Abstract
Magnesium ions (Mg2+) play an important role in mammalian cell function; however, relatively little is known about the mechanisms of Mg2+ regulation in disease states. An advance in this field would come from the development of selective, reversible fluorescent chemosensors, capable of repeated measurements. To this end, the rational design and fluorescence-based photophysical characterisation of two spiropyran-based chemosensors for Mg2+ are presented. The most promising analogue, chemosensor 1, exhibits 2-fold fluorescence enhancement factor and 3-fold higher binding affinity for Mg2+ (Kd 6.0 µM) over Ca2+ (Kd 18.7 µM). Incorporation of spiropyran-based sensors into optical fibre sensing platforms has been shown to yield significant signal-to-background changes with minimal sample volumes, a real advance in biological sensing that enables measurement on subcellular-scale samples. In order to demonstrate chemosensor compatibility within the light intense microenvironment of an optical fibre, photoswitching and photostability of 1 within a suspended core optical fibre (SCF) was subsequently explored, revealing reversible Mg2+ binding with improved photostability compared to the non-photoswitchable Rhodamine B fluorophore. The spiropyran-based chemosensors reported here highlight untapped opportunities for a new class of photoswitchable Mg2+ probe and present a first step in the development of a light-controlled, reversible dip-sensor for Mg2+.
1. Introduction
Magnesium ions (Mg2+) play an important role in mammalian cell function [1,2,3,4], as an enzymatic cofactor [5], regulator of cellular ion channels [6,7,8] and energy metabolism [9]. Conditions such as Type 2 Diabetes [10,11,12], Alzheimer’s [13,14] and cardiovascular disease [15,16] have been linked with magnesium deficiency, however the mechanisms of Mg2+ regulation in such disease states are poorly understood [17,18]. Fluorescent chemosensors, in combination with specialised imaging technologies, provide a useful tool to study the role of metal ions in cellular processes as they enable detection in and around cells with spatial and temporal resolution [19].
Mag-fura-2 (FURAPTRA) [20,21] is one such commercially available chemosensor for Mg2+, based on a benzofuran fluorophore scaffold and functionalised with the O-aminophenol-N,N,O-triacetic acid (APTRA) Mg2+ chelator [22]. Mag-fura-2 has been shown to detect cytosolic Mg2+ in various cells [23,24,25], however as it is structurally analogous to the calcium (Ca2+) chelator fura-2, intracellular Mg2+ detection is complicated by an affinity for Ca2+ [26,27]. More recently, other probes based on fluorophore scaffolds such as fluorescein and rhodamine have been reported to display improved selectivity [28,29,30]. For example, the KMG series of chemosensors possess a bidentate, charged beta-diketone binding domain, which gives rise to excellent Mg2+ selectivity over Ca2+ (Figure 1B) [29]. Similar selectivity has been observed with the MGQ series, which possess a range of tridentate carboxy binding domains (Figure 1C) [30]. These studies represent significant developments in the field of Mg2+ detection, but further work is required in order to advance this area [31,32].
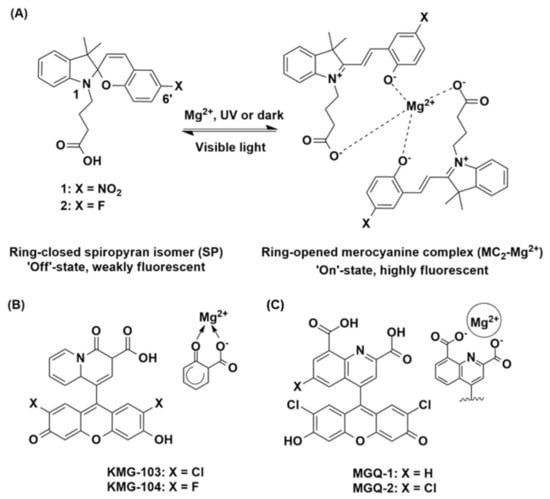
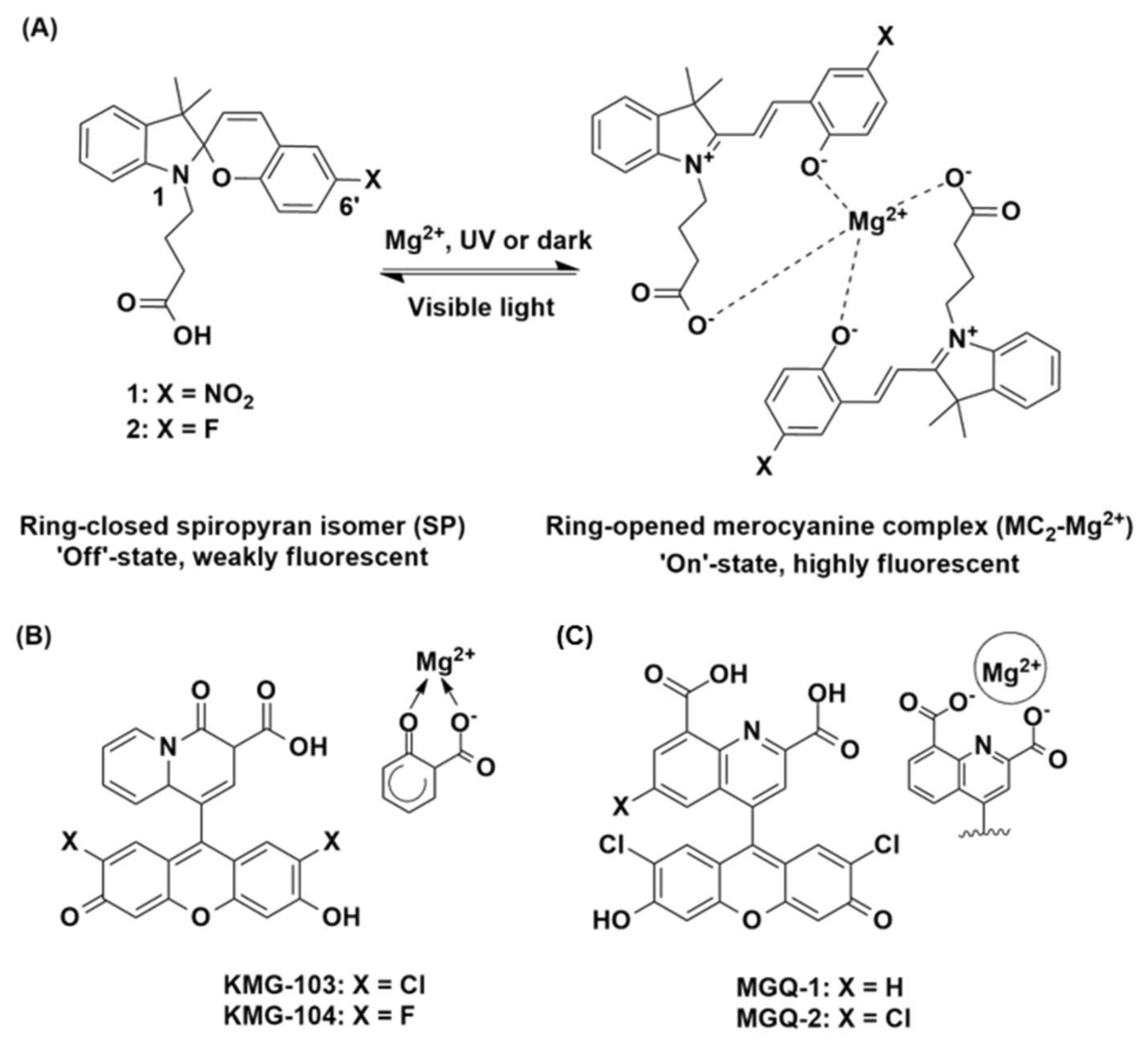
Figure 1.
(A) Structures of 1 and 2 as the ring-closed, weakly fluorescent spiropyran (SP, ‘off’) isomer, and the proposed ring-opened, highly fluorescent merocyanine (MC2-Mg2+, ‘on’) complex. Structures of the (B) KMG [29] and (C) MGQ [30] chemosensors with the proposed Mg2+-binding domains highlighted.
