1. Introduction
Residual oxygen (O
2) is an important parameter reflecting the quality and safety of packaged foods, and also a shelf-life limiting factor [
1]. Elevated O
2 levels in packs, due to inadequate packaging material or process, improper storage, handling, accidental damage of packaged products, can lead to premature deterioration and spoilage of the food by oxidation, chemical degradation, microbial growth or their products [
2,
3,
4]. Oxidation also changes the sensory properties (taste, texture and aroma) of certain foods and decreases their nutritional value (e.g., potato snacks). All this negatively impacts the perceptions of both the consumers and producers of such products [
5].
To increase the quality and prolong shelf-life of food products, active packaging is used. The main concepts of active packaging include freezing, chilling, acidification, vacuum and modified atmosphere packaging (MAP), and the use of scavengers, moisture absorbents, antimicrobial and antioxidant releasing components [
1]. MAP is one of the most widely used concepts, which involves the tailoring of gas composition inside packages to optimal levels, which effectively maintain the quality and appearance of the food product, inhibit microbial growth and prevent contamination [
6]. The majority of MAP products are packaged under low O
2 atmosphere (<0.5%), except for fresh red meats for which high O
2 levels (>40%) help ensure good appearance and red colour [
7,
8].
Due to the above reasons, control or residual O
2 in packaged foods is of major importance, and ideally has to be implemented for every pack and throughout the whole production and distribution chain. Traditionally, quality control of residual O
2 in MAP foods was conducted by sampling and destructive methods. Thus, headspace analysis with Dansensor
TM analyser entails the removal of packages at random from the product line, puncturing the package through a septum with a thin needle, withdrawing a small volume of headspace gas and measuring its O
2 and CO
2 content with O
2 and CO
2 sensors located inside the instrument [
9]. Such methods are essentially destructive (although measurements can be repeated several times), create wastage, and provide only snapshot readings at a time of analysis in the selected packs. This approach is inefficient to detect all below quality packages with incorrect gas levels, which have been shown to be frequent [
2], and it has relatively low sample throughput. The performance of MAP apparatus can also be controlled with an on-line gas analyser to ensure the correct levels of gas are being fed into the packages, however this strategy does not account for all faults in packaging such as poor sealing or damage to packaging during processing or transport allowing ingress of oxygen.
Phosphorescence based O
2 sensor systems have the ability to implement efficient non-destructive monitoring of residual O
2 in packaged food products (and ultimately in every pack), and they can perform a number of different analytical tasks at different stages of package lifespan [
1]. Sensor active element consists of an O
2-sensitive dye embedded in a polymeric matrix, so that its emission intensity and lifetime are quenched by O
2. Such sensors provide reversible and quantitative optical response to O
2 concentration (partial pressure) in the surrounding medium, gaseous or liquid [
10,
11]. Solid-state O
2 sensors are usually fabricated using solution based processes such as drying from a cocktail in an organic solvent [
1]. Conventional sensor materials and fabrication methods include the casting of polymeric cocktails on planar support (polyester film), polymerization or curing of liquid precursors (e.g., silicones and sol-gels [
12]), dye incorporation into host matrices by adsorption [
13], covalent attachment [
14], etc. [
15,
16]. Although efficient in some applications, existing O
2 sensor systems are not very suitable for large-scale applications such as food packaging due to their complex composition and fabrication processes, which make them rather expensive, variable and inflexible. Very few sensor types are characterised to a sufficient degree with respect to their stability in contact with food products and safety for food producers and consumers.
To be usable and viable in large scale applications such as food packaging, O
2 sensors should be simple, scalable, reproducible, stable and cost less than 1 cent each [
17]. We have recently reported that non-woven polyolefin fabric materials (polypropylene (PP) and polyethylene (PE) based), as well as poly(phenylene sulphide) films (PPS) can be used as simple polymer host and support materials, which allow fabrication of simple and robust O
2 sensors by solvent crazing [
18,
19] and polymer swelling [
20] methods. These food-compatible polymer materials possess many desirable qualities, such as gas permeability, process ability, simple composition and uniformity, storage stability and low cost. However, they have not been characterised or assessed in sufficient detail for food packaging applications.
In this study, we conducted a comparative evaluation of several types of such O2 sensor materials, particularly analysing migration of their components and stability of optical characteristics and O2 calibration upon prolonged exposure to various food simulants, as well as in MAP meat and cheese products.
2. Materials and Methods
Pt (II)-benzoporphyrin dye (PtBP) was from Luxcel Biosciences (Cork, Ireland). Analytical grade tetrahydrofuran (THF), ethyl acetate (EtAc), 2-butanone, 96% ethanol, acetic acid, L-lactic acid, NaCl, NaHCO3 and sucrose were from Sigma-Aldrich (Dublin, Ireland).
2.1. O2 Sensor Materials
Five different types of O
2-sensitive materials were investigated, as well as one commercial sensor (Optech
® Platinum sensor stickers, Mocon, MN, USA) which was used as a reference. Their names (Types 1–5), material composition, and fabrication method are described in
Table 1, while detailed fabrication procedures can be found in the references provided. All these sensors were produced in small batches as strips or sheets, then cut into individual sensors (typically 5–10 mm in diameter), and then stored at room temperature and used as required.
