Advances and Perspectives in Chemical Imaging in Cellular Environments Using Electrochemical Methods
Abstract
1. Introduction
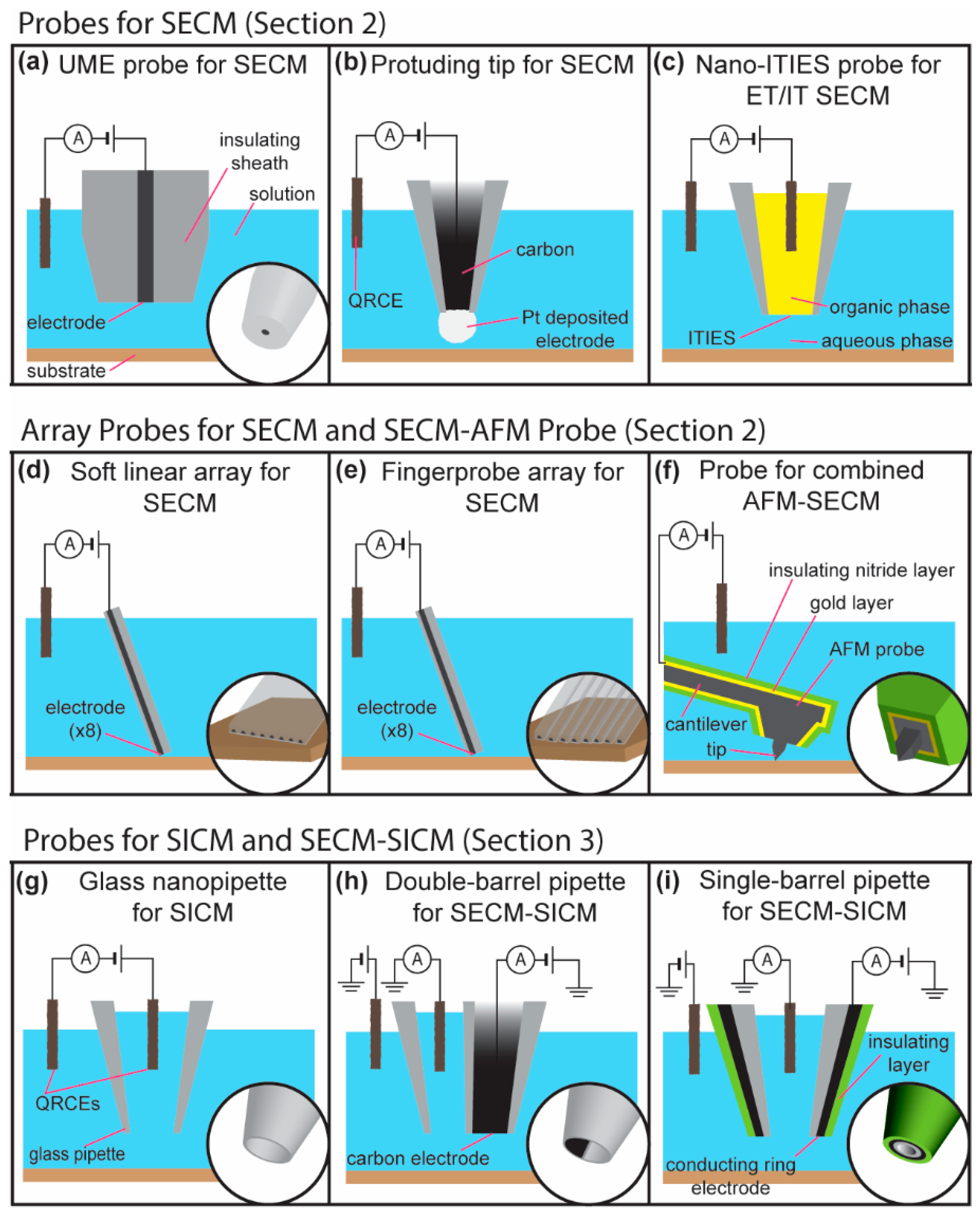
2. Scanning Electrochemical Microscopy
2.1. Constant-Distance Imaging Modes
2.2. Scanning Electrochemical Microscopy Using Microelectrode Array Probes
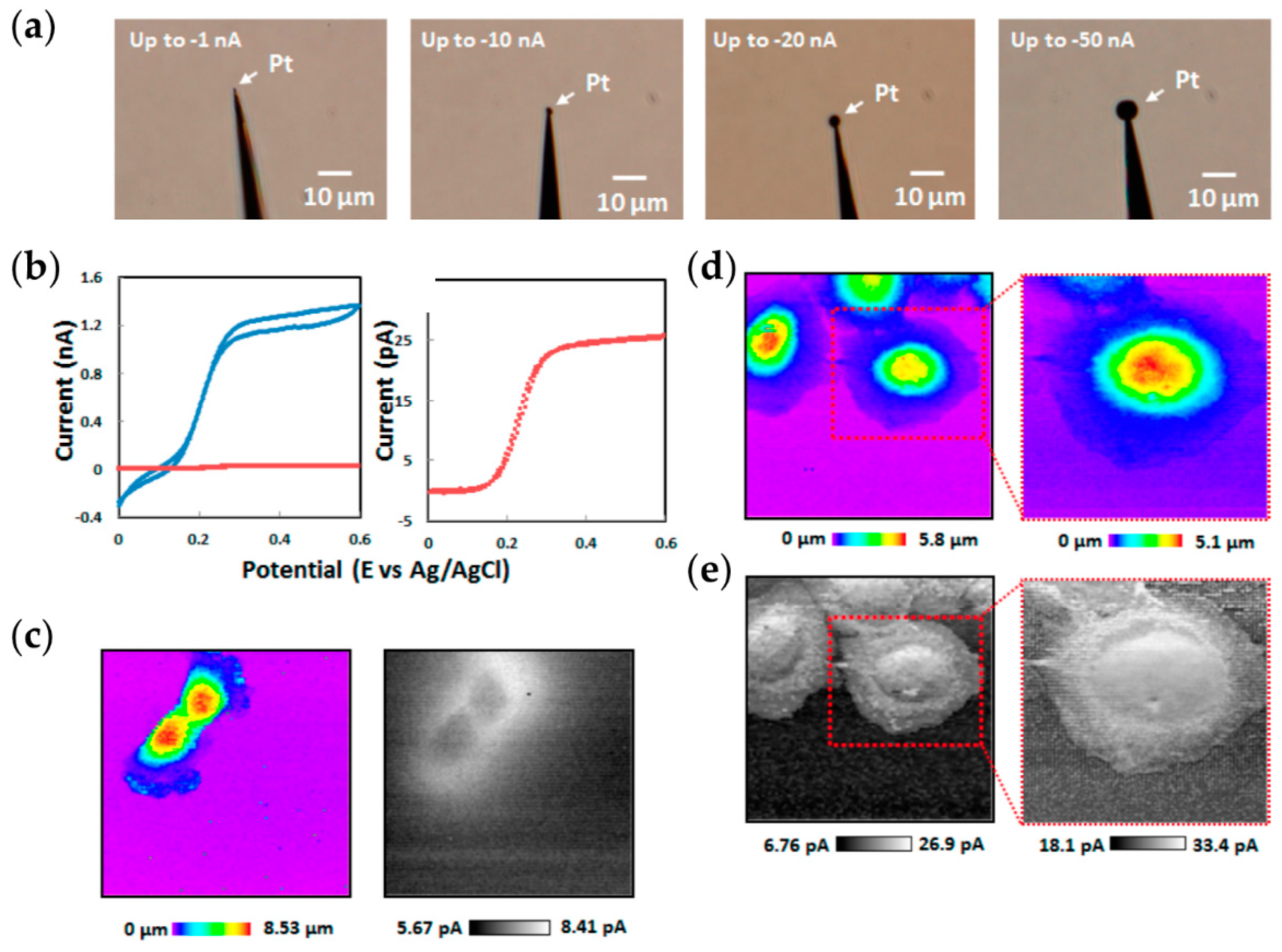
2.3. Nanoscale Imaging Using SECM
3. Scanning Ion Conductance Microscopy
3.1. Combined SECM-SICM
3.2. High-Resolution SECM-SICM
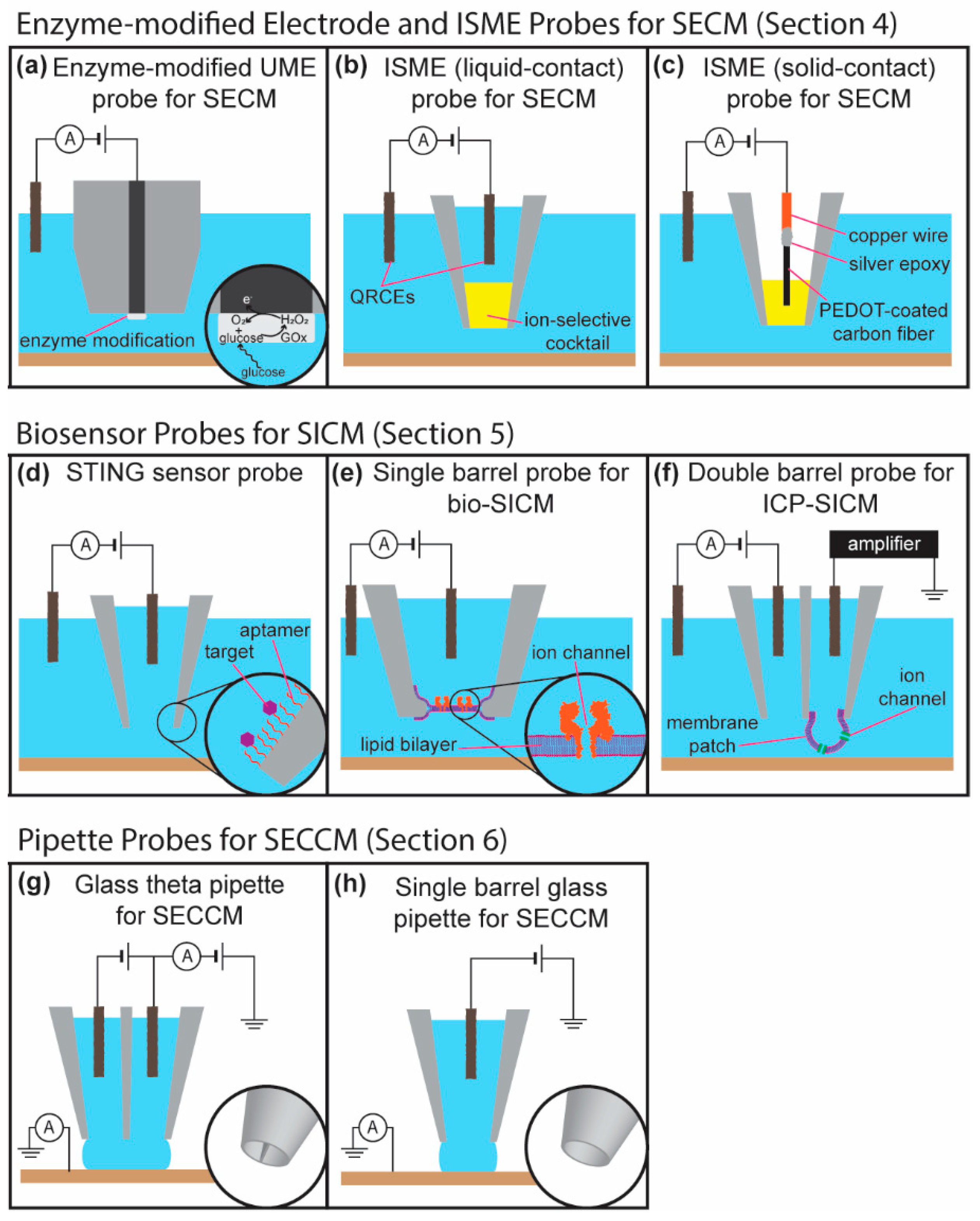
4. Functional and Chemical Specific Probes for SECM
4.1. Enzyme Modified Probes for Chemical Imaging in Scanning Electrochemical Microscopy
4.2. Potential for Biosensor Probes in Scanning Electrochemical Microscopy
4.3. Scanning Ion-Selective Electrode Technique (SIET)
5. Biosensor Probes in Scanning Ion Conductance Microscopy
5.1. Functionalized Glass Nanopipettes for Ion Gating Based Sensors
5.2. Ion Channel Probe-Based Scanning Ion Conductance Microscopy
6. Advanced Scanning Modes of SEPM Including Fast Scanning and Imaging Movies
6.1. Hopping Imaging Modes of SICM and SECM
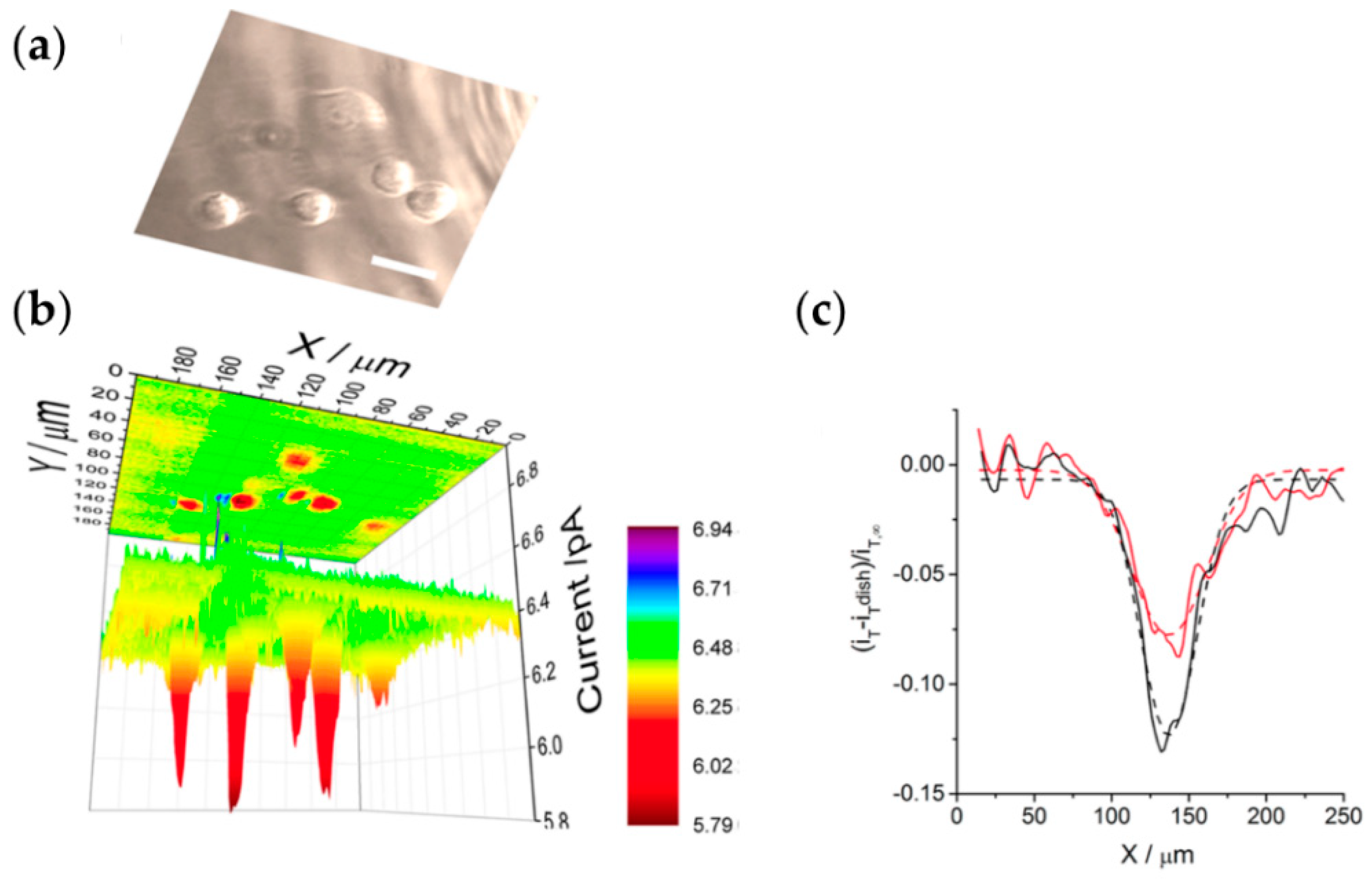
6.2. Fast Scanning and Imaging Movies Obtained with SEPM
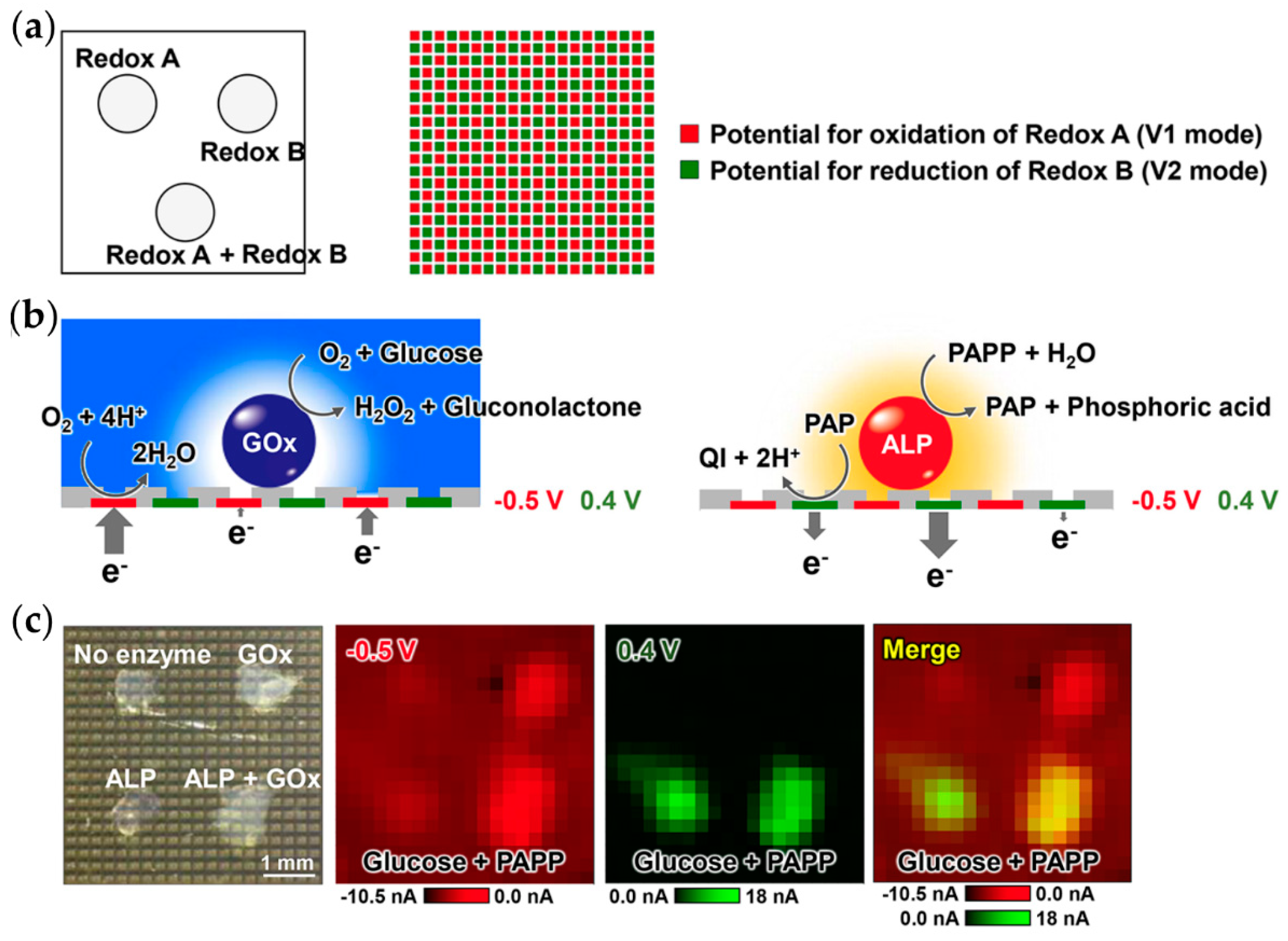
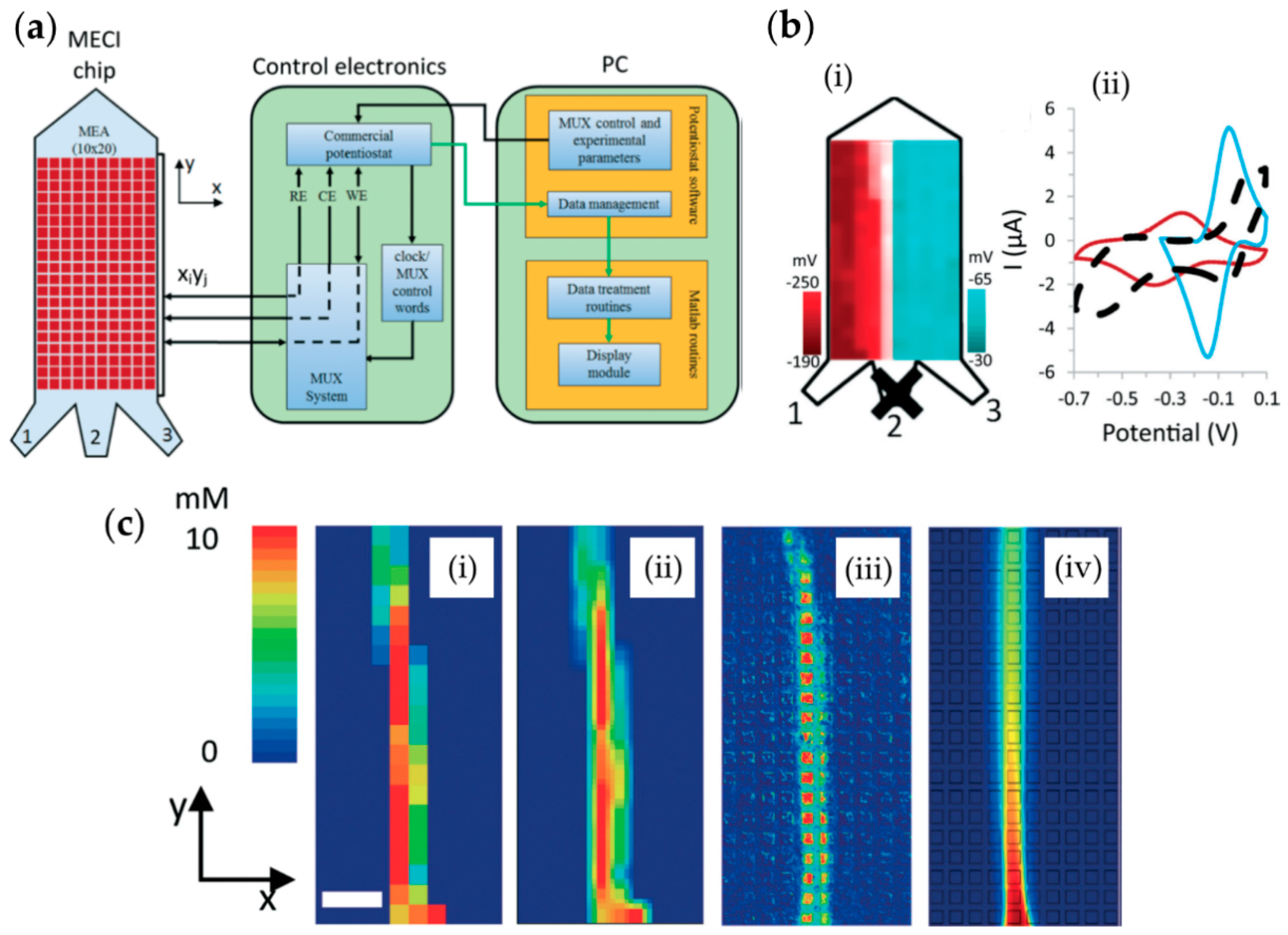
7. Microelectrode Arrays and Large-Scale Integration Chips
8. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Ning, X.; Ma, Q.; Qin, D.; Lu, X. Recent advances in electrochemistry by scanning electrochemical microscopy. TrAC Trends Anal. Chem. 2016, 80, 242–254. [Google Scholar] [CrossRef]
- Zoski, C.G. Review—Advances in Scanning Electrochemical Microscopy (SECM). J. Electrochem. Soc. 2016, 163, H3088–H3100. [Google Scholar] [CrossRef]
- Izquierdo, J.; Knittel, P.; Kranz, C. Scanning electrochemical microscopy: An analytical perspective. Anal. Bioanal. Chem. 2018, 410, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Bergner, S.; Vatsyayan, P.; Matysik, F.-M. Recent advances in high resolution scanning electrochemical microscopy of living cells—A review. Anal. Chim. Acta 2013, 775, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.; Nebel, M.; Schuhmann, W. Scanning Electrochemical Microscopy in Neuroscience. Annu. Rev. Anal. Chem. 2010, 3, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Page, A.; Perry, D.; Unwin, P.R. Multifunctional scanning ion conductance microscopy. Proc. R. Soc. Lond. Ser. A 2017, 473, 20160889. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kumatani, A.; Shiku, H.; Matsue, T. Scanning Probe Microscopy for Nanoscale Electrochemical Imaging. Anal. Chem. 2017, 89, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Kranz, C. Recent advancements in nanoelectrodes and nanopipettes used in combined scanning electrochemical microscopy techniques. Analyst 2014, 139, 336–352. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Momotenko, D.; Page, A.; Perry, D.; Unwin, P.R. Frontiers in Nanoscale Electrochemical Imaging: Faster, Multifunctional, and Ultrasensitive. Langmuir 2016, 32, 7993–8008. [Google Scholar] [CrossRef] [PubMed]
- Kueng, A.; Kranz, C.; Mizaikoff, B. Scanning Probe Microscopy with Integrated Biosensors. Sens. Lett. 2003, 1, 2–15. [Google Scholar] [CrossRef][Green Version]
- Bard, A.J.; Fan, F.R.F.; Kwak, J.; Lev, O. Scanning electrochemical microscopy. Introduction and principles. Anal. Chem. 1989, 61, 132–138. [Google Scholar] [CrossRef]
- Engstrom, R.C.; Pharr, C.M. Scanning Electrochemical Microscopy. Anal. Chem. 1989, 61, 1099–1104. [Google Scholar] [CrossRef]
- Bard, A.J.; Fan, F.-R.F.; Pierce, D.T.; Unwin, P.R.; Wipf, D.O.; Zhou, F. Chemical Imaging of Surfaces with the Scanning Electrochemical Microscope. Science 1991, 254, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Payne, N.A.; Stephens, L.I.; Mauzeroll, J. The Application of Scanning Electrochemical Microscopy to Corrosion Research. Corrosion 2017, 73, 759–780. [Google Scholar] [CrossRef]
- Perry, A.R.; Lazenby, R.A.; Adobes-Vidal, M.; Peruffo, M.; McKelvey, K.; Snowden, M.E.; Unwin, P.R. Hopping intermittent contact-scanning electrochemical microscopy (HIC-SECM) as a new local dissolution kinetic probe: Application to salicylic acid dissolution in aqueous solution. CrystEngComm 2015, 17, 7835–7843. [Google Scholar] [CrossRef]
- Ishimatsu, R.; Kim, J.; Jing, P.; Striemer, C.C.; Fang, D.Z.; Fauchet, P.M.; McGrath, J.L.; Amemiya, S. Ion-Selective Permeability of an Ultrathin Nanoporous Silicon Membrane as Probed by Scanning Electrochemical Microscopy Using Micropipet-Supported ITIES Tips. Anal. Chem. 2010, 82, 7127–7134. [Google Scholar] [CrossRef] [PubMed]
