xu, F.; Li, J.; Yuan, Y.; He, X.; et al. Lightweight, Superelastic, and Mechanically Flexible Graphene/Polyimide Nanocomposite Foam for Strain Sensor Application. ACS Nano 2015, 9, 8933–8941. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, S.; Zeng, X.; Huang, W.; Gong, Z.; Zhang, G.; Sun, R.; Wong, C.P. Highly Stretchable and Sensitive Strain Sensor Based on Facilely Prepared Three-Dimensional Graphene Foam Composite. ACS Appl. Mater. Interfaces 2016, 8, 18954–18961. [Google Scholar] [CrossRef] [PubMed]
- Ananthanarayanan, A.; Wang, X.; Routh, P.; Sana, B.; Lim, S.; Kim, D.H.; Lim, K.H.; Li, J.; Chen, P. Facile synthesis of graphene quantum dots from 3D graphene and their application for Fe3+ sensing. Adv. Funct. Mater. 2014, 24, 3021–3026. [Google Scholar] [CrossRef]
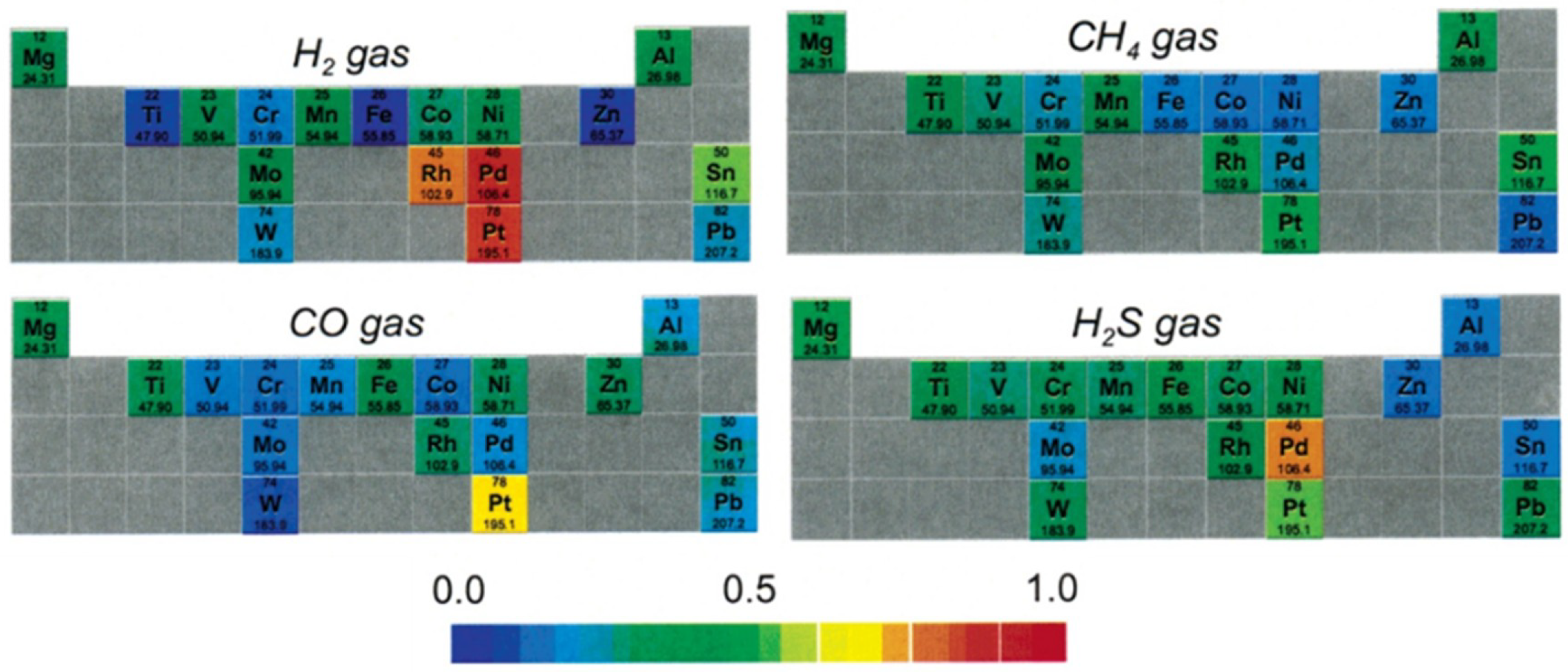
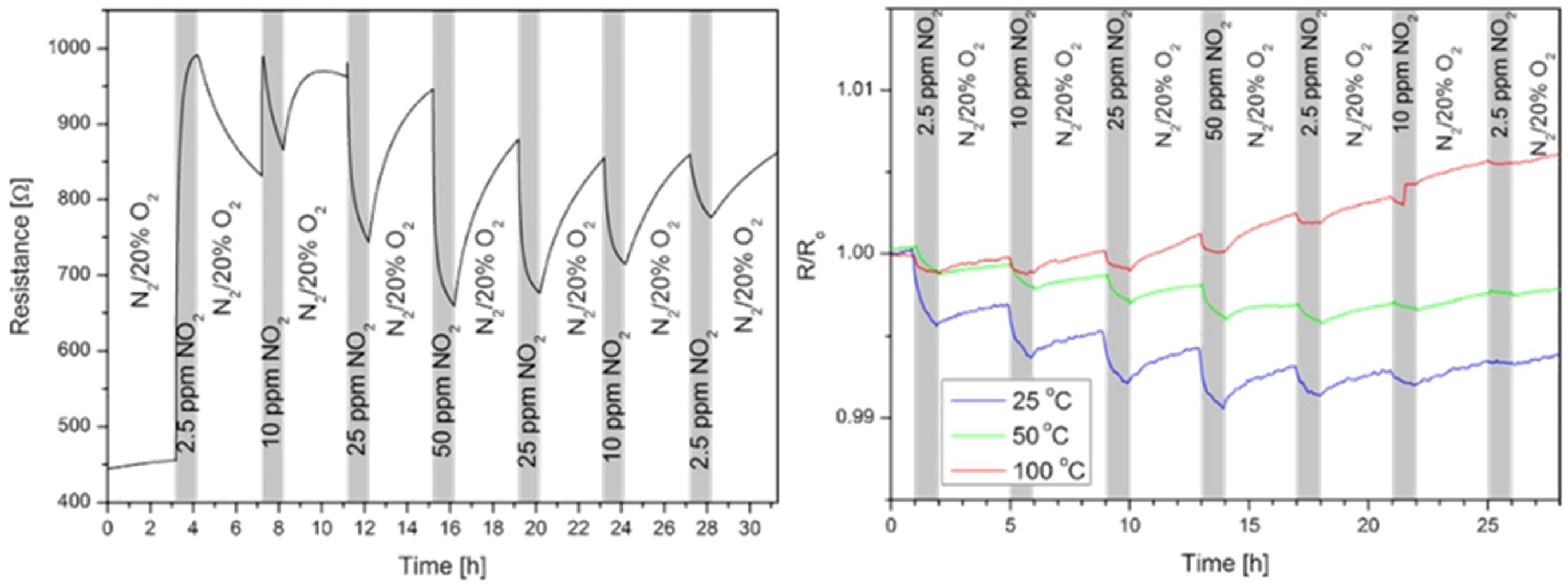
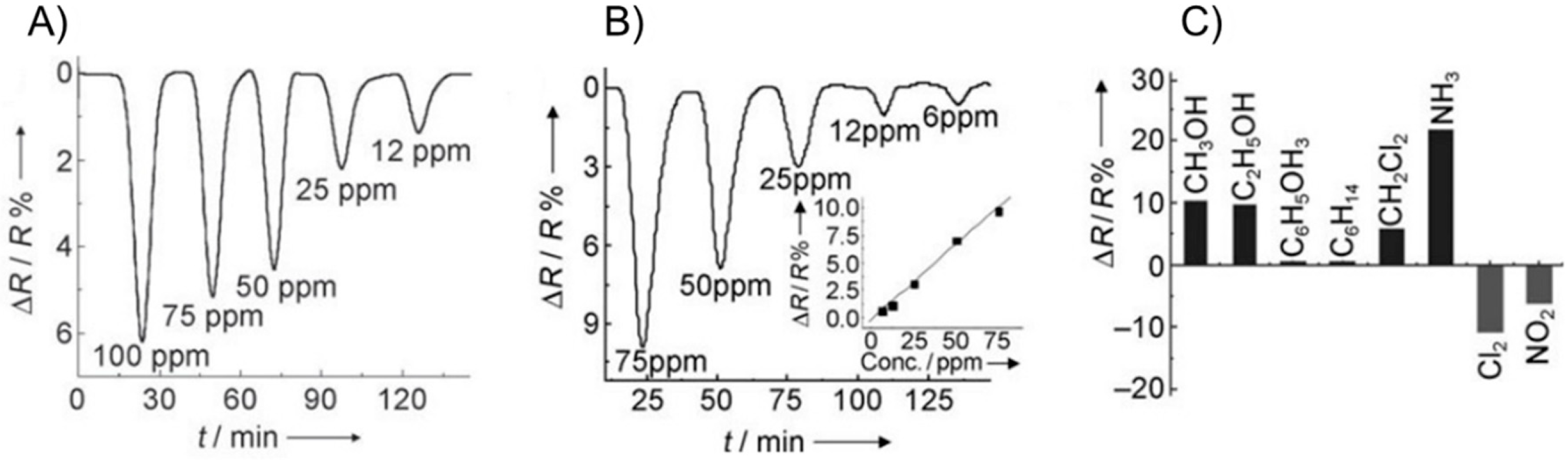
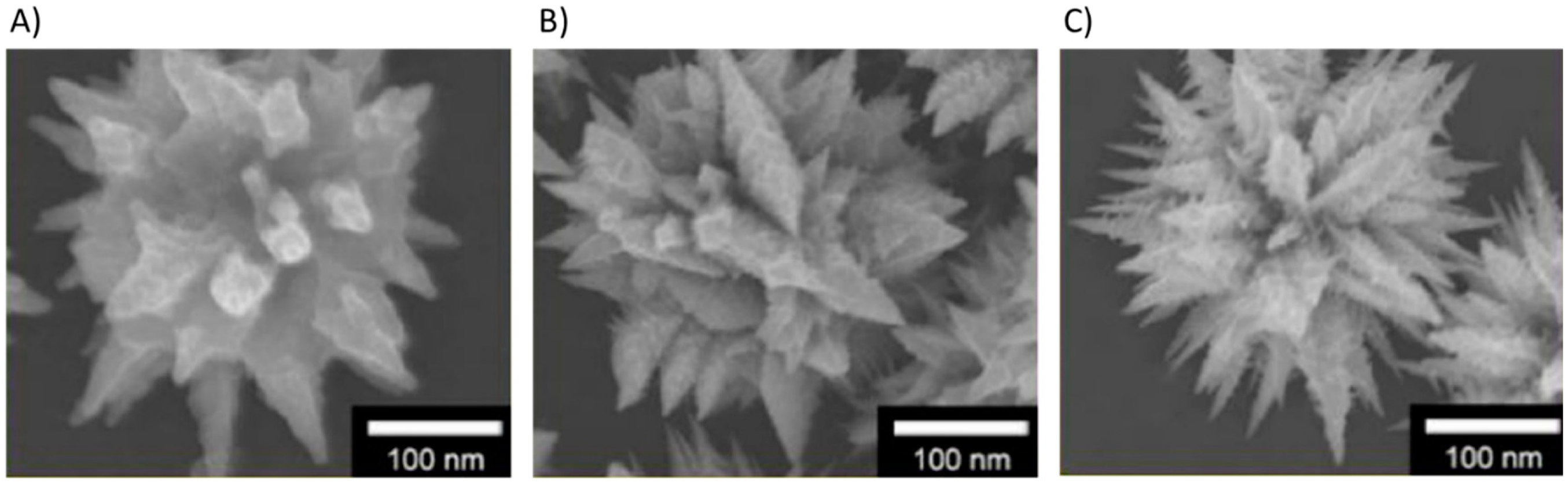
| Carbon Nanostructure | Functionalization | Analyte (Detection Limit) | References |
|---|---|---|---|
| 1D carbon nanostructure | |||
| Multi-walled CNTs | Au nanoparticles | NO2 (0.1 ppm), CO (2 ppm), C6H6 (-) | [9] |