2.2. Sensor Exposure to Food Simulants
Sensors of approximately 6–9 mm in diameter were cut out from the sheets and placed individually in 2 mL glass HPLC vials. Two millilitres of simulant solution (SS) were added to each vial: 95% EtOH (SS1, positive control), 10% EtOH (SS2), 5% Acetic Acid (SS3), 3% Lactic Acid (SS4), 3% NaHCO3 (SS5), H2O (SS6), 3% NaCl (SS7) and 20% Sucrose (SS8). The vials were sealed, incubated, shaken at 40 °C over a 21-day period and assayed periodically.
The leakage of PtBP dye from the sensors was analysed on an 1100 Series HPLC system (Agilent, Santa Clara, CA, USA) consisting of a quaternary pump, diode array photometric detector, an auto-sampler and an Eclipse XDB-C18 reverse phase column (150 × 4.6 mm, 5 µm, Agilent, Santa Clara, CA, USA). Ten microlitres of the simulant sample were injected into a Mobile phase A (H2O, 0.1% TFA) and eluted with an ascending gradient of THF (0–70% over 22 min). A calibration curve was generated with PtBP standards in THF (quantified on an 8453 UV-Vis spectrophotometer, Agilent, Santa Clara, CA, USA).
2.3. Sensor Exposure to Fresh Meat and Cheese Samples
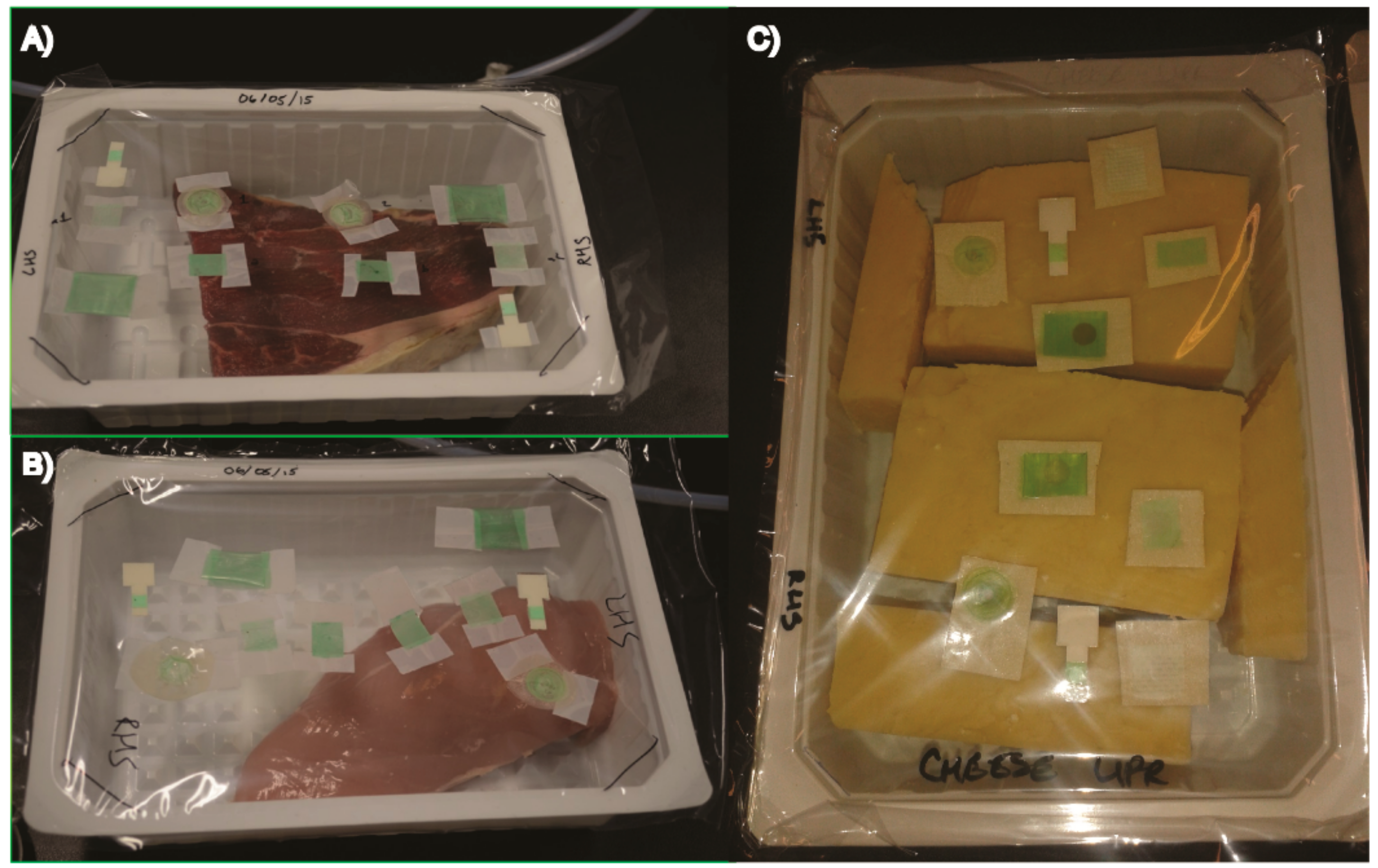
Pieces of fresh beef steak and chicken breast obtained from commercial source (Lidl, Cork, Ireland) were placed in standard MAP trays (EVOH/PE, O2 permeability 8–12 cm3 m−2 day−1 at STP). The O2 sensors were adhered in duplicate with a piece of Scotch tape to the inner side of a packaging laminate consisting of a 20 µm oriented polypropylene (OPP) and a 50 µm co-extruded PE/EVOH/PE (O2 permeability 3 cm3 m−2 day−1 at STP, Cryovac/WR Grace, Kriens, Switzerland). The laminate film with sensors was then used to heat-seal the trays with or without meat samples on a VS 100 BS packaging system (Gustav Muller and Co., Bad Homburg, Germany). MAP was performed under 55% O2 for beef samples and under 0% O2 for chicken samples.