For example, the ability to turn metal ion sensing on and off, with an external and non-invasive stimulus such as light, would extend the range of sensing capability for live-cell imaging. A photoswitchable spiropyran presents as one such sensing moiety, where these structures form the basis of a chemosensor when functionalised with a suitable ionophore that is capable of complexing with a metal ion [33,34]. Photo-controlled switching between the weakly fluorescent spiropyran (SP) and highly fluorescent merocyanine (MC) isomers occurs on irradiation with UV light or in the presence of the target metal ion, while visible light reverses the isomerisation (see 1 and 2 Figure 1A) [35,36,37]. Recently, we have reported the combination of spiropyran-based chemosensors with microstructured optical fibres for the nanoscale detection of metal ions [38,39,40]. These systems have been shown to yield significant signal-to-background changes with minimal sample volumes, a real advance in biological sensing that enables measurement on subcellular-scale samples. Importantly, spiropyrans are among the most stable photoswitches in the light intense microenvironment of an optical fibre [41]. The fibre also provides a platform for sensing metal ions in confined spaces such as the medium surrounding cell clusters, oocytes and embryos, and in the in vivo environment [42,43,44].
Here we present two rationally designed, spiropyran-based chemosensors for Mg2+ (1 and 2, Figure 1A). Chemosensor 1 consists of a photoswitchable spiropyran fluorophore scaffold, functionalised with a butanoic acid at the N1-indole. The molecule is designed to chelate Mg2+ through the free carboxyl group, in combination with the phenoxide of the ring-opened merocyanine isomer. We envisaged that 1 would bind Mg2+ in a 2:1 chemosensor to metal ion ratio (depicted in Figure 1A), in a design inspired by the Mg2+-selective, literature chemosensors described above [28,29,30]. An electron withdrawing NO2 group was incorporated at the C6’ position of the benzopyran ring, since such groups are known to further promote metal ion binding by stabilising the ring-opened MC isomer [45]. The fluorinated analogue 2 was also investigated as a moderately electron withdrawing fluoro-substituent is known to give rise to relatively high fluorescence yields compared to a nitro analogue [39]. It is interesting to note that while absorbance-based photophysical studies have previously been reported on 1 [33,46], the fluorescence properties of this chemosensor in the presence of Mg2+ are unknown. As such, this work reports on the rational design and photophysical characterisation of the first spiropyran-based fluorescence chemosensors for Mg2+ and demonstrates the photo-compatibility of chemosensor 1 within a suspended core optical fibre (SCF) as a first step in the development of a light-controlled, reversible dip-sensor for Mg2+.
2. Materials and Methods
All 13C-NMR and 1H-NMR spectra were recorded on an Agilent Technologies 500 MHz NMR with DD2 console in CD3CN or DMSO-d6 (Cambridge Isotope Laboratories, Cambridge, MA, USA). Chemical shifts (δ) are reported in ppm. Chemical shifts of CD3CN (δC = 118.26 ppm), DMSO-d6 (δC = 39.52 ppm) or TMS (δH = 0.0 ppm) were used as internal standards in all 13C-NMR and 1H-NMR experiments, respectively. High resolution mass spectrometry was performed on the Agilent 6230 TOF LC-MS. HPLC grade acetonitrile was used in all related experiments. All metal ions used in this work were in the form of perchlorate salt, except for Cs2SO4. All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification.
2.1. Chemical Syntheses
2.1.1. Ethyl 4-(3′,3′-Dimethyl-6-fluorospiro[chromene-2,2′-indolin]-1′-yl)butanoate (4)
To a solution of 5-fluoro-2-hydroxybenzaldehyde 8 (44 mg, 0.31 mmol) in dry ethanol (5 mL) was added 1-(4-ethoxy-4-oxobutyl)-2,3,3-trimethyl-3H-indolium bromide 6 (100 mg, 0.28 mmol) and the reaction heated at reflux for 18 h. The solvent was removed in vacuo to give an orange crude oil (150 mg). The crude compound was purified on normal-phase silica by gradient column chromatography in 0–10% methanol in dichloromethane to give 4 as a yellow oil (44 mg, 39%). 1H-NMR (500 MHz, DMSO-d6) δ 7.10–7.06 (m, 3H, ArH), 6.97 (d, 1H, ArH, J = 10.5 Hz), 6.90 (td, 1H, ArH, J = 8.5, 3.5 Hz), 6.75 (t, 1H, ArH, J = 7.5 Hz), 6.65 (dd, 1H, ArH, J = 8.5, 4.5 Hz), 6.61 (d, 1H, ArH, J = 8.0 Hz), 5.84 (d, 1H, ArH, J = 7.5 Hz), 4.00 (q, 2H, CH2, J = 7.5 Hz), 3.19–3.12 (m, 1H, CHH), 3.10–3.04 (m, 1H, CHH), 2.35–2.30 (m, 2H, CH2), 1.86–1.80 (m, 1H, CHH), 1.78–1.72 (m, 1H, CHH), 1.19 (s, 3H, CH3), 1.13 (t, 3H, CH3, J = 7.5 Hz), 1.07 (s, 3H, CH3) ppm; 13C-NMR (126 MHz, DMSO-d6) δ 172.6, 156.8, 155.0, 149.8, 147.0, 135.9, 128.5, 128.4, 127.4, 121.6, 121.1, 118.6, 112.9, 112.7, 106.3, 104.3, 59.7, 51.8, 42.3, 31.0, 25.7, 23.7, 19.7, 14.0 ppm; HRMS-ESI (m/z) calculated for C24H26FNO3 [M + Na]+ 418.1789, found 418.1769.
2.1.2. Ethyl 4-(3′,3′-Dimethyl-6-nitrospiro[chromene-2,2′-indolin]-1′-yl)butanoate (3)
To a solution of 2-hydroxy-5-nitrobenzaldehyde 7 (52 mg, 0.31 mmol) in dry ethanol (5 mL) was added 6 (100 mg, 0.28 mmol) and the solution heated at reflux for 18 h. The solvent was removed in vacuo to give a brown crude solid (156 mg). The crude compound was purified on normal-phase silica by gradient column chromatography in 0–10% methanol in dichloromethane to give 3 as a purple solid (64 mg, 54%). 1H-NMR (500 MHz, DMSO-d6) δ 8.21 (d, 1H, ArH, J = 3.0 Hz,), 7.99 (dd, 1H, ArH, J = 2.5, 8.5 Hz), 7.20 (d, 1H, ArH, J = 10.0 Hz), 7.13–7.10 (m, 2H, ArH), 6.85 (d, 1H, ArH, J = 9.0 Hz), 6.79 (t, 1H, ArH, J = 7.5 Hz), 6.66 (d, 1H, ArH, J = 8.0 Hz), 5.98 (d, 1H, ArH, J = 10.0 Hz), 4.00 (q, 2H, CH2, J = 7.0 Hz), 3.20–3.10 (m, 2H, CH2), 2.36–2.30 (m, 2H, CH2), 1.86–1.81 (m, 1H, CHH), 1.76–1.71 (m, 1H, CHH), 1.19 (m, 3H, CH3), 1.13–1.09 (m, 6H, 2 × CH3) ppm; 13C-NMR (126 MHz, DMSO-d6) δ 172.6, 159.1, 146.7, 140.5, 135.6, 129.6, 128.1, 127.6, 125.7, 122.8, 121.7, 121.6, 119.1, 115.4, 106.5, 106.4, 59.8, 52.3, 42.3, 30.9, 25.8, 23.6, 19.6, 14.0 ppm; HRMS-ESI (m/z) calculated for C24H26N2O5 found [M + Na]+ 445.1734, found 445.1720.
