Research on a Humidity Sensor Based on Polymerizable Deep Eutectic System-Modified Filter Paper
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
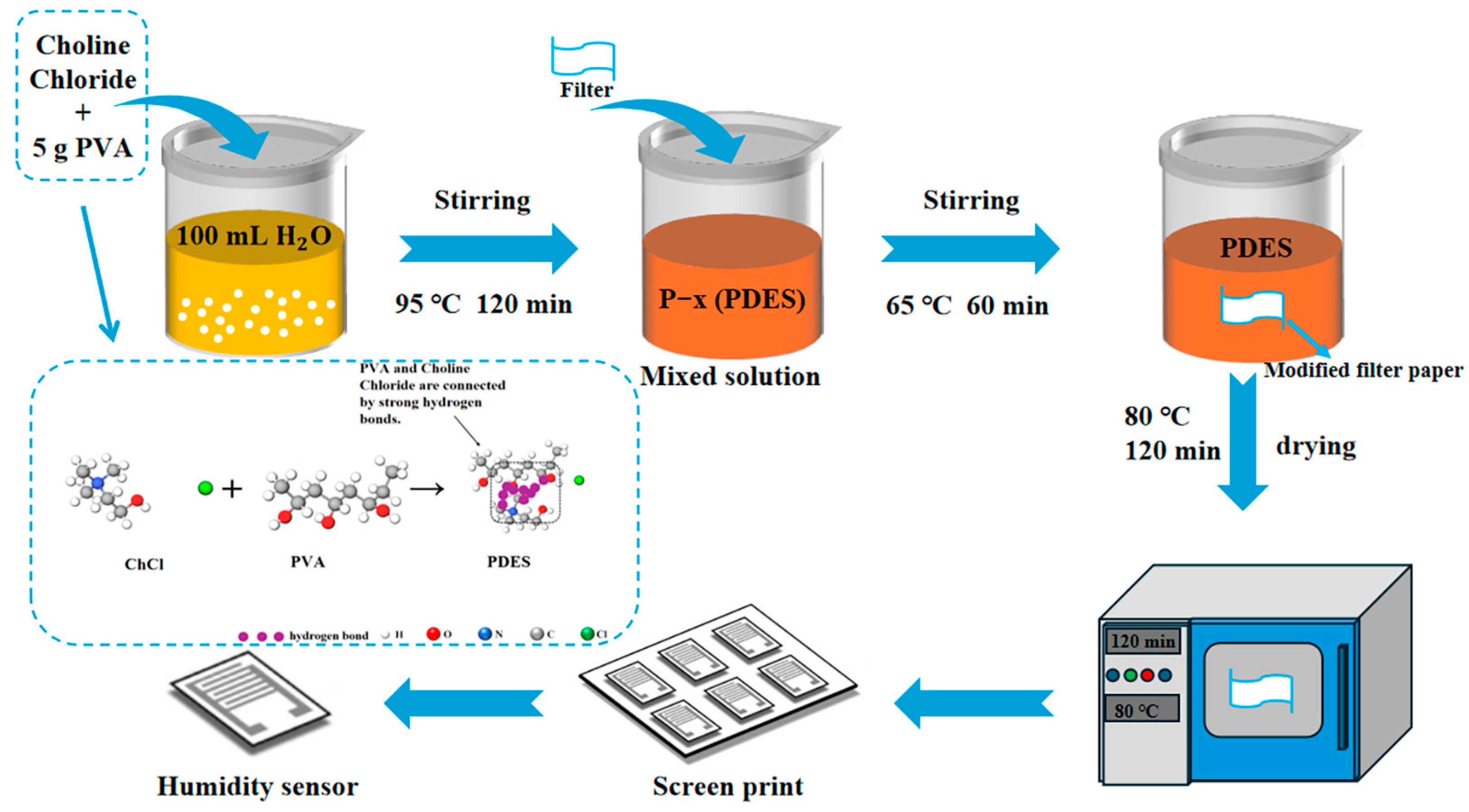
2.2. Materials Preparation
2.3. Sensor Fabrication
2.4. Characterizations
2.5. Measurement of Humidity Sensors
3. Results and Discussion
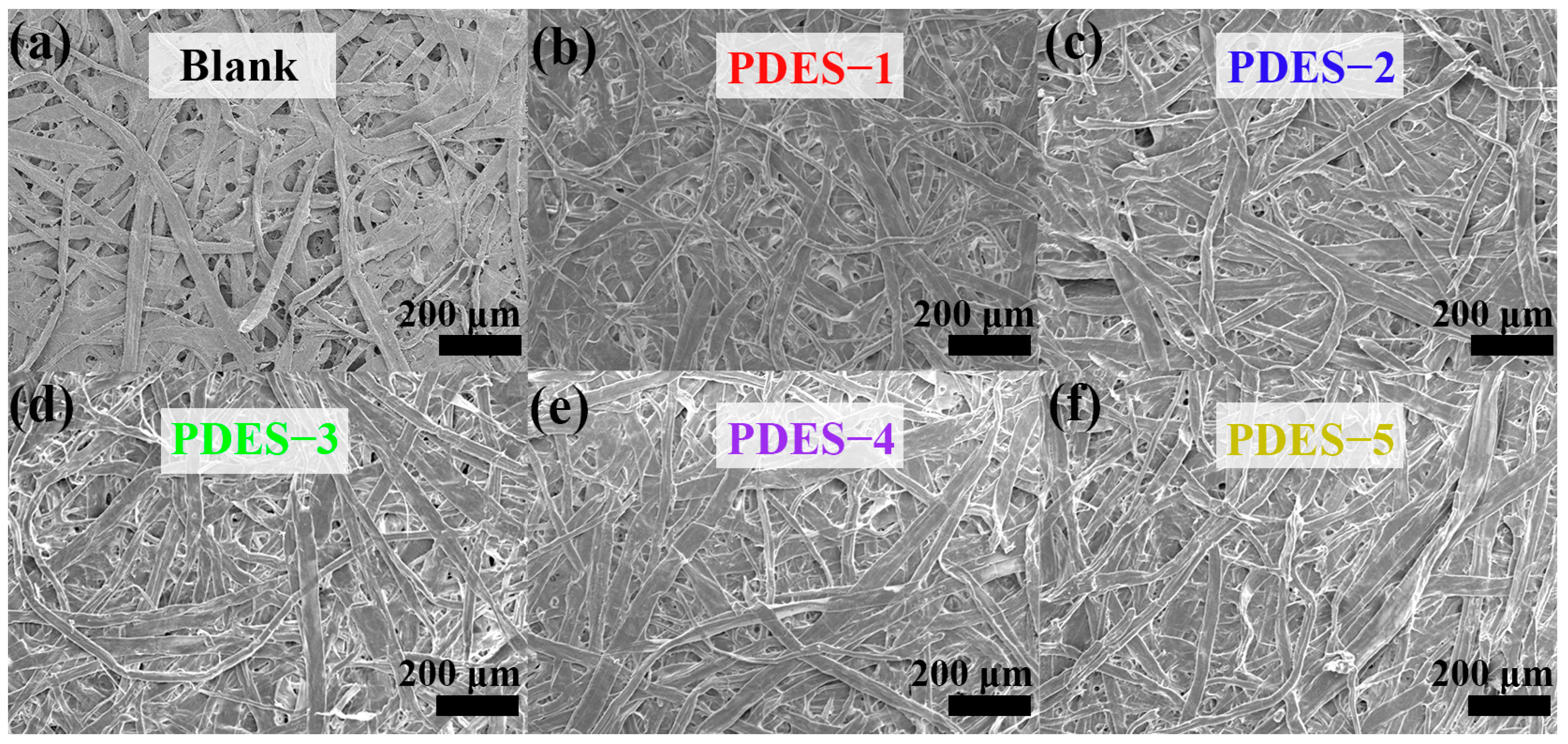
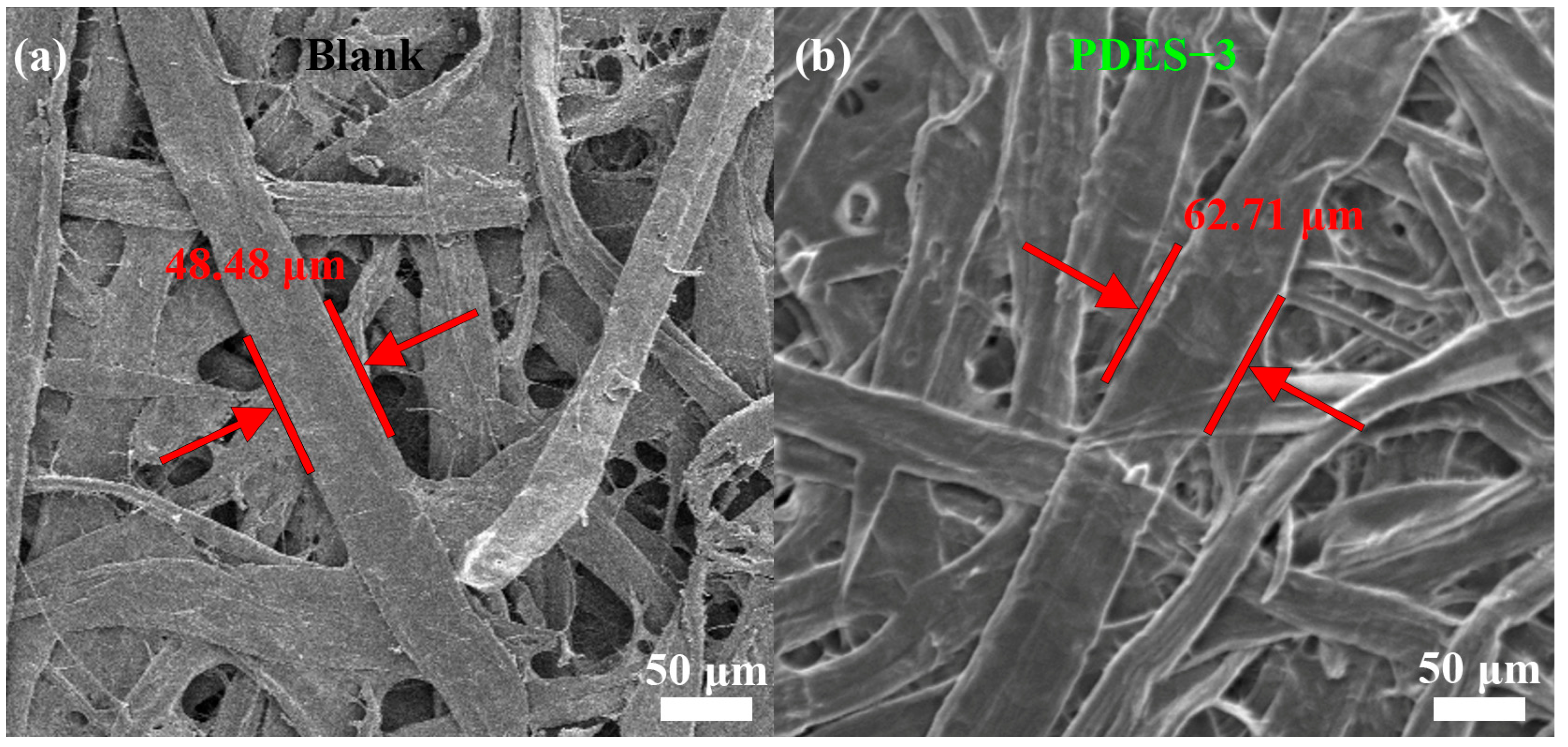
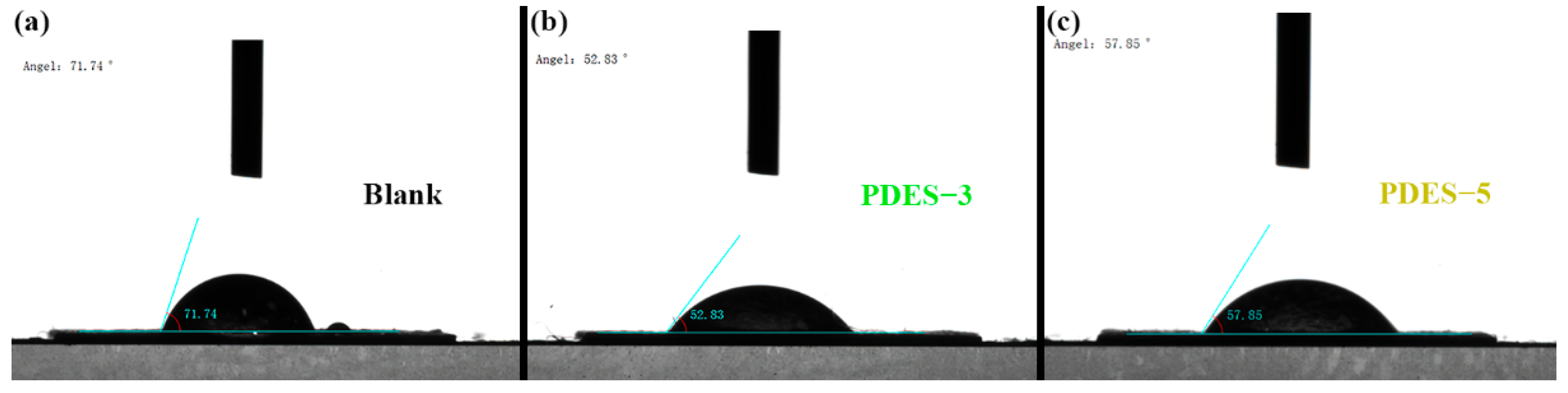
3.1. Morphological Features
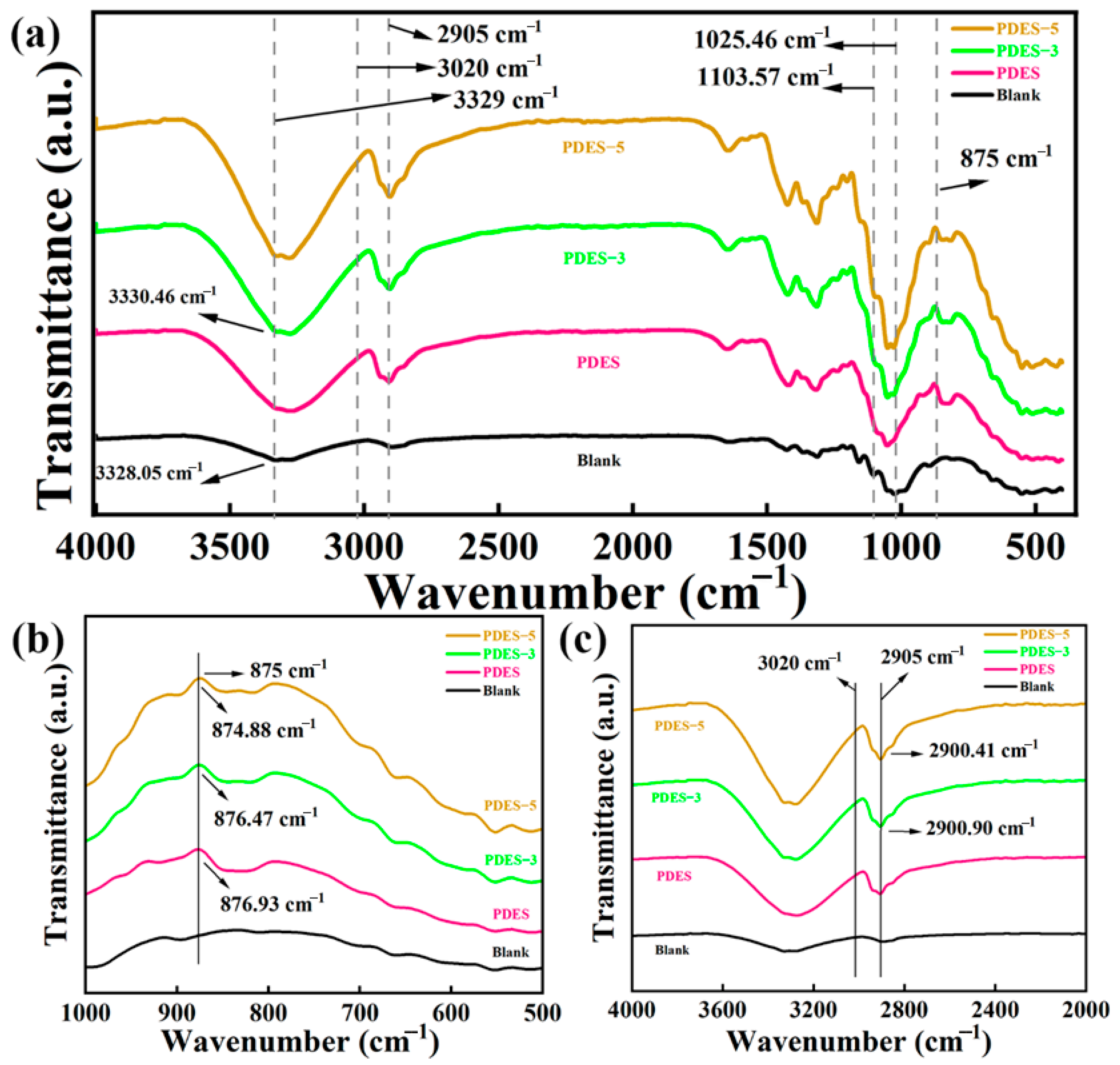
3.2. Structural Characterization
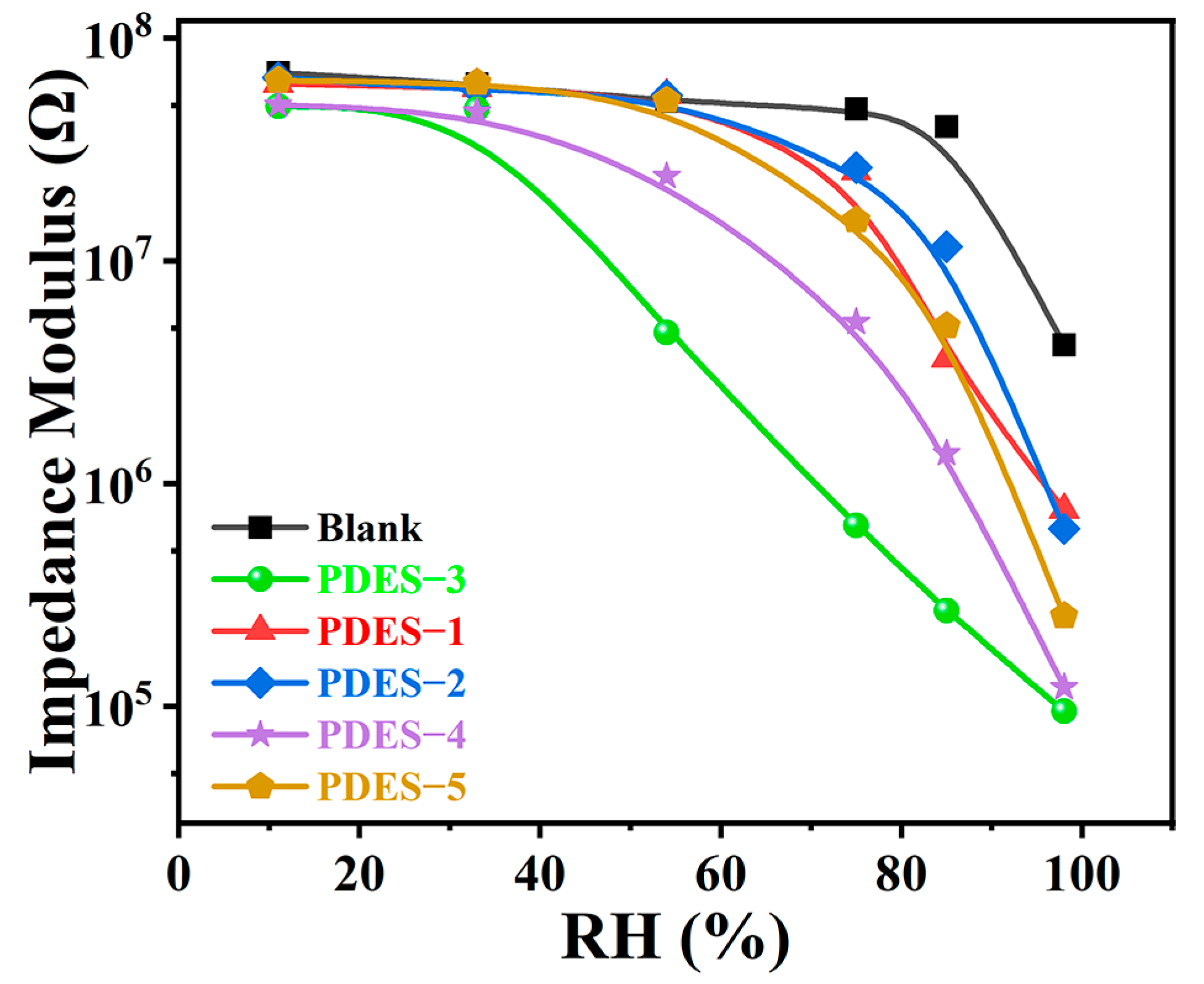
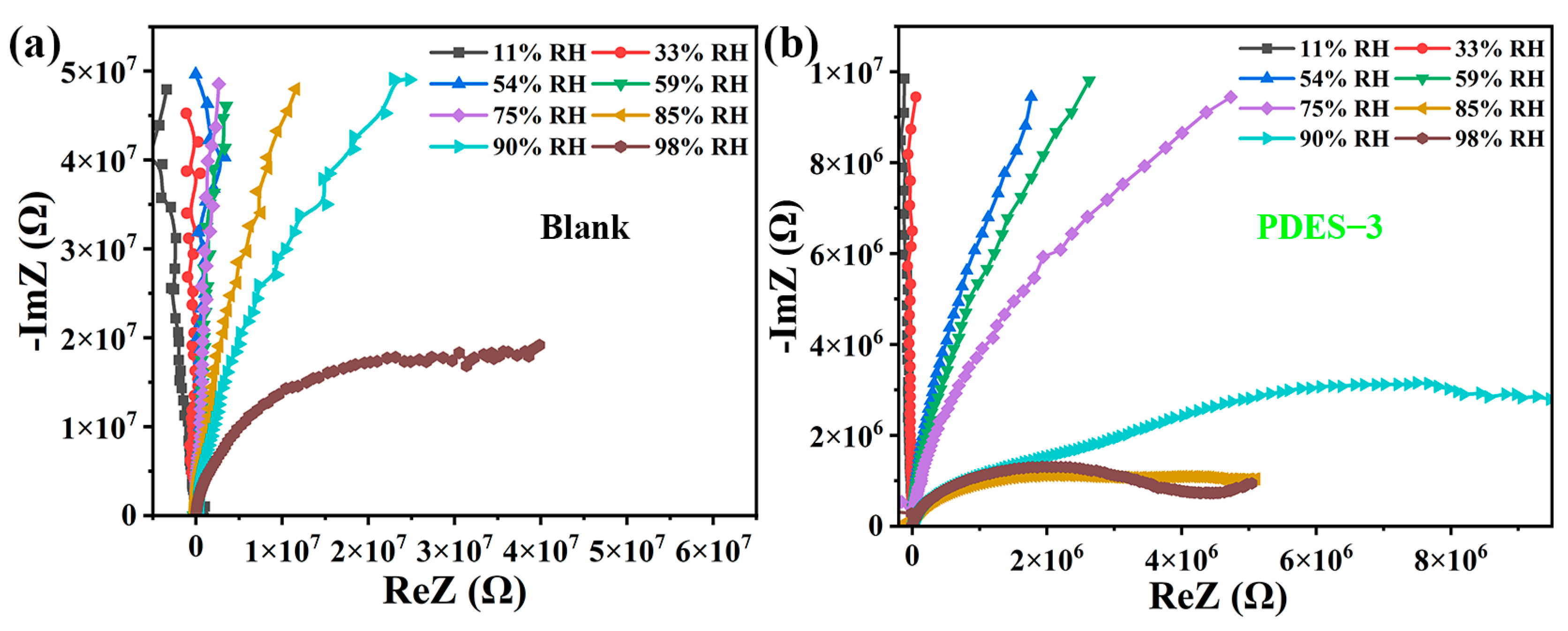
3.3. Humidity-Sensing Performance
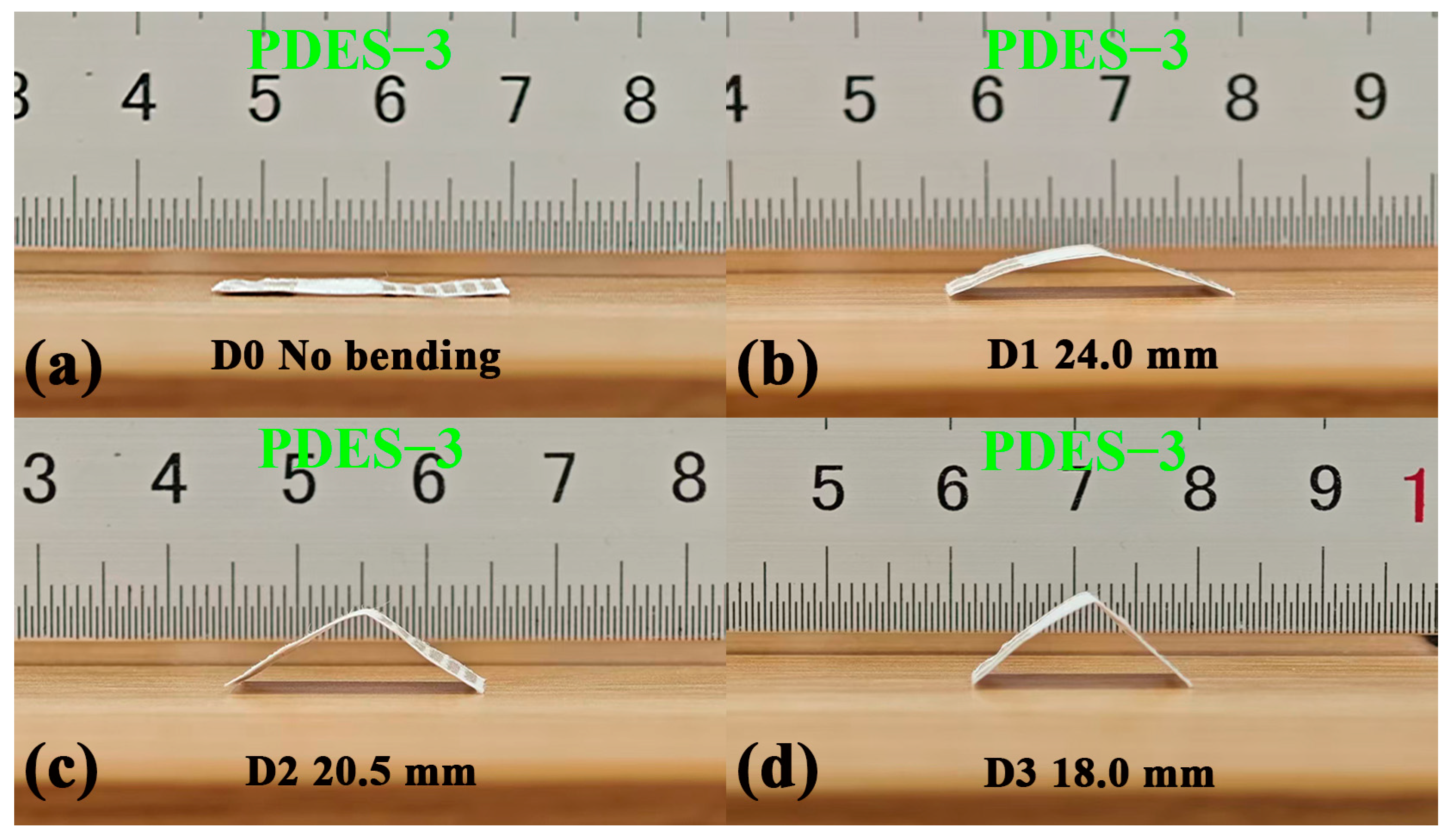
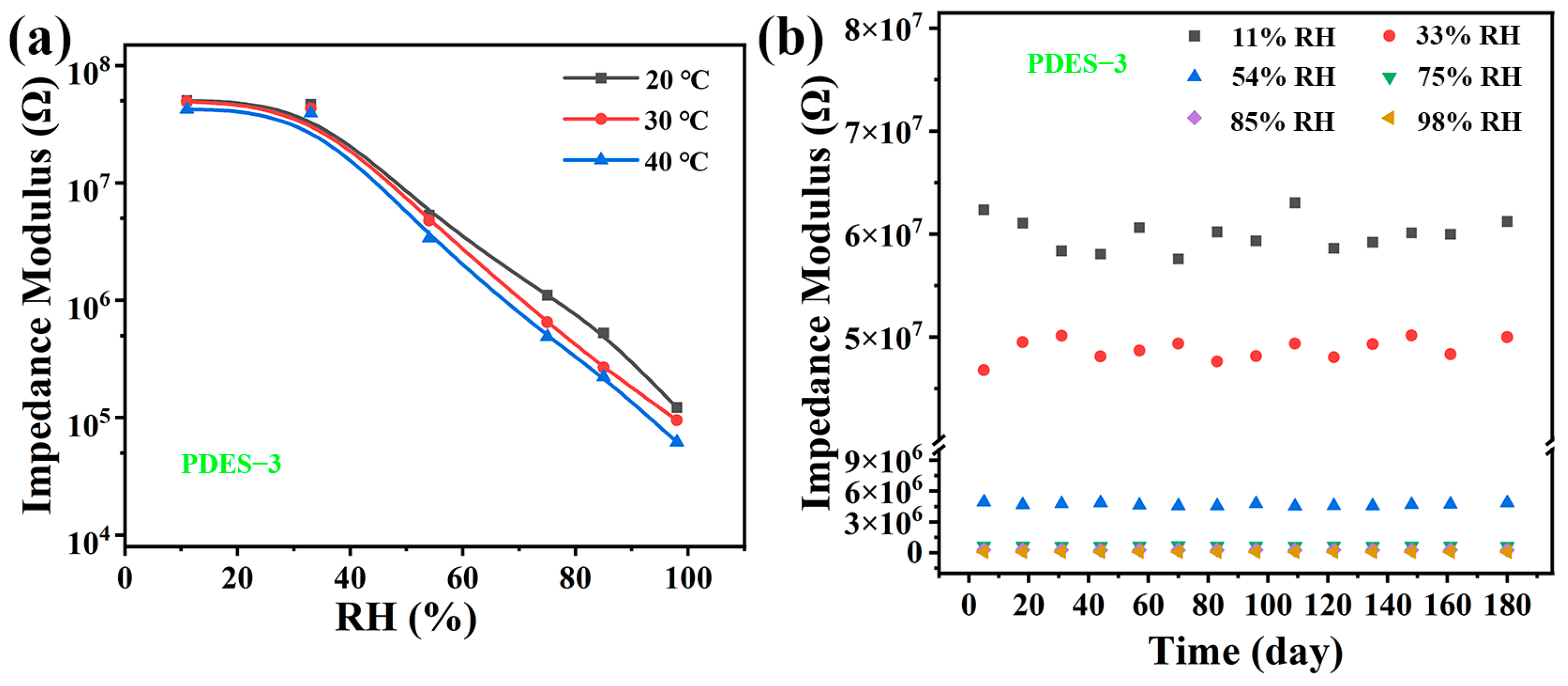
3.4. Flexibility Characteristics
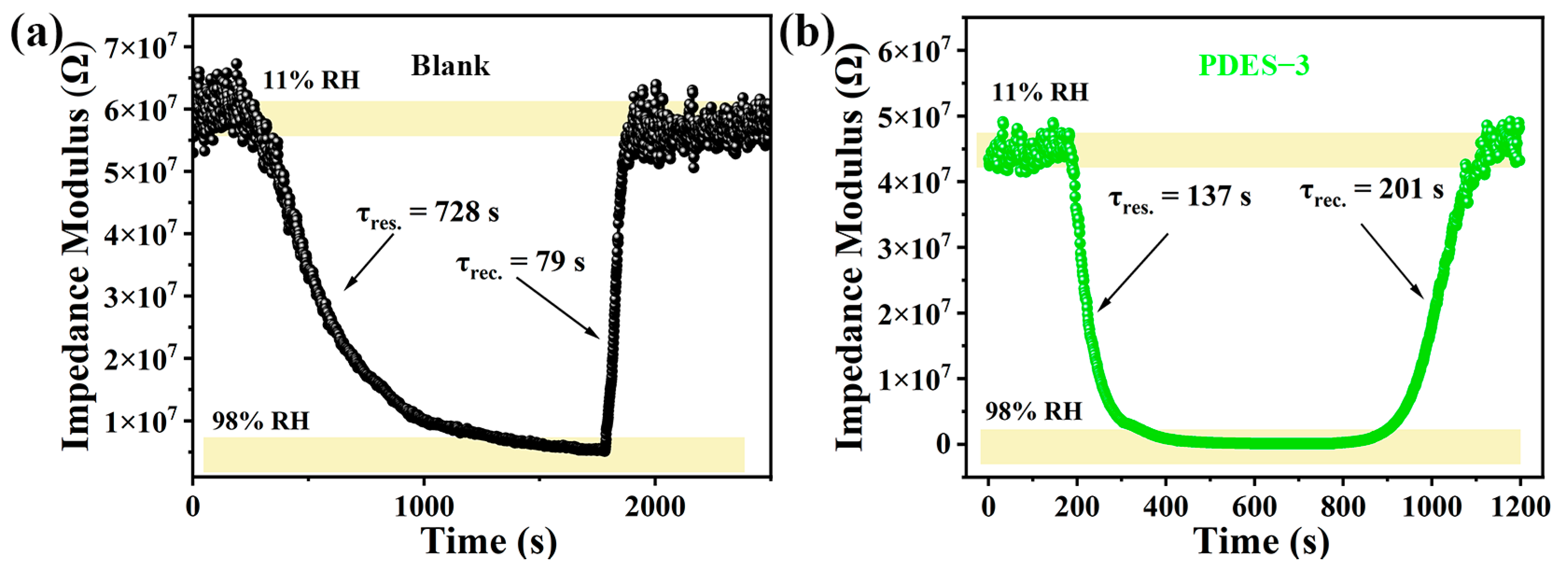
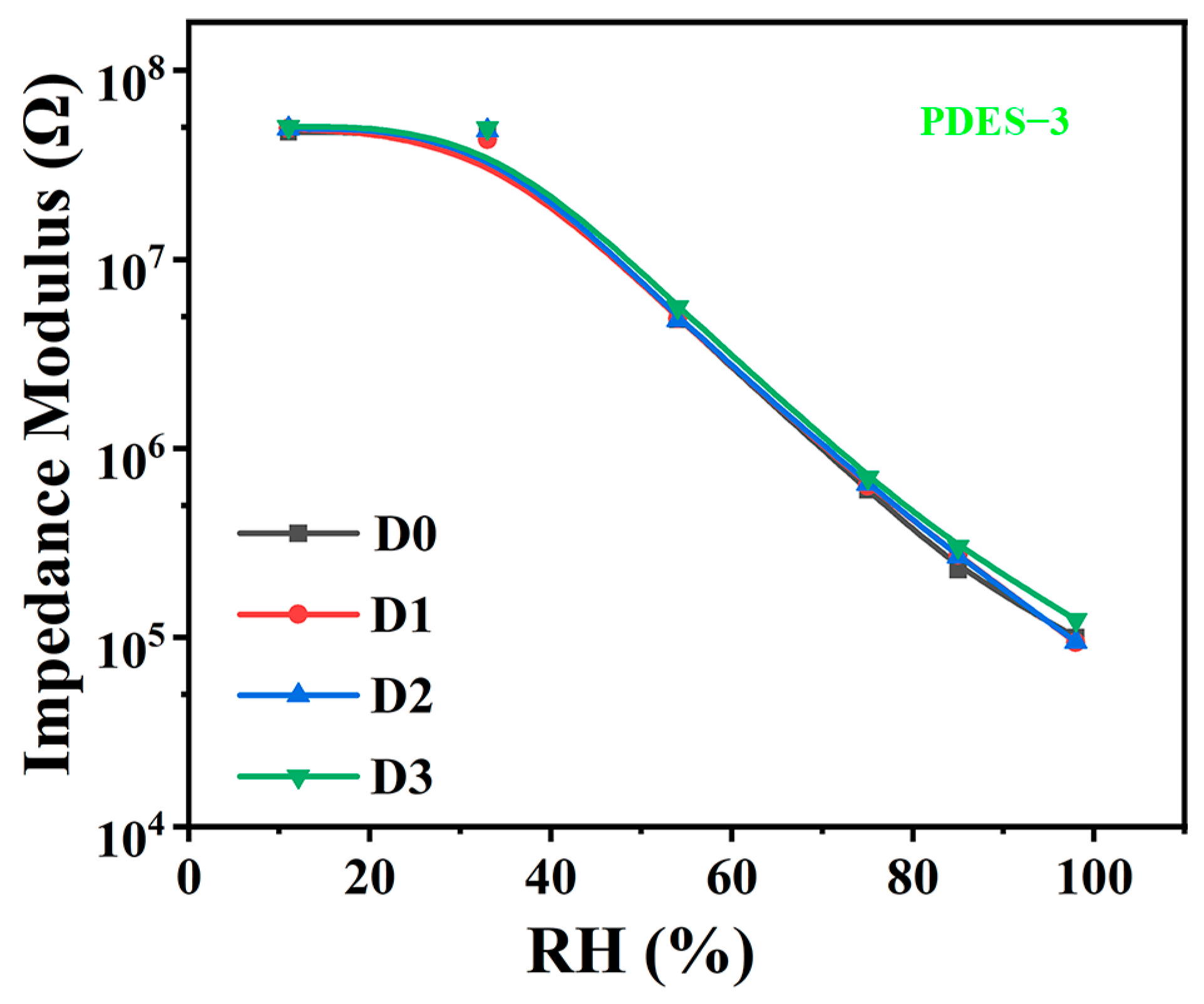
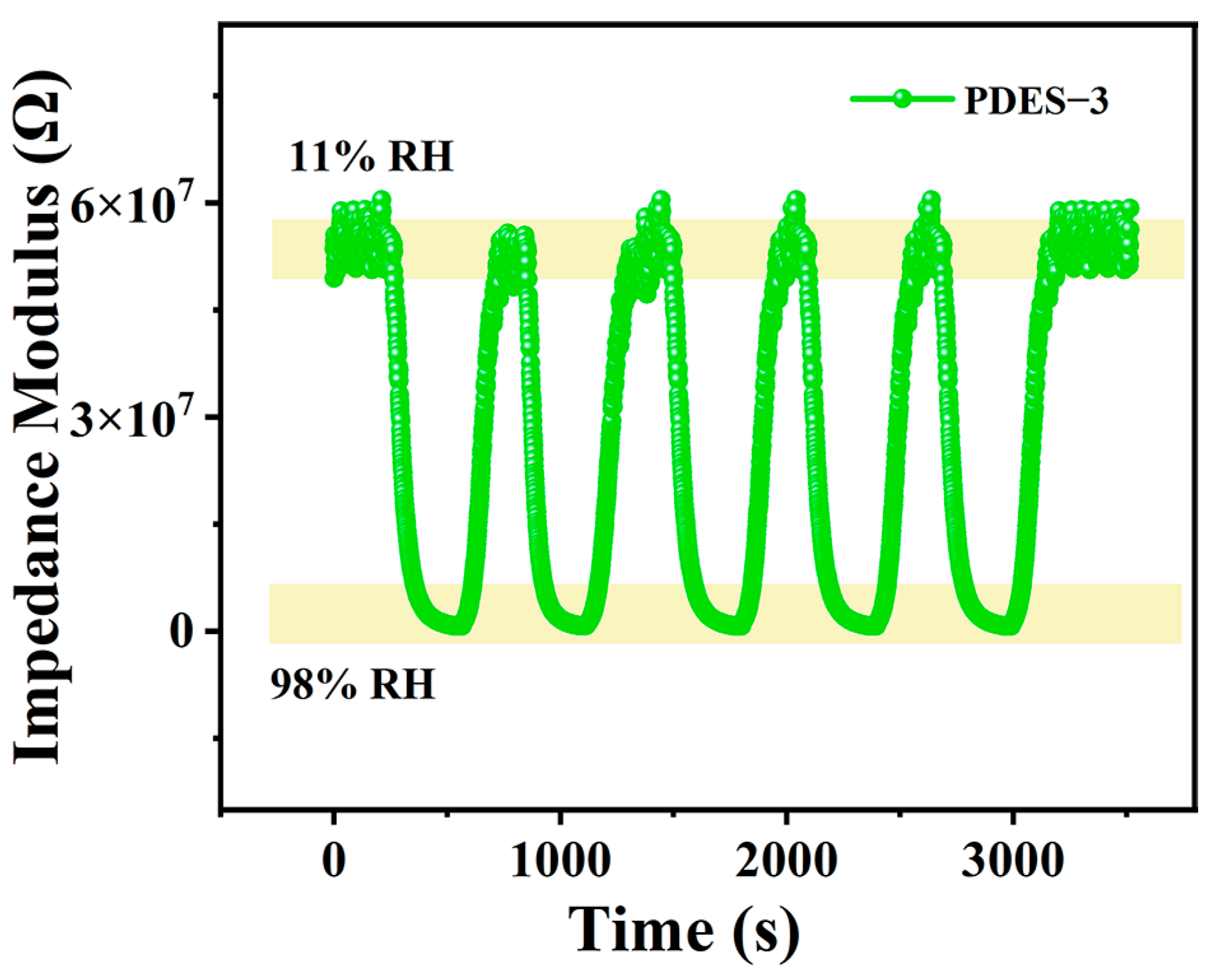
3.5. Stability and Repeatability
3.6. Sensing Mechanism
3.7. Performance Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sadeq, H.Z.; Michael, W.S.; Reza, G.; Chen, Z. Thermoelectric coolers as thermal management systems for medical applications: Design, optimization, and advancement. Nano Energy 2021, 90, 2211–2855. [Google Scholar]
- Zhou, J.; Wu, Q.; Chen, X.; Fei, Q.; Zhang, G.; Rong, W.M.; Xiao, F.H.; Yao, L.; Dong, Y.H.; Lin, L. Two-component ratiometric sensor for Cu2+ detection on paper-based device. Anal. Bioanal. Chem. 2019, 411, 6165–6172. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, P.; Wang, D.; Luo, Y.; Li, M.; Lee, H.; Gerhardt, R.A. Novel paper-based flexible ammonia gas sensor via silver and SWNT−PABS inkjet printing. Sens. Actuators B 2014, 197, 308–313. [Google Scholar] [CrossRef]
- Morais, R.M.; Klem, M.S.; Nogueira, G.L.; Gomes, T.C.; Alves, N. Low-cost humidity sensor based on PANI/PEDOT:PSS printed on paper. IEEE Sens. J. 2018, 18, 2647–2651. [Google Scholar] [CrossRef]
