Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging
Abstract
:1. Introduction
2. Metal Oxide Nanostructures Growth and Fabrication Methods
3. Food Quality Control
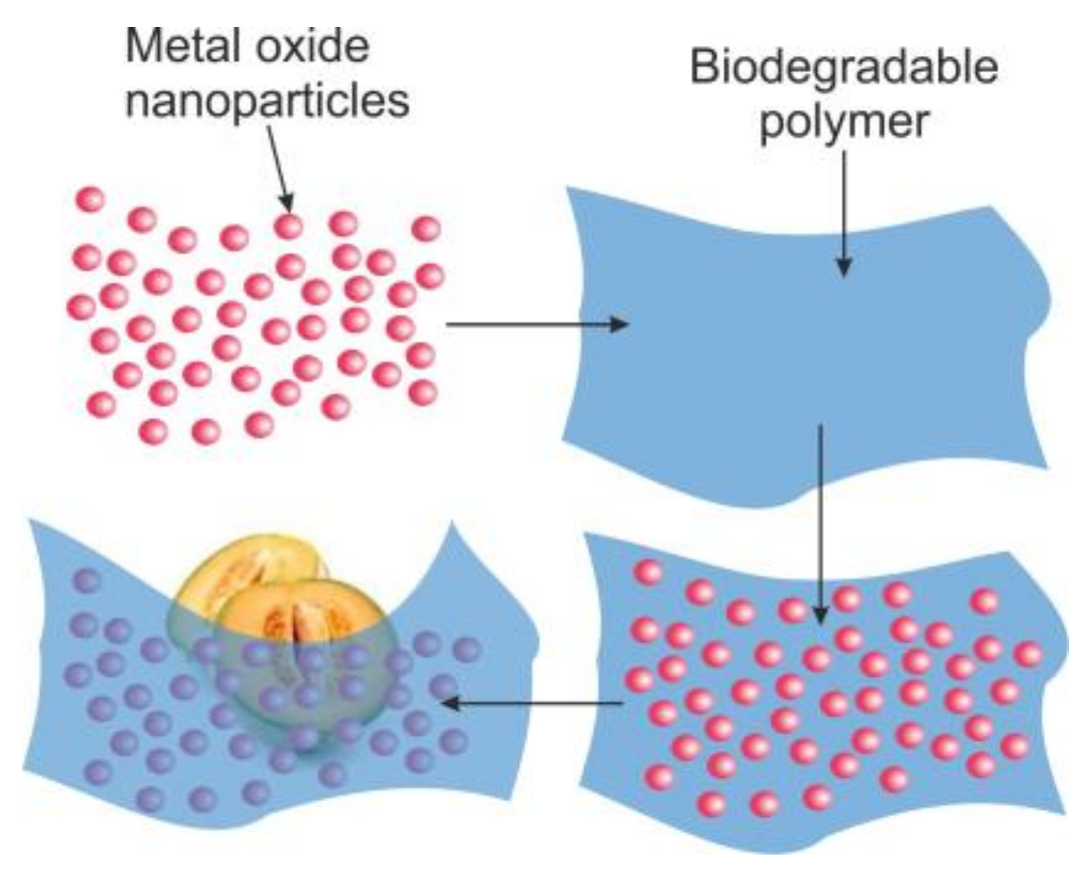
4. Food Packaging and Antimicrobial Actions
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Di Stefano, V.; Avellone, G.; Bongiorno, D.; Cunsolo, V.; Muccilli, V.; Sforza, S.; Dossena, A.; Drahos, L.; Vékey, K. Applications of liquid chromatography–mass spectrometry for food analysis. J. Chromatogr. A 2012, 1259, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Vezočnik, V.; Hodnik, V.; Anderluh, G. Surface plasmon resonance analysis of food toxins and toxicants. In Analysis of Food Toxins and Toxicants; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2017; pp. 195–216. [Google Scholar]
- McCarthy, M.J.; McCarthy, K.L. Advanced Sensors, Quality Attributes, and Modeling in Food Process Control; Springer: Boston, MA, USA, 2013; pp. 499–517. [Google Scholar]
- Galstyan, V.; Comini, E.; Kholmanov, I.; Ponzoni, A.; Sberveglieri, V.; Poli, N.; Faglia, G.; Sberveglieri, G. A composite structure based on reduced graphene oxide and metal oxide nanomaterials for chemical sensors. Beilstein J. Nanotechnol. 2016, 7, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V.; Comini, E.; Ponzoni, A.; Sberveglieri, V.; Sberveglieri, G. ZnO quasi-1D nanostructures: Synthesis, modeling, and properties for applications in conductometric chemical sensors. Chemosensors 2016, 4, 6. [Google Scholar] [CrossRef]
- Galstyan, V. Porous TiO2-based gas sensors for cyber chemical systems to provide security and medical diagnosis. Sensors 2017, 17, 2947. [Google Scholar] [CrossRef] [PubMed]
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 082001. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Bai, J.; Plotto, A.; Dea, S. Electronic noses and tongues: Applications for the food and pharmaceutical industries. Sensors 2011, 11, 4744–4766. [Google Scholar] [CrossRef] [PubMed]
- Sliwinska, M.; Wisniewska, P.; Dymerski, T.; Namiesnik, J.; Wardencki, W. Food analysis using artificial senses. J. Agric. Food Chem. 2014, 62, 1423–1448. [Google Scholar] [CrossRef] [PubMed]
- He, X.J.; Hwang, H.M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A. Diverse applications of electronic-nose technologies in agriculture and forestry. Sensors 2013, 13, 2295–2348. [Google Scholar] [CrossRef] [PubMed]
- Baietto, M.; Wilson, A. Electronic-nose applications for fruit identification, ripeness and quality grading. Sensors 2015, 15, 899–931. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, B.S. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, N.; Ranjan, S.; Mundekkad, D.; Ramalingam, C.; Shanker, R.; Kumar, A. Nanotechnology in agro-food: From field to plate. Food Res. Int. 2015, 69, 381–400. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Hariharan, V.; Sekar, C. High-sensitivity humidity sensor based on SnO2 nanoparticles synthesized by microwave irradiation method. Mater. Sci. Eng. C 2011, 31, 840–844. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.J.; Feng, Z.M.; Lu, G.Y.; Sun, T.; Xu, Y. Crystalline-phase-induced formation of fibre-intube TiO2-SnO2 fibres for a humidity sensor. CrystEngComm 2017, 19, 5528–5531. [Google Scholar] [CrossRef]
- Hsu, C.L.; Su, I.L.; Hsueh, T.J. Tunable schottky contact humidity sensor based on S-doped ZnO nanowires on flexible pet substrate with piezotronic effect. J. Alloys Compd. 2017, 705, 722–733. [Google Scholar] [CrossRef]
- Reig, C.S.; Lopez, A.D.; Ramos, M.H.; Ballester, V.A.C. Nanomaterials: A map for their selection in food packaging applications. Packag. Technol. Sci. 2014, 27, 839–866. [Google Scholar] [CrossRef]
- Andrews, D.; Scholes, G.; Wiederrecht, G. Comprehensive Nanoscience and Technology, Volume 1: Nanomaterials; Elsevier Science Bv: Amsterdam, The Netherlands, 2011; pp. 1–635. [Google Scholar]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 nanotubes: Recent advances in synthesis and gas sensing properties. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.J.S. Gas sensing applications of 1d-nanostructured zinc oxide: Insights from density functional theory calculations. Prog. Mater. Sci. 2012, 57, 437–486. [Google Scholar] [CrossRef]
- Galstyan, V.; Vomiero, A.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 nanotubular and nanoporous arrays by electrochemical anodization on different substrates. RSC Adv. 2011, 1, 1038–1044. [Google Scholar] [CrossRef]
- Choi, M.J.; Cho, C.J.; Kim, K.C.; Pyeon, J.J.; Park, H.H.; Kim, H.S.; Han, J.H.; Kim, C.G.; Chung, T.M.; Park, T.J.; et al. SnO2 thin films grown by atomic layer deposition using a novel sn precursor. Appl. Surf. Sci. 2014, 320, 188–194. [Google Scholar] [CrossRef]
- Shaik, U.P.; Krishna, M.G. Single step formation of indium and tin doped ZnO nanowires by thermal oxidation of indium-zinc and tin-zinc metal films: Growth and optical properties. Ceram. Int. 2014, 40, 13611–13620. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Du, G.; Chen, N.; Liu, S.; Wang, S.; Huang, H.; Lu, C.; Niu, X. Enhanced electrical and optical properties of boron-doped ZnO films grown by low pressure chemical vapor deposition for amorphous silicon solar cells. Ceram. Int. 2016, 42, 1361–1365. [Google Scholar] [CrossRef]