The trays with packaged meat and sensors were stored at 4 °C in both upright (sensor exposed to headspace gas) and inverted (sensor in direct contact with meat and meat juice) position. The sensors were measured with OptechTM reader (Mocon, MN, USA) every second day over six days (a typical shelf-life for these products). At the end of the study, packs were opened, sensors removed, washed thoroughly with water and then analysed for changes in their O2 calibration. For the upright samples, sensors of several different types were inserted in duplicates in headspace area of each pack. For the inverted samples, two sensors were inserted in each pack, which was then flipped over and handled such that the sensors remained in constant contact with meat sample.
Confirmatory analysis of headspace O2 and CO2 levels in the packs with meat and sensors and also in control empty packs was performed at the beginning and end of this study. A special septum was applied on the sealing laminate and then pierced with a needle probe of the DansensorTM Checkmate 3 headspace analyser (Mocon, MN, USA), which extracted and analysed headspace gas for O2 and CO2 levels. Readings can be taken three times before package failure; the sample volume taken is 6 mL; and the accuracy is ±0.01% O2 and ±0.08% CO2.
Similarly, trays with cheese samples were prepared and tested. Cheddar cheese (Lidl, Cork, Ireland) was sliced and divided between two MAP containers with an additional MAP container left empty as control. The sensors were adhered to the sealing film with tape and sealed in an atmosphere of 32% CO2 and 68% N2. Blank containers were also included for testing the MAP process efficiency. The containers were then stored at 3 °C in both upright and inverted orientation and tested intermittently over a 32-day shelf life span.
2.4. Changes in Sensor Characteristics after Exposure to Simulants and MAP Meat
Changes in phosphorescence intensity and lifetime signals after sensor exposure to food simulants were assessed by placing the sensors in a clear 20 mL polystyrene vial (Sarstedt, Drinagh, Ireland) with controlled temperature and gas composition (0 kPa or 21 kPa O2), and measuring each sensors five times in different positions with an OptechTM handheld reader (Mocon) or with an optical fibre reader FirestingTM (PyroScience, Aachen, Germany). Average values and standard deviations were calculated and compared to those of the control sensors.
For the sensors exposed to meat in MAP trays, full O2 calibrations in dry and humid gases were also examined and compared with untreated sensors. The sensor was placed in a flow-through cell which had a window for optical contact with the OptechTM instrument. The flow cell was submerged in a Refrigerated Circulator, Model 911 (Polysciences, Hirschberg, Germany) equilibrated at 20 °C and flushed sequentially with different O2/N2 gas mixtures produced by a precision gas mixer LN Industries SA (Champagne, Switzerland). At each O2 concentration (0–100 kPa range), phosphorescence lifetime signals were measured, mean values calculated and used to plot O2 calibrations and assess the changes induced by contact with food.
3. Results and Discussion
Phosphorescent O2 sensors are designed to record small deviations in O2 concentration within packages in a contactless non-destructive manner. However, potential interactions with food products and variability between individual sensors (usually batch or factory calibrated) can compromise system performance, stability of measurements and also raise safety concerns of the consumer, if significant leaching of sensor components and food contamination are likely to occur. Type 1–5 O2 sensors analysed in this study had simple chemical composition and contained only the carrier polymer and the reporter dye, PtBP. However, the dye was embedded in the polymer matrix by physical means rather than chemical (covalently bound), therefore there is a possibility for the dye to leak from the polymer upon contact with food.
3.1. Effects of Sensor Exposure to Food Simulants
The initial testing of the different sensor types was carried out with a standard set of food simulants, which mimic a variety of food substances (see experimental), as well as 95% ethanol as positive control [
23]. Sensors were submerged in the simulants and incubated for 21 days at 40 °C while shaking. Periodic sampling and HPLC analysis of supernatants occurred every seven days. For all the simulants, no measurable dye leeching was observed, except in the Type 1 sensor which showed some leaching (1.47 μg/mL) in 10% EtOH. We attributed this to the residual aggregated dye left on the surface after the impregnation and aqueous washing.