- Alatraktchi, F.A.; Noori, J.S.; Tanev, G.P.; Mortensen, J.; Dimaki, M.; Johansen, H.K.; Madsen, J.; Molin, S.; Svendsen, W.E. Paper-based sensors for rapid detection of virulence factor produced by Pseudomonas aeruginosa. PLoS ONE 2018, 13, e0194157. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Xu, K.; Zhang, M. Multiple hydrogen bonds reinforced conductive hydrogels with robust elasticity and ultra-durability as multifunctional ionic skins. Chem. Eng. J. 2023, 451, 138525. [Google Scholar] [CrossRef]
- Tao, L.Q.; Zhang, K.N.; Tian, H.; Liu, Y.; Wang, D.Y.; Yang, Y.Q.; Ren, T.L. Graphene-paper pressure sensor for detecting human motions. ACS Nano 2017, 11, 8790–8795. [Google Scholar] [CrossRef] [PubMed]
- Pataniya, M.; Sumesh, C.K.; Tannarana, M. Flexible paper-based piezo-resistive sensor functionalised by 2D-WSe2 nanosheets. Nanotechnology 2020, 31, 435503. [Google Scholar] [CrossRef]
- Qu, Y.; He, W.; Li, S.; Liu, T.; Wang, S.; Yin, Q.; Zhu, Z. Paper-based colorimetric sensor for hydrogen peroxide based upon a graphene oxide/platinum-cobalt nanocomposite. Anal. Lett. 2024, 58, 152–166. [Google Scholar] [CrossRef]
- Eryanto, G.; Tseng, S.F. Self-powered paper-based humidity sensors with MgCl2/CNTs composites. Sens. Actuators A 2024, 376, 115606. [Google Scholar] [CrossRef]
- Park, H.; Kim, W.; Lee, S.W.; Park, J.; Lee, G.; Yoon, D.S.; Lee, W.; Park, J. Flexible and disposable paper-based gas sensor using reduced graphene oxide/chitosan composite. J. Mater. Sci. Technol. 2022, 101, 165–172. [Google Scholar] [CrossRef]
- Chavda, H.V.; Patel, C.N. Preparation and characterization of swellable polymer-based superporous hydrogel composite of poly(acrylamide-co-acrylic acid). Trends Biomater. Artif. Organs 2010, 24, 83–89. [Google Scholar]