- Oyamatsu, D.; Hirano, Y.; Kanaya, N.; Mase, Y.; Nishizawa, M.; Matsue, T. Imaging of enzyme activity by scanning electrochemical microscope equipped with a feedback control for substrate-probe distance. Bioelectrochemistry 2003, 60, 115–121. [Google Scholar] [CrossRef]
- Gyurcsányi, R.E.; Jágerszki, G.; Kiss, G.; Tóth, K. Chemical imaging of biological systems with the scanning electrochemical microscope. Bioelectrochemistry 2004, 63, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Heinze, J. Ultramicroelectrodes in Electrochemistry. Angew. Chem Int. Ed. Engl. 1993, 32, 1268–1288. [Google Scholar] [CrossRef]
- Kwak, J.; Bard, A.J. Scanning electrochemical microscopy. Theory of the feedback mode. Anal. Chem. 1989, 61, 1221–1227. [Google Scholar] [CrossRef]
- Martin, R.; Unwin, P. Scanning electrochemical microscopy Kinetics of chemical reactions following electron-transfer measured with the substrate-generation-tip-collection mode. J. Chem. Soc. Faraday Trans. 1998, 94, 753–759. [Google Scholar] [CrossRef]
- Eckhard, K.; Chen, X.; Turcu, F.; Schuhmann, W. Redox competition mode of scanning electrochemical microscopy (RC-SECM) for visualisation of local catalytic activity. Phys. Chem. Chem. Phys. 2006, 8, 5359–5365. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, J.; Alpuche-Avilés, M.A.; Bard, A.J. Interrogation of Surfaces for the Quantification of Adsorbed Species on Electrodes: Oxygen on Gold and Platinum in Neutral Media. J. Am. Chem. Soc. 2008, 130, 16985–16995. [Google Scholar] [CrossRef] [PubMed]
- Wuu, Y.; Fan, F.F.; Bard, A.J. High Resolution Deposition of Polyaniline on Pt with the Scanning Electrochemical Microscope. J. Electrochem. Soc. 1989, 136, 885–886. [Google Scholar] [CrossRef]
- Denuault, G.; Frank, M.H.T.; Peter, L.M. Scanning electrochemical microscopy: Potentiometric probing of ion fluxes. Faraday Discuss. 1992, 94, 23–35. [Google Scholar] [CrossRef]
- Díaz-Ballote, L.; Alpuche-Aviles, M.; Wipf, D.O. Fast-scan cyclic voltammetry–scanning electrochemical microscopy. J. Electroanal. Chem. 2007, 604, 17–25. [Google Scholar] [CrossRef]
- O’Connell, M.A.; Wain, A.J. Combined electrochemical-topographical imaging: A critical review. Anal. Methods 2015, 7, 6983–6999. [Google Scholar] [CrossRef]
- Eckhard, K.; Schuhmann, W. Alternating current techniques in scanning electrochemical microscopy (AC-SECM). Analyst 2008, 133, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Wipf, D.O.; Bard, A.J. Scanning electrochemical microscopy. 15. Improvements in imaging via tip-position modulation and lock-in detection. Anal. Chem. 1992, 64, 1362–1367. [Google Scholar] [CrossRef]
- Hengstenberg, A.; Kranz, C.; Schuhmann, W. Facilitated Tip-Positioning and Applications of Non-Electrode Tips in Scanning Electrochemical Microscopy Using a Shear Force Based Constant-Distance Mode. Chem. A Eur. J. 2000, 6, 1547–1554. [Google Scholar] [CrossRef]
- McKelvey, K.; Edwards, M.A.; Unwin, P.R. Intermittent Contact-Scanning Electrochemical Microscopy (IC-SECM): A New Approach for Tip Positioning and Simultaneous Imaging of Interfacial Topography and Activity. Anal. Chem. 2010, 82, 6334–6337. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, V.; Marchuk, K.; Yu, Y.; Titus, E.J.; Wilson, A.J.; Armstrong, C.M.; Zhang, B.; Willets, K.A. Visualizing and Calculating Tip–Substrate Distance in Nanoscale Scanning Electrochemical Microscopy Using 3-Dimensional Super-Resolution Optical Imaging. Anal. Chem. 2017, 89, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.A.; Whitworth, A.L.; Unwin, P.R. Quantitative Analysis and Application of Tip Position Modulation-Scanning Electrochemical Microscopy. Anal. Chem. 2011, 83, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Kranz, C.; Schuhmann, W.; Gaub, H.E. Topography feedback mechanism for the scanning electrochemical microscope based on hydrodynamic forces between tip and sample. Rev. Sci. Instrum. 1995, 66, 2857–2860. [Google Scholar] [CrossRef]