| Multi-walled CNTs | COOH | O2 (0.3%) | [10] |
| Single-walled CNTs | FeOOH | O3 (4.1 ppb) | [11] |
| Single-walled CNTs | CuCl | CO (20 ppm) | [13] |
| Multi-walled CNTs | SnO2 | ethanol (30 ppm), methanol (30 ppm), H2S (9 ppm) | [14] |
| Multi-walled CNTs | maleic acid, acetylene | NH3 (10 ppm) | [15] |
| Multi-walled CNTs | Pt nanoparticles | H2 (4%) | [16] |
| Single-walled CNTs | Pd doping/sputtering | H2 (0.5%) | [17] |
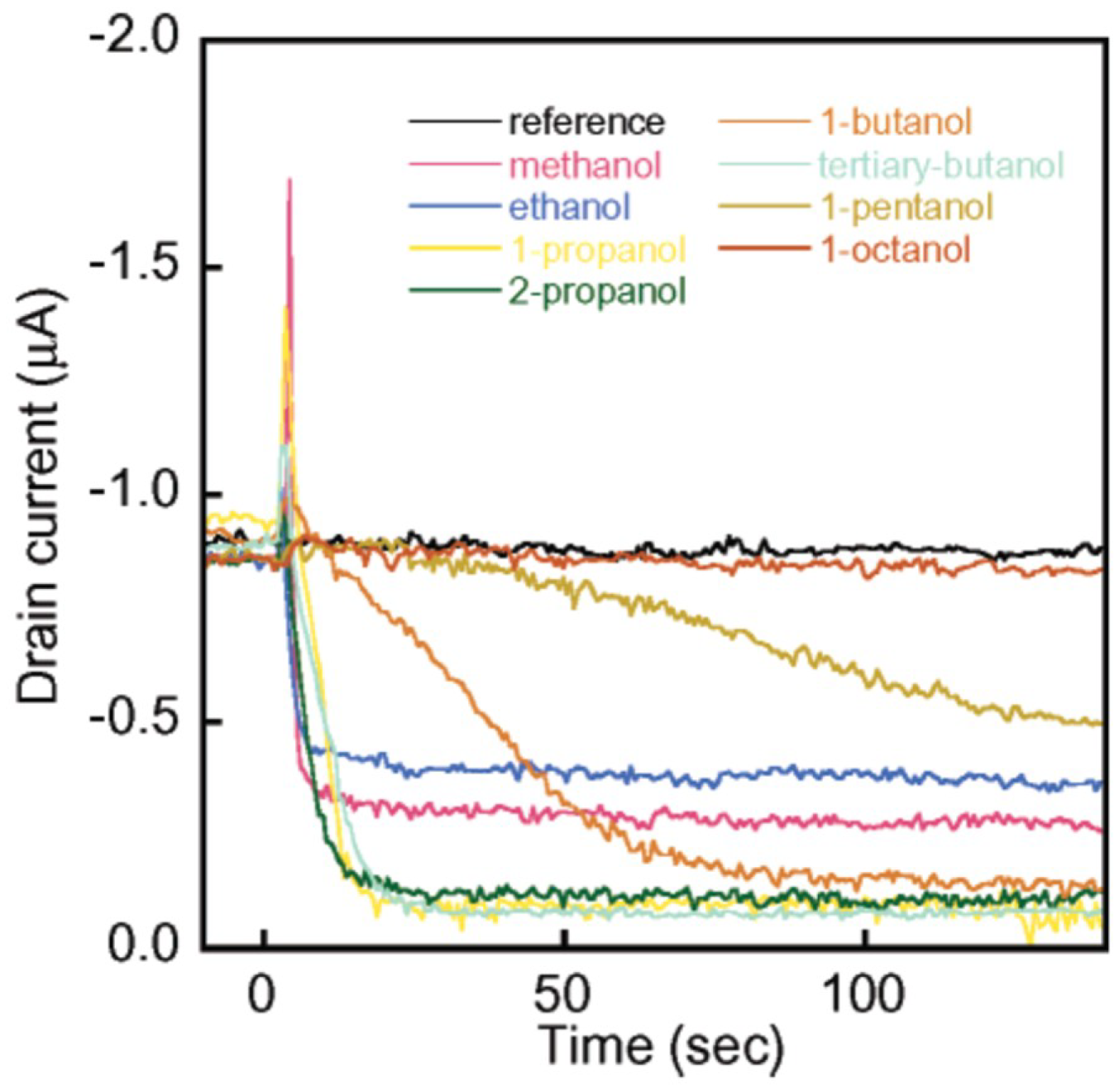
| Single-walled CNTs | - | ethanol, methanol, 1-propanol, 2-propanol, 1-butanol, tertiary-butanol, 1-pentanol, 1-octanol (2 mmHg for all analytes) | [18] |
| Multi-walled CNTs | ethyl cellulose | benzene (2.5 ppm) | [19] |
| Single-walled CNTs | poly(vinylpyrrolidone) | isopropyl (100 ppm) | [20] |
| Single-walled CNTs | LaFeO3 | methanol (1 ppm) | [23] |
| Multi-walled CNTs | - | dopamine (500 nM), glutamate (10 µM) | [26] |
| Multi-walled CNTs | Pt-Ni alloy | uric acid (0.03 µM) | [29] |
| Single-walled CNTs | - | O2 (1 × 10−10 torr) | [48] |
| Single-walled CNTs | 3-aminopropyltrimethysilane | dimethyl methylphosphonate (5 ppm) | [49] |