- Russler, A.; Wieland, M.; Bacher, M.; Henniges, U.; Miethe, P.; Liebner, F.; Potthast, A.; Rosenau, T. AKD-modification of bacterial cellulose aerogels in supercritical CO2. Cellulose 2012, 19, 1337–1349. [Google Scholar] [CrossRef]
- Arnata, I.W.; Suprihatin, S.; Fahma, F.; Nur, R.; Candra, S.T. Cationic modification of nanocrystalline cellulose from sago fronds. Cellulose 2020, 27, 3121–3141. [Google Scholar] [CrossRef]
- Sawhney, D.; Vaid, S.; Bangotra, R.; Sharma, S.; Dutt, H.C.; Kapoor, N.; Mahajan, R.; Bajaj, B.K. Proficient bioconversion of rice straw biomass to bioethanol using a novel combinatorial pretreatment approach. Bioresour. Technol. 2023, 375, 128791. [Google Scholar] [CrossRef]
- Alshammari, O.A.O.; Almulgabsagher, G.A.A.; Ryder, K.S.; Surbhi, S.; Chander, D.H.; Nisha, K.; Ritu, M.; Kumar, B.B. Effect of solute polarity on extraction efficiency using deep eutectic solvents. Green Chem. 2021, 23, 5097–5105. [Google Scholar] [CrossRef]
- Song, X.; Hu, W.; Huang, W.; Hui, W.; Hai, Y.S.; Tao, Y.S.; Sheng, L.F. Methanolysis of polycarbonate into bisphenol A using choline chloride-based deep eutectic solvents. Chem. Eng. J. 2020, 388, 124324. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, X.; Ju, Z.; Bo, L.; Yu, Y.H.; Li, X.J.; Zhang, Q.; Feng, H.Y.; Jiang, Z.S. Alcoholysis of polyethylene terephthalate to dioctyl terephthalate using choline chloride-based deep eutectic solvents. Green Chem. 2019, 21, 897–906. [Google Scholar] [CrossRef]
- Angsantikul, P.; Peng, K.; Curreri, A.M.; Ye, C.; Kevin, Z.C.; Jessica, E.; Samir, M. Ionic liquids and deep eutectic solvents for enhanced delivery of antibodies in the gastrointestinal tract. Adv. Funct. Mater. 2020, 31, 2002912. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Guo, Q.; Zhang, X.; Tian, M. Polymerizable deep eutectic solvent-based skin-like elastomers with dynamic schemochrome and self-healing ability. Small 2022, 18, 2201012. [Google Scholar] [CrossRef]