- Hu, P.; Han, N.; Zhang, D.; Ho, J.C.; Chen, Y. Highly formaldehyde-sensitive, transition-metal doped ZnO nanorods prepared by plasma-enhanced chemical vapor deposition. Sens. Actuators B Chem. 2012, 169, 74–80. [Google Scholar] [CrossRef]
- Manuel, M.-M.; Ana, B.; Zineb, S.; Juan, P.E.; Angel, B.; Jose, C.; Gonzalez-Elipe, A.R. Vertical and tilted Ag-NPS@ZnO nanorods by plasma-enhanced chemical vapour deposition. Nanotechnology 2012, 23, 255303. [Google Scholar]
- Haddad, K.; Abokifa, A.; Kavadiya, S.; Chadha, T.S.; Shetty, P.; Wang, Y.; Fortner, J.; Biswas, P. Growth of single crystal, oriented SnO2 nanocolumn arrays by aerosol chemical vapour deposition. CrystEngComm 2016, 18, 7544–7553. [Google Scholar] [CrossRef]
- Panigrahi, J.; Behera, D.; Mohanty, I.; Subudhi, U.; Nayak, B.B.; Acharya, B.S. Radio frequency plasma enhanced chemical vapor based ZnO thin film deposition on glass substrate: A novel approach towards antibacterial agent. Appl. Surf. Sci. 2011, 258, 304–311. [Google Scholar] [CrossRef]
- Wang, J.; Dong, X.; Zhang, B.; Zhang, Y.; Wang, H.; Shi, Z.; Zhang, S.; Yin, W.; Du, G. P-type NiZnO thin films grown by photo-assist metal–organic chemical vapor deposition. J. Alloys Compd. 2013, 579, 160–164. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, T.; Wu, S.; Ji, X.; Zhang, Q. Na-doped ZnO nanorods fabricated by chemical vapor deposition and their optoelectrical properties. J. Alloys Compd. 2017, 690, 189–194. [Google Scholar] [CrossRef]
- Comini, E.; Baratto, C.; Faglia, G.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Quasi-one dimensional metal oxide semiconductors: Preparation, characterization and application as chemical sensors. Prog. Mater. Sci. 2009, 54, 1–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Ren, W.; Ye, Z.-G. Well-ordered ZnO nanotube arrays and networks grown by atomic layer deposition. Appl. Surf. Sci. 2015, 340, 120–125. [Google Scholar] [CrossRef]
- Lim, Y.T.; Son, J.Y.; Rhee, J.S. Vertical ZnO nanorod array as an effective hydrogen gas sensor. Ceram. Int. 2013, 39, 887–890. [Google Scholar] [CrossRef]
- Huang, Y.J.; Pandraud, G.; Sarro, P.M. The atomic layer deposition array defined by etch-back technique: A new method to fabricate TiO2 nanopillars, nanotubes and nanochannel arrays. Nanotechnology 2012, 23, 485306. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, R.; Ghosh, A.; Dwivedi, S.M.M.D.; Chakrabartty, S.; Chinnamuthu, P.; Mondal, A. Performance of erbium-doped TiO2 thin film grown by physical vapor deposition technique. Appl. Phys. A 2017, 123, 573. [Google Scholar] [CrossRef]
- Comini, E.; Baratto, C.; Concina, I.; Faglia, G.; Falasconi, M.; Ferroni, M.; Galstyan, V.; Gobbi, E.; Ponzoni, A.; Vomiero, A.; et al. Metal oxide nanoscience and nanotechnology for chemical sensors. Sens. Actuators B Chem. 2013, 179, 3–20. [Google Scholar] [CrossRef]
- George, A.; Kumari, P.; Soin, N.; Roy, S.S.; McLaughlin, J.A. Microstructure and field emission characteristics of ZnO nanoneedles grown by physical vapor deposition. Mater. Chem. Phys. 2010, 123, 634–638. [Google Scholar] [CrossRef]
- Dlugosch, T.; Chnani, A.; Muralidhar, P.; Schirmer, A.; Biskupek, J.; Strehle, S. Thermal oxidation synthesis of crystalline iron-oxide nanowires on low-cost steel substrates for solar water splitting. Semicond. Sci. Technol. 2017, 32, 084001. [Google Scholar] [CrossRef]
- Kim, D.; Leem, J.Y. Catalyst-free synthesis of ZnO nanorods by thermal oxidation of Zn films at various temperatures and their characterization. J. Nanosci. Nanotechnol. 2017, 17, 5826–5829. [Google Scholar] [CrossRef]
- Comini, E.; Galstyan, V.; Faglia, G.; Bontempi, E.; Sberveglieri, G. Highly conductive titanium oxide nanotubes chemical sensors. Microporous Mesoporous Mater. 2015, 208, 165–170. [Google Scholar] [CrossRef]
- Ellis, B.L.; Knauth, P.; Djenizian, T. Three-dimensional self-supported metal oxides for advanced energy storage. Adv. Mater. 2014, 26, 3368–3397. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. Synthesis of self-ordered and well-aligned Nb2O5 nanotubes. CrystEngComm 2014, 16, 10273–10279. [Google Scholar] [CrossRef]
- Galstyan, V.; Vomiero, A.; Concina, I.; Braga, A.; Brisotto, M.; Bontempi, E.; Faglia, G.; Sberveglieri, G. Vertically aligned TiO2 nanotubes on plastic substrates for flexible solar cells. Small 2011, 7, 2437–2442. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V.; Comini, E.; Baratto, C.; Faglia, G.; Sberveglieri, G. Nanostructured ZnO chemical gas sensors. Ceram. Int. 2015, 41, 14239–14244. [Google Scholar] [CrossRef]
- Rana, A.u.H.S.; Chang, S.-B.; Chae, H.U.; Kim, H.-S. Structural, optical, electrical and morphological properties of different concentration sol-gel ZnO seeds and consanguineous ZnO nanostructured growth dependence on seeds. J. Alloys Compd. 2017, 729, 571–582. [Google Scholar] [CrossRef]
- Valerio, L.R.; Mamani, N.C.; de Zevallos, A.O.; Mesquita, A.; Bernardi, M.I.B.; Doriguetto, A.C.; de Carvalho, H.B. Preparation and structural-optical characterization of dip-coated nanostructured co-doped ZnO dilute magnetic oxide thin films. Rsc Adv. 2017, 7, 20611–20619. [Google Scholar] [CrossRef]