After the exposure to the food simulants, changes in sensor O2 calibration were analysed by measuring their phosphorescence lifetime signals at 0 and 21 kPa O2. It is worth noting that changes in these lifetimes signify a change in the quenching constant and/or unquenched lifetime, which could be attributed to polymer swelling and partial dissolution and redistribution of the dye molecules within host matrix. Some variation of lifetime readings for the different samples could be due to variation of their temperature, which was not tightly controlled or compensated for.
As we expected from the low rates of dye leaching, changes were relatively minor or none. Type 2 sensor showed best stability with practically no notable drift in the lifetime signal at 21 kPa and 0 kPa O
2 in all the simulants. The Type 1 sensor showed a minor rise in lifetime signal (<0.5 μs) in some simulants at 21 kPa, but not at 0 kPa. Type 3 and 4 sensors show similar minor lifetime changes both in air and nitrogen, which we can attribute to washing out of residual surfactant retained in these sensors after their fabrication [
21]. Type 5 sensors also showed no dye leaching in any of the simulants indicating that the dye is firmly embedded in PPS. However, Type 5 sensors showed higher heterogeneity and variability of lifetime readings, both within one sensor and between different sensors.
At the same time, in the positive control (95% EtOH) was observed significant dye leaching for all the sensors, except for the Type 5 (
Table 2). After seven days immersed in the 95% EtOH, the Type 1, 3 and 4 sensors became unusable: their phosphorescence lifetime could not be measured due to low intensity signals. Based on the results of these tests, the Type 1, 2 and 5 sensors were chosen for further studies involving exposure to meat products.