- Ballesteros Katemann, B.; Schulte, A.; Schuhmann, W. Constant-Distance Mode Scanning Electrochemical Microscopy (SECM)—Part I: Adaptation of a Non-Optical Shear-Force-Based Positioning Mode for SECM Tips. Chem. A Eur. J. 2003, 9, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Comstock, D.J.; Elam, J.W.; Pellin, M.J.; Hersam, M.C. Integrated Ultramicroelectrode−Nanopipet Probe for Concurrent Scanning Electrochemical Microscopy and Scanning Ion Conductance Microscopy. Anal. Chem. 2010, 82, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Shevchuk, A.I.; Novak, P.; Murakami, Y.; Shiku, H.; Korchev, Y.E.; Matsue, T. Simultaneous Noncontact Topography and Electrochemical Imaging by SECM/SICM Featuring Ion Current Feedback Regulation. J. Am. Chem. Soc. 2010, 132, 10118–10126. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.E.; Macpherson, J. V Peer Reviewed: Atomic Force Microscopy Probes Go Electrochemical. Anal. Chem. 2002, 74, 576A–584A. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, J.; Agrawal, A.; An, S.; Choudhary, E.; Szalai, V.A. Fabrication of Scanning Electrochemical Microscopy-Atomic Force Microscopy Probes to Image Surface Topography and Reactivity at the Nanoscale. Anal. Chem. 2017, 89, 2687–2691. [Google Scholar] [CrossRef] [PubMed]
- Seifert, J.; Rheinlaender, J.; Novak, P.; Korchev, Y.E.; Schäffer, T.E. Comparison of Atomic Force Microscopy and Scanning Ion Conductance Microscopy for Live Cell Imaging. Langmuir 2015, 31, 6807–6813. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Holzinger, A.; Knittel, P.; Poltorak, L.; Gamero-Quijano, A.; Rickard, W.D.A.; Walcarius, A.; Herzog, G.; Kranz, C.; Arrigan, D.W.M. Visualization of Diffusion within Nanoarrays. Anal. Chem. 2016, 88, 6689–6695. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.L.; Unwin, P.R.; Gardner, J.W.; Rieley, H. A multi-electrode probe for parallel imaging in scanning electrochemical microscopy. Electrochem. Commun. 2004, 6, 91–97. [Google Scholar] [CrossRef]
- Cortés-Salazar, F.; Momotenko, D.; Girault, H.H.; Lesch, A.; Wittstock, G. Seeing Big with Scanning Electrochemical Microscopy. Anal. Chem. 2011, 83, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-E.; Lesch, A.; Li, C.-L.; Girault, H.H. Mapping the antioxidant activity of apple peels with soft probe scanning electrochemical microscopy. J. Electroanal. Chem. 2017, 786, 120–128. [Google Scholar] [CrossRef]
- Lesch, A.; Momotenko, D.; Cortés-Salazar, F.; Roelfs, F.; Girault, H.H.; Wittstock, G. High-throughput scanning electrochemical microscopy brushing of strongly tilted and curved surfaces. Electrochim. Acta 2013, 110, 30–41. [Google Scholar] [CrossRef]
- Lesch, A.; Chen, P.-C.; Roelfs, F.; Dosche, C.; Momotenko, D.; Cortés-Salazar, F.; Girault, H.H.; Wittstock, G. Finger Probe Array for Topography-Tolerant Scanning Electrochemical Microscopy of Extended Samples. Anal. Chem. 2014, 86, 713–720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, T.E.; Lu, Y.J.; Sun, C.L.; Pick, H.; Chen, J.P.; Lesch, A.; Girault, H.H. Soft Electrochemical Probes for Mapping the Distribution of Biomarkers and Injected Nanomaterials in Animal and Human Tissues. Angew. Chem. Int. Ed. 2017, 56, 16498–16502. [Google Scholar] [CrossRef] [PubMed]
- Clausmeyer, J.; Schuhmann, W. Nanoelectrodes: Applications in electrocatalysis, single-cell analysis and high-resolution electrochemical imaging. TrAC Trends Anal. Chem. 2016, 79, 46–59. [Google Scholar] [CrossRef]
- Nioradze, N.; Chen, R.; Kim, J.; Shen, M.; Santhosh, P.; Amemiya, S. Origins of Nanoscale Damage to Glass-Sealed Platinum Electrodes with Submicrometer and Nanometer Size. Anal. Chem. 2013, 85, 6198–6202. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hu, K.; Yu, Y.; Mirkin, M.V.; Amemiya, S. Focused-Ion-Beam-Milled Carbon Nanoelectrodes for Scanning Electrochemical Microscopy. J. Electrochem. Soc. 2016, 163, H3032–H3037. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shen, M.; Nioradze, N.; Amemiya, S. Stabilizing Nanometer Scale Tip-to-Substrate Gaps in Scanning Electrochemical Microscopy Using an Isothermal Chamber for Thermal Drift Suppression. Anal. Chem. 2012, 84, 3489–3492. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Renault, C.; Nioradze, N.; Arroyo-Currás, N.; Leonard, K.C.; Bard, A.J. Nanometer Scale Scanning Electrochemical Microscopy Instrumentation. Anal. Chem. 2016, 88, 10284–10289. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yu, Y.; Zacher, B.J.; Mirkin, M. V Scanning Electrochemical Microscopy of Individual Catalytic Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 14120–14123. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Renault, C.; Nioradze, N.; Arroyo-Currás, N.; Leonard, K.C.; Bard, A.J. Electrocatalytic Activity of Individual Pt Nanoparticles Studied by Nanoscale Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2016, 138, 8560–8568. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Balla, R.J.; Lima, A.; Amemiya, S. Characterization of Nanopipet-Supported ITIES Tips for Scanning Electrochemical Microscopy of Single Solid-State Nanopores. Anal. Chem. 2017, 89, 9946–9952. [Google Scholar] [CrossRef] [PubMed]