- Chen, W.; Sun, W.; Li, J.C.; Qiu, Y.X.; Qiu, L.Y.; Ma, J.; Li, N.; Ji, X.X. Multifunctional conductive elastomers based on tannic acid and polymerizable 1-butyl-3-methylimidazolium chloride/acrylic acid deep eutectic solvent. ACS Appl. Polym. Mater. 2024, 6, 180–191. [Google Scholar] [CrossRef]
- Zhao, H.; Lin, X.; Qi, R.; Dai, J.; Liu, S.; Fei, T.; Zhang, T. A composite structure of in situ cross-linked poly(ionic liquid)s and paper for humidity-monitoring application. IEEE Sens. J. 2018, 19, 833–837. [Google Scholar] [CrossRef]
- Tao, L.J.; Zu, Z.M.; Hong, N.P.; Lin, H.J. Application of deep eutectic solvents in polymer synthesis. Prog. Chem. 2022, 34, 2159–2172. [Google Scholar]
- Holman, J.B.; Shi, Z.; Fadahunsi, A.A.; Li, C.; Ding, W. Advances on microfluidic paper-based electroanalytical devices. Biotechnol. Adv. 2023, 63, 108093. [Google Scholar] [CrossRef] [PubMed]
- Ana, R.; Ana, M.A.; Alexandre, P.; Anarita, D.C. Polymer science and engineering using deep eutectic solvents. Polymers 2019, 11, 912. [Google Scholar] [CrossRef] [PubMed]
- Analisa, R.; Yeop, A.B.; Jacob, J.A.; Eric, D.B.; Jennifer, B.T.; Jennifer, L.A. Pen-on-paper flexible electronics. Adv. Mater. 2011, 23, 3426–3430. [Google Scholar]
- Yuan, H.Z.; Fei, L.H.; Liang, X.J.; Lie, S.H.; Bo, L.; Sheng, C.S.; Ying, C.; Ji, M.Y.; Xue, F. Ultralow-cost, highly sensitive, and flexible pressure sensors based on carbon black and airlaid paper. ACS Appl. Mater. Interfaces 2019, 11, 33370–33379. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Long, Z.; Nan, H.Y.; Guang, W.S.; Bo, P.G.; Qiang, L.L. Directly writing flexible temperature sensor with graphene nanoribbons for disposable healthcare devices. RSC Adv. 2020, 10, 22222–22229. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoon, S.; Cho, Y.H.; Gyewon, K.; Han, K.K. Paintable and writable electrodes using black conductive ink on traditional Korean paper (Hanji). RSC Adv. 2020, 10, 24631–24641. [Google Scholar] [CrossRef]