- Richard, D.; Romero, M.; Faccio, R. Experimental and theoretical study on the structural, electrical and optical properties of tantalum-doped ZnO nanoparticles prepared via sol-gel acetate route. Ceram. Int. 2018, 44, 703–711. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, C.; Yang, L.; Jia, W.; Zhou, J.; Xu, H.; Cao, D. H2 response characteristics for sol–gel-derived WO3-SnO2 dual-layer thin films. Ceram. Int. 2017, 43, 6693–6699. [Google Scholar] [CrossRef]
- Yao, S.; Qu, F.; Wang, G.; Wu, X. Facile hydrothermal synthesis of WO3 nanorods for photocatalysts and supercapacitors. J. Alloys Compd. 2017, 724, 695–702. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, C.; Han, T.; Peng, L. Hydrothermal synthesis, characterization and gas sensing properties of the WO3 nanofibers. Mater. Lett. 2016, 169, 17–20. [Google Scholar] [CrossRef]
- Yu, Z.; Qu, X.; Yang, W.; Peng, J.; Xu, Z. A facile hydrothermal synthesis and memristive switching performance of rutile TiO2 nanowire arrays. J. Alloys Compd. 2016, 688, 37–43. [Google Scholar] [CrossRef]
- Yin, M.; Yao, Y.; Fan, H.; Liu, S. WO3-SnO2 nanosheet composites: Hydrothermal synthesis and gas sensing mechanism. J. Alloys Compd. 2018, 736, 322–331. [Google Scholar] [CrossRef]
- Sun, K.C.; Qadir, M.B.; Jeong, S.H. Hydrothermal synthesis of TiO2 nanotubes and their application as an over-layer for dye-sensitized solar cells. RSC Adv. 2014, 4, 23223–23230. [Google Scholar] [CrossRef]
- Perillo, P.M.; Rodríguez, D.F. Low temperature trimethylamine flexible gas sensor based on TiO2 membrane nanotubes. J. Alloys Compd. 2016, 657, 765–769. [Google Scholar] [CrossRef]
- Liu, L.; Song, P.; Yang, Z.; Wang, Q. Highly sensitive and selective trimethylamine sensors based on WO3 nanorods decorated with Au nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 90, 109–115. [Google Scholar] [CrossRef]
- Chu, X.; Zhou, S.; Dong, Y.; Sun, W.; Ge, X. Trimethylamine gas sensor based on Cr3+ doped ZnO nanorods/nanoparticles prepared via solvothermal method. Mater. Chem. Phys. 2011, 131, 27–31. [Google Scholar] [CrossRef]
- Meng, F.; Zheng, H.; Sun, Y.; Li, M.; Liu, J. Trimethylamine sensors based on Au-modified hierarchical porous single-crystalline ZnO nanosheets. Sensors 2017, 17, 1478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, P.; Li, Z.; Zhang, S.; Yang, Z.; Wang, Q. Enhanced trimethylamine sensing performance of single-crystal MoO3 nanobelts decorated with au nanoparticles. J. Alloys Compd. 2016, 685, 1024–1033. [Google Scholar] [CrossRef]
- Woo, H.-S.; Na, C.; Kim, I.-D.; Lee, J.-H. Highly sensitive and selective trimethylamine sensor using one-dimensional ZnO-Cr2O3 hetero-nanostructures. Nanotechnology 2012, 23, 245501. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Kim, I.-D.; Lee, J.-H. Selective and sensitive detection of trimethylamine using ZnO-In2O3 composite nanofibers. Sens. Actuators B Chem. 2013, 181, 463–470. [Google Scholar] [CrossRef]
- Kwak, C.-H.; Woo, H.-S.; Lee, J.-H. Selective trimethylamine sensors using Cr2O3-decorated SnO2 nanowires. Sens. Actuators B Chem. 2014, 204, 231–238. [Google Scholar] [CrossRef]
- Lou, Z.; Li, F.; Deng, J.; Wang, L.; Zhang, T. Branch-like hierarchical heterostructure (α-Fe2O3/TiO2): A novel sensing material for trimethylamine gas sensor. ACS Appl. Mater. Interfaces 2013, 5, 12310–12316. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.-N.; Kim, B.; Shin, H.-S. Evaluation of a freshness indicator for quality of fish products during storage. Food Sci. Biotechnol. 2014, 23, 1719–1725. [Google Scholar] [CrossRef]
- Du, J.; Wang, H.; Zhao, R.; Xie, Y.; Yao, H. Surfactant-assisted synthesis of the pencil-like zinc oxide and its sensing properties. Mater. Lett. 2013, 107, 259–261. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Lu, H.; Li, L.; Zheng, J.; Zhang, J.; Li, H.; Zhu, Z. Highly sensitive and selective dimethylamine sensors based on hierarchical ZnO architectures composed of nanorods and nanosheet-assembled microspheres. Sens. Actuators B Chem. 2012, 171, 1101–1109. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Portable electronic nose based on electrochemical sensors for food quality assessment. Sensors 2017, 17, 2715. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ban, H.; Yang, M. Highly sensitive NH3 gas sensors based on novel polypyrrole-coated SnO2 nanosheet nanocomposites. Sens. Actuators B Chem. 2016, 224, 449–457. [Google Scholar] [CrossRef]
- Jain, S.; Karmakar, N.; Shah, A.; Kothari, D.C.; Mishra, S.; Shimpi, N.G. Ammonia detection of 1-d ZnO/polypyrrole nanocomposite: Effect of CSA doping and their structural, chemical, thermal and gas sensing behavior. Appl. Surf. Sci. 2017, 396, 1317–1325. [Google Scholar] [CrossRef]
- Tai, H.; Jiang, Y.; Xie, G.; Yu, J.; Chen, X.; Ying, Z. Influence of polymerization temperature on NH3 response of pani/ TiO2 thin film gas sensor. Sens. Actuators B Chem. 2008, 129, 319–326. [Google Scholar] [CrossRef]
- Das, M.; Sarkar, D. One-pot synthesis of zinc oxide-polyaniline nanocomposite for fabrication of efficient room temperature ammonia gas sensor. Ceram. Int. 2017, 43, 11123–11131. [Google Scholar] [CrossRef]
- Shingange, K.; Tshabalala, Z.P.; Ntwaeaborwa, O.M.; Motaung, D.E.; Mhlongo, G.H. Highly selective NH3 gas sensor based on au loaded ZnO nanostructures prepared using microwave-assisted method. J. Colloid Interface Sci. 2016, 479, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jiang, K.; Chen, S.; Lou, Z.; Huang, T.; Chen, D.; Shen, G. SnO2/SnS2 nanotubes for flexible room-temperature NH3 gas sensors. RSC Adv. 2017, 7, 52503–52509. [Google Scholar] [CrossRef]
