Arrays of TiO2 Nanowires as Photoelectrochemical Sensors for Hydrazine Detection
Abstract
:1. Introduction
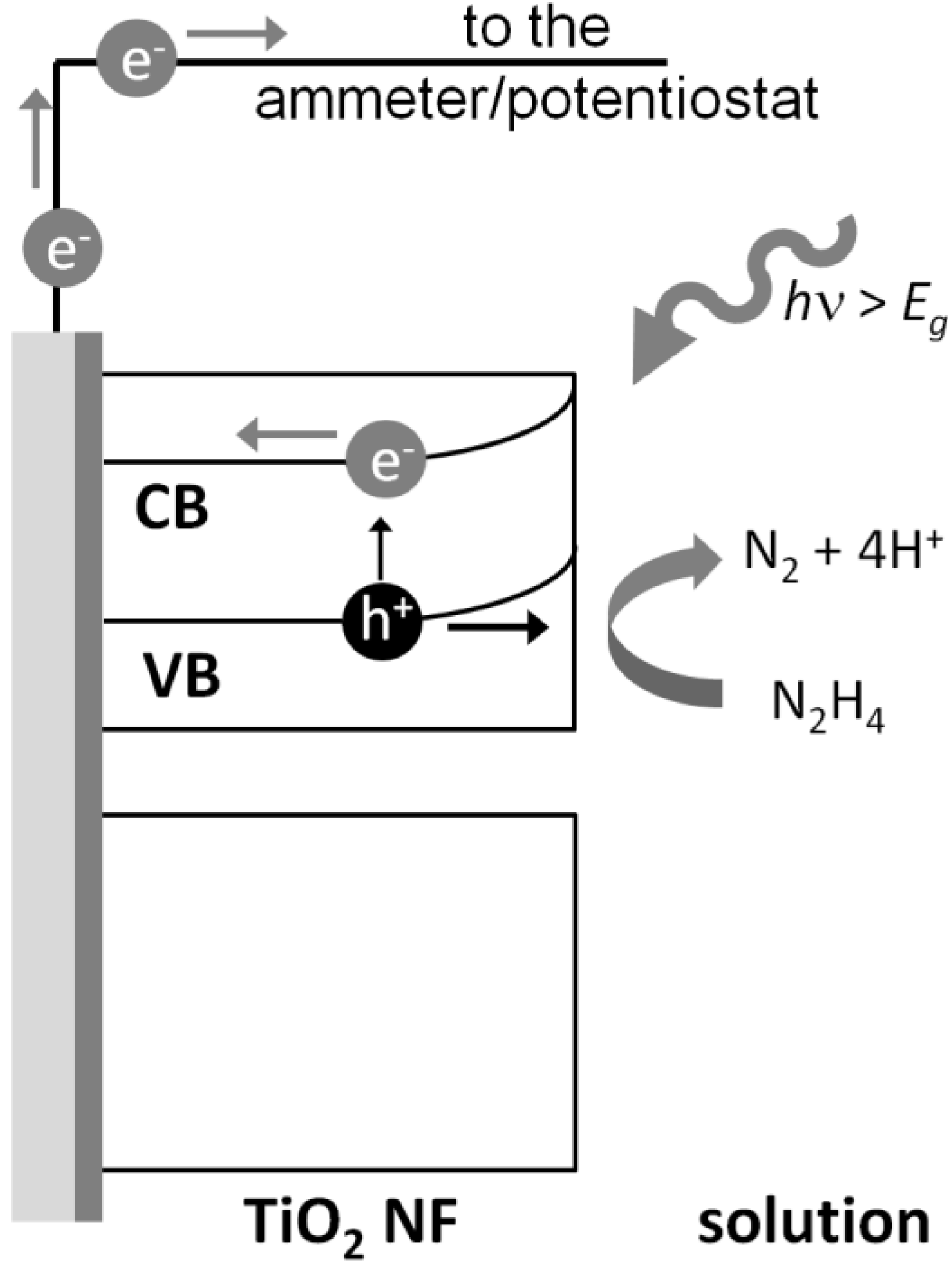
2. Experimental Section
2.1. Chemicals and Materials
2.2. Template Synthesis
2.3. Electrodes Fabrication
2.4. Sensor Characterizations