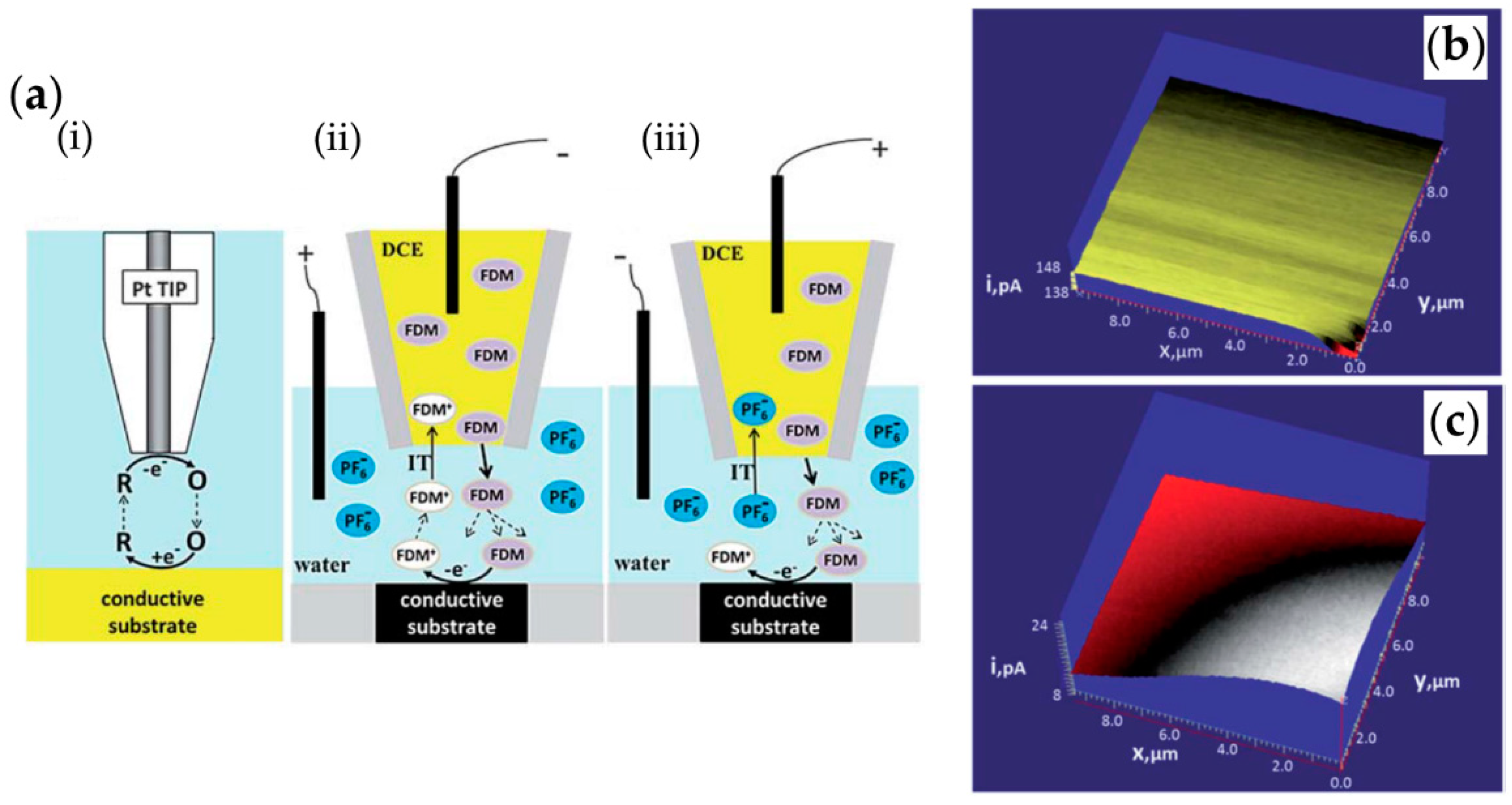
- Wang, Y.; Kececi, K.; Velmurugan, J.; Mirkin, M. V Electron transfer/ion transfer mode of scanning electrochemical microscopy (SECM): A new tool for imaging and kinetic studies. Chem. Sci. 2013, 4, 3606–3616. [Google Scholar] [CrossRef]
- Hansma, P.K.; Drake, B.; Marti, O.; Gould, S.A.; Prater, C.B. The scanning ion-conductance microscope. Science 1989, 243, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Rheinlaender, J.; Schäffer, T.E. Lateral Resolution and Image Formation in Scanning Ion Conductance Microscopy. Anal. Chem. 2015, 87, 7117–7124. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Mizutani, Y.; Iwata, F.; Ushiki, T. Scanning ion conductance microscopy for visualizing the three-dimensional surface topography of cells and tissues. Semin. Cell Dev. Biol. 2018, 73, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Momotenko, D.; McKelvey, K.; Kang, M.; Meloni, G.N.; Unwin, P.R. Simultaneous Interfacial Reactivity and Topography Mapping with Scanning Ion Conductance Microscopy. Anal. Chem. 2016, 88, 2838–2846. [Google Scholar] [CrossRef] [PubMed]
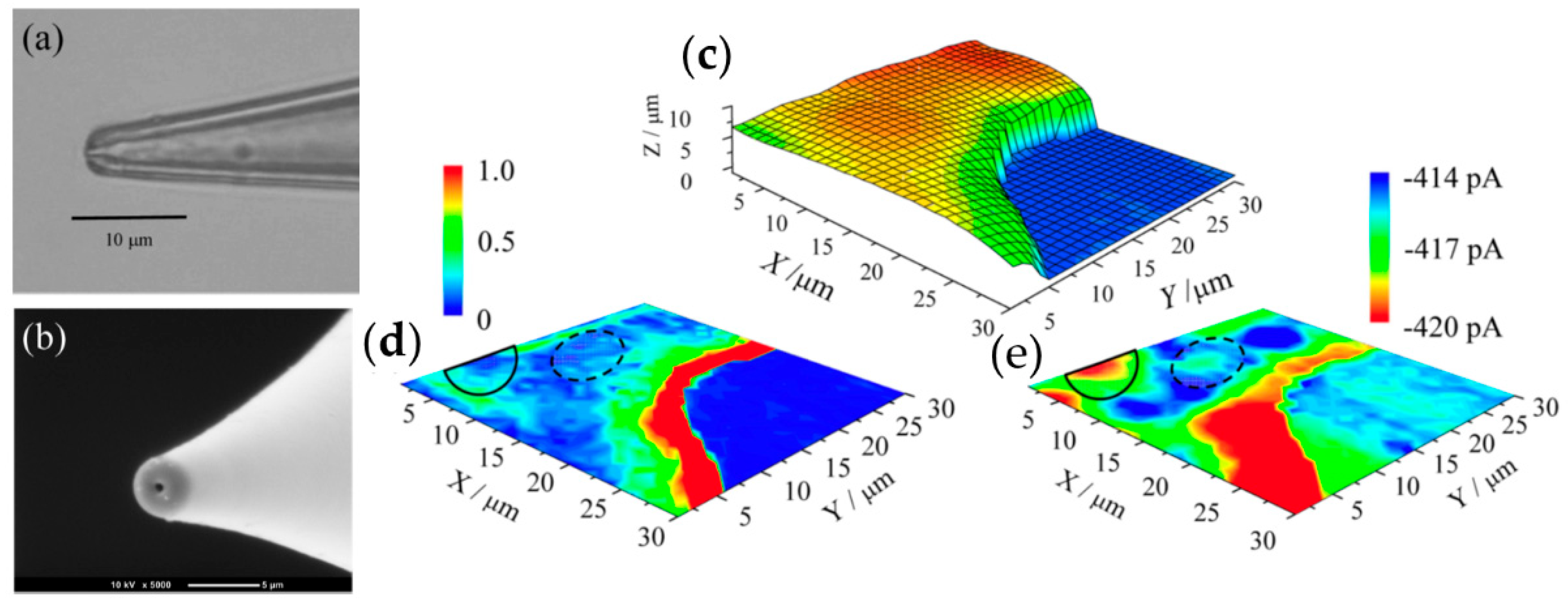
- McKelvey, K.; Kinnear, S.L.; Perry, D.; Momotenko, D.; Unwin, P.R. Surface Charge Mapping with a Nanopipette. J. Am. Chem. Soc. 2014, 136, 13735–13744. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, K.; Perry, D.; Byers, J.C.; Colburn, A.W.; Unwin, P.R. Bias Modulated Scanning Ion Conductance Microscopy. Anal. Chem. 2014, 86, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Baker, L.A. Effects of pipette modulation and imaging distances on ion currents measured with Scanning Ion Conductance Microscopy (SICM). Analyst 2011, 136, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.; Page, A.; Chen, B.; Frenguelli, B.G.; Unwin, P.R. Differential-Concentration Scanning Ion Conductance Microscopy. Anal. Chem. 2017, 89, 12458–12465. [Google Scholar] [CrossRef] [PubMed]
- Thakar, R.; Weber, A.E.; Morris, C.A.; Baker, L.A. Multifunctional carbon nanoelectrodes fabricated by focused ion beam milling. Analyst 2013, 138, 5973–5982. [Google Scholar] [CrossRef] [PubMed]
- Lazenby, R.A.; McKelvey, K.; Peruffo, M.; Baghdadi, M.; Unwin, P.R. Nanoscale intermittent contact-scanning electrochemical microscopy. J. Solid State Electrochem. 2013, 17, 2979–2987. [Google Scholar] [CrossRef]
- Takahashi, Y.; Shevchuk, A.I.; Novak, P.; Zhang, Y.; Ebejer, N.; Macpherson, J.V.; Unwin, P.R.; Pollard, A.J.; Roy, D.; Clifford, C.A.; et al. Multifunctional Nanoprobes for Nanoscale Chemical Imaging and Localized Chemical Delivery at Surfaces and Interfaces. Angew. Chem. Int. Ed. 2011, 50, 9638–9642. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sa, N.; Thakar, R.; Baker, L.A. Nanopipette delivery: Influence of surface charge. Analyst 2015, 140, 4835–4842. [Google Scholar] [CrossRef] [PubMed]
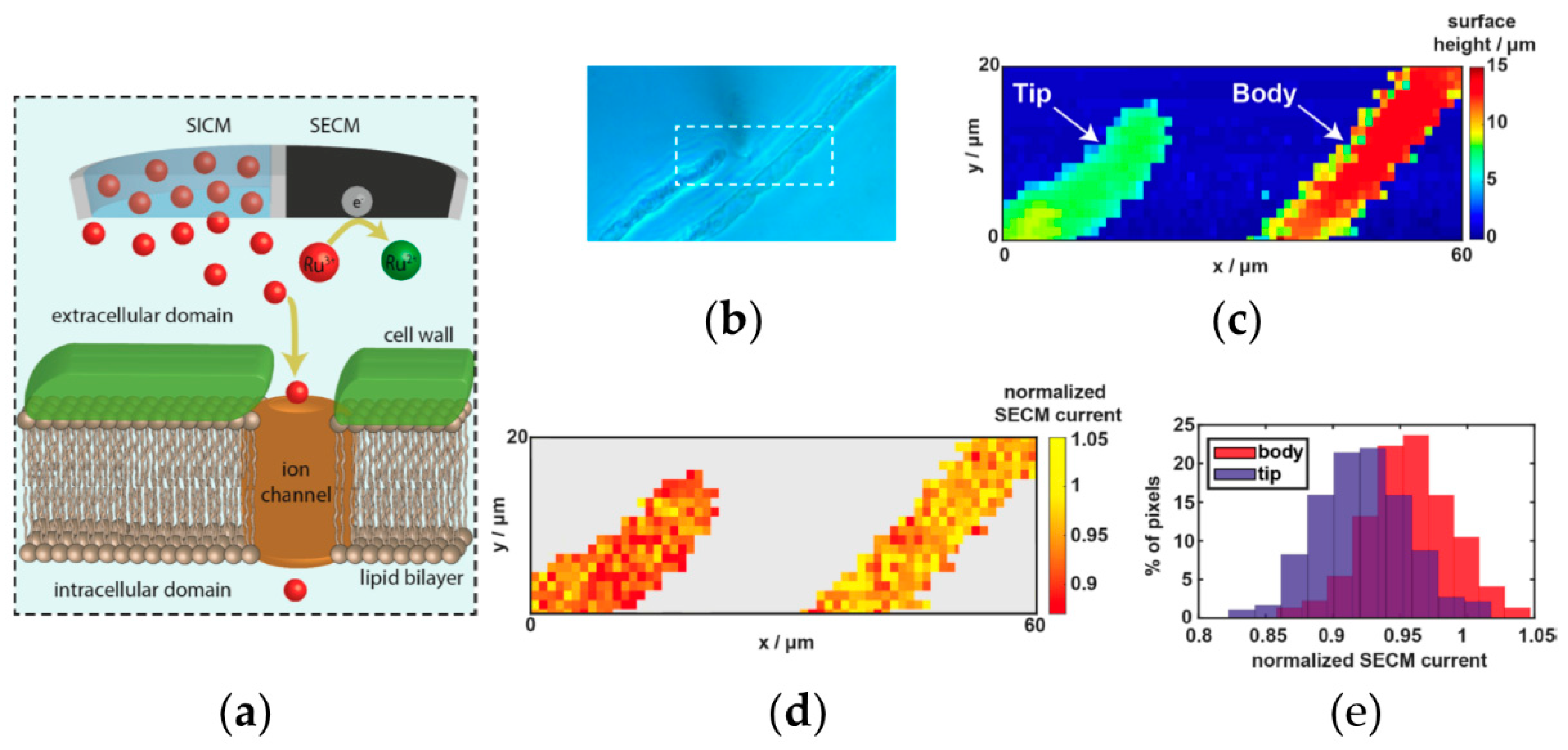
- Page, A.; Kang, M.; Armitstead, A.; Perry, D.; Unwin, P.R. Quantitative Visualization of Molecular Delivery and Uptake at Living Cells with Self-Referencing Scanning Ion Conductance Microscopy-Scanning Electrochemical Microscopy. Anal. Chem. 2017, 89, 3021–3028. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.; Momotenko, D.; Lazenby, R.A.; Kang, M.; Unwin, P.R. Characterization of Nanopipettes. Anal. Chem. 2016, 88, 5523–5530. [Google Scholar] [CrossRef] [PubMed]
- Tognoni, E.; Baschieri, P.; Ascoli, C.; Pellegrini, M.; Pellegrino, M. Characterization of tip size and geometry of the pipettes used in scanning ion conductance microscopy. Micron 2016, 83, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Actis, P.; Tokar, S.; Clausmeyer, J.; Babakinejad, B.; Mikhaleva, S.; Cornut, R.; Takahashi, Y.; López Córdoba, A.; Novak, P.; Shevchuck, A.I.; et al. Electrochemical Nanoprobes for Single-Cell Analysis. ACS Nano 2014, 8, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Gao, Y.; Wang, Y.; Yu, Y.; Zhao, X.; Rotenberg, S.A.; Gökmeşe, E.; Mirkin, M.V.; Friedman, G.; Gogotsi, Y. Platinized carbon nanoelectrodes as potentiometric and amperometric SECM probes. J. Solid State Electrochem. 2013, 17, 2971–2977. [Google Scholar] [CrossRef]
- O’Connell, M.A.; Lewis, J.R.; Wain, A.J. Electrochemical imaging of hydrogen peroxide generation at individual gold nanoparticles. Chem. Commun. 2015, 51, 10314–10317. [Google Scholar] [CrossRef] [PubMed]