- Warren, M.W.; Larick, D.K.; Ball, H.R. Volatiles and sensory characteristics of cooked egg-yolk, white and their combinations. J. Food Sci. 1995, 60, 79–84. [Google Scholar] [CrossRef]
- Perillo, P.; Rodríguez, D. TiO2 nanotubes membrane flexible sensor for low-temperature H2S detection. Chemosensors 2016, 4, 15. [Google Scholar] [CrossRef]
- Yu, T.; Cheng, X.; Zhang, X.; Sui, L.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. Highly sensitive H2S detection sensors at low temperature based on hierarchically structured nio porous nanowall arrays. J. Mater. Chem. A 2015, 3, 11991–11999. [Google Scholar] [CrossRef]
- Li, Z.; Wang, N.; Lin, Z.; Wang, J.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z. Room-temperature high-performance H2S sensor based on porous CuO nanosheets prepared by hydrothermal method. ACS Appl. Mater. Interfaces 2016, 8, 20962–20968. [Google Scholar] [CrossRef] [PubMed]
- Yeh, B.-Y.; Jian, B.-S.; Wang, G.-J.; Tseng, W.J. CuO/V2O5 hybrid nanowires for highly sensitive and selective h2s gas sensor. RSC Adv. 2017, 7, 49605–49612. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, H.; Chen, C.; Liang, J.; Luo, Y.; Zhang, M.; Cai, M. Co3O4-SnO2 nanobox sensor with a pn junction and semiconductor-conductor transformation for high selectivity and sensitivity detection of H2S. CrystEngComm 2017, 19, 5742–5748. [Google Scholar] [CrossRef]
- Guo, W.; Mei, L.; Wen, J.; Ma, J. High-response H2S sensor based on ZnO/SnO2 heterogeneous nanospheres. RSC Adv. 2016, 6, 15048–15053. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Hong, Y.J.; Chan Kang, Y.; Lee, J.-H. High performance chemiresistive H2S sensors using Ag-loaded SnO2 yolk-shell nanostructures. RSC Adv. 2014, 4, 16067–16074. [Google Scholar] [CrossRef]
- Yang, T.; Yang, Q.; Xiao, Y.; Sun, P.; Wang, Z.; Gao, Y.; Ma, J.; Sun, Y.; Lu, G. A pulse-driven sensor based on ordered mesoporous Ag2O/SnO2 with improved H2S-sensing performance. Sens. Actuators B Chem. 2016, 228, 529–538. [Google Scholar] [CrossRef]
- Kheel, H.; Sun, G.-J.; Lee, J.K.; Lee, S.; Dwivedi, R.P.; Lee, C. Enhanced H2S sensing performance of TiO2-decorated α-Fe2O3 nanorod sensors. Ceram. Int. 2016, 42, 18597–18604. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Xiao, S.; Wang, X.; Sun, L.; Li, H.; Xie, W.; Li, Q.; Zhang, Q.; Wang, T. Low-temperature H2S detection with hierarchical Cr-doped WO3 microspheres. ACS Appl. Mater. Interfaces 2016, 8, 9674–9683. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Shen, W.; Chen, X.; Corriou, J.-P. A fast response and recovery H2S gas sensor based on free-standing TiO2 nanotube array films prepared by one-step anodization method. Ceram. Int. 2017, 43, 14200–14209. [Google Scholar] [CrossRef]
- Hosseini, Z.S.; Mortezaali, A.; Iraji zad, A.; Fardindoost, S. Sensitive and selective room temperature H2S gas sensor based on Au sensitized vertical ZnO nanorods with flower-like structures. J. Alloys Compd. 2015, 628, 222–229. [Google Scholar] [CrossRef]
- Nimbalkar, A.R.; Patil, M.G. Synthesis of highly selective and sensitive Cu-doped ZnO thin film sensor for detection of H2S gas. Mater. Sci. Semicond. Process. 2017, 71, 332–341. [Google Scholar] [CrossRef]
- Hyun, S.K.; Sun, G.-J.; Lee, J.K.; Lee, C.; In Lee, W.; Kim, H.W. Ethanol gas sensing using a networked pbo-decorated SnO2 nanowires. Thin Solid Films 2017, 637, 21–26. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Baratto, C.; Ponzoni, A.; Ferroni, M.; Poli, N.; Bontempi, E.; Brisotto, M.; Faglia, G.; Sberveglieri, G. Large surface area biphase titania for chemical sensing. Sens. Actuators B Chem. 2015, 209, 1091–1096. [Google Scholar] [CrossRef]
- Choi, S.; Bonyani, M.; Sun, G.-J.; Lee, J.K.; Hyun, S.K.; Lee, C. Cr2O3 nanoparticle-functionalized WO3 nanorods for ethanol gas sensors. Appl. Surf. Sci. 2018, 432, 241–249. [Google Scholar] [CrossRef]
- Tan, J.; Dun, M.; Li, L.; Zhao, J.; Tan, W.; Lin, Z.; Huang, X. Synthesis of hollow and hollowed-out Co3O4 microspheres assembled by porous ultrathin nanosheets for ethanol gas sensors: Responding and recovering in one second. Sens. Actuators B Chem. 2017, 249, 44–52. [Google Scholar] [CrossRef]
- Ben Amor, M.; Boukhachem, A.; Labidi, A.; Boubaker, K.; Amlouk, M. Physical investigations on Cd doped NiO thin films along with ethanol sensing at relatively low temperature. J. Alloys Compd. 2017, 693, 490–499. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Koo, W.-T.; Jang, J.-S.; Kim, D.-H.; Kim, M.-H.; Kim, I.-D. Nanoscale PtO2 catalysts-loaded SnO2 multichannel nanofibers toward highly sensitive acetone sensor. ACS Appl. Mater. Interfaces 2018, 10, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-Y.; Wang, M.-D.; Wang, Y.-F.; Hu, J.-Y.; Zhu, Y.; Zhang, Y.X.; Li, Z.-J.; Yao, H.-C. Iron and carbon codoped WO3 with hierarchical walnut-like microstructure for highly sensitive and selective acetone sensor. Sens. Actuators B Chem. 2018, 256, 27–37. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Yang, Q.; Sun, P.; Gao, Y.; Liu, F.; Zheng, J.; Lu, G. Ultrasensitive and low detection limit of acetone gas sensor based on W-doped NiO hierarchical nanostructure. Sens. Actuators B Chem. 2015, 220, 59–67. [Google Scholar] [CrossRef]