2.5. Photoelectrochemical Hydrazine Determination
3. Results and Discussion
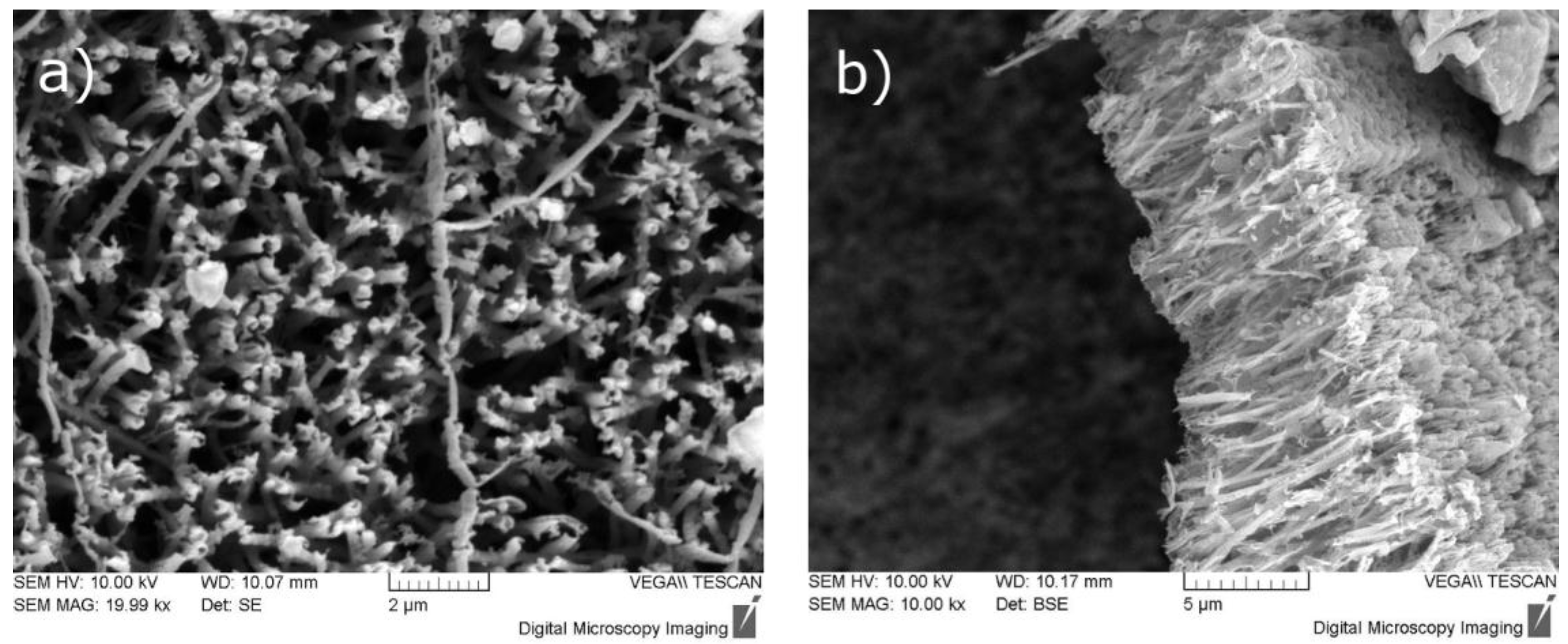
3.1. Electrode Fabrication
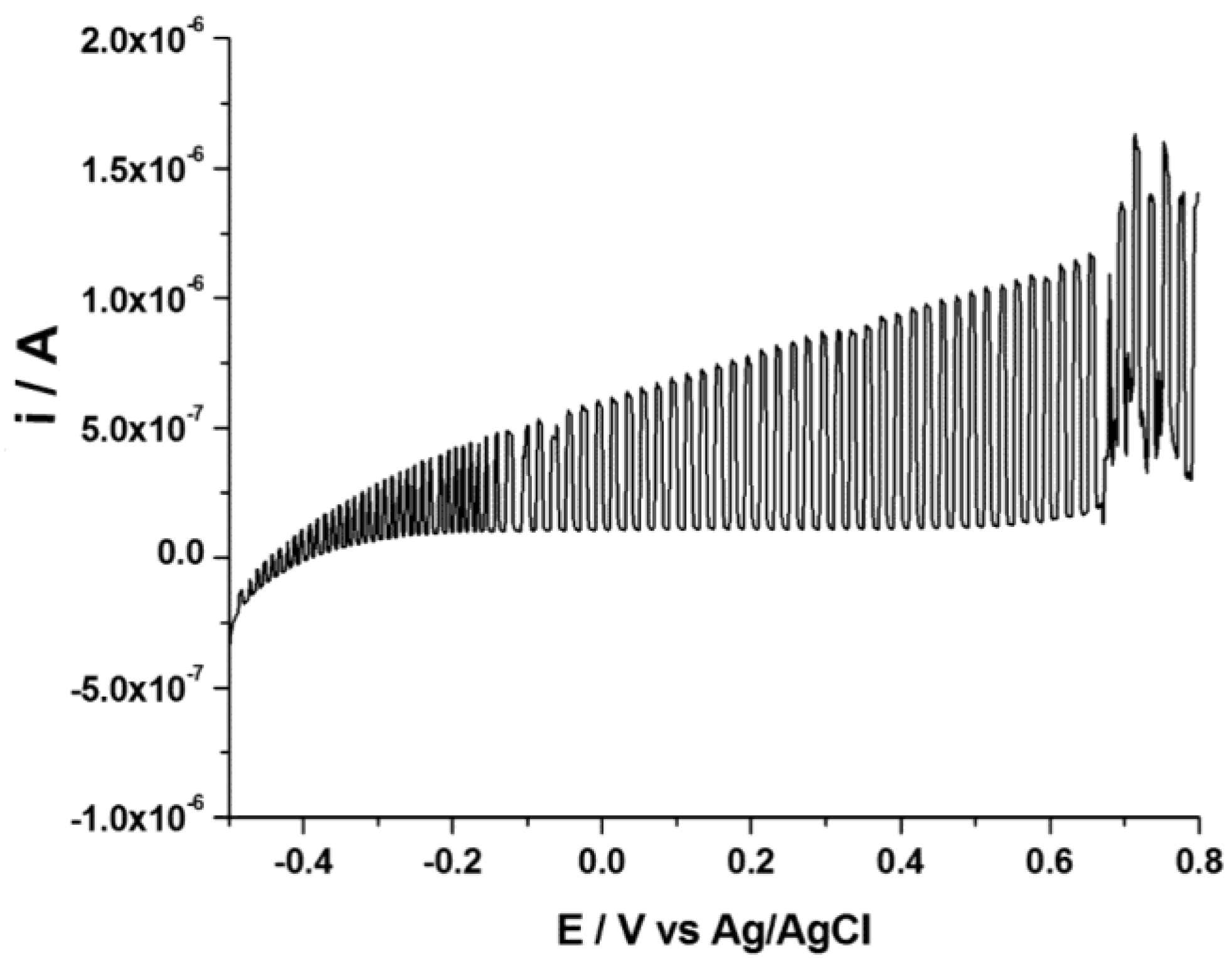
3.2. Influence of Bias Potential on the Hydrazine Determination
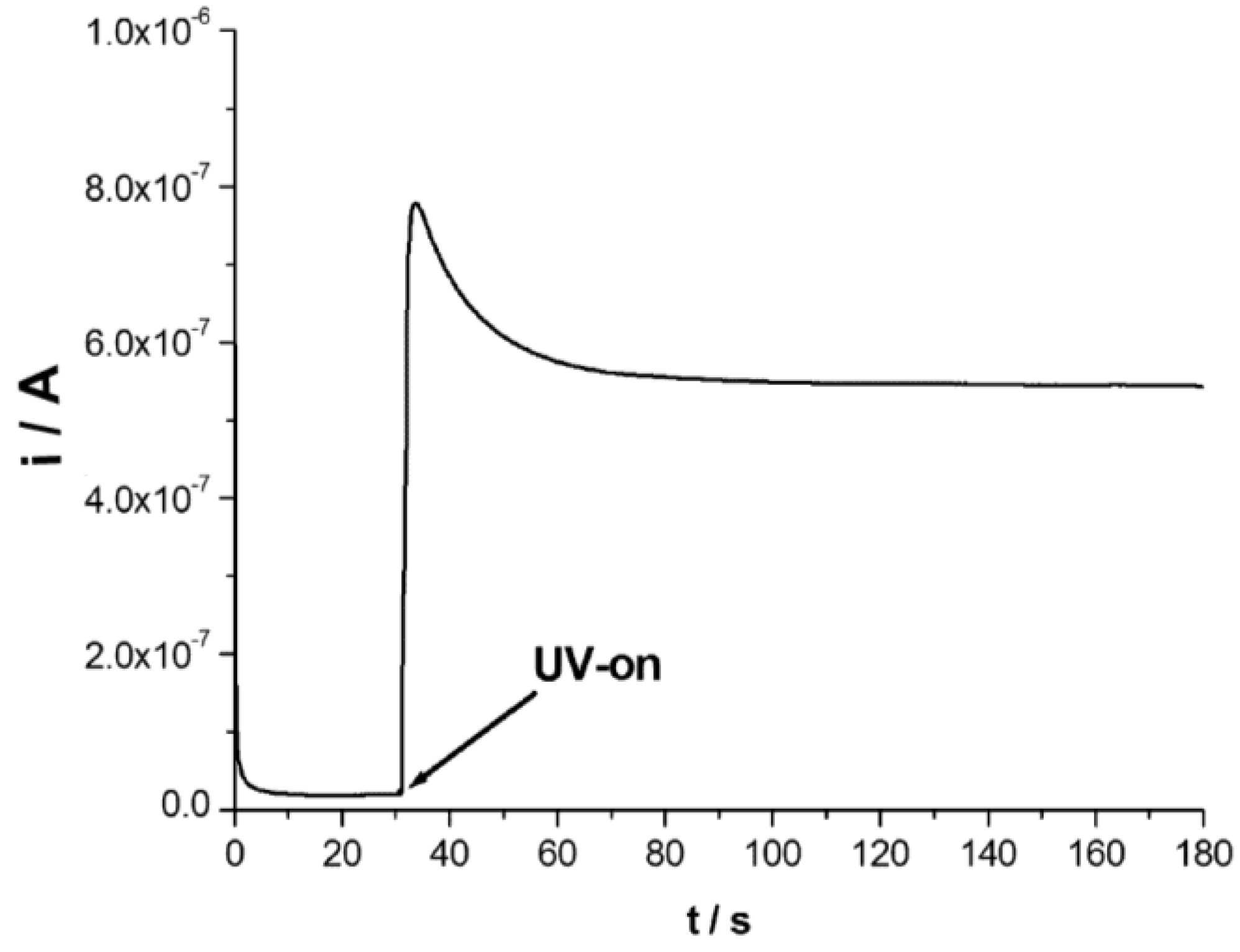