| Multi-walled CNTs | - | NO2 (10 ppb) | [39,40] |
| Single-walled CNTs | Br | ethanol (608 ppb), HCl (769 ppb), NH3 (1645 ppb), sulfuric acid (286 ppb) | [58] |
| Double- and Multi-walled CNTs | - | NO2 (0.1 ppm) | [59] |
| Multi-walled CNTs | thiol | formaldehyde (10 ppm) | [60] |
| Multi-walled CNTs | amine | formaldehyde (20 ppb) | [61] |
| Single-walled CNTs | Au, Pt, Pd, Rh | H2 (0.4%), CH4 (0.5%), CO (2%), H2S (50 ppm) | [65] |
| Single-walled CNTs | - | dimethyl methylphosphonate (1 ppb) | [66] |
| Multi-walled CNTs | glucose oxidase | glucose (0.08 mM) | [67] |
| Single-walled carbon nanohorns | glucose oxidase, nafion | glucose (6 µM) | [68] |
| Multi-walled CNTs | horseradish peroxidase, glucose oxidase | glucose (0.5 µM) | [69] |
| Multi-walled CNTs | Pt nanoparticles | glucose (1 × 10−5 mol/L) | [70] |
| Single-walled CNTs | Nafion | dopamine (250 nM), serotonin (130 nM) | [73] |
| Multi-walled CNTs | 1-butyl-3-methylimidazolium hexafluorophosphate | dopamine (60 nM), serotonin (8 nM) | [74] |
| Multi-walled CNTs | RuOx | insulin (1 nM) | [71] |
| Multi-walled CNTs | Ni(OH)2-Nafion | insulin (85 nM) | [72] |