- Mishra, R.K.; Murali, G.; Kim, T.-H.; Kim, J.H.; Lim, Y.J.; Kim, B.-S.; Sahay, P.P.; Lee, S.H. Nanocube In2O3@RGO heterostructure based gas sensor for acetone and formaldehyde detection. RSC Adv. 2017, 7, 38714–38724. [Google Scholar] [CrossRef]
- Konduru, T.; Rains, G.; Li, C. A customized metal oxide semiconductor-based gas sensor array for onion quality evaluation: System development and characterization. Sensors 2015, 15, 1252–1273. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Serra, D.; Suraci, F.; Di Sanzo, R.; Fuda, S.; Postorino, S. The potential of e-nose aroma profiling for identifying the geographical origin of licorice (Glycyrrhiza glabra L.) roots. Food Chem. 2014, 165, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, G.; Cerretani, L.; Procida, G.; Cichelli, A. Composition of commercial truffle flavored oils with GC–MS analysis and discrimination with an electronic nose. Food Chem. 2014, 146, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.H.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-dimensional nanostructured materials for gas sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar] [CrossRef]
- Barsan, N.; Schweizer-Berberich, M.; Gopel, W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: A status report. Fresenius J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Lelono, D.; Triyana, K.; Hartati, S.; Istiyanto, J.E. Classification of indonesia black teas based on quality by using electronic nose and principal component analysis. In Advances of Science and Technology for Society; Nuringtyas, T.R., Roto, R., Widyaparaga, A., Mahardika, M., Kusumaadmaja, A., Sholihun, Hadi, N., Eds.; AIP: College Park, MD, USA, 2016; Volume 1755. [Google Scholar]
- Severini, C.; Derossi, A.; Fiore, A.G.; Ricci, I.; Marone, M. The electronic nose system: Study on the global aromatic profile of espresso coffee prepared with two types of coffee filter holders. Eur. Food Res. Technol. 2016, 242, 2083–2091. [Google Scholar] [CrossRef]
- Gobbi, E.; Falasconi, M.; Zambotti, G.; Sberveglieri, V.; Pulvirenti, A.; Sberveglieri, G. Rapid diagnosis of enterobacteriaceae in vegetable soups by a metal oxide sensor based electronic nose. Sens. Actuators B Chem. 2015, 207, 1104–1113. [Google Scholar] [CrossRef]
- Ghosh, A.; Ray, H.; Ghosh, T.K.; Das, A.; Bhattacharyya, N. Generic handheld e-nose platform for quality assessment of agricultural produces and biomedical applications. In Proceedings of the Nose2014: 4th International Conference on Environmental Odour Monitoring and Control, Venice, Italy, 14–17 September 2014; Volume 40, pp. 259–264. [Google Scholar]
- Sanaeifar, A.; Mohtasebi, S.S.; Ghasemi-Varnamkhasti, M.; Ahmadi, H. Application of mos based electronic nose for the prediction of banana quality properties. Measurement 2016, 82, 105–114. [Google Scholar] [CrossRef]
- Trirongjitmoah, S.; Juengmunkong, Z.; Srikulnath, K.; Somboon, P. Classification of garlic cultivars using an electronic nose. Comput. Electron. Agric. 2015, 113, 148–153. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, J.; Gao, L. Qualification and quantisation of processed strawberry juice based on electronic nose and tongue. LWT-Food Sci. Technol. 2015, 60, 115–123. [Google Scholar] [CrossRef]
- Güney, S.; Atasoy, A. Study of fish species discrimination via electronic nose. Comput. Electron. Agric. 2015, 119, 83–91. [Google Scholar] [CrossRef]
- Severini, C.; Ricci, I.; Marone, M.; Derossi, A.; De Pilli, T. Changes in the aromatic profile of espresso coffee as a function of the grinding grade and extraction time: A study by the electronic nose system. J. Agric. Food Chem. 2015, 63, 2321–2327. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Ejaz, N.; Ejaz, W.; Kim, H. Meat and fish freshness inspection system based on odor sensing. Sensors 2012, 12, 15542–15557. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ye, L.; Wang, J.; Wei, Z.; Cheng, S. Quality tracing of peanuts using an array of metal-oxide based gas sensors combined with chemometrics methods. Postharvest Biol. Technol. 2017, 128, 98–104. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.; Liu, L.; Zhang, R. A novel method for qualitative analysis of edible oil oxidation using an electronic nose. Food Chem. 2016, 202, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.-Y.; Cai, Q.; Zhang, Y.-M. Rapid classification of hairtail fish and pork freshness using an electronic nose based on the PCA method. Sensors 2012, 12, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Dymerski, T.; Gebicki, J.; Wardencki, W.; Namiesnik, J. Application of an electronic nose instrument to fast classification of polish honey types. Sensors 2014, 14, 10709–10724. [Google Scholar] [CrossRef] [PubMed]
- Yemmireddy, V.K.; Hung, Y.-C. Effect of binder on the physical stability and bactericidal property of titanium dioxide (TiO2) nanocoatings on food contact surfaces. Food Control 2015, 57, 82–88. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S. Morphology and thermal degradation studies of melt-mixed poly(hydroxybutyrate-co-valerate) (PHBV)/poly(ε-caprolactone) (PCL) biodegradable polymer blend nanocomposites with TiO2 as filler. J. Mater. Sci. 2015, 50, 3812–3824. [Google Scholar] [CrossRef]