- Şen, M.; Takahashi, Y.; Matsumae, Y.; Horiguchi, Y.; Kumatani, A.; Ino, K.; Shiku, H.; Matsue, T. Improving the Electrochemical Imaging Sensitivity of Scanning Electrochemical Microscopy-Scanning Ion Conductance Microscopy by Using Electrochemical Pt Deposition. Anal. Chem. 2015, 87, 3484–3489. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Rocha-Santos, T.A.; Duarte, A.C.; Rocha-Santos, T.A. Review of analytical figures of merit of sensors and biosensors in clinical applications. TrAC Trends Anal. Chem. 2010, 29, 1172–1183. [Google Scholar] [CrossRef]
- Ganesana, M.; Lee, S.T.; Wang, Y.; Venton, B.J. Analytical Techniques in Neuroscience: Recent Advances in Imaging, Separation, and Electrochemical Methods. Anal. Chem. 2017, 89, 314–341. [Google Scholar] [CrossRef] [PubMed]
- Huffman, M.L.; Venton, B.J. Carbon-Fiber Microelectrodes for In Vivo Applications. Analyst 2009, 134, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Myung, D.; Shim, J.H.; Kim, M.H.; Lee, Y. A dual electrochemical microsensor for simultaneous imaging of oxygen and pH over the rat kidney surface. Analyst 2013, 138, 5258–5264. [Google Scholar] [CrossRef] [PubMed]
- Nadappuram, B.P.; McKelvey, K.; Al Botros, R.; Colburn, A.W.; Unwin, P.R. Fabrication and Characterization of Dual Function Nanoscale pH-Scanning Ion Conductance Microscopy (SICM) Probes for High Resolution pH Mapping. Anal. Chem. 2013, 85, 8070–8074. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-E.; Cortés-Salazar, F.; Lesch, A.; Qiao, L.; Bondarenko, A.; Girault, H.H. Multiple scanning electrochemical microscopy mapping of tyrosinase in micro-contact printed fruit samples on polyvinylidene fluoride membrane. Electrochim. Acta 2015, 179, 57–64. [Google Scholar] [CrossRef]
- Horrocks, B.R.; Schmidtke, D.; Heller, A.; Bard, A.J. Scanning electrochemical microscopy. 24. Enzyme ultramicroelectrodes for the measurement of hydrogen peroxide at surfaces. Anal. Chem. 1993, 65, 3605–3614. [Google Scholar] [CrossRef] [PubMed]
- Polcari, D.; Kwan, A.; Van Horn, M.R.; Danis, L.; Pollegioni, L.; Ruthazer, E.S.; Mauzeroll, J. Disk-Shaped Amperometric Enzymatic Biosensor for in Vivo Detection of d-serine. Anal. Chem. 2014, 86, 3501–3507. [Google Scholar] [CrossRef] [PubMed]
- Polcari, D.; Perry, S.C.; Pollegioni, L.; Geissler, M.; Mauzeroll, J. Localized Detection of d-Serine by using an Enzymatic Amperometric Biosensor and Scanning Electrochemical Microscopy. ChemElectroChem 2017, 4, 920–926. [Google Scholar] [CrossRef]
- Ciobanu, M.; Taylor, D.E.; Wilburn, J.P.; Cliffel, D.E. Glucose and Lactate Biosensors for Scanning Electrochemical Microscopy Imaging of Single Live Cells. Anal. Chem. 2008, 80, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Morales, L.Z.; Loziuk, P.L.; Corder, A.K.; Toups, J.V.; Roberts, J.G.; McCaffrey, K.A.; Sombers, L.A. Enzyme-Modified Carbon-Fiber Microelectrode for the Quantification of Dynamic Fluctuations of Nonelectroactive Analytes Using Fast-Scan Cyclic Voltammetry. Anal. Chem. 2013, 85, 8780–8786. [Google Scholar] [CrossRef] [PubMed]
- Soldà, A.; Valenti, G.; Marcaccio, M.; Giorgio, M.; Pelicci, P.G.; Paolucci, F.; Rapino, S. Glucose and Lactate Miniaturized Biosensors for SECM-Based High-Spatial Resolution Analysis: A Comparative Study. ACS Sens. 2017, 2, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Creager, S.E.; Olsen, K.G. Self-assembled monolayers and enzyme electrodes: Progress, problems and prospects. Anal. Chim. Acta 1995, 307, 277–289. [Google Scholar] [CrossRef]
- Zhao, F.; Conzuelo, F.; Hartmann, V.; Li, H.; Stapf, S.; Nowaczyk, M.M.; Rögner, M.; Plumeré, N.; Lubitz, W.; Schuhmann, W. A novel versatile microbiosensor for local hydrogen detection by means of scanning photoelectrochemical microscopy. Biosens. Bioelectron. 2017, 94, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Wilburn, J.P.; Ciobanu, M.; Cliffel, D.E. Scanning Electrochemical Microscopy of Individual Pancreatic Islets. J. Electrochem. Soc. 2016, 163, H3077–H3082. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Wagan, S.; Dávila Morris, M.; Taylor, J.; White, R.J. Achieving Reproducible Performance of Electrochemical, Folding Aptamer-Based Sensors on Microelectrodes: Challenges and Prospects. Anal. Chem. 2014, 86, 11417–11424. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Kelley, S.O. Tuning the Bacterial Detection Sensitivity of Nanostructured Microelectrodes. Anal. Chem. 2013, 85, 7333–7338. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Bard, A.J.; Nagy, G.; Toth, K. Scanning Electrochemical Microscopy. 28. Ion-Selective Neutral Carrier-Based Microelectrode Potentiometry. Anal. Chem. 1995, 67, 1346–1356. [Google Scholar] [CrossRef]