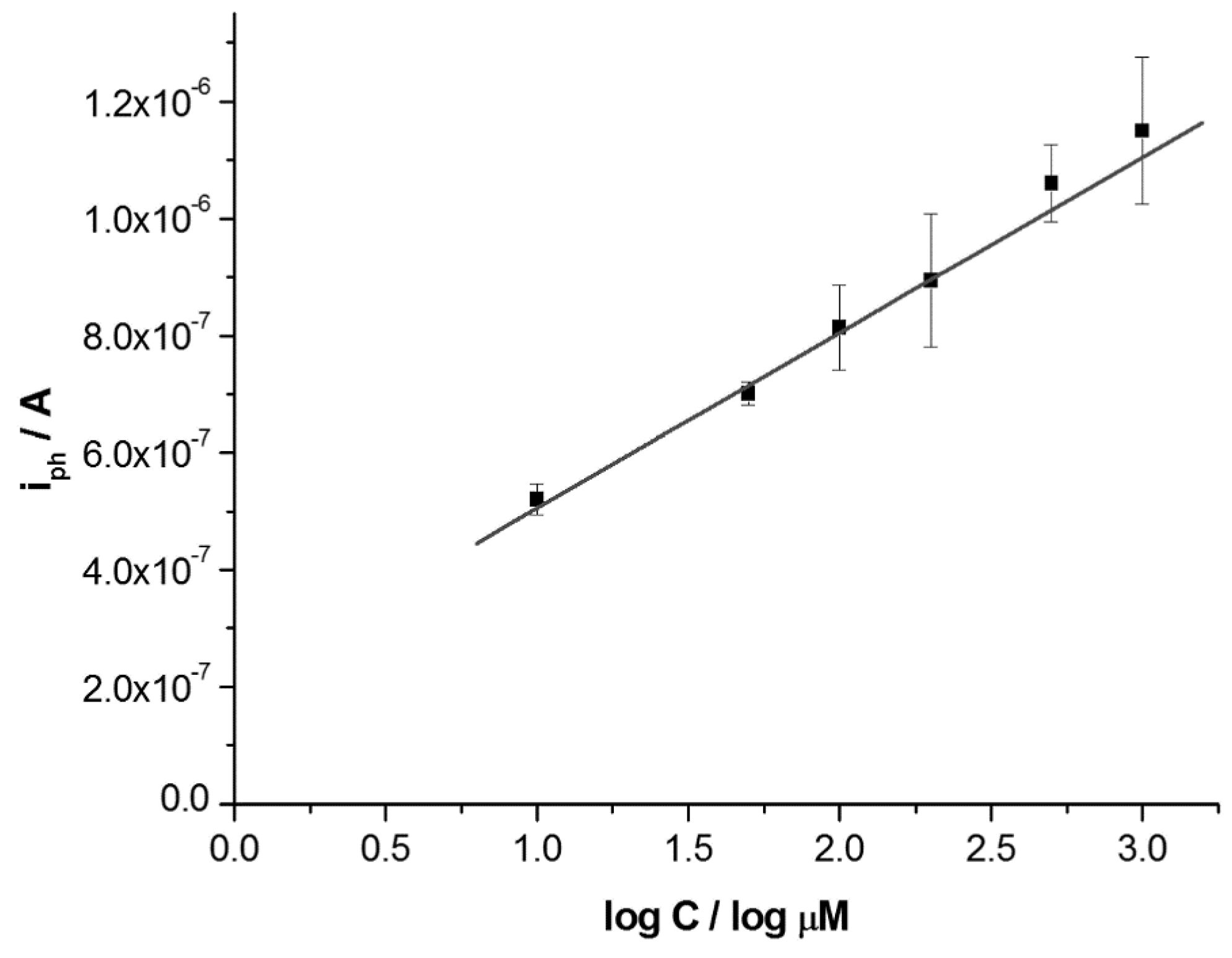
3.3. Photoelectrochemical Determination of Hydrazine
| Method | Electrode | LOD (μM) | Reference |
|---|---|---|---|
| Chromatography | // | 0.03 | [5] |
| Spectrophotometry | // | 30* (45) | [2] |
| Chemiluminescence | // | 0.5* (0.75) | [4] |
| Electrocatalysis | NPHMGCa | 8 | [5] |
| Electrocatalysis | PCVMGCb | 4.2 | [6] |
| Electrocatalysis | NHCFMGCc | 90.6 | [7] |
| Electrocatalysis | MNPMBDDd | 1–3.27 | [26–28] |
| Photoelectrochemistry | Ti/TiO2e | 8.54 | [13] |
| Photoelectrochemistry | TiO2NWA | 1.91 | present work |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United States Environmental Protection Agency. Hydrazine Hazard Summary-Created in April 1992; Revised in January 2000. Available online: http://www.epa.gov/ttnatw01/hlthef/hydrazin.html (accessed on 6 May 2015).