- Lin, Q.B.; Li, H.; Zhong, H.N.; Zhao, Q.; Xiao, D.H.; Wang, Z.W. Migration of ti from nano-TiO2-polyethylene composite packaging into food simulants. Food Addit. Contam. Part A-Chem. Anal. Control Expos. Risk Assess. 2014, 31, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Yemmireddy, V.K.; Farrell, G.D.; Hung, Y.C. Development of titanium dioxide (TiO2) nanocoatings on food contact surfaces and method to evaluate their durability and photocatalytic bactericidal property. J. Food Sci. 2015, 80, N1903–N1911. [Google Scholar] [CrossRef] [PubMed]
- Pathakoti, K.; Morrow, S.; Han, C.; Pelaez, M.; He, X.; Dionysiou, D.D.; Hwang, H.-M. Photoinactivation of escherichia coli by sulfur-doped and nitrogen–fluorine-codoped TiO2 nanoparticles under solar simulated light and visible light irradiation. Environ. Sci. Technol. 2013, 47, 9988–9996. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; dos Santos, V.; Fernandez-Garcia, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [PubMed]
- Farhoodi, M. Nanocomposite materials for food packaging applications: Characterization and safety evaluation. Food Eng. Rev. 2016, 8, 35–51. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M.; Salama, H.H.; El-Sayed, H.S.; Dufresne, A. Evaluation of bionanocomposites as packaging material on properties of soft white cheese during storage period. Carbohydr. Polym. 2015, 132, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Dufresne, A. Enhancement of egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr. Polym. 2016, 151, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Krehula, L.K.; Papić, A.; Krehula, S.; Gilja, V.; Foglar, L.; Hrnjak-Murgić, Z. Properties of uv protective films of poly(vinyl-chloride)/TiO2 nanocomposites for food packaging. Polym. Bull. 2017, 74, 1387–1404. [Google Scholar] [CrossRef]
- He, Q.Y.; Huang, Y.; Lin, B.B.; Wang, S.Y. A nanocomposite film fabricated with simultaneously extracted protein-polysaccharide from a marine alga and TiO2 nanoparticles. J. Appl. Phycol. 2017, 29, 1541–1552. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of ecofriendly uv-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.S.; Qin, Y.; Ye, Q.Y. Effect of nano-TiO2-ldpe packaging on microbiological and physicochemical quality of pacific white shrimp during chilled storage. Int. J. Food Sci. Technol. 2015, 50, 1567–1573. [Google Scholar] [CrossRef]
- Bodaghi, H.; Mostofi, Y.; Oromiehie, A.; Zamani, Z.; Ghanbarzadeh, B.; Costa, C.; Conte, A.; Del Nobile, M.A. Evaluation of the photocatalytic antimicrobial effects of a TiO2 nanocomposite food packaging film by in vitro and in vivo tests. LWT-Food Sci. Technol. 2013, 50, 702–706. [Google Scholar] [CrossRef]
- Bodaghi, H.; Mostofi, Y.; Oromiehie, A.; Ghanbarzadeh, B.; Hagh, Z.G. Synthesis of clay-TiO2 nanocomposite thin films with barrier and photocatalytic properties for food packaging application. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- De Chiara, M.L.V.; Pal, S.; Licciulli, A.; Amodio, M.L.; Colelli, G. Photocatalytic degradation of ethylene on mesoporous TiO2/SiO2 nanocomposites: Effects on the ripening of mature green tomatoes. Biosyst. Eng. 2015, 132, 61–70. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Sani, M.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Li, X.; Zhou, X.; Xu, X.; Jian, H.; Li, M.; Guo, K.; Guan, J.; Yan, S. Fabrication and characterization of nanocomposite film made from a jackfruit filum polysaccharide incorporating TiO2 nanoparticles by photocatalysis. RSC Adv. 2017, 7, 16931–16937. [Google Scholar] [CrossRef]
- Naik, K.; Kowshik, M. Anti-quorum sensing activity of AgCl-TiO2 nanoparticles with potential use as active food packaging material. J. Appl. Microbiol. 2014, 117, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.; Tegla, D.; Giurgiulescu, L.; Cozmuta, A.M.; Nicula, C.; Cozmuta, L.M.; Vagelas, I. Development of Ag/TiO2-SiO2-coated food packaging film and its role in preservation of green lettuce during storage. Carpathian J. Food Sci. Technol. 2015, 7, 88–96. [Google Scholar]
- Cozmuta, A.M.; Peter, A.; Cozmuta, L.M.; Nicula, C.; Crisan, L.; Baia, L.; Turila, A. Active packaging system based on Ag/TiO2 nanocomposite used for extending the shelf life of bread. Chemical and microbiological investigations. Packag. Technol. Sci. 2015, 28, 271–284. [Google Scholar] [CrossRef]
- Peter, A.; Mihaly-Cozmuta, L.; Mihaly-Cozmuta, A.; Nicula, C.; Ziemkowska, W.; Basiak, D.; Danciu, V.; Vulpoi, A.; Baia, L.; Falup, A.; et al. Changes in the microbiological and chemical characteristics of white bread during storage in paper packages modified with Ag/TiO2–SiO2, Ag/N–TiO2 or Au/TiO2. Food Chem. 2016, 197, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Li, L.; Zhang, H.; Yuan, M.L.; Qin, Y.Y. Evaluation of pla nanocomposite films on physicochemical and microbiological properties of refrigerated cottage cheese. J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A.A. Modification of physicochemical and thermal properties of starch films by incorporation of TiO2 nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Shaili, T.; Abdorreza, M.N.; Fariborz, N. Functional, thermal, and antimicrobial properties of soluble soybean polysaccharide biocomposites reinforced by nano TiO2. Carbohydr. Polym. 2015, 134, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Vejdan, A.; Ojagh, S.M.; Adeli, A.; Abdollahi, M. Effect of TiO2 nanoparticles on the physico-mechanical and ultraviolet light barrier properties of fish gelatin/agar bilayer film. LWT-Food Sci. Technol. 2016, 71, 88–95. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Hassan, E.A.; Abou-Zeid, R.E.; Dufresne, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H.; Abd Salam, N.R.; Zainal, N.; Basha, R.K.; Talib, R.A. Antimicrobial activity of TiO2 nanoparticle-coated film for potential food packaging applications. Int. J. Photoenergy 2014. [Google Scholar] [CrossRef]