- Izquierdo, J.; Kiss, A.; Santana, J.J.; Nagy, L.; Bitter, I.; Isaacs, H.S.; Nagy, G.; Souto, R.M. Development of Mg2+ Ion-Selective Microelectrodes for Potentiometric Scanning Electrochemical Microscopy Monitoring of Galvanic Corrosion Processes. J. Electrochem. Soc. 2013, 160, C451–C459. [Google Scholar] [CrossRef]
- Ummadi, J.G.; Downs, C.J.; Joshi, V.S.; Ferracane, J.L.; Koley, D. Carbon-Based Solid-State Calcium Ion-Selective Microelectrode and Scanning Electrochemical Microscopy: A Quantitative Study of pH-Dependent Release of Calcium Ions from Bioactive Glass. Anal. Chem. 2016, 88, 3218–3226. [Google Scholar] [CrossRef] [PubMed]
- Filotás, D.; Asserghine, A.; Nagy, L.; Nagy, G. Short-term influence of interfering ion activity change on ion-selective micropipette electrode potential; another factor that can affect the time needed for imaging in potentiometric SECM. Electrochem. Commun. 2017, 77, 62–64. [Google Scholar] [CrossRef]
- Kiss, A.; Nagy, G. Deconvolution of potentiometric SECM images recorded with high scan rate. Electrochim. Acta 2015, 163, 303–309. [Google Scholar] [CrossRef]
- Yamada, H.; Haraguchi, D.; Yasunaga, K. Fabrication and characterization of a K+-selective nanoelectrode and simultaneous imaging of topography and local K+ flux using scanning electrochemical microscopy. Anal. Chem. 2014, 86, 8547–8552. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.A.S.; Özel, R.E.; Mak, W.H.; Mulato, M.; Singaram, B.; Pourmand, N. Single Cell “Glucose Nanosensor” Verifies Elevated Glucose Levels in Individual Cancer Cells. Nano Lett. 2016, 16, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Vilozny, B.; Actis, P.; Seger, R.A.; Vallmajo-Martin, Q.; Pourmand, N. Reversible Cation Response with a Protein-Modified Nanopipette. Anal. Chem. 2011, 83, 6121–6126. [Google Scholar] [CrossRef] [PubMed]
- Actis, P.; Mak, A.C.; Pourmand, N. Functionalized nanopipettes: Toward label-free, single cell biosensors. Bioanal. Rev. 2010, 1, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Umehara, S.; Karhanek, M.; Davis, R.W.; Pourmand, N. Label-free biosensing with functionalized nanopipette probes. Proc. Natl. Acad. Sci. USA 2009, 106, 4611–4616. [Google Scholar] [CrossRef] [PubMed]
- Actis, P.; Rogers, A.; Nivala, J.; Vilozny, B.; Seger, R.A.; Jejelowo, O.; Pourmand, N. Reversible thrombin detection by aptamer functionalized STING sensors. Biosens. Bioelectron. 2011, 26, 4503–4507. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Friedman, A.K.; Baker, L.A. Nanopore Sensing. Anal. Chem. 2017, 89, 157–188. [Google Scholar] [CrossRef] [PubMed]
- Coronado, R.; Latorre, R. Phospholipid bilayers made from monolayers on patch-clamp pipettes. Biophys. J. 1983, 43, 231–236. [Google Scholar] [CrossRef]
- Alvarez, O. Ion Channel Reconstitution. In Ion Channel Reconstitution; Miller, C., Ed.; Springer: New York, NY, USA, 1986; pp. 115–130. ISBN 978-1-4757-1363-3. [Google Scholar]
- Zhou, Y.; Bright, L.K.; Shi, W.; Aspinwall, C.A.; Baker, L.A. Ion Channel Probes for Scanning Ion Conductance Microscopy. Langmuir 2014, 30, 15351–15355. [Google Scholar] [CrossRef] [PubMed]
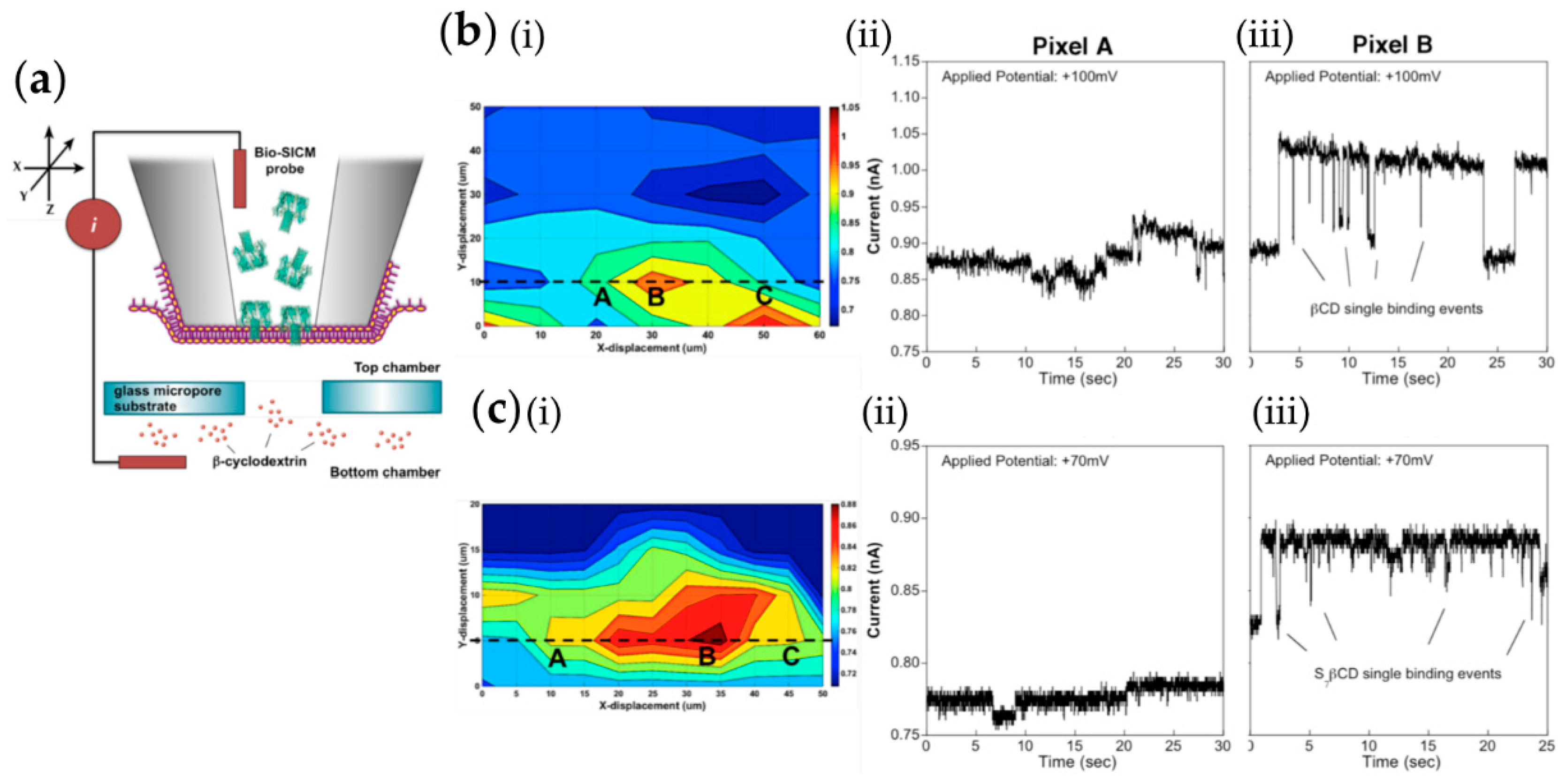
- Macazo, F.C.; White, R.J. Bioinspired Protein Channel-Based Scanning Ion Conductance Microscopy (Bio-SICM) for Simultaneous Conductance and Specific Molecular Imaging. J. Am. Chem. Soc. 2016, 138, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zeng, Y.; Zhou, L.; Xiao, Y.; Cummins, T.R.; Baker, L.A. Membrane patches as ion channel probes for scanning ion conductance microscopy. Faraday Discuss. 2016, 193, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Lazenby, R.A.; Macazo, F.C.; Wormsbecher, R.F.; White, R.J. Quantitative Framework for Stochastic Nanopore Sensors Using Multiple Channels. Anal. Chem. 2018, 90, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Bayley, H.; Braha, O.; Gu, L.Q. Stochastic sensing with protein pores. Adv. Mater. 2000, 12, 139–142. [Google Scholar] [CrossRef]
- Shi, W.; Zeng, Y.; Zhu, C.; Xiao, Y.; Cummins, T.R.; Hou, J.; Baker, L.A. Characterization of Membrane Patch-Ion Channel Probes for Scanning Ion Conductance Microscopy. Small 2018, 14, 1702945. [Google Scholar] [CrossRef] [PubMed]