2.1.3. 4-(6-Fluoro-3′,3′-dimethylspiro[chromene-2,2′-indolin]-1′-yl)butanoic acid (2)
To a solution of 4 (200 mg, 0.51 mmol) in methanol (10 mL) was added 2 M aqueous NaOH (5 mL) and the reaction heated at 50 °C for 5 h. The solvent was removed in vacuo to give an orange solid (1.362 g). A sample of the crude material (100 mg) was purified by reverse-phase HPLC eluting water and acetonitrile (60–100%) to give a purple solid consisting of 2 in mixture with its MC isomer (41 mg); 1H-NMR (500 MHz, CD3CN/DMSO-d6) selected data for SP isomer from mixture δ 7.08–6.48 (m, aromH), 6.84 (d, 1H, CH, J = 10.0 Hz), 5.83 (d, 1H, CH, J = 10.0 Hz), 3.18–3.12 (m, 2H, CH2), 2.31 (d, 2H, CH2, J = 8.0 Hz), 1.74–1.69 (m, CH2), 1.18 (s, 3H, CH3), 1.06 (s, 3H, CH3); selected data for the MC isomer from mixture δ 7.08–6.48 (m, aromH), 4.46 (d, 1H, CHH, J = 10.0 Hz), 4.12 (m, 1H, CHH), 2.06–2.03 (m, 2H, CH2), 1.74–1.69 (m, CH2), 1.23 (s, 3H, CH3), 1.19 (s, 3H, CH3); HRMS-ESI (m/z) calculated for C22H22FNO3 [M + H]+ 368.1656, found 368.1646.
2.1.4. 4-(3′,3′-Dimethyl-6-nitrospiro[chromene-2,2′-indolin]-1′-yl)butanoic acid (1)
To a solution of 3 (224 mg, 0.53 mmol) in methanol (10 mL) was added 2M aqueous NaOH (5 mL) and the reaction heated at 50 °C for 5 h. The solvent was removed in vacuo to give an orange solid (1.441 g). The crude material was purified by reverse-phase HPLC eluting with water and acetonitrile (60–100%) to give 1 as an orange solid (104 mg, 50%). 1H-NMR (500 MHz, CD3CN) δ 8.06 (d, 1H, ArH, J = 2.5 Hz,), 7.98 (dd, 1H, ArH, J = 3.0, 9.0 Hz), 7.14 (t, 1H, ArH, J = 7.5 Hz), 7.10 (d, 1H, ArH, J = 10.0 Hz), 7.02 (d, 1H, ArH, J = 10.0 Hz), 6.83 (t, 1H, ArH, J = 7.5 Hz), 6.70 (d, 1H, ArH, J = 9.0 Hz), 6.68 (d, 1H, ArH, J = 7.5 Hz), 5.96 (d, 1H, ArH, J = 10.5 Hz), 3.24–3.11 (m, 2H, CH2), 2.31–2.27 (m, 2H, CH2), 1.92–1.76 (m, 2H, CH2), 1.24 (s, 3H, CH3), 1.14 (s, 3H, CH3) ppm; 13C-NMR (126 MHz, CD3CN) δ 174.6, 160.4, 148.2, 142.1, 137.10, 129.1, 128.7, 126.6, 123.7, 122.8, 122.7, 120.4, 120.0, 116.2, 107.9, 107.8, 53.3, 43.6, 31.5, 26.3, 24.7, 20.00 ppm; HRMS-ESI (m/z) calculated for C22H22N2O5 [M + H]+ 395.1579, found 395.1587.
2.2. Spectroscopic Analyses of Chemosensors 1–4
Stock solutions of spiropyrans (5 mM) were prepared in HPLC-grade acetonitrile. Stock solutions of metal ion salts (10 mM) were prepared in water from dried perchlorate salts of Li+, Na+, K+, Mg2+, Ca2+, Mn2+, Cu2+, Zn2+, and Cs+ (from Cs2SO4). For the selectivity studies, solutions were prepared (in triplicate) on the same microplate tray from 2 µL spiropyran and 2 µL of ion stock solutions, such that each replicate contained a 2:1 molar ratio of ion: spiropyran. 196 µL of HPLC grade acetonitrile was then added to dilute each replicate, such that the final concentrations of spiropyran and ions in each solution were 50 µM and 100 µM, respectively. The microplate tray was then incubated in the dark for 10 mins before reading. Absorbance and fluorescence spectra were recorded between 300 and 700 nm, and 555 and 800 nm, respectively, at 25 °C using a BioTek Synergy H4 Hybrid Multi-Mode Microplate Reader. The scanning resolution was 5 nm, with a band pass of 9 nm. Fluorescence excitation was at 532 nm, with 100 gain setting for all chemosensors. All absorbance and fluorescence measurements were repeated in triplicate. The error bars presented represent a standard deviation about the mean value. Further details of the solution-based fluorescence experiments are reported in the Supporting Information.
2.3. Photoswitching in Optical Fibre
A 5 mW laser was coupled into the core of a suspended core fibre (SCF) via dichroic mirror at a wavelength of 532 nm using 60× objective as described previously [39]. One end of the fibre was filled by capillary action by dipping in an acetonitrile solution containing chemosensor 1 (500 µM) and Mg2+ (1 mM). The chemosensor-filled SCF was then exposed to a 532 nm green laser for 2 min, and fluorescence was measured after excitation with the same 532 nm laser (10 × 50 ms). Next, the SCF was exposed to UV light (365 nm) for 5 min to facilitate photoswitching to the ring-opened MC(1)-Mg2+ complex, and fluorescence was measured again after excitation with the 532 nm green laser (10 × 50 ms). This process was repeated for 10 cycles to investigate photoswitching of 1 in the presence of Mg2+. Fluorescence of Rhodamine B was similarly recorded; however, UV irradiation was not required for the ‘on’ cycles as this fluorophore does not photoswitch.
3. Results and Discussion
3.1. DFT Modelling
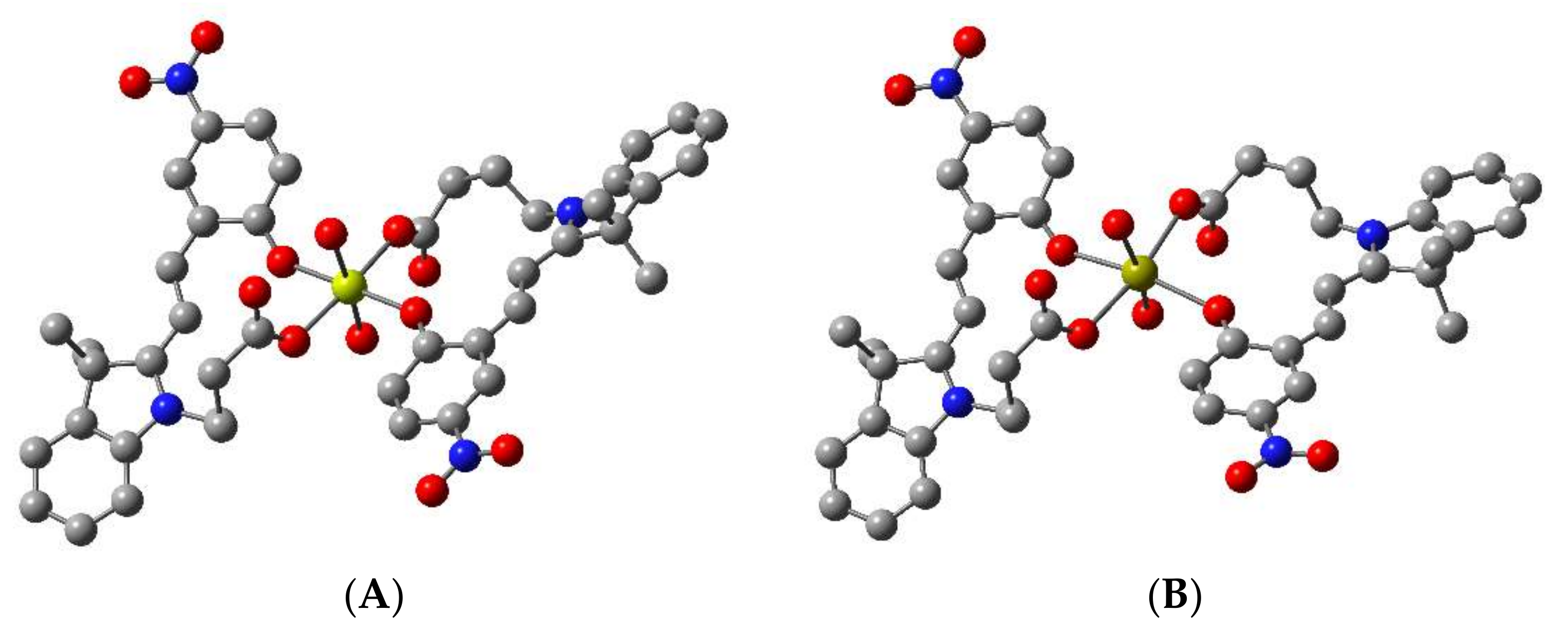
Structures of chemosensors 1 and 2, bound with both Mg2+ and Ca2+, were calculated by density functional theory based on a 2:1 binding of ring opened merocyanine isomer to metal ion (see Figure 1). This proposed coordination geometry has precedent for similarly functionalised spiropyran-based chemosensors with divalent metal ions [47,48,49]. Two water molecules were included in the initial calculations to provide additional coordinating sites, based on the metal-bound crystal structure reported for a similar spiropyran [47]. Geometry optimised structures were obtained using the B3LYP functionals [50] and 6-311G** basis set for all atoms, within the Gaussian09 package [51].