- Safavi, A.; Ensafi, A.A. Kinetic spectrophotometric determination of hydrazine. Anal. Chim. Acta 1995, 300, 307–311. [Google Scholar] [CrossRef]
- Rajasekharan, V.; Ramachandran, C.G. The titration of isoniazid and other hydrazine derivatives with chloramine-t. Anal. Chim. Acta 1971, 57, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Baezzat, M.R. Flow injection chemiluminescence determination of hydrazine. Anal. Chim. Acta 1998, 358, 121–125. [Google Scholar] [CrossRef]
- Evgen’ev, M.I.; Evgen’eva, I.I.; Ismailova, R.N. Extraction-chromatographic determination of hydrazine in natural water as a 5,7-dinitrobenzofurazan derivative using diode-array detection. J. Anal. Chem. 2000, 55, 933–937. [Google Scholar] [CrossRef]
- Abbaspour, A.; Khajehzadeh, A.; Ghaffarinejad, A. Electrocatalytic oxidation and determination of hydrazine on nickel hexacyanoferrate nanoparticles-modified carbon ceramic electrode. J. Electroanal. Chem. 2009, 631, 52–57. [Google Scholar] [CrossRef]
- Golabi, S.M.; Zare, H.R.; Hamzehloo, M. Electrocatalytic oxidation of hydrazine at a pyrocatechol violet (PCV) hemically modified electrode. Microchem. J. 2001, 69, 13–23. [Google Scholar] [CrossRef]
- Shankaran, D.R.; Narayanan, S.S. Amperometric sensor for hydrazine determination based on mechanically immobilized nickel hexacyanoferrate modified electrode. Russ. J. Electrochem. 2001, 37, 1149–1153. [Google Scholar] [CrossRef]
- Liu, S.; Liu, B.; Nakata, K.; Ochiai, T.; Murakami, T.; Fujishima, A. Electrospinning preparation and photocatalytic activity of porous TiO2 nanofibers. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Reddy, K.R.; Nakata, K.; Ochiai, T.; Murakami, T.; Tryk, D.A.; Fujishima, A. Facile fabrication and photocatalytic application of Ag nanoparticles-TiO2 nanofiber composites. J. Nanosci. Nanotechnol. 2011, 11, 3692–3695. [Google Scholar] [CrossRef] [PubMed]
- Haddour, N.; Chauvin, J.; Gondran, C.; Cosnier, S. Photoelectrochemical immunosensor for label-free detection and quantification of anti-cholera toxin antibody. J. Am. Chem. Soc. 2006, 128, 9693–9698. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Tang, L.; Jiang, X.; Chen, H.; Yang, M.; Jin, L.; Zhang, S.; Wang, C.; Zhang, W. A photoelectrochemical immunosensor based on Au-doped TiO2 nanotube arrays for the detection of α-synuclein. Chem. Eur. J. 2010, 16, 14439–14446. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Lei, J.; Wang, P.; Ju, H. Photoelectrochemistry of free-base-porphyrin-functionalized zinc oxide nanoparticles and their applications in biosensing. Chem. Eur. J. 2011, 17, 9440–9447. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Teng, F.; Zhang, P.; Zhao, C.; Pan, X.; Zhang, Z.; Xie, E. Enhanced photoelectrochemical sensor based on ZnO–SnO2 composite nanotubes. J. Alloy. Compd. 2014, 614, 373–378. [Google Scholar] [CrossRef]
- Ojani, R.; Zarei, E. A new simple electrochemically assisted photocatalysis sensor of hydrazine using Ti/TiO2 electrode. J. Braz. Chem. Soc. 2013, 24, 657–662. [Google Scholar]
- Hu, Y.; Xue, Z.; He, H.; Ai, R.; Liu, X.; Lu, X. Photoelectrochemical sensing for hydroquinone based on porphyrin-functionalized Au nanoparticles on grapheme. Biosens. Bioelectron. 2013, 47, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwar, K. Photoelectrochemistry and the environment. J. Appl. Electrochem. 1995, 25, 1067–1082. [Google Scholar]