| Single-walled CNTs | Pd nanoparticles | glucose (0.2 µM) | [106] |
| Single-layered graphene | SnO2 | CO (30 ppm) | [12] |
| SiO2-Graphite | CuO | glucose (0.02 mmol/L) | [25] |
| Single-layered graphene | - | NO2 (100 ppm; 2.5 ppm; 100 ppm), NH3 (500 ppb), CO2 (10 ppm) | [82,83,84,87,89,90] |
| Multi-layered graphene | - | CO2 (3 ppm), liquid petroleum gas (4 ppm) | [88] |
| Graphene oxide | COOH, OH | NO2 (20 ppb), NH3 (500 ppm), 2,4-dinitrotoluene (28 ppb) | [91,92,94,95] |
| Graphene oxide | ascorbic acid, thiol | ethanol (100 ppm), NO2 (100 ppm) | [93] |
| Reduced graphene oxide | - | NH3 (1%; 100 ppm), Cl2 (100 ppm), NO2 (100 ppm; 1 ppm), methanol (500 ppm), ethanol (500 ppm), isopropanol (500 ppm) | [96,97,98,99,100] |
| Reduced graphene oxide | sulfophenyl, ethylenediamine | NO2 (3.6 ppm) | [101] |
| Reduced graphene oxide | Octadecylamine, Pd- and Pt-doping, MnO2 and TiO2 nanoparticle | NO2 (25 ppm), CH4 (1000 ppm), H2 (500 ppm) | [102] |
| Multi-layered graphene | Poly(vinylpyrrolidone), glucose oxidase | glucose (2 mM) | [103] |
| Multi-layered graphene | glucose oxidase, Pt, chitosan | glucose (0.6 µM) | [104] |