The optimised structures for chemosensor 1 bound to Mg2+ and Ca2+ are presented in Figure 2. The resulting complexes of 1 bound with Mg2+ and Ca2+ are both hexa-coordinated, such that the ion is bound by two, bidentate MC ligands, with two water molecules fulfilling the coordination of the metal in each case. The Mg2+···O distances are between 2.07 and 2.13 Å, and Ca2+···O distances are between 2.37 and 2.46 Å. The reaction energies for the formation of the M[1(MC)2(H2O)2] species were calculated to be −299.0 kJ/mol and −137.8 kJ/mol for Mg2+ and Ca2+, respectively. Similar results were observed for the fluorinated chemosensor 2 (see Figure S9). Taken together, the more negative ΔG298 for the formation of the Mg[1(MC)2(H2O)2] and Mg[2(MC)2(H2O)2], and the shorter Mg2+···O distances compared with Ca2+ analogues in this study suggest stronger binding of the Mg2+ compared to the Ca2+ by chemosensors 1 and 2.
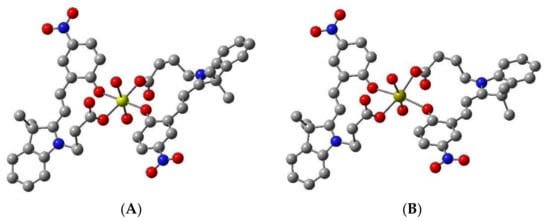
Figure 2.
B3LYP/6-311G** optimised structure of 1 bound to (A) Mg2+ (yellow) and (B) Ca2+ (gold), respectively, in a 2:1 ratio, showing oxygen atoms (red) chelating to the metal ion. Hydrogen atoms are omitted for clarity.
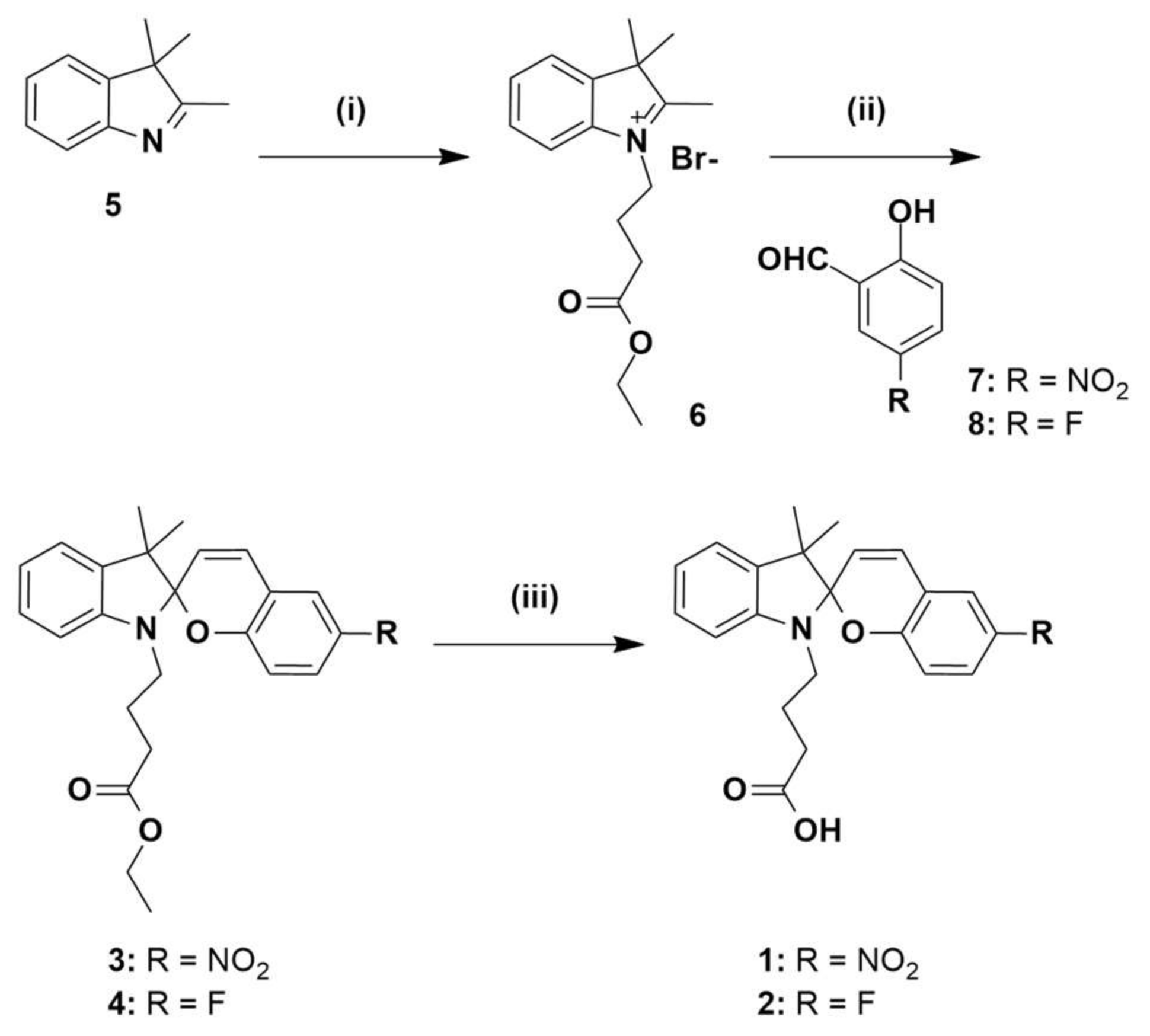
3.2. Synthesis
Compounds 1 and 2 were prepared as outlined in Scheme 1. Commercially available 3,3-dimethyl-2-methyleneindoline 5 was alkylated with ethyl-4-bromobutanoate to give the previously reported indoline 6 [38]. Separate condensation reactions of 2-hydroxy-5-nitrobenzaldehyde 7 or 5-fluoro-2-hydroxybenzaldehyde 8, with indoline 6 in refluxing ethanol, gave the ester-protected spiropyrans 3 and 4, respectively which were fully characterised by 1H, 13C-NMR and HRMS (see Figures S1–S4 and S8). Spiropyrans 3 and 4 were then separately treated with 2 M aqueous NaOH in order to hydrolyse the ester protecting groups, and the crude, free-acid products were then purified by reverse-phase HPLC, respectively. The resulting compounds were characterised by 1H, 13C-NMR and HRMS, with details reported in Supporting Information (Figures S5–S8). The proton NMR spectrum of 1 shows the chemosensor fully in the ring-closed SP form, as is consistent with previous studies [33]. Interestingly, the proton NMR spectrum of 2 suggests a mixture of SP and MC isomers. Based on this, subsequent work was focused on chemosensor 1.
