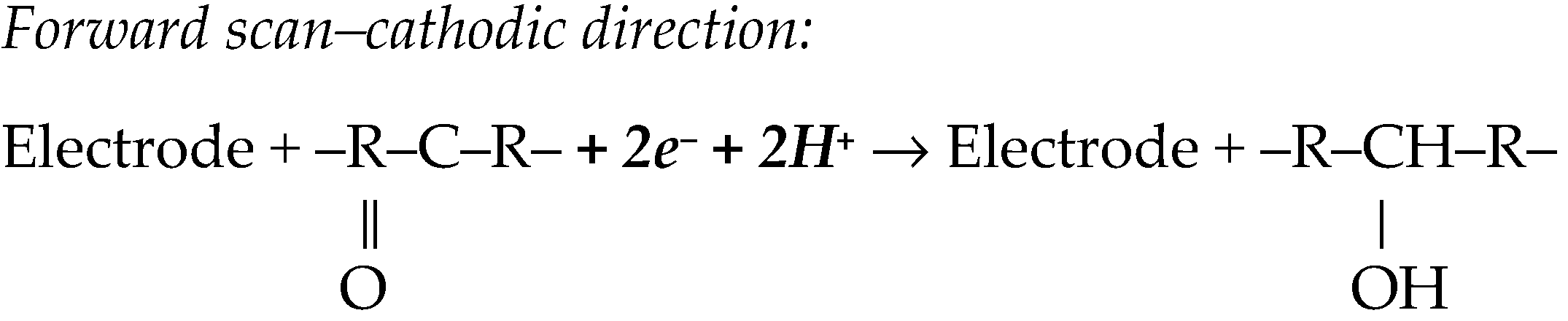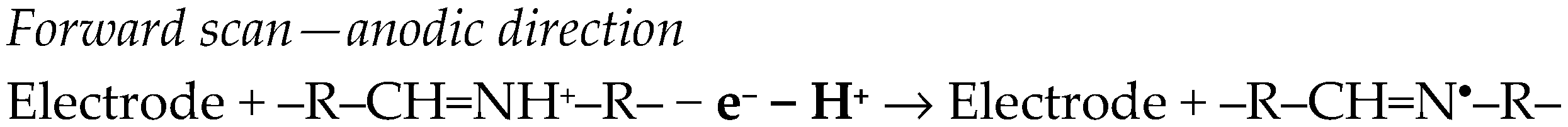