- Huang, Z.; Tang, Y.; Guo, H.; Feng, X.; Zhang, T.; Li, P.; Qian, B.; Xie, Y. 3D printing of ceramics and graphene circuits-on-ceramics by thermal bubble inkjet technology and high-temperature sintering. Ceram. Int. 2020, 46, 10096–10104. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Tan, K.; Dong, S.; He, Y.; Ye, L.; Zhao, W.; Gu, L.; Prati, S.; Bai, J. Deep eutectic solvent-based green gel for removal of polymeric coating from mural paintings. J. Cult. Herit. 2025, 71, 51–60. [Google Scholar] [CrossRef]
- Mi, X.; Zhang, C.; Zhang, N.; Wang, Z.; Wang, J. Recyclable flexible pressure, temperature and humidity multimodal sensors based on micro-pyramidal structures and PVA/choline chloride/ethylene glycol. Sens. Actuators B 2025, 440, 137866. [Google Scholar] [CrossRef]
- Oliveira, A.E.; Pereira, A.C.; Resende, M.A. Fabrication of Low-Cost Screen-Printed Electrode in Paper Using Conductive Inks of Graphite and Silver/Silver Chloride. Electroanalysis 2022, 35, 202200093. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Q.; Wang, Q.; Li, Z.; Liang, J.; Wu, W. All-printed flexible self-powered humidity sensor. Sens. Actuators B 2025, 440, 137868. [Google Scholar] [CrossRef]
- Tan, C.; Cao, Y.; Xie, N.; Zhang, M.; Liu, L.; Yu, H.; Wang, C.; Jiang, Y.; Wu, Y.; Yuan, Z.; et al. Intelligent respiratory rate detection using disposable paper-based humidity sensor and precise peak-seeking algorithm. Sens. Actuators B 2025, 436, 137738. [Google Scholar] [CrossRef]