| Multi-layered graphene | Pd nanoflower, Nafion, glucose oxidase | glucose (1 nM) | [105] |
| Graphene oxide | Cu nanoparticles | glucose (0.35 µM) | [107] |
| Reduced graphene oxide | Au-Pt alloy, chitosan-glucose oxidase | glucose (5 µM) | [108] |
| Graphene oxide | poly(3,4-ethylenedioxythiophene) | dopamine (0.33 µM) | [109] |
| Multi-layered graphene | E. coli O157:H7 specific antibodies | E. coli bacteria (10 to 100 cells/mL) | [110] |
| Reduced graphene oxide | Al2O3, Au nanoparticles | E. coli bacteria (100 to 100,000 cells/mL) | [111] |
| Reduced graphene oxide | H1N1 specific monoclonal antibodies | H1N1 influenza virus (1 to 104 virus/mL) | [112] |
| Carbon paste | NiO nanoparticles, 1-butyl-3-methylimidazolium tetrafluoroborate | ascorbic acid (0.04 µM) | [27] |
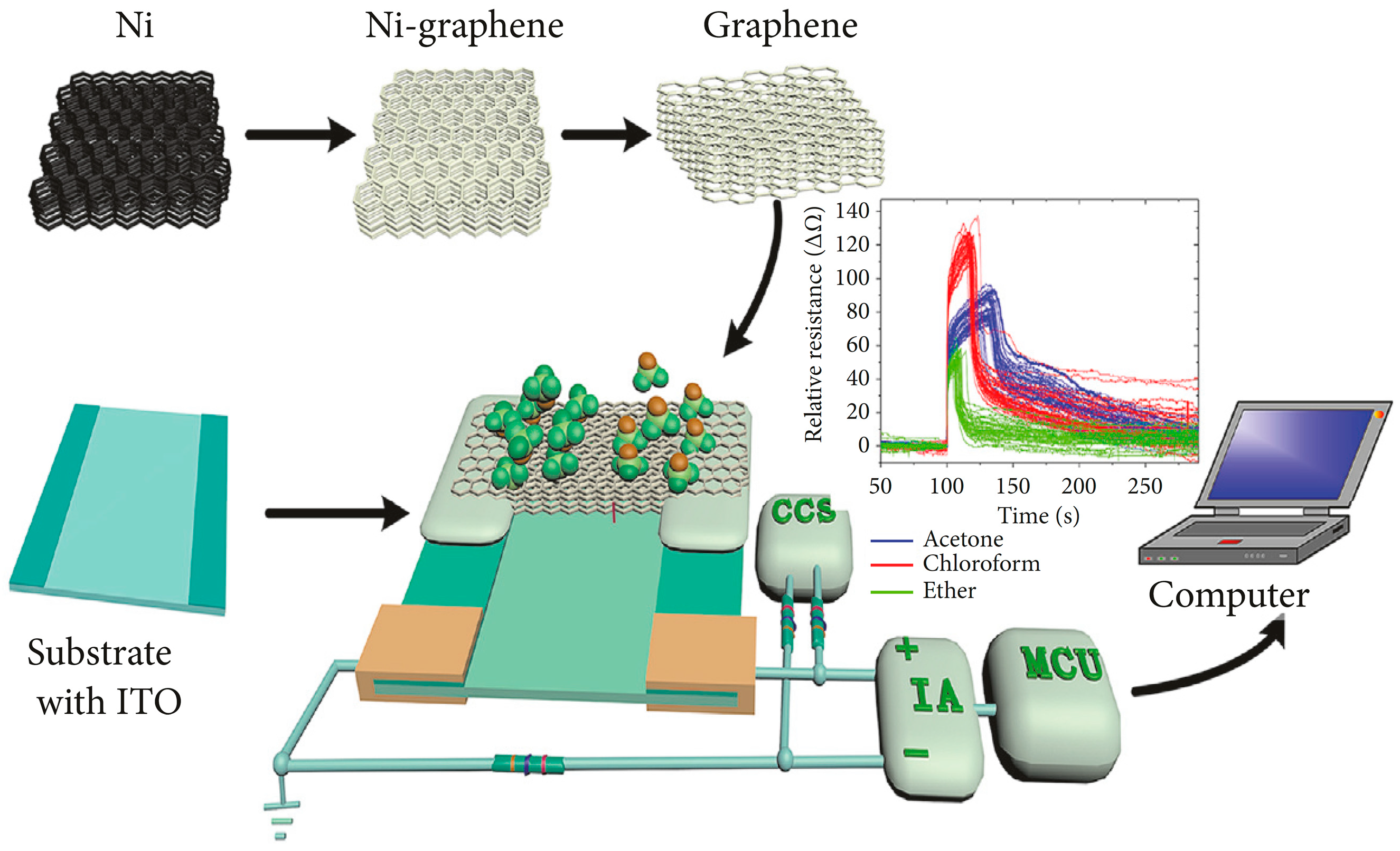
| Graphene foam | - | NH3 (20 ppm), dopamine (25 nM) and selectivity measurement for chloroform, acetone, ether | [124,125,143] |
| Graphene foam | Co3O4 | ethanol (50 ppm; 15 ppm) | [127,130] |
| Graphene foam | α-Fe2O3 | NO2 (0.12 µM) | [131] |