- Macazo, F.C.; White, R.J. Monitoring Charge Flux to Quantify Unusual Ligand-Induced Ion Channel Activity for Use in Biological Nanopore-Based Sensors. Anal. Chem. 2014, 86, 5519–5525. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Li, C.; Shevchuk, A.I.; Stepanyan, R.; Caldwell, M.; Hughes, S.; Smart, T.G.; Gorelik, J.; Ostanin, V.P.; Lab, M.J.; et al. Nanoscale live-cell imaging using hopping probe ion conductance microscopy. Nat. Methods 2009, 6, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Lazenby, R.A.; McKelvey, K.; Unwin, P.R. Hopping intermittent contact-scanning electrochemical microscopy (HIC-SECM): Visualizing interfacial reactions and fluxes from surfaces to bulk solution. Anal. Chem. 2013, 85, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Nebel, M.; Eckhard, K.; Erichsen, T.; Schulte, A.; Schuhmann, W. 4D Shearforce-Based Constant-Distance Mode Scanning Electrochemical Microscopy. Anal. Chem. 2010, 82, 7842–7848. [Google Scholar] [CrossRef] [PubMed]
- Li, M.S.M.; Filice, F.P.; Ding, Z. Determining live cell topography by scanning electrochemical microscopy. J. Electroanal. Chem. 2016, 779, 176–186. [Google Scholar] [CrossRef]
- Filice, F.P.; Li, M.S.M.; Henderson, J.D.; Ding, Z. Mapping Cd2+-induced membrane permeability changes of single live cells by means of scanning electrochemical microscopy. Anal. Chim. Acta 2016, 908, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.D.; Filice, F.P.; Li, M.S.M.; Ding, Z. Tracking live cell response to cadmium (II) concentrations by scanning electrochemical microscopy. J. Inorg. Biochem. 2016, 158, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kuss, S.; Trinh, D.; Mauzeroll, J. High-Speed Scanning Electrochemical Microscopy Method for Substrate Kinetic Determination: Application to Live Cell Imaging in Human Cancer. Anal. Chem. 2015, 87, 8102–8106. [Google Scholar] [CrossRef] [PubMed]
- Kuss, S.; Trinh, D.; Danis, L.; Mauzeroll, J. High-Speed Scanning Electrochemical Microscopy Method for Substrate Kinetic Determination: Method and Theory. Anal. Chem. 2015, 87, 8096–8101. [Google Scholar] [CrossRef] [PubMed]
- Kuss, S.; Kuss, C.; Trinh, D.; Schougaard, S.B.; Mauzeroll, J. Forced convection during scanning electrochemical microscopy imaging over living cells: Effect of topographies and kinetics on the microelectrode current. Electrochim. Acta 2013, 110, 42–48. [Google Scholar] [CrossRef]
- Ida, H.; Takahashi, Y.; Kumatani, A.; Shiku, H.; Matsue, T. High Speed Scanning Ion Conductance Microscopy for Quantitative Analysis of Nanoscale Dynamics of Microvilli. Anal. Chem. 2017, 89, 6015–6020. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Nagy, G. New SECM scanning algorithms for improved potentiometric imaging of circularly symmetric targets. Electrochim. Acta 2014, 119, 169–174. [Google Scholar] [CrossRef]
- Zhuang, J.; Jiao, Y.; Mugabo, V. A new scanning mode to improve scanning ion conductance microscopy imaging rate with pipette predicted movement. Micron 2017, 101, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ebejer, N.; Schnippering, M.; Colburn, A.W.; Edwards, M.A.; Unwin, P.R. Localized High Resolution Electrochemistry and Multifunctional Imaging: Scanning Electrochemical Cell Microscopy. Anal. Chem. 2010, 82, 9141–9145. [Google Scholar] [CrossRef] [PubMed]
- Bentley, C.L.; Kang, M.; Unwin, P.R. Nanoscale Structure Dynamics within Electrocatalytic Materials. J. Am. Chem. Soc. 2017, 139, 16813–16821. [Google Scholar] [CrossRef] [PubMed]
- Bentley, C.L.; Kang, M.; Maddar, F.M.; Li, F.; Walker, M.; Zhang, J.; Unwin, P.R. Electrochemical maps and movies of the hydrogen evolution reaction on natural crystals of molybdenite (MoS2): Basal vs. edge plane activity. Chem. Sci. 2017, 8, 6583–6593. [Google Scholar] [CrossRef] [PubMed]
- Momotenko, D.; Byers, J.C.; McKelvey, K.; Kang, M.; Unwin, P.R. High-Speed Electrochemical Imaging. ACS Nano 2015, 9, 8942–8952. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Trouillon, R.; Lin, Y.; Svensson, M.I.; Ewing, A.G. Individually Addressable Thin-Film Ultramicroelectrode Array for Spatial Measurements of Single Vesicle Release. Anal. Chem. 2013, 85, 5600–5608. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Trouillon, R.; Dunevall, J.; Ewing, A.G. Spatial Resolution of Single-Cell Exocytosis by Microwell-Based Individually Addressable Thin Film Ultramicroelectrode Arrays. Anal. Chem. 2014, 86, 4515–4520. [Google Scholar] [CrossRef] [PubMed]