- Korotcenkov, G. Paper-based humidity sensors as promising flexible devices: State of the art—Part 1. General considerations. Nanomaterials 2023, 13, 1110. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, E.; Kim, J.M.; Ayoung, P.; Thiruvengadam, P.; Sik, E.M.; Su, H.M.; Woo, L.S. Simple, fast and easy assay for transition-metal-catalyzed coupling reactions using a paper-based colorimetric iodide sensor. Chem. Commun. 2012, 48, 8751–8753. [Google Scholar] [CrossRef]
- Zheng, L.; Hua, H.; Zhang, Z.; Zhu, Y.; Wang, L.; Li, Y. PVA/ChCl deep eutectic polymer blends for transparent strain sensors with antifreeze, flexible, and recyclable properties. ACS Appl. Mater. Interfaces 2022, 14, 49212–49223. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Yan, L. Deep eutectic solvent-induced microphase separation and entanglement of PVA chains for tough and reprocessable eutectogels for sensors. Langmuir 2022, 38, 12189–12197. [Google Scholar] [CrossRef]
- Che, W.; He, X.; Zheng, X.; Wang, K.; Shi, J.; Song, Z.; Liu, B.; Zeng, X.; Zuo, M. Rational design of anti-freezing dual-network hydrogels with superior mechanical and self-healing properties for advanced flexible electronics. Chem. Eng. J. 2025, 515, 163607. [Google Scholar] [CrossRef]
- Guan, X.; Hou, Z.N.; Wang, K.; Zhao, H.R.; Liu, S.; Fei, T.; Zhang, T. Flexible humidity sensor based on modified cellulose paper. Sens. Actuators B 2021, 339, 129879. [Google Scholar] [CrossRef]
- Zhu, P.; Kuang, Y.; Wei, Y.; Wu, Z.; Guo, C.F.; Wang, S.; Yin, Q.; Zhu, Z. Electrostatic self-assembly enabled flexible paper-based humidity sensor with high sensitivity and superior durability. Chem. Eng. J. 2021, 404, 127105. [Google Scholar] [CrossRef]
- Zhang, D.; Zong, X.; Wu, Z.; Su, Y.; Wang, D.; Zhang, X.; Zhang, Y. Hierarchical self-assembled SnS2 nanoflower/Zn2SnO4 hollow-sphere nanohybrid for humidity-sensing applications. ACS Appl. Mater. Interfaces 2018, 10, 32631–32639. [Google Scholar] [CrossRef]