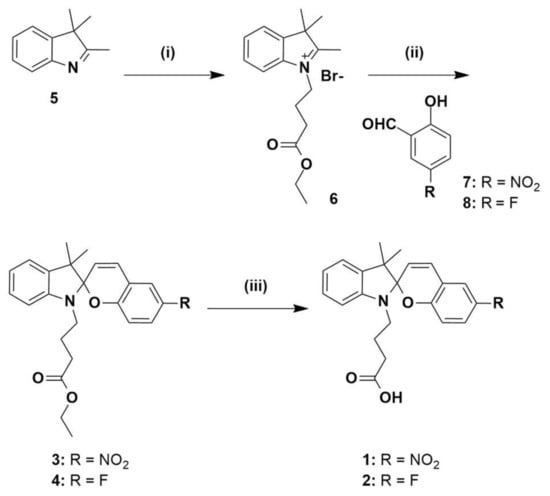
Scheme 1.
Synthesis of chemosensors 1 and 2. Reagents and conditions: (i) ethyl-4-bromobutyrate, CHCl3, reflux 24 h; (ii) EtOH, reflux 18 h; (iii) 2 M NaOH, MeOH, 50 °C, 5 h.
3.3. Sensor Characterisation
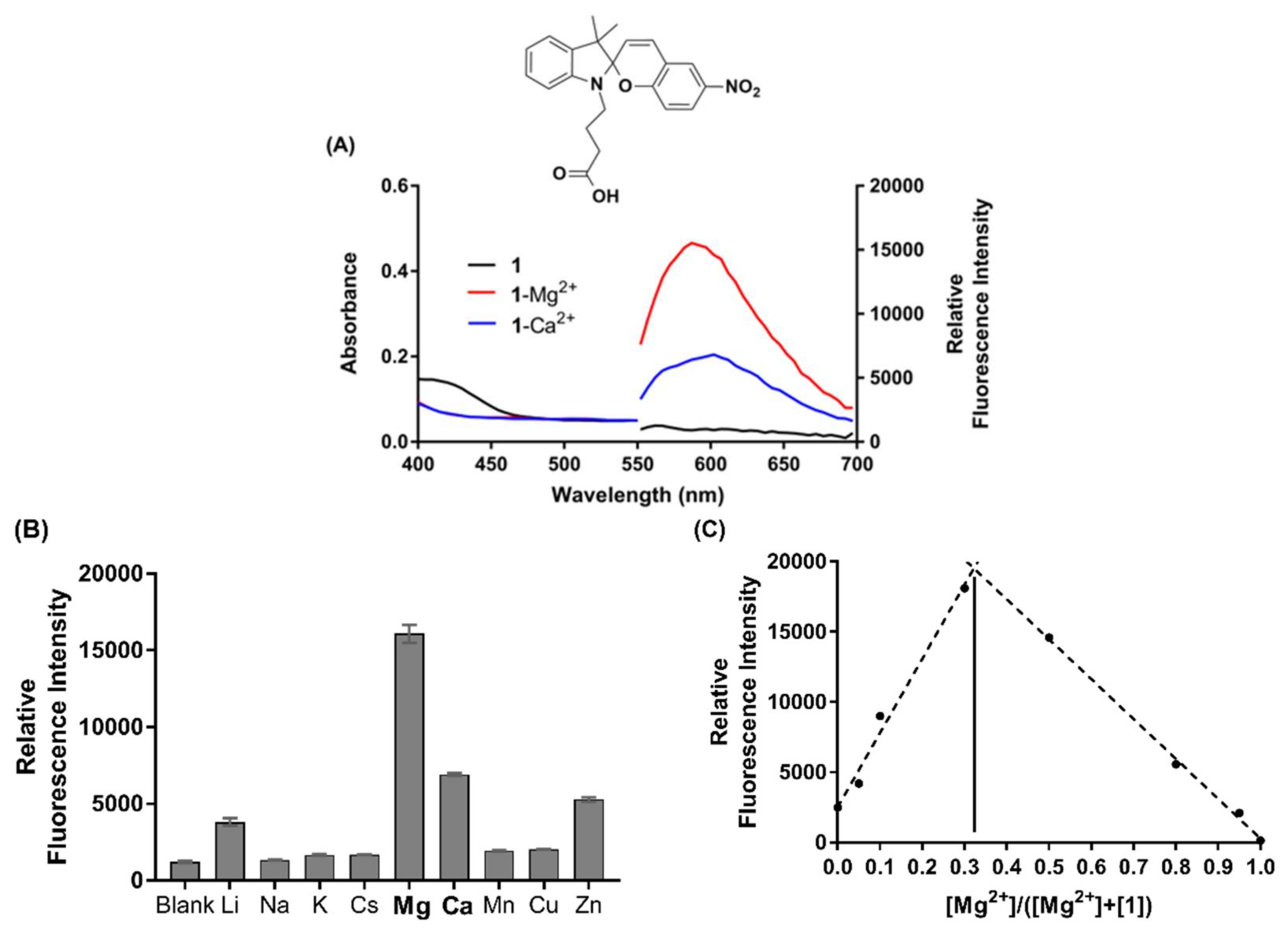
Absorbance and fluorescence emission spectra of chemosensor 1 (50 µM in acetonitrile) were first measured in the presence of an excess of Mg2+ (100 µM), as well as other biologically relevant metal ions (Li+, Na+, K+, Cs+, Ca2+, Mn2+, Cu2+ and Zn2+), to characterise its selectivity profile. Spectra were recorded in ambient light conditions, in order to characterise the metal-induced SP to MC isomerisation [36,37]. Results displayed in Figure 3 show that chemosensor 1 has the highest fluorescence emission in the presence of Mg2+, 2-fold higher than that of Ca2+ and 3–4-fold higher than for Li+ and Zn2+. In the presence of excess Mg2+, 1 gives an emission maximum at 590 nm, hypsochromically shifted compared to the chemosensor in the presence of Ca2+ (605 nm) or in the absence of metal ions (605 nm). These maxima are characteristic of the MC-form of the spiropyran, and thus, metal ion binding induces isomerisation of chemosensor 1 to the more coloured MC(1)-M2+ complex [36]. As has been reported previously [33], chemosensor 1 exhibits a significant increase in the characteristic MC absorbance in the prescence of Cu2+ (Figure S10), while the absorbance spectra of the 1-Mg2+ and 1-Ca2+ species are similar to the SP form of 1 under ambient light conditions (see Figure 3A) [36].
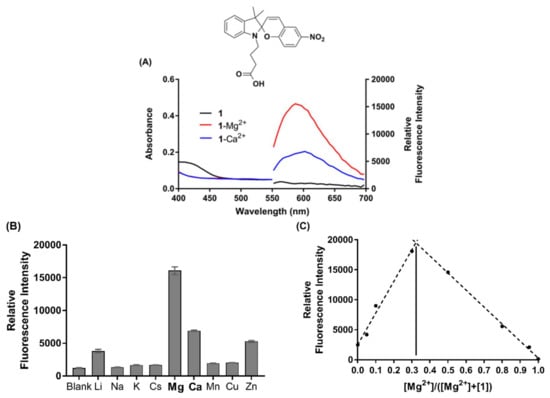
Figure 3.
(A) Absorbance and fluorescence emission spectra of chemosensor 1 in the absence (black, 50 µM) and presence of excess Mg2+ (red, 100 µM) and Ca2+ (blue, 100 µM), respectively. (B) Selectivity profile of 1 (50 µM) in the presence of various biologically relevant metal ions (100 µM). (C) Job’s plot analysis of MC(1)-Mg2+ complex, where [SP] + [Mg2+] = 100 µM in acetonitrile. Excitation was at 532 nm, and all experiments were performed under ambient light conditions.
Data pertaining to the photophysical characterisation of chemosensor 1 is presented in Table 1. The binding stoichiometry of 1 in the presence of excess Mg2+ was next defined by Job’s plot analysis, as displayed in Figure 3C. Chemosensor 1 binds Mg2+ in a 2:1 ratio (Job’s plot apex at 0.33), in agreement with the design proposal and DFT modelling discussed above. Similarly, Job’s analysis showed that 1 also binds Ca2+ in a 2:1 ratio (apex at 0.33, Figure S11). Dissociation constants (Kd) for the binding of 1 with Mg2+ and Ca2+, respectively, were determined by fitting a saturation binding model to concentration curves of the chemosensor (50 µM) with increasing concentrations of metal ions (1–200 µM) (Figure S12). The dissociation constant for chemosensor 1 with Mg2+ was calculated to be 6.0 µM, and a 3-fold weaker affinity was observed with Ca2+ (Kd = 18.7 µM). Finally, the quantum yields of fluorescence for chemosensor 1 in the presence of Mg2+ (Φ = 0.20) and Ca2+ (Φ = 0.06) were determined via the method described in the Supporting Information, using Rhodamine B as the calibration standard (Figure S13) [52].