- Wigström, J.; Dunevall, J.; Najafinobar, N.; Lovrić, J.; Wang, J.; Ewing, A.G.; Cans, A.-S. Lithographic Microfabrication of a 16-Electrode Array on a Probe Tip for High Spatial Resolution Electrochemical Localization of Exocytosis. Anal. Chem. 2016, 88, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Schierbaum, N.; Hack, M.; Betz, O.; Schäffer, T.E. Macro-SICM: A Scanning Ion Conductance Microscope for Large-Range Imaging. Anal. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Ino, K.; Li, C.-Z.; Kanno, Y.; Inoue, K.Y.; Suda, A.; Kunikata, R.; Matsudaira, M.; Takahashi, Y.; Shiku, H.; et al. Electrochemical Imaging of Dopamine Release from Three-Dimensional-Cultured PC12 Cells Using Large-Scale Integration-Based Amperometric Sensors. Anal. Chem. 2015, 87, 6364–6370. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Sen, M.; Shiku, H.; Matsue, T. Micro/nanoelectrochemical probe and chip devices for evaluation of three-dimensional cultured cells. Analyst 2017, 142, 4343–4354. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Ino, K.; Abe, H.; Sakamoto, C.; Onodera, T.; Inoue, K.Y.; Suda, A.; Kunikata, R.; Matsudaira, M.; Shiku, H.; et al. Electrochemicolor Imaging Using an LSI-Based Device for Multiplexed Cell Assays. Anal. Chem. 2017, 89, 12778–12786. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Reitz, A.; Mathault, J.; Mehou-Loko, S.; Amirdehi, M.A.; Miled, A.; Greener, J. Electrochemical imaging for microfluidics: A full-system approach. Lab Chip 2016, 16, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Wydallis, J.B.; Feeny, R.M.; Wilson, W.; Kern, T.; Chen, T.; Tobet, S.; Reynolds, M.M.; Henry, C.S. Spatiotemporal norepinephrine mapping using a high-density CMOS microelectrode array. Lab Chip 2015, 15, 4075–4082. [Google Scholar] [CrossRef] [PubMed]
- Mensack, M.M.; Wydallis, J.B.; Lynn, N.S.; Dandy, D.S.; Henry, C.S. Spatially resolved electrochemical sensing of chemical gradients. Lab Chip 2013, 13, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.E.; Joo, H.R.; Fan, J.L.; Liu, D.F.; Barnett, A.H.; Chen, S.; Geaghan-Breiner, C.; Karlsson, M.P.; Karlsson, M.; Lee, K.Y.; et al. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. bioRxiv 2018, 242693. [Google Scholar] [CrossRef]
- Szostak, K.M.; Grand, L.; Constandinou, T.G. Neural interfaces for intracortical recording: Requirements, fabrication methods, and characteristics. Front. Neurosci. 2017, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Felix, S.H.; Shah, K.G.; Tolosa, V.M.; Sheth, H.J.; Tooker, A.C.; Delima, T.L.; Jadhav, S.P.; Frank, L.M.; Pannu, S.S. Insertion of Flexible Neural Probes Using Rigid Stiffeners Attached with Biodissolvable Adhesive. J. Vis. Exp. 2013, 79, e50609. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.T.-C.; Monbouquette, H.G. Implantable microprobe with arrayed microsensors for combined amperometric monitoring of the neurotransmitters, glutamate and dopamine. J. Electroanal. Chem. 2012, 682, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Şen, M.; Ino, K.; Inoue, K.Y.; Arai, T.; Nishijo, T.; Suda, A.; Kunikata, R.; Shiku, H.; Matsue, T. LSI-based amperometric sensor for real-time monitoring of embryoid bodies. Biosens. Bioelectron. 2013, 48, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Ino, K.; Inoue, K.Y.; Şen, M.; Suda, A.; Kunikata, R.; Matsudaira, M.; Abe, H.; Li, C.-Z.; Shiku, H.; et al. Feedback mode-based electrochemical imaging of conductivity and topography for large substrate surfaces using an LSI-based amperometric chip device with 400 sensors. J. Electroanal. Chem. 2015, 741, 109–113. [Google Scholar] [CrossRef]
- Kanno, Y.; Ino, K.; Sakamoto, C.; Inoue, K.Y.; Matsudaira, M.; Suda, A.; Kunikata, R.; Ishikawa, T.; Abe, H.; Shiku, H.; et al. Potentiometric bioimaging with a large-scale integration (LSI)-based electrochemical device for detection of enzyme activity. Biosens. Bioelectron. 2016, 77, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.N.; Herbst, A.D.; Kim, S.J.; Minch, B.A.; Lindau, M. Parallel recording of neurotransmitters release from chromaffin cells using a 10 × 10 CMOS IC potentiostat array with on-chip working electrodes. Biosens. Bioelectron. 2013, 41, 736–744. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazenby, R.A.; White, R.J. Advances and Perspectives in Chemical Imaging in Cellular Environments Using Electrochemical Methods. Chemosensors 2018, 6, 24. https://doi.org/10.3390/chemosensors6020024
Lazenby RA, White RJ. Advances and Perspectives in Chemical Imaging in Cellular Environments Using Electrochemical Methods. Chemosensors. 2018; 6(2):24. https://doi.org/10.3390/chemosensors6020024
Chicago/Turabian StyleLazenby, Robert A., and Ryan J. White. 2018. "Advances and Perspectives in Chemical Imaging in Cellular Environments Using Electrochemical Methods" Chemosensors 6, no. 2: 24. https://doi.org/10.3390/chemosensors6020024
APA StyleLazenby, R. A., & White, R. J. (2018). Advances and Perspectives in Chemical Imaging in Cellular Environments Using Electrochemical Methods. Chemosensors, 6(2), 24. https://doi.org/10.3390/chemosensors6020024