- Gaspar, C.; Olkkonen, J.; Passoja, S.; Lehto, M.; Hakonen, H.; Töyryläinen, J.; Lampinen, J. Paper as active layer in inkjet-printed capacitive humidity sensors. Sensors 2017, 17, 1464. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Chen, S.; Zhu, R. Measurement of electric double layer capacitance using dielectrophoresis-based particle manipulation. Anal. Chem. 2021, 93, 5882–5889. [Google Scholar] [CrossRef]
- Weng, Z.; Su, Y.; Wang, D.; Li, F.; Du, J.; Cheng, H. Graphene-cellulose paper flexible supercapacitors. Adv. Energy Mater. 2011, 1, 917–922. [Google Scholar] [CrossRef]
- Hu, J.; Wu, C.; Liu, B.; Zhang, Y.; Wu, Z.; Wang, S.; Yin, Q.; Zhu, Z. Dual-mode paper-based humidity sensor: Optical and electrical dual-signal sensing platform for multifunctional applications. Sens. Actuators B 2025, 440, 137890. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, Y.; Yan, M.; Wang, S.; Yuan, Z.; Zhao, Q.; Sun, P.; Xie, G.; Du, X.; Tai, H. Facile, flexible, cost-saving and environment-friendly paper-based humidity sensor for multifunctional applications. ACS Appl. Mater. Interfaces 2019, 11, 21840–21849. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Zhou, J.; Lu, A. Flexible and transparent cellulose-based ionic film as a humidity sensor. ACS Appl. Mater. Interfaces 2020, 12, 7631–7638. [Google Scholar] [CrossRef]
- Guan, X.; Yu, Y.; Hou, Z.; Wu, K.; Zhao, H.; Liu, S.; Fei, T.; Zhang, T. A flexible humidity sensor based on self-supported polymer film. Sens. Actuators B 2022, 358, 131438. [Google Scholar] [CrossRef]
- Suman, J.; Adib, T.; Aarsh, P.; Masoud, M.S. Laser-assisted dry printing eco-friendly paper-based humidity and temperature sensors. Laser Appl. 2025, 37, 012025. [Google Scholar]
- Lin, X.Z.; Xue, H.; Li, F.; Zhao, H.R.; Zhang, T. An electrolyte-mediated paper-based humidity sensor fabricated by an office inkjet printer. IEEE Electron. Device Lett. 2024, 45, 244–247. [Google Scholar] [CrossRef]