Table 1.
Photophysical properties of Chemosensor 1.
The absorbance and fluorescence emission spectra for the fluorinated chemosensor 2 were similarly analysed. Fluorescence emission spectra gave two distinct emission maxima, which are attributable to the SP (~560 nm) and MC (~605 nm) isomers of chemosensor 2 [36] and suggest the presence of both these species in solution (Figure S14), as per the earlier proton NMR spectrum. The chemosensor shows a similar fluorescence response in the presence of Mg2+ and Zn2+ with non-significant response for all other ions. In particular, a diminished fluorescence is observed in the presence of Ca2+ compared to chemosensor 1. In all cases, while the presence of metal ions appears to affect the fluorescence intensity of chemosensor 2, no shifts in the emission maxima are observed. Interestingly, chemosensor 2 exhibits no absorbance in the characteristic MC region and unlike chemosensor 1, no absorbance was observed for the 2-Cu2+ species (Figure S10). Job’s plot analysis of chemosensor 2 suggests multiple potential binding stoichiometries may be present (Figure S15). Based on these results, subsequent optical fibre-based studies were only performed on chemosensor 1. Finally, fluorescence emission spectra of the ester-protected precursors 3 and 4 revealed a loss of fluorescence intensity in the presence of all ions (see Figure S16), indicating that the free carboxylic acid (of 1 and 2) is important for metal ion chelation, and hence chemosensor brightness and selectivity as per the earlier discussion and modelling results.
3.4. Photoswitching in an Optical Fibre
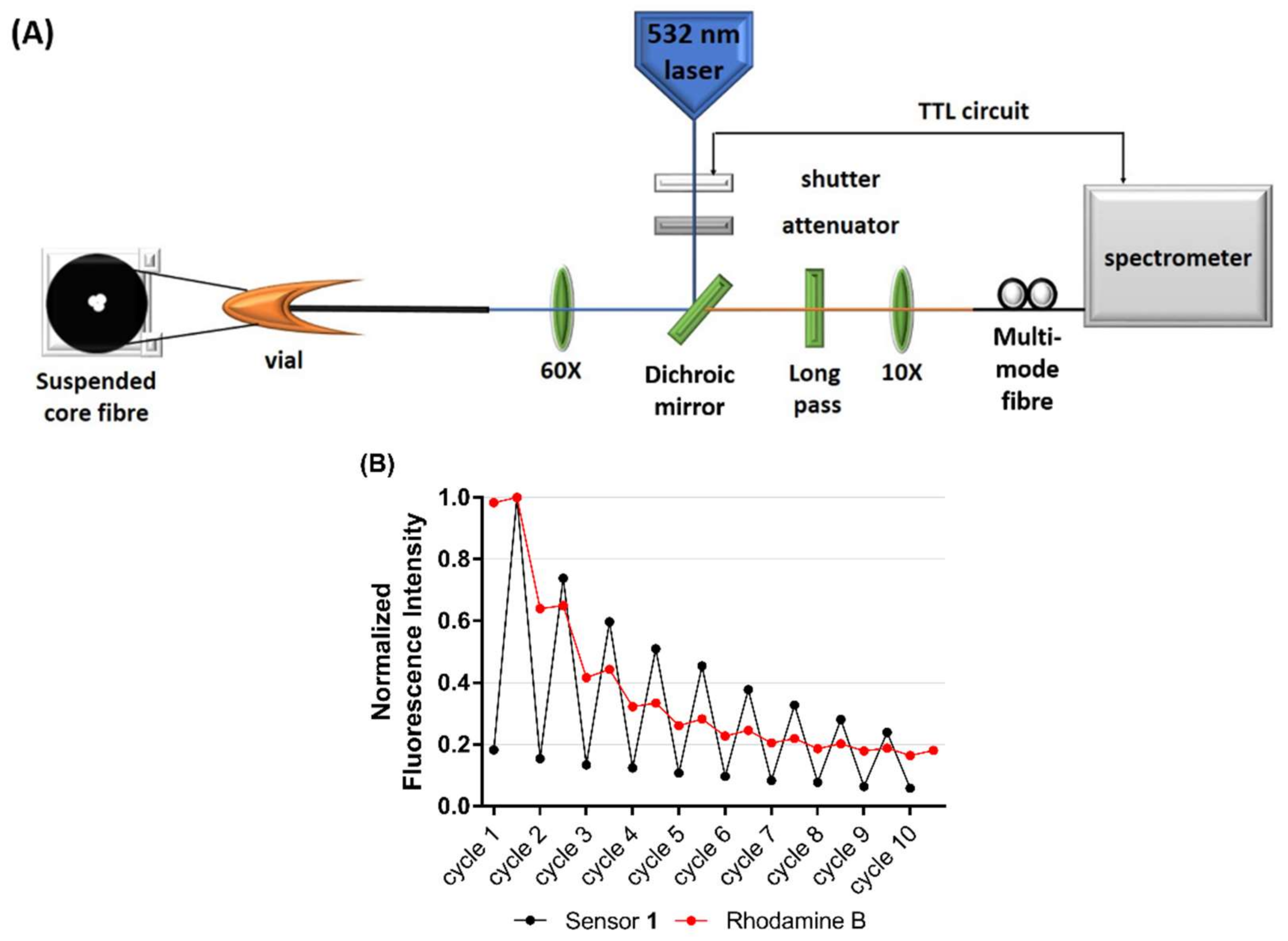
In the final part of the study, chemosensor 1 was combined with a microstructured optical fibre in order to demonstrate compatibility and as a first step in the development of a light-controlled, dip-sensor for Mg2+. Photostability and photo-reversibility of analyte binding to 1 were defined on a silica-based, in-house fabricated suspended core optical fibre (SCF) [53,54], which allows rapid photoswitching using a laser. Interaction between the light and 1 in microstructured optical fibres is extended along the entire length of the fiber, while maintaining the integrity of the device. In SCFs, the glass core is suspended in air by thin struts, allowing a portion of the guided light to extend outside the fiber core into the surrounding holes which serve as low-volume sample chambers (see Figure 4A, schematic).
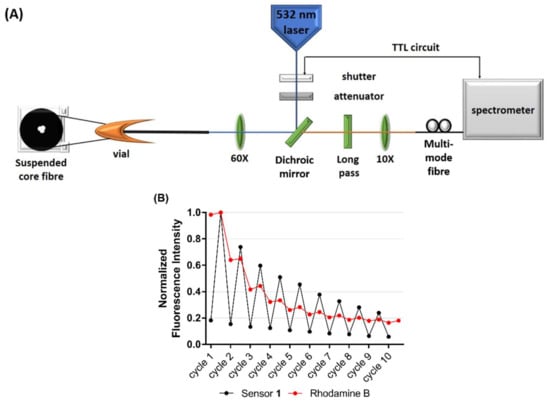
Figure 4.
(A) Schematic of the optical setup used to measure fluorescence from a suspended core microstructured optical fibre (SCF). (B) Photostability of chemosensor 1 (500 µM) in the presence of Mg2+ (1 mM) (black), compared to the photostability of Rhodamine B (red) in an SCF. Excitation was at 532 nm, with 10 × 50 ms pulses at 1 mW power. Results are normalised to the highest fluorescence measurement, respectively.