| Graphene foam | prussian blue nanoparticles, CuNi nanoparticles, Co nanoparticles | H2O2 (0.1 µM), glucose (2.3 µM), aminoacids (0.02mM) | [132] |
| Graphene foam | Au | carcinoembryonic antigen (0.024 pg/mL) | [133] |
| Graphene foam | Co3O4 nanowires | glucose (25 nM) | [134] |
| Graphene foam | N-doped carbon nanotubes, glucose oxidase | glucose (5 µM) | [135] |
| Graphene foam | Ni nanoparticles | glucose (4.8 µM) | [136] |
| Graphene foam | Mn3O4 | glucose (10 µM), H2O2 (1 µM) | [137] |
| Graphene foam | polydopamine, horseradish peroxidase, methylene blue | H2O2 (58 nM) | [138] |
| Graphene foam | PtRu nanoparticles | H2O2 (0.04 µM) | [139] |
| Graphene foam | CuO nanoparticles | glucose (1 µM) | [140] |
| Graphene foam | CuO nanoflower | ascorbic acid (0.43 µM) | [142] |
| Graphene foam | polydimethylsiloxane | human blood pressure (60 kPa−1) | [123] |
| Graphene foam | ZnO nanowires | uric acid (0.5 µM), dopamine (0.5 µM), ascorbic acid (5 µM) | [144] |
| Graphene foam | polydimethylsiloxane | strain (gauge factor 98.66) | [145,147] |
| Graphene foam | Polydimethylsiloxane, polyimide | strain (low pressure: 0.18 kPa−1; large pressure: 0.023 kPa−1) | [146] |