3.2. Effects of Sensor Contact with MAP Meats
The selected sensor types were attached in duplicates to the inner side of the sealing laminate film using Scotch tape, and then the packages with food products were flushed and sealed under suitable MA. Optech
TM-Platinum stickers were also included in each pack. Photographs of the trays with sensors and food samples are shown in
Figure 1. Once sealed, the meat packages were transferred to a cold room and one of each was stored upright and inverted at 4 °C.
Measurements of residual O2 and CO2 content in headspace gas taken by DansensorTM Checkmate 3 instrument, revealed that O2 and CO2 levels in meat and chicken packs remained stable and in the required range, reducing slightly over the seven-day storage period.
Upon sealing and storing the packages with MAP food samples, phosphorescence lifetime signals from the sensors were measured using the Optech
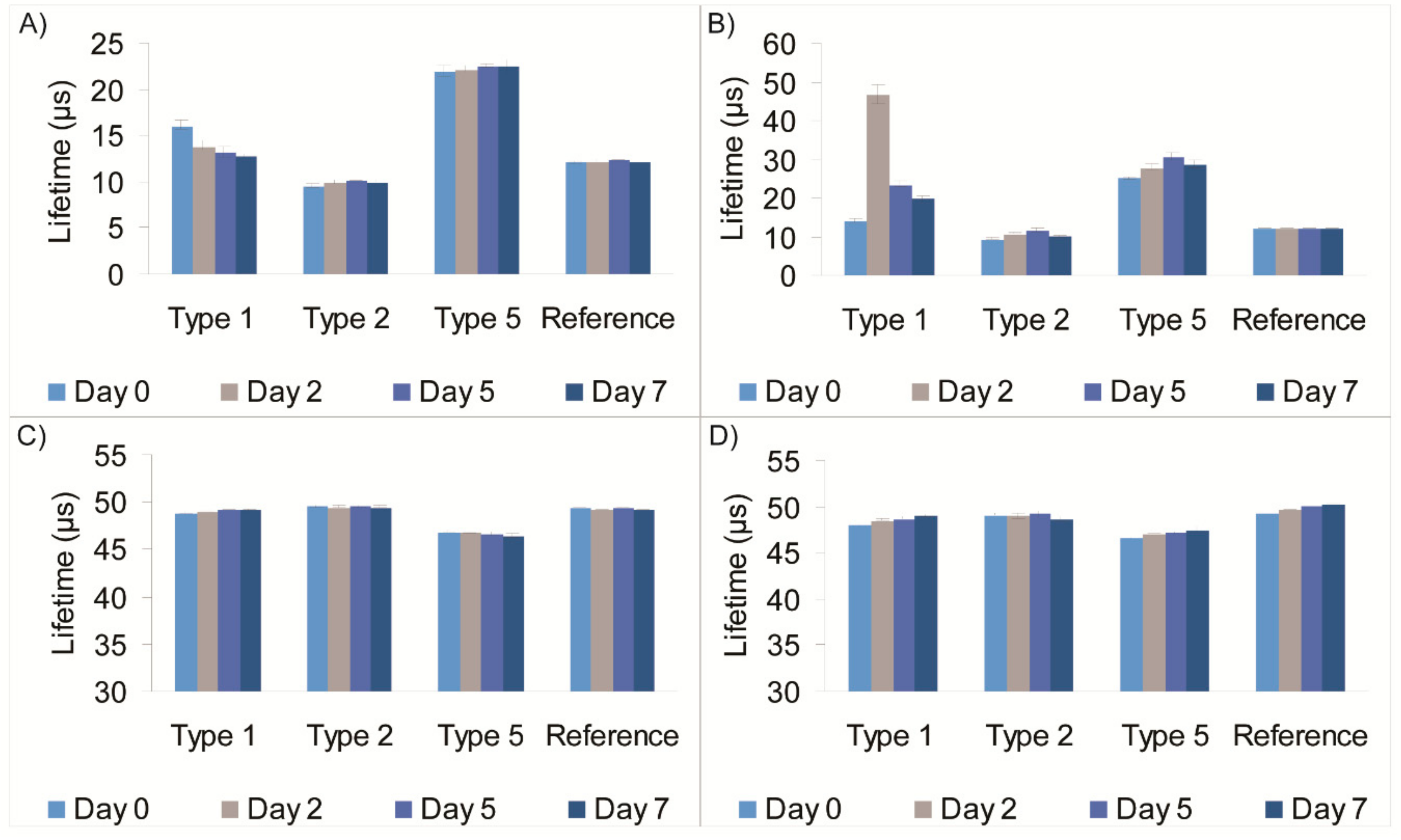
TM handheld instrument—on Days 0, 2, 5 and 7. Results of these measurements for the worst-performing of the four sensor types are shown in
Figure 2.
The screening of the meat packages showed the Type 2 sensor having the least variation in lifetime signals (<0.3 µs) over the seven-day period in the chicken packages and the upright beef package. A higher variation was observed in the inverted beef package, which we attribute to the blood juices from the product soaking the sensor after Day 0. Likewise, the Type 1 sensor showed small variations in lifetime signal in chicken