Photoswitching between the weakly fluorescent SP isomer and the highly fluorescent MC(1)-Mg2+ complex was achieved by irradiation with UV (‘on’-cycle, 365 nm lamp) and visible light (‘off’-cycle, 532 nm green laser), respectively, within the SCF platform. A significant decrease in signal was observed in the first 4 experimental cycles, falling to 50% of the initial fluorescence, as seen in Figure 4B. This decrease is likely the result of the sensor switching back to the weakly-fluorescent SP isomer under the influence of the 532 nm laser light and/or light induced parasitic side reactions that led to the formation of non-switchable by-products, a process that is not completely understood and beyond the scope of this work [55]. In comparison, the fluorescence of Rhodamine B (a fluorophore capable of excitation under the same 532 nm laser but not photoswitchable) fell to 30% of the initial fluorescence by the same photoswitching cycle in a similar experiment. These results demonstrate that chemosensor 1 is capable of photoswitching over multiple cycles in the SCF platform, however future work is needed to optimise the laser power and intensity to reduce the initial photo-decolouration.
4. Conclusions
Here we present the rational design and photophysical characterisation of spiropyran-based chemosensors for Mg2+, 1 and 2. Fluorescence characterisation revealed that the C6’-nitro functionalised chemosensor 1 exhibits a 2-fold fluorescence enhancement factor for Mg2+ over Ca2+ ions, comparable to the commercially available mag-fura-2 [21]. Importantly, the dissociation constant (Kd) of 1-Mg2+ was calculated to be 6.0 µM, with a 3-fold weaker affinity observed for 1-Ca2+ (18.7 µM). As proposed by DFT modelling, stoichiometric studies support 2:1 chemosensor to metal ion binding of 1 with Mg2+. Interestingly, structural and fluorescence characterisation of the C6’-fluorinated analogue 2 suggests the presence of both SP and MC species in solution, while stoichiometric studies indicate a complex metal binding relationship with Mg2+. Subsequent studies were thus focused on 1, which was combined with a suspended core optical fiber (SCF) as a first step towards the development of a light-controlled, reversible dip-sensor for Mg2+. Fibre-based photoswitching experiments revealed reversible Mg2+ binding with improved photostability, as compared to the non-photoswitchable Rhodamine B fluorophore.
Supplementary Materials
The following are available online at http://www.mdpi.com/2227-9040/6/2/17/s1, Figures S1–S7: NMR spectra; Figure S8: HRMS spectra; Figure S9: DFT images of 2; Figure S10: Absorbance spectra for 1 and 2; Figure S11: Job’s Plot of 1 with Ca2+; Figure S12: Determination of Kd constants; Figure S13: Determination of Quantum Yield values; Figure S14: Fluorescence spectra of 2; Figure S15: Job’s Plot of 2 with Mg2+; Figure S16: Selectivity profiles of 3 and 4.
Acknowledgments
The authors (G.M.S., S.H., A.B., H.E.-H and A.D.A) acknowledge funding support from the Centre of Nanoscale BioPhotonics, through the Australian Research Council (ARC) CE140100 003. This work was performed in part at the OptoFab node of the Australian National Fabrication Facility utilising Commonwealth and South Australian State Government funding. The authors would like to acknowledge Yow Yu Ting and Nicole Tan Jia Ling for assistance with fluorescence characterisation. This work was supported with high-performance computing resources provided by the Phoenix HPC service at the University of Adelaide and the A*STAR Computational Resource Center (A*CRC).
Author Contributions
G.M.S., S.H. and A.D.A. conceived and designed the experiments; A.M.M. performed DFT calculations; G.M.S. synthesised and characterised all compounds; A.B. performed optical fibre experiments; G.M.S., S.H. and A.B. analysed data; H.E.-H. contributed analytical tools; G.M.S., S.H. and A.D.A. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Glasdam, S.M.; Glasdam, S.; Peters, G.H. The importance of magnesium in the human body: A systematic literature review. Adv. Clin. Chem. 2016, 73, 169–193. [Google Scholar] [PubMed]
- Wolf, F.I.; Trapani, V. Cell (patho)physiology of magnesium. Clin. Sci. 2008, 114, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Gunther, T. Concentration, compartmentation and metabolic function of intracellular free Mg2+. Magnes. Res. 2006, 19, 225–236. [Google Scholar] [PubMed]
- Romani, A.M. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.F. The role of magnesium in clinical biochemistry: An overview. Ann. Clin. Biochem. 1991, 28 Pt 1, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Krishnamoorthy, G.; Yang, Y.; Hu, L.; Chaturvedi, N.; Harilal, D.; Qin, J.; Cui, J. Mechanism of magnesium activation of calcium-activated potassium channels. Nature 2002, 418, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Mubagwa, K.; Gwanyanya, A.; Zakharov, S.; Macianskiene, R. Regulation of cation channels in cardiac and smooth muscle cells by intracellular magnesium. Arch. Biochem. Biophys. 2007, 458, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.J.; Hermosura, M.C.; Inabe, K.; Perraud, A.L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.P.; Penner, R.; Scharenberg, A.M.; et al. Ltrpc7 is a mg.Atp-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.H.; Hong, S.; Pedersen, P.L. Chemical mechanism of atp synthase. Magnesium plays a pivotal role in formation of the transition state where atp is synthesized from adp and inorganic phosphate. J. Biol. Chem. 1999, 274, 28853–28856. [Google Scholar] [CrossRef] [PubMed]
- Sarrafzadegan, N.; Khosravi-Boroujeni, H.; Lotfizadeh, M.; Pourmogaddas, A.; Salehi-Abargouei, A. Magnesium status and the metabolic syndrome: A systematic review and meta-analysis. Nutrition 2016, 32, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Mooren, F.C. Magnesium and disturbances in carbohydrate metabolism. Diabetes Obes. Metab. 2015, 17, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.L. Magnesium, the metabolic syndrome, insulin resistance, and type 2 diabetes mellitus. Crit. Rev. Food Sci. Nutr. 2008, 48, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Zurlo, A.; Solmi, M.; Luchini, C.; Trevisan, C.; Bano, G.; Manzato, E.; Sergi, G.; Rylander, R. Magnesium status in alzheimer’s disease: A systematic review. Am. J. Alzheimer’s Dis. Other Dement. 2016, 31, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Belvedere, M.; Di Bella, G.; Dominguez, L.J. Altered ionized magnesium levels in mild-to-moderate alzheimer’s disease. Magnes. Res. 2011, 24, S115–S121. [Google Scholar] [PubMed]
- Kolte, D.; Vijayaraghavan, K.; Khera, S.; Sica, D.A.; Frishman, W.H. Role of magnesium in cardiovascular diseases. Cardiol. Rev. 2014, 22, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Kupetsky-Rincon, E.A.; Uitto, J. Magnesium: Novel applications in cardiovascular disease--a review of the literature. Ann. Nutr. Metab. 2012, 61, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Geiger, H.; Wanner, C. Magnesium in disease. Clin. Kidney J. 2012, 5, i25–i38. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Romani, A.M.P. Role of cellular magnesium in human diseases. Austin J. Nutr. Food Sci. 2014, 2, 1051. [Google Scholar] [PubMed]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Raju, B.; Murphy, E.; Levy, L.A.; Hall, R.D.; London, R.E. A fluorescent indicator for measuring cytosolic free magnesium. Am. J. Physiol.-Cell Physiol. 1989, 256, C540–C548. [Google Scholar] [CrossRef] [PubMed]