- Zhang, X.D.; Xiao, G.; Wang, Y.Q.; Zhao, Y.; Su, H.J.; Tan, T.W. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Singh, R.P.; Pandey, A.; Pandey, A. Photocatalytic antibacterial performance of TiO2 and Ag-doped TiO2 against S. aureus. P. aeruginosa and E. coli. Beilstein J. Nanotechnol. 2013, 4, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubbu, G.D.; Baskar, R.; Anusuya, T.; Seshan, C.A.; Chelliah, R. Toxicity mechanism of titanium dioxide and zinc oxide nanoparticles against food pathogens. Colloids Surf. B Biointerfaces 2016, 148, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Marcous, A.; Rasouli, S.; Ardestani, F. Low-density polyethylene films loaded by titanium dioxide and zinc oxide nanoparticles as a new active packaging system against escherichia coli O157:H7 in fresh calf minced meat. Packag. Technol. Sci. 2017, 30, 693–701. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Mokarram, R.R.; Hashemi, M.; Coma, V. Preparation and characterization of active emulsified films based on chitosan-carboxymethyl cellulose containing zinc oxide nano particles. Int. J. Biol. Macromol. 2017, 99, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Noshirvani, N.; Ghanbarzadeh, B.; Rezaei Mokarram, R.; Hashemi, M. Novel active packaging based on carboxymethyl cellulose-chitosan-ZnO nps nanocomposite for increasing the shelf life of bread. Food Packag. Shelf Life 2017, 11, 106–114. [Google Scholar] [CrossRef]
- Rescek, A.; Krehula, L.K.; Katancic, Z.; Hrnjak-Murgic, Z. Active bilayer pe/pcl films for food packaging modified with zinc oxide and casein. Croat. Chem. Acta 2015, 88, 461–473. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Threepopnatkul, P.; Wongnarat, C.; Intolo, W.; Suato, S.; Kulsetthanchalee, C. Effect of TiO2 and ZnO on thin film properties of pet/pbs blend for food packaging applications. Energy Procedia 2014, 56, 102–111. [Google Scholar] [CrossRef]
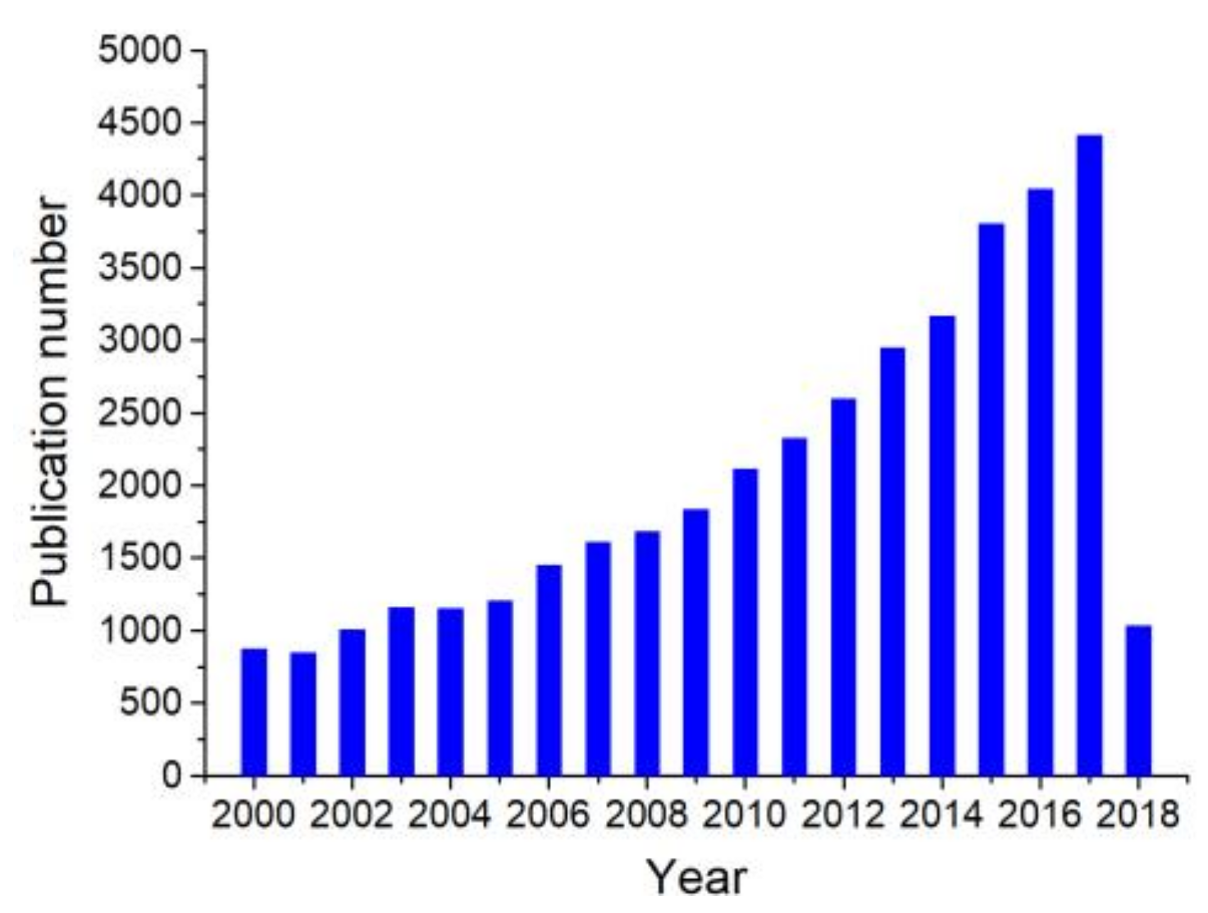

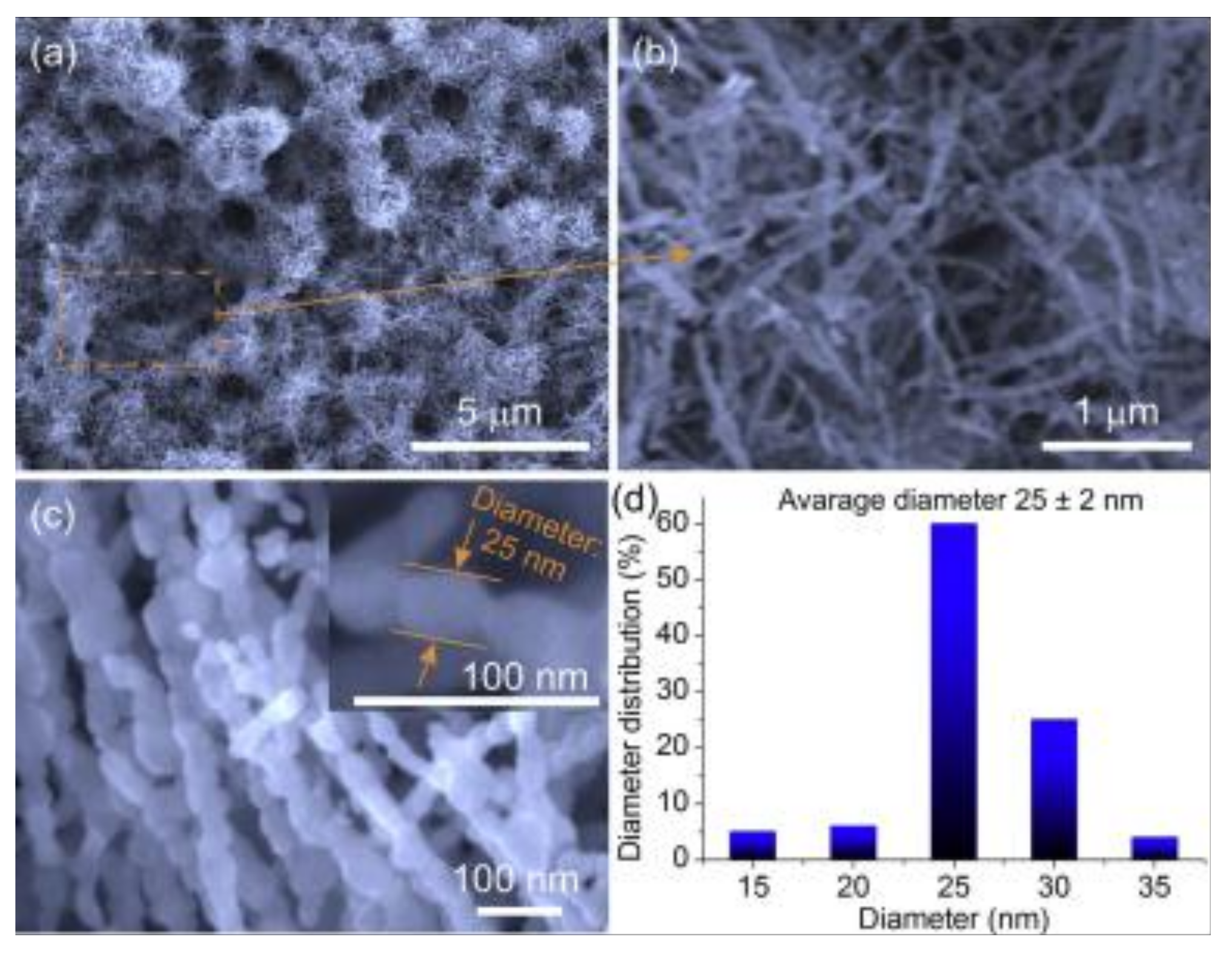
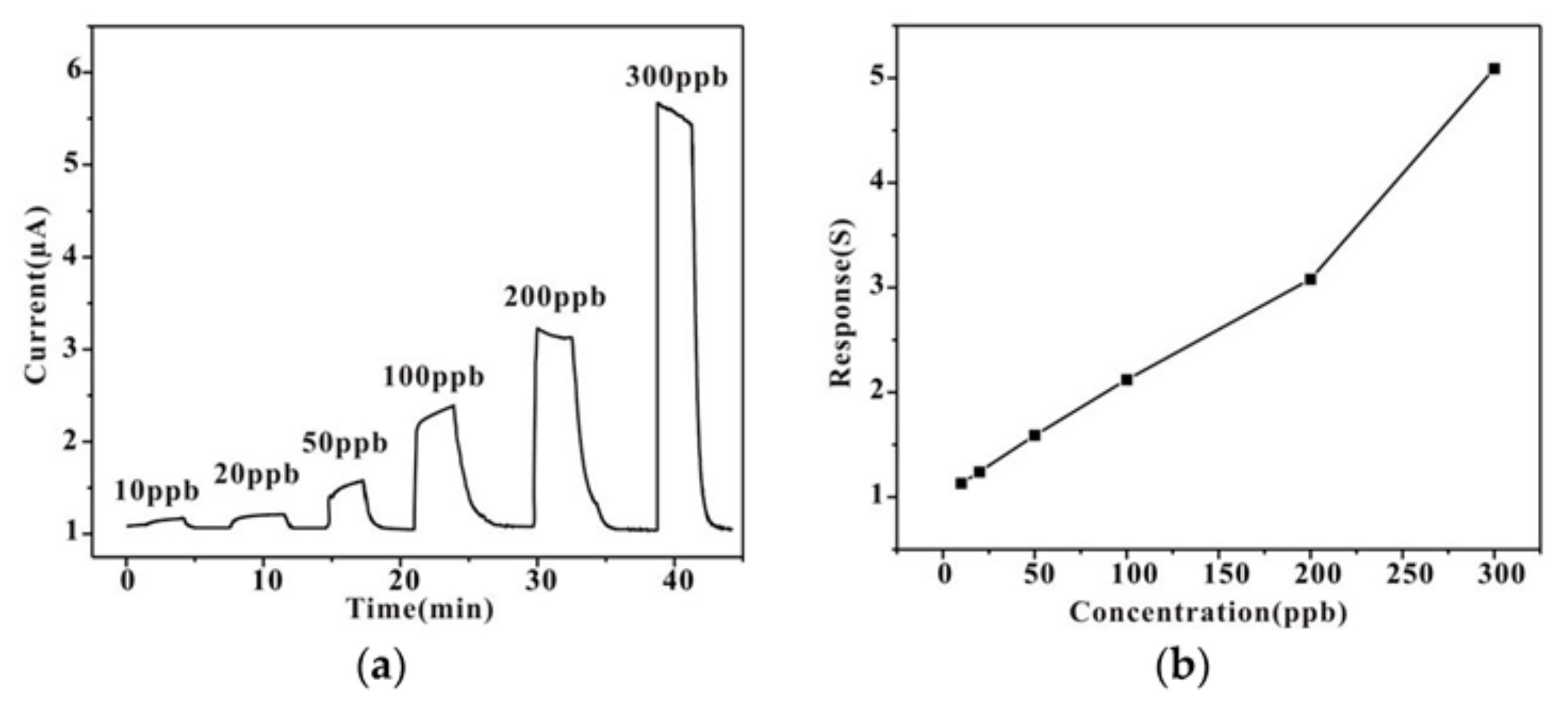
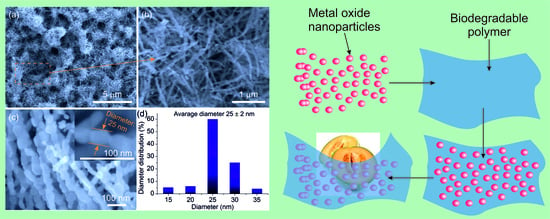
| Material and Morphology | Target Gas and Concentration | Operating Temperature (°C) | Reference |
|---|---|---|---|
| TiO2 nanotubes | TMA, 40–400 ppm | - | [55] |
| Au-WO3 nanorods | TMA, 100 ppm | 280 | [56] |
| Au-MoO3 nanobelts | TMA, 5–100 ppm | 280 | [59] |
| Cr3+-ZnO nanorod | TMA, 0.01–100 ppm | 255 | [57] |
| ZnO-Cr2O3 | TMA, 5 ppm | 400 | [60] |
| ZnO-In2O3 nanofibers | TMA, 0.05–5 ppm | 375 | [61] |
| Cr2O3-SnO2 nanowire | TMA, 0.25–5 ppm | 450 | [62] |
| Au-ZnO porous nanosheets | TMA, 10–300 ppm | 260 | [58] |
| α-Fe2O3/TiO2 nanofibers/nanorods | TMA, 10–200 ppm | 250–320 | [63] |
| ZnO pencil-like | DMA, 5–300 ppm | 340 | [65] |
| ZnO nanorod/nanosheet | DMA, 1–1000 ppm | 370 | [66] |
| polypyrrole-SnO2 nanosheets | NH3, 1–10.7 ppm | RT | [68] |
| polypyrrole-ZnO nanorods | NH3, 50 ppm | RT | [69] |
| Polyaniline-TiO2 thin films | NH3, 23–141 ppm | RT | [70] |
| Polyaniline-ZnO nanoparticles | NH3, 20–100 ppm | RT | [71] |
| Au-ZnO nanorods | NH3, 5–100 ppm | RT | [72] |
| SnO2/SnS2 nanotubes/nanoparticles | NH3, 20–500 ppm | RT | [73] |
| TiO2 nanotubes | H2S, 1–50 ppm | 300 | [85] |
| TiO2 nanotubes | H2S, 6–38 ppm | 70 | [75] |
| NiO porous | H2S, 1 ppb–100 ppm | RT-92 | [76] |
| CuO porous nanosheets | H2S, 10 ppb–60 ppm | RT | [77] |
| TiO2-Fe2O3 nanoparticle-nanorods | H2S, 1–200 ppm | 300 | [83] |
| Au-ZnO nanorods | H2S, 3 ppm | 25 | [86] |
| Cu-ZnO thin film | H2S, 5–50 ppm | 250 | [87] |
| ZnO/SnO2, nanospheres | H2S, 0.5–100 ppm | 300 | [80] |
| Ag-SnO2, yolk-shell | H2S, 0.25–5 ppm | 350 | [81] |
| Ag2O/SnO2 porous | H2S, 300 ppb | 100 | [82] |
| Co3O4-SnO2 nanobox | H2S, 50 ppm | 180 | [79] |
| CuO/V2O5 nanowires | H2S, 7–23 ppm | 220 | [78] |
| PbO-SnO2 nanowires | Ethanol, 5–200 ppm | 300 | [88] |
| Reduced graphene oxide-ZnO, nanoparticles, chain-like agglomerates | Ethanol, 100–250 ppm | 250 | [4] |
| TiO2 nanotubes | Ethanol, 10–50 ppm | 200–500 | [89] |
| Cr2O3-WO3, nanoparticle, nanorods | Ethanol, 5–200 ppm | 300 | [90] |
| Co3O4 porous nanosheets | Ethanol, 1–100 ppm | 220 | [91] |
| Cd-NiO thin film | Ethanol, 1000 ppm | 100 | [92] |
| PtO2-SnO2 nanofiber | Acetone, 0.6–5 ppm | 400 | [93] |
| Fe-C-WO3 walnut-like particles | Acetone, 0.2–10 ppm | 300 | [94] |
| W-NiO, flower-like spheres | Acetone, 10–1000 ppm | 250 | [95] |
| In2O3-reduced graphene oxide, nanocubes | Acetone, 5–25 ppm | 175 | [96] |
| Sensing Material | Target Gases | Application | Reference |
|---|---|---|---|
| SnO2, WO3 | Ethanol, acetone, acetonitrile, ethyl acetate | Onion quality evaluation | [97] |
| SnO2 | Ethanol, ethyl acetate, isobutyl alcohol, etc. | To identify geographical origin of Licorice roots | [98] |
| SnO2, SnO2-SiO2, Ag-SnO2, Au-SnO2, Pd-SnO2, WO3 | Acetaldehyde,ethanol, acetone, etc. | To distinguish truffle-flavored oils | [99] |