| Graphene foam-graphene quantum dots | - | Fe3+ (7.22 µM) | [148] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, R.G.; Wróbel, P.S.; Bachmatiuk, A.; Sun, J.; Gemming, T.; Liu, Z.; Rümmeli, M.H. Carbon Nanostructures as a Multi-Functional Platform for Sensing Applications. Chemosensors 2018, 6, 60. https://doi.org/10.3390/chemosensors6040060
Mendes RG, Wróbel PS, Bachmatiuk A, Sun J, Gemming T, Liu Z, Rümmeli MH. Carbon Nanostructures as a Multi-Functional Platform for Sensing Applications. Chemosensors. 2018; 6(4):60. https://doi.org/10.3390/chemosensors6040060
Chicago/Turabian StyleMendes, Rafael Gregorio, Paweł S. Wróbel, Alicja Bachmatiuk, Jingyu Sun, Thomas Gemming, Zhongfan Liu, and Mark Hermann Rümmeli. 2018. "Carbon Nanostructures as a Multi-Functional Platform for Sensing Applications" Chemosensors 6, no. 4: 60. https://doi.org/10.3390/chemosensors6040060
APA StyleMendes, R. G., Wróbel, P. S., Bachmatiuk, A., Sun, J., Gemming, T., Liu, Z., & Rümmeli, M. H. (2018). Carbon Nanostructures as a Multi-Functional Platform for Sensing Applications. Chemosensors, 6(4), 60. https://doi.org/10.3390/chemosensors6040060