- Fluorescent Mg2+ indicators. The Molecular Probes Handbook—A Guide to Fluorescent Probes and Labeling Technologies, 11st ed.; ThermoFisher Scientific: Waltham, MA, USA, 2010; pp. 862–864. [Google Scholar]
- Levy, L.A.; Murphy, E.; Raju, B.; London, R.E. Measurement of cytosolic free magnesium ion concentration by fluorine-19 nmr. Biochemistry 1988, 27, 4041–4048. [Google Scholar] [CrossRef] [PubMed]
- Schachter, M.; Gallagher, K.L.; Sever, P.S. Measurement of intracellular magnesium in a vascular smooth muscle cell line using a fluorescent probe. Biochim. Biophys. Acta (BBA) Gen. Subj. 1990, 1035, 378–380. [Google Scholar] [CrossRef]
- Murphy, E.; Freudenrich, C.C.; Levy, L.A.; London, R.E.; Lieberman, M. Monitoring cytosolic free magnesium in cultured chicken heart cells by use of the fluorescent indicator furaptra. Proc. Natl. Acad. Sci. USA 1989, 86, 2981–2984. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, R.; Walter, A. Determination of cytosolic Mg2+ activity and buffering in BC3H-1 cells with MAG-fura-2. Mol. Cell. Biochem 1994, 136, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zaguilán, R.; Parnami, J.; Martinez, G.M. Mag-fura-2 (furaptra) exhibits both low (µm) and high (nm) affinity for Ca2+. Cell. Physiol. Biochem. 1998, 8, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Hurley, T.W.; Ryan, M.P.; Brinck, R.W. Changes of cytosolic Ca2+ interfere with measurements of cytosolic Mg2+ using mag-fura-2. Am. J. Physiol.-Cell Physiol. 1992, 263, C300–C307. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Fujii, T.; Komatsu, H.; Citterio, D.; Hotta, K.; Suzuki, K.; Oka, K. Newly developed Mg2+-selective fluorescent probe enables visualization of mg2+ dynamics in mitochondria. PLoS ONE 2011, 6, e23684. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Iwasawa, N.; Citterio, D.; Suzuki, Y.; Kubota, T.; Tokuno, K.; Kitamura, Y.; Oka, K.; Suzuki, K. Design and synthesis of highly sensitive and selective fluorescein-derived magnesium fluorescent probes and application to intracellular 3d Mg2+ imaging. J. Am. Chem. Soc. 2004, 126, 16353–16360. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Sadhu, K.K.; Mizukami, S.; Kikuchi, K. Highly selective tridentate fluorescent probes for visualizing intracellular Mg2+ dynamics without interference from Ca2+ fluctuation. Chem. Commun. 2017, 53, 10644–10647. [Google Scholar] [CrossRef] [PubMed]
- Trapani, V.; Farruggia, G.; Marraccini, C.; Iotti, S.; Cittadini, A.; Wolf, F.I. Intracellular magnesium detection: Imaging a brighter future. Analyst 2010, 135, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Trapani, V.; Schweigel-Rontgen, M.; Cittadini, A.; Wolf, F.I. Intracellular magnesium detection by fluorescent indicators. Methods Enzymol. 2012, 505, 421–444. [Google Scholar] [PubMed]
- Natali, M.; Giordani, S. Interaction studies between photochromic spiropyrans and transition metal cations: The curious case of copper. Org. Biomol. Chem. 2012, 10, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Natali, M.; Giordani, S. Molecular switches as photocontrollable “smart” receptors. Chem. Soc. Rev. 2012, 41, 4010–4029. [Google Scholar] [CrossRef] [PubMed]
- Lukyanov, B.S.; Lukyanova, M.B. Spiropyrans: Synthesis, properties, and application. Chem. Heterocycl. Compd. 2005, 41, 281–311. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, M.I.; Pimienta, V.; Metelitsa, A.V.; Minkin, V.I.; Micheaua, J.C. Thermodynamic and kinetic analysis of metal ion complexation by photochromic spiropyrans. Russ. Chem. Bull. 2009, 58, 1329–1337. [Google Scholar] [CrossRef]
- Heng, S.; Mak, A.M.; Kostecki, R.; Zhang, X.Z.; Pei, J.X.; Stubing, D.B.; Ebendorff-Heidepriem, H.; Abell, A.D. Photoswitchable calcium sensor: ‘On’-’off’ sensing in cells or with microstructured optical fibers. Sens. Actuators B Chem. 2017, 252, 965–972. [Google Scholar] [CrossRef]
- Heng, S.; McDevitt, C.A.; Kostecki, R.; Morey, J.R.; Eijkelkamp, B.A.; Ebendorff-Heidepriem, H.; Monro, T.M.; Abell, A.D. Microstructured optical fiber-based biosensors: Reversible and nanoliter-scale measurement of zinc ions. ACS Appl. Mater. Interfaces 2016, 8, 12727–12732. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.; Nguyen, M.-C.; Kostecki, R.; Monro, T.M.; Abell, A.D. Nanoliter-scale, regenerable ion sensor: Sensing with a surface functionalized microstructured optical fibre. RSC Adv. 2013, 3, 8308–8317. [Google Scholar] [CrossRef]
- Stubing, D.B.; Heng, S.; Monro, T.M.; Abell, A.D. A comparative study of the fluorescence and photostability of common photoswitches in microstructured optical fibre. Sens. Actuators B Chem. 2017, 239, 474–480. [Google Scholar] [CrossRef][Green Version]
- Monro, T.M.; Warren-Smith, S.; Schartner, E.P.; François, A.; Heng, S.; Ebendorff-Heidepriem, H.; Afshar, S. Sensing with suspended-core optical fibers. Opt. Fiber Technol. 2010, 16, 343–356. [Google Scholar] [CrossRef]
- Schartner, E.P.; Henderson, M.R.; Purdey, M.; Dhatrak, D.; Monro, T.M.; Gill, P.G.; Callen, D.F. Cancer detection in human tissue samples using a fiber-tip pH probe. Cancer Res. 2016, 76, 6795–6801. [Google Scholar] [CrossRef] [PubMed]
- Purdey, M.S.; Schartner, E.P.; Sutton-McDowall, M.L.; Ritter, L.J.; Thompson, J.; Monro, T.M.; Abell, A.D. Localised hydrogen peroxide sensing for reproductive health. In Proceedings of the SPIE Optics + Optoelectronics, Prague, Czech Republic, 13–16 April 2015. [Google Scholar]
- Collins, G.E.; Choi, L.-S.; Ewing, K.J.; Michelet, V.; Bowen, C.M.; Winkler, J.D. Photoinduced switching of metal complexation by quinolinospiropyranindolines in polar solvents. Chem. Commun. 1999, 321–322. [Google Scholar] [CrossRef]
- Garcia, A.A.; Cherian, S.; Park, J.; Gust, D.; Jahnke, F.; Rosario, R. Photon-controlled phase partitioning of spiropyrans. J. Phys. Chem. A 2000, 104, 6103–6107. [Google Scholar] [CrossRef]
- Baldrighi, M.; Locatelli, G.; Desper, J.; Aakeroy, C.B.; Giordani, S. Probing metal ion complexation of ligands with multiple metal binding sites: The case of spiropyrans. Chem.-Eur. J. 2016, 22, 13976–13984. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.; Mak, A.M.; Stubing, D.B.; Monro, T.M.; Abell, A.D. Dual sensor for cd(ii) and ca(ii): Selective nanoliter-scale sensing of metal ions. Anal. Chem. 2014, 86, 3268–3272. [Google Scholar] [CrossRef] [PubMed]
- Filley, J.; Ibrahim, M.A.; Nimlos, M.R.; Watt, A.S.; Blake, D.M. Magnesium and calcium chelation by a bis-spiropyran. J. Photochem. Photobiol. A Chem. 1998, 117, 193–198. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Frisch, M.J.E.A. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Magde, D.; Rojas, G.E.; Seybold, P.G. Solvent dependence of the fluorescence lifetimes of xanthene dyes. Photochem. Photobiol. 1999, 70, 737–744. [Google Scholar] [CrossRef]
- Zhao, J.B.; Jin, D.Y.; Schartner, E.P.; Lu, Y.Q.; Liu, Y.J.; Zvyagin, A.V.; Zhang, L.X.; Dawes, J.M.; Xi, P.; Piper, J.A.; et al. Single-nanocrystal sensitivity achieved by enhanced upconversion luminescence. Nat. Nanotechnol. 2013, 8, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Schartner, E.P.; Tsiminis, G.; Francois, A.; Kostecki, R.; Warren-Smith, S.C.; Nguyen, L.V.; Heng, S.; Reynolds, T.; Klantsataya, E.; Rowland, K.J.; et al. Taming the light in microstructured optical fibers for sensing. Int. J. Appl. Glass Sci. 2015, 6, 229–239. [Google Scholar] [CrossRef]
- Wiedemann, U.; Alt, W.; Meschede, D. Switching photochromic molecules adsorbed on optical microfibres. Opt. Express 2012, 20, 12710–12720. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).