packages, however noticeable lifetime variations were observed in the beef samples: a variation of 1.5 µs in the upright and 5.1 µs in the inverted Type 1 sample. The smaller variation can be attributed to moisture condensation around the sensor and/or sensor soaking in fatty meat juice (plasticising effect), while the larger variation could be due to growth of aerobic bacteria at the meat/sensor interface. The Type 5 sensor also performed well, with a variation of 2.5 µs found in the inverted beef package.
It is worth noting that the sensor lifetimes in beef and chicken packs correspond to the different O
2 levels in packs (
Table 3). Therefore, changes in sensor signals that occur in the different products are not so easy to compare. To better assess these effects, we carried out full O
2 calibrations of used and control sensors under standard conditions: dry gas, 0–100 kPa O
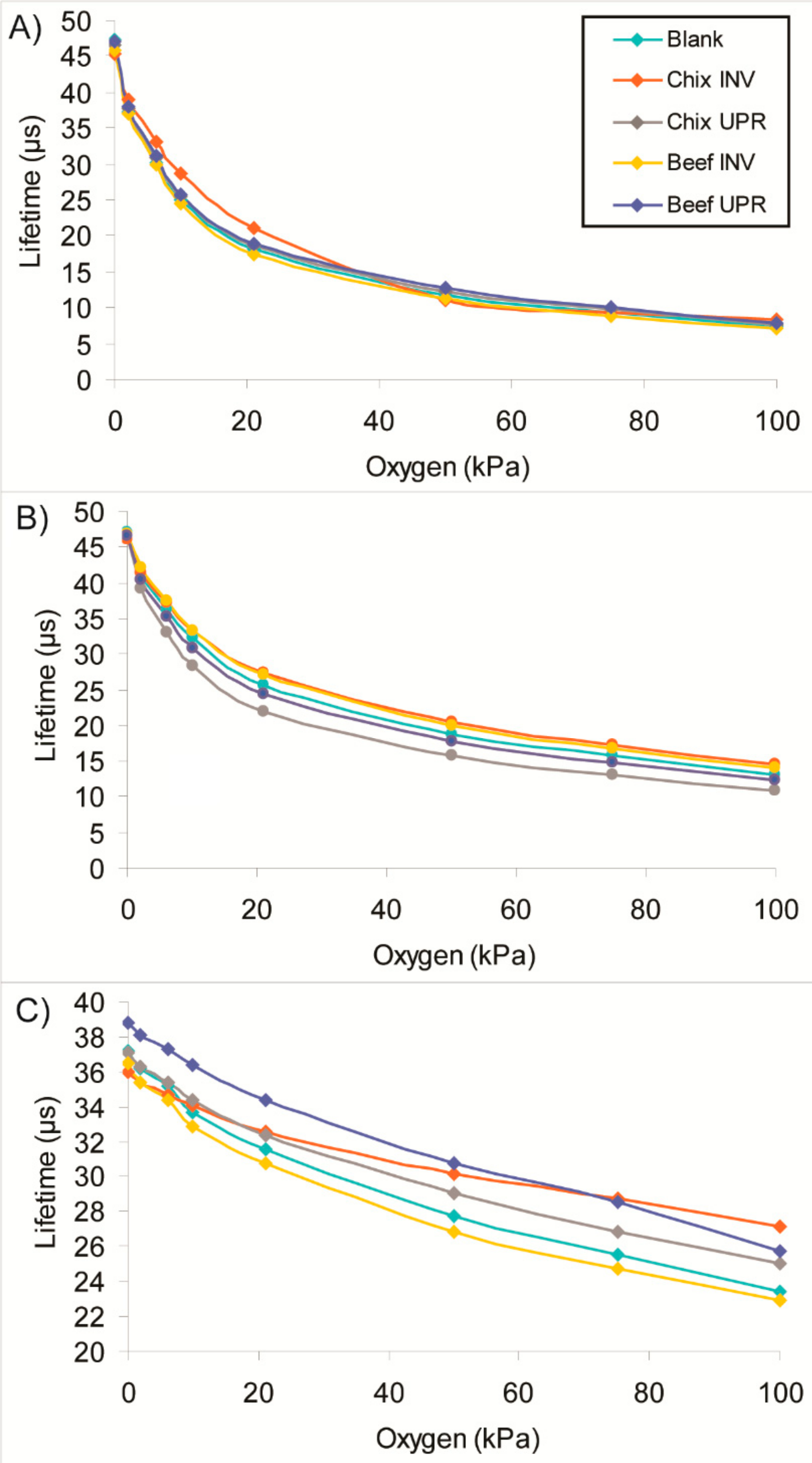
2 range, 20 °C. For this, sensors were removed from the packages on Day 7 were thoroughly rinsed with water to remove any residual meat juices, dried overnight and then calibrated.
Figure 3 shows that the Type 2 sensors performed well: after the exposure to food calibrations showed only slight deviations, compared to the same unused sensor or sensor placed in the empty package. The Type 1 and 5 sensors showed greater deviation from the original calibration with deviations of up to 3.7 µs. The greatest deviation in the Type 5 sensor was upon direct contact with the chicken, however this was not the case for the Type 1 sensor. No significant change in response time was observed for all the exposed sensors; it was typically <10 s.