- Zhang, X.; Guo, Y.; Liu, M.; Zhang, S. Photoelectrochemically active species and photoelectrochemical biosensors. RSC Adv. 2013, 3, 2846–2857. [Google Scholar] [CrossRef]
- Voccia, D.; Palchetti, I. Photoelectrochemical biosensors for nucleic acid detection. J. Nanosci. Nanotechnol. 2015, 15, 3320–3332. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, M.; Gambirasi, A.; Favaro, M.; Ugo, P. Electrochemical synthesis and characterization of hierarchically branched ZnO nanostructures on ensembles of gold nanowires. Electrochim. Acta 2012, 78, 539–546. [Google Scholar] [CrossRef]
- Ongaro, M.; Mardegan, A.; Stortini, A.M.; Signoretto, M.; Ugo, P. Arrays of templated TiO2 nanofibres as improved photoanodes for water splitting under visible light. Nanotechnology 2015, 26, 165402. [Google Scholar] [CrossRef] [PubMed]
- Signoretto, M.; Ghedini, E.; Pinna, F.; Nichele, V.; Crocella, V.; Cerrato, G. Effect of textural properties on the drug delivery behaviour of nanoporous TiO2 matrices. Microporous Mesoporous Mater. 2011, 139, 189–196. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmidt, B.; Kunze, J.; Schmuki, P. Photoresponse in the visible range from Cr-doped TiO2 nanotubes. Chem. Phys. Lett. 2007, 433, 323–326. [Google Scholar] [CrossRef]
- Spadavecchia, F.; Ardizzone, S.; Cappelletti, G.; Falciola, L.; Ceotto, M.; Lotti, D. Investigation and optimization of photocurrent transient measurements on nano-TiO2. J. Appl. Electrochem. 2013, 43, 217–225. [Google Scholar] [CrossRef]
- Channon, R.B.; Newland, J.C.; Bristow, A.W.T.; Ray, A.D.; Macpherson, J.V. Selective detection of hydrazine in the presence of excess electrochemically active pharmaceutical ingredients using boron doped diamond metal nanoparticle functionalised electrodes. Electroanalysis 2013, 25, 2613–2619. [Google Scholar] [CrossRef]
- Sun, H.; Dong, L.; Yu, H.; Huo, M. Direct electrochemical oxidation and detection of hydrazine on a boron doped diamond (BDD) electrode. Russ. J. Electrochem. 2013, 49, 883–887. [Google Scholar] [CrossRef]
- Batchelor-McAuley, C.; Banks, C.E.; Simm, A.O.; Jones, T.G.J.; Compton, R.G. The electroanalytical detection of hydrazine: A comparison of the use of palladium nanoparticles supported on boron-doped diamond and palladium plated BDD microdisc array. Analyst 2006, 131, 106–110. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ongaro, M.; Signoretto, M.; Trevisan, V.; Stortini, A.M.; Ugo, P. Arrays of TiO2 Nanowires as Photoelectrochemical Sensors for Hydrazine Detection. Chemosensors 2015, 3, 146-156. https://doi.org/10.3390/chemosensors3020146
Ongaro M, Signoretto M, Trevisan V, Stortini AM, Ugo P. Arrays of TiO2 Nanowires as Photoelectrochemical Sensors for Hydrazine Detection. Chemosensors. 2015; 3(2):146-156. https://doi.org/10.3390/chemosensors3020146
Chicago/Turabian StyleOngaro, Michael, Michela Signoretto, Valentina Trevisan, Angela Maria Stortini, and Paolo Ugo. 2015. "Arrays of TiO2 Nanowires as Photoelectrochemical Sensors for Hydrazine Detection" Chemosensors 3, no. 2: 146-156. https://doi.org/10.3390/chemosensors3020146
APA StyleOngaro, M., Signoretto, M., Trevisan, V., Stortini, A. M., & Ugo, P. (2015). Arrays of TiO2 Nanowires as Photoelectrochemical Sensors for Hydrazine Detection. Chemosensors, 3(2), 146-156. https://doi.org/10.3390/chemosensors3020146