| Mater. | Prep. | T. R. (% RH) | Sens. | τres./τrec. (s) | Ref. |
|---|---|---|---|---|---|
| Printing paper | Paste | 41–91 | 1647 a, – | 19/472 | [48] |
| Cellulose/KOH | Immersion | 11–97 | >200 b, – | 6/10.8 | [49] |
| EPTAC/CNFs | Solution blending | 11–98 | 8.41 c, 54% RH | 25/188 | [41] |
| Crosslinked polyelectrolyte | Click syndicate | 11–95 | 103.75 c, 95% RH | 12.5/700 | [50] |
| Printing paper | Dry additive nanomanufacturing | 20–90 | 2.46 d, 70% RH | − | [51] |
| Printing paper/NaCl | Inkjet printing | 11–95 | 72.36 c, 95% RH | 1623/70 | [52] |
| PDES−3 | One-step solution blending | 11–98 |
10.36 c, 54% RH; 519.2 c, 95% RH | 137/201 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, M.; Zhang, B.; Lu, Q.; Xiao, Y.; Shen, H.; Ni, Y.; Liu, Y.; Song, H. Research on a Humidity Sensor Based on Polymerizable Deep Eutectic System-Modified Filter Paper. Chemosensors 2025, 13, 354. https://doi.org/10.3390/chemosensors13090354
Shen M, Zhang B, Lu Q, Xiao Y, Shen H, Ni Y, Liu Y, Song H. Research on a Humidity Sensor Based on Polymerizable Deep Eutectic System-Modified Filter Paper. Chemosensors. 2025; 13(9):354. https://doi.org/10.3390/chemosensors13090354
Chicago/Turabian StyleShen, Mengyao, Bo Zhang, Qi Lu, Yanan Xiao, Hao Shen, Yi Ni, Yuechen Liu, and Haitao Song. 2025. "Research on a Humidity Sensor Based on Polymerizable Deep Eutectic System-Modified Filter Paper" Chemosensors 13, no. 9: 354. https://doi.org/10.3390/chemosensors13090354
APA StyleShen, M., Zhang, B., Lu, Q., Xiao, Y., Shen, H., Ni, Y., Liu, Y., & Song, H. (2025). Research on a Humidity Sensor Based on Polymerizable Deep Eutectic System-Modified Filter Paper. Chemosensors, 13(9), 354. https://doi.org/10.3390/chemosensors13090354