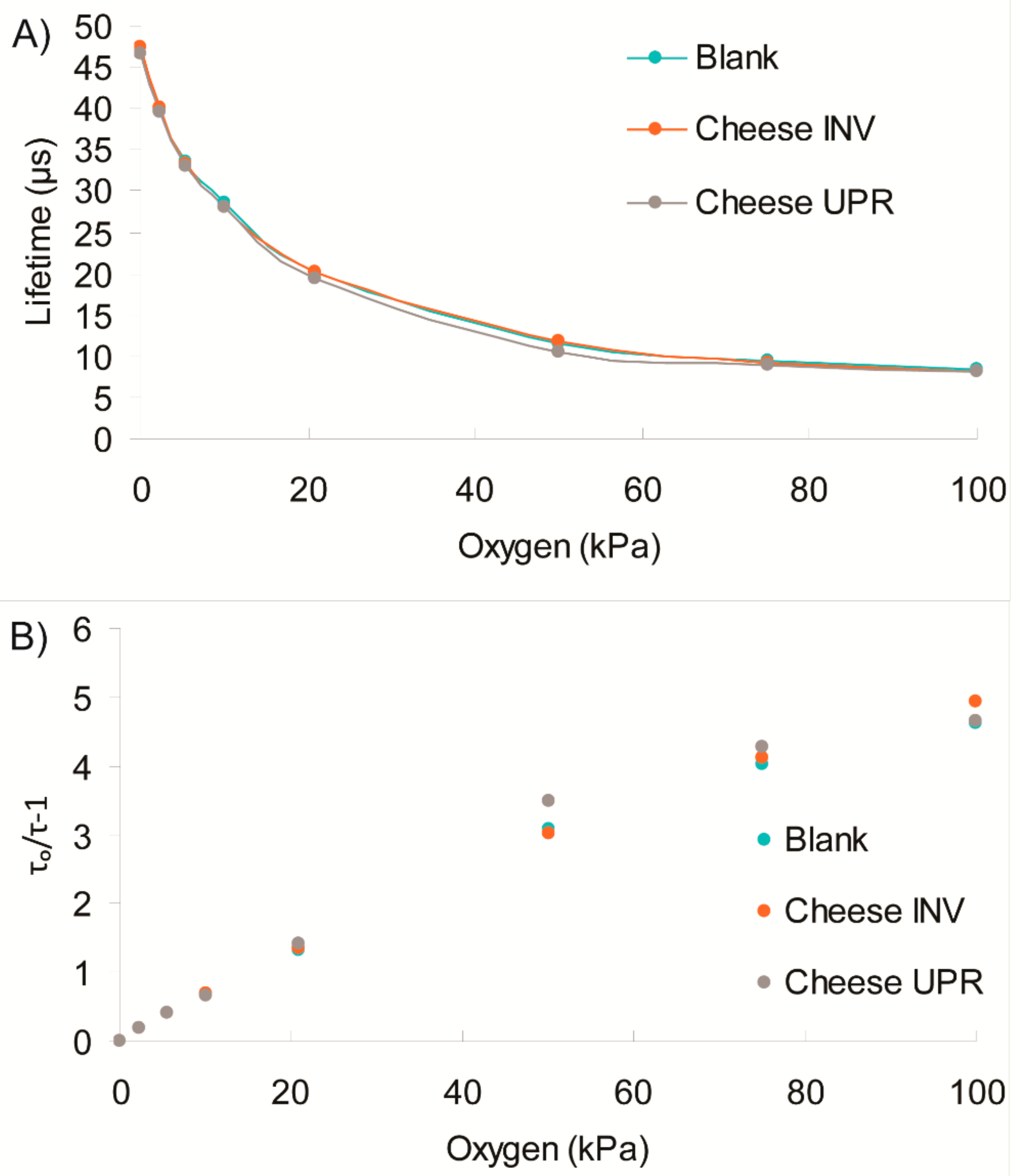
3.3. Effects of Sensor Contact with MAP Cheese
The O
2 sensors are intended for applications in various types of food packaging. Therefore, selected sensors underwent additional contact testing with MAP cheddar cheese for 32 days. Headspace gas analysis with Dansensor
TM at the beginning and the end of the study confirmed stable low levels of O
2 and significant decrease of CO
2 over 32-day storage period for the cheese samples with sensors (
Table 3). Screening of the O
2 sensors placed in these packs (both in the upward and inverted packs and with the commercial OpTech
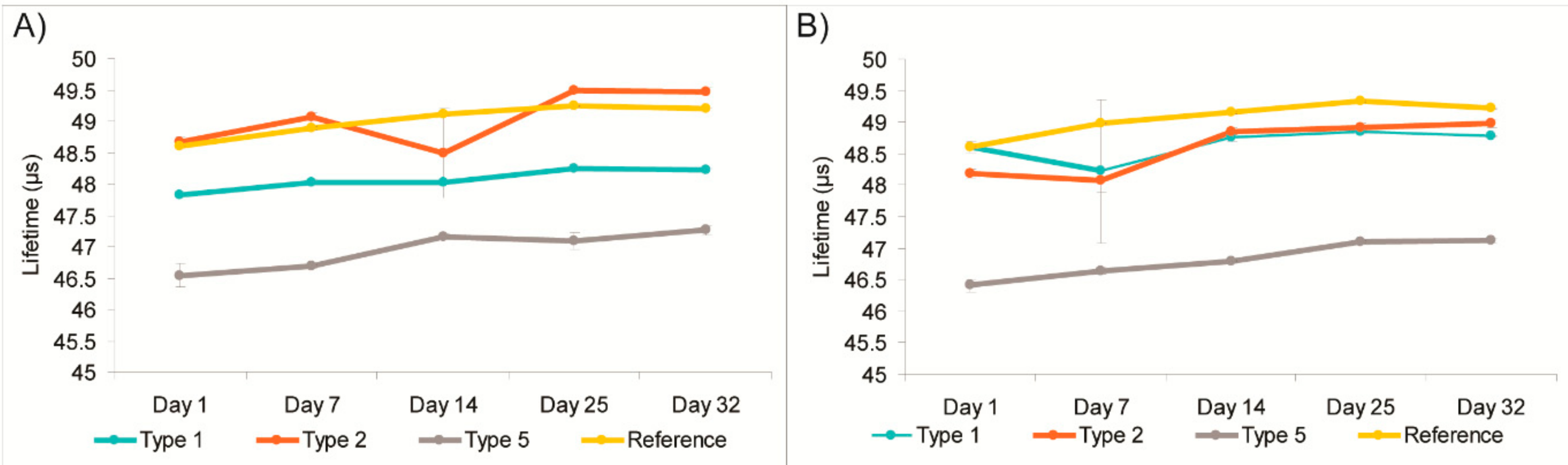
TM sticker) revealed a slight upward drift in lifetime signal over 32-day storage: 0.7–0.2 μs for the new sensors and 0.5 μs for Optech (
Figure 4). This drift is attributed mainly to the natural decrease in residual O
2 levels in these packs—from approximately 0.1% to 0.02%. Outliers (e.g., Type 2 readings on Day 14, and Type 1 and 2 readings on Day 7) can be due to temperature variation in these packs and/or measurement error. At the same time, the sensors from blank container showed lower sensor readings than those in the cheese containing packages. The OpTech
TM sensors gave ~46 μs instead of the 48–49 μs signals in the cheese packages, indicating O
2 ingress had occurred. Comparing these results with Dansensor
TM data, we could conclude that the blank package had not sealed correctly.
Upon removal from packages on Day 32, all the sensors were washed with water, dried overnight and then underwent full O
2 calibration (0–100 kPa) in dry gas at 20 °C. As seen with the meat products, Type 2 sensors performed best.