| SnO2 | Aromatic compounds | Classification of Indonesian black tea | [104] |
| SnO2 | Alcohols, aldehydes, ketones, etc. | To study the global aromatic profile of coffee | [105] |
| SnO2, MoO3 | Acids, alcohols | Diagnosis of Enterobacteriaceae in vegetable soups | [106] |
| SnO2 | Aromatic compounds | To study the quality of tea | [107] |
| Metal oxide sensors, Hanwei Electronics Co., Ltd. | Alcohols, NH3 | Prediction of banana quality | [108] |
| Metal oxide sensors, Hanwei Electronics Co., Ltd. and Figaro Inc. | Sulfur compounds, H2S, NH3 | Classification of garlic cultivars | [109] |
| Metal oxide sensors, PEN2, Airsense Analytics, Germany | Alcohol, NH3, aromatic compounds | Strawberry juice quality control | [110] |
| Metal oxide sensors, Figaro USA Inc. | VOCs | Fish species discrimination | [111] |
| Metal oxide sensors, Alpha M.O.S., France | Organic acids, caffeine | Aromatic profile of Espresso coffee | [112] |
| Metal oxide sensors, Ogam Technology, Futurlec, e2v and Figaro Engineering | VOCs, NH3, alcohol, etc. | Meat and fish freshness control | [113] |
| Metal oxide sensors, Alpha M.O.S., France | Ethanol, NH3, VOCs | To trace peanuts quality | [114] |
| Metal oxide sensors, Win Muster Airsense Analytics Inc., Germany | Alcohol, methane, aromatic compounds | Analysis of edible oil oxidation | [115] |
| Metal oxide sensors, Hangzhou Ke Na Sensors Inc., and Figaro Inc. | TMA, DMA, Ethanol | Freshness control of hairtail fish and pork | [116] |
| Metal oxide sensors, Figaro Inc., Japan | Ethanol, NH3, VOCs | Classification of honey | [117] |
| Material | Application/ Mode of Action | Reference |
|---|---|---|
| CS/PVA/TiO2 | Food packaging | [125] |
| CH/CMC/ZnO | Food packaging | [126] |
| PVC/TiO2 | Food packaging | [127] |
| TiO2-protein/polysaccharide (marine red alga) | Quality and shelf life of cherry tomatoes | [128] |
| Starch/TiO2 | UV-protective food packaging material | [129] |
| TiO2-LDPE | Quality and shelf-life of Pacific white shrimp | [130] |
| TiO2-LDPE | Antimicrobial actions in fruit and vegetable | [131] |
| LDPE/clay-TiO2 | Antimicrobial action, horticulture packaging | [132] |
| TiO2/SiO2 | Degrade ethylene, quality and shelf life of mature green tomatoes | [133] |
| Chitosan/TiO2 | Ethylene photodegradation, prolongation of tomato storage | [134] |
| WPI/CNF/TiO2/REO | Microbial and sensory qualities of lamb meat, antimicrobial action | [135] |
| JFPS-TiO2 | Antimicrobial action, active food packaging | [136] |
| AgCl-TiO2 | Anti-quorum sensing, food packaging | [137] |
| Ag/TiO2-SiO2 | Preservation of fresh green lettuce, reduction of lettuce spoilage, antimicrobial action | [138] |
| Ag/TiO2 | Shelf life and microbial safety of white bread, inhibition of yeast, mold and Gram-positive bacteria | [139] |
| Ag/TiO2-SiO2, Ag/N-TiO2 or Au/TiO2 | Shelf life of white bread, antimicrobial action | [140] |
| PLA/TiO2 and PLA/TiO2+Ag | Shelf-life of Yunnan cottage cheese | [141] |
| Starch/TiO2 | Reduced water solubility, water vapor permeability and moisture uptake of the film, food packaging function | [142] |
| Soybean polysaccharide/TiO2 | Heat seal strength, antimicrobial action, food coating and packaging | [143] |
| Fish gelatin/agar bilayer/TiO2 | Food packaging, food safety | [144] |
| Wheat gluten/CNC/TiO2 | Food packaging, food safety | [145] |
| TiO2/LDPE | Food packaging, food safety | [146] |
| ZnO/LDPE, TiO2/LDPE, and ZnO-TiO2/LDPE | Antimicrobial property against E. coli in fresh calf minced meat, shelf life of calf meat | [150] |
| Carboxymethyl cellulose-chitosan-oleic acid/ZnO | Antimicrobial action against the fungi Aspergillus niger, increased extensibility | [151] |
| CMC-CH-OL-ZnO | Shelf life of sliced white bread, active against yeast and molds | [152] |
| polyethylene/polycaprolactone-ZnO | Shelf life of food, antimicrobial actions, food packaging and transport | [153] |
| Chitosan-TiO2 | Food packaging, effective packaging of red grapes, shelf life of grapes | [147] |
| TiO2 and Ag-TiO2 | Photocatalytic antibacterial performance, food packaging | [148] |
| Chitosan-ZnO/polyethylene | Food packaging,antimicrobial properties, shelf life of food | [154] |
| Wheat gluten/nanocellulose/TiO2 coated in craft paper | Food packaging, antimicrobial action | [145] |
| TiO2, ZnO-poly(ethyleneterephthalate) and poly(butylene succinate) | Food packaging | [155] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galstyan, V.; Bhandari, M.P.; Sberveglieri, V.; Sberveglieri, G.; Comini, E. Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging. Chemosensors 2018, 6, 16. https://doi.org/10.3390/chemosensors6020016
Galstyan V, Bhandari MP, Sberveglieri V, Sberveglieri G, Comini E. Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging. Chemosensors. 2018; 6(2):16. https://doi.org/10.3390/chemosensors6020016
Chicago/Turabian StyleGalstyan, Vardan, Manohar P. Bhandari, Veronica Sberveglieri, Giorgio Sberveglieri, and Elisabetta Comini. 2018. "Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging" Chemosensors 6, no. 2: 16. https://doi.org/10.3390/chemosensors6020016
APA StyleGalstyan, V., Bhandari, M. P., Sberveglieri, V., Sberveglieri, G., & Comini, E. (2018). Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging. Chemosensors, 6(2), 16. https://doi.org/10.3390/chemosensors6020016