Figure 5 shows that their calibration remained almost unchanged after prolonged exposure to food product in MAP cheese packs. The Type 1 and 5 sensors showed more pronounced deviations from the original calibration—up to 1.2 µs and 1.8 µs, respectively. As before, no difference in response times was seen.
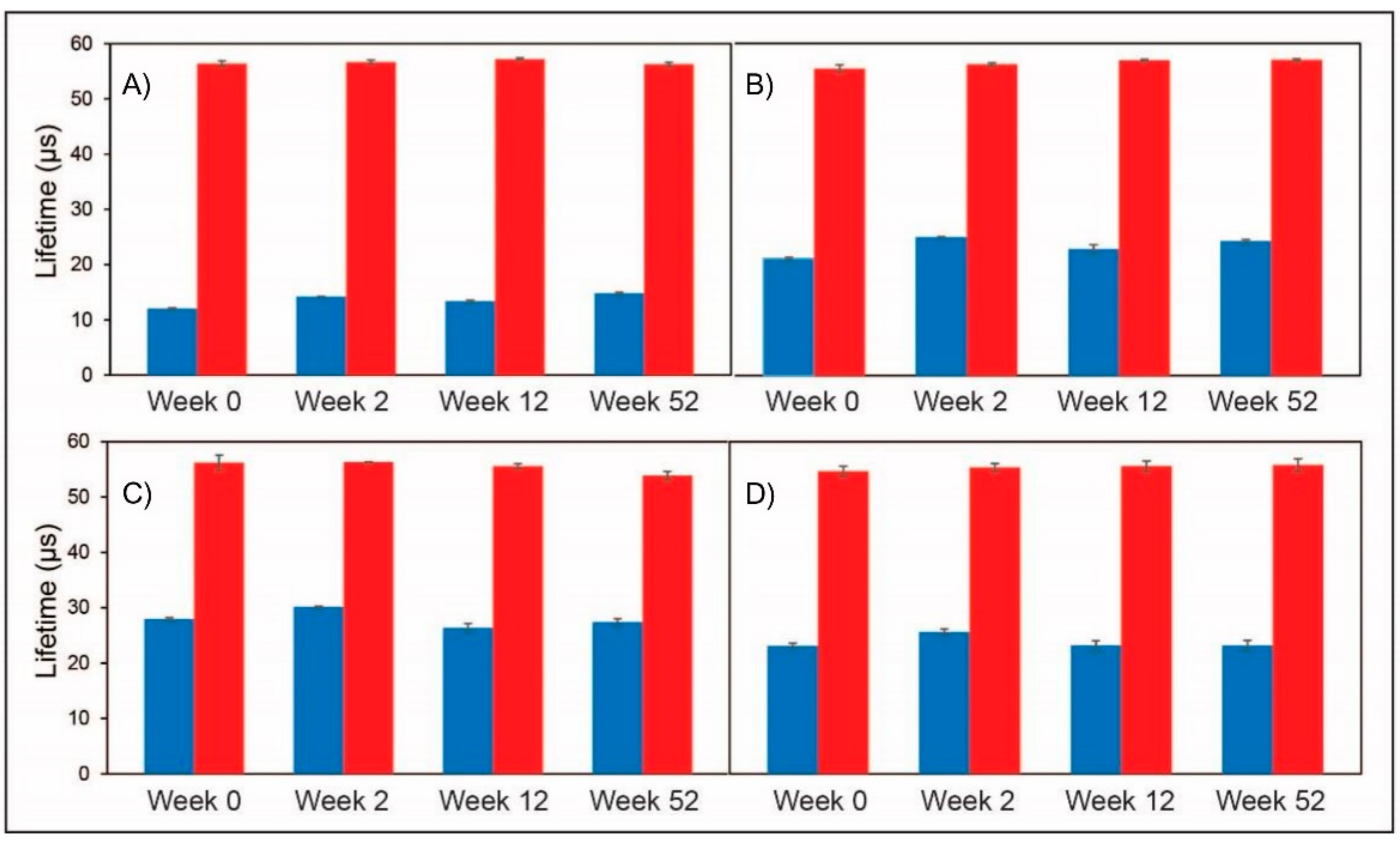
Finally, Types 1–4 sensors were assessed for potential ageing, upon their storage at ambient temperature in air atmosphere for 52 weeks (
Figure 6). Again, the Type 2 sensors performed best; changes were less than 0.5 µs at 0 kPa over 12 months. The Type 1, 3 and 4 sensors showed somewhat greater deviations of up to 1–2 µs. The Type 1 sensor showed the lifetime signal increase incrementally over the 12 months from the one-month time-point while Type 3 and 4 sensors showed fluctuating lifetimes from two weeks onward culminating in a change in lifetime signal of greater than 2 µs overall at the 12 month time-point. Small variations in the signals at 21 kPa could be due to differing sites of measurement.
Overall, the PtBP based oxygen sensors studied here, particularly Type 2 sensors, demonstrated useful performance and working characteristics for their use in food packaging applications on disposable basis. Currently, they are better suited and validated for such applications than alternative sensor materials, such as those based on ruthenium dyes and mesoporous silica matrices [
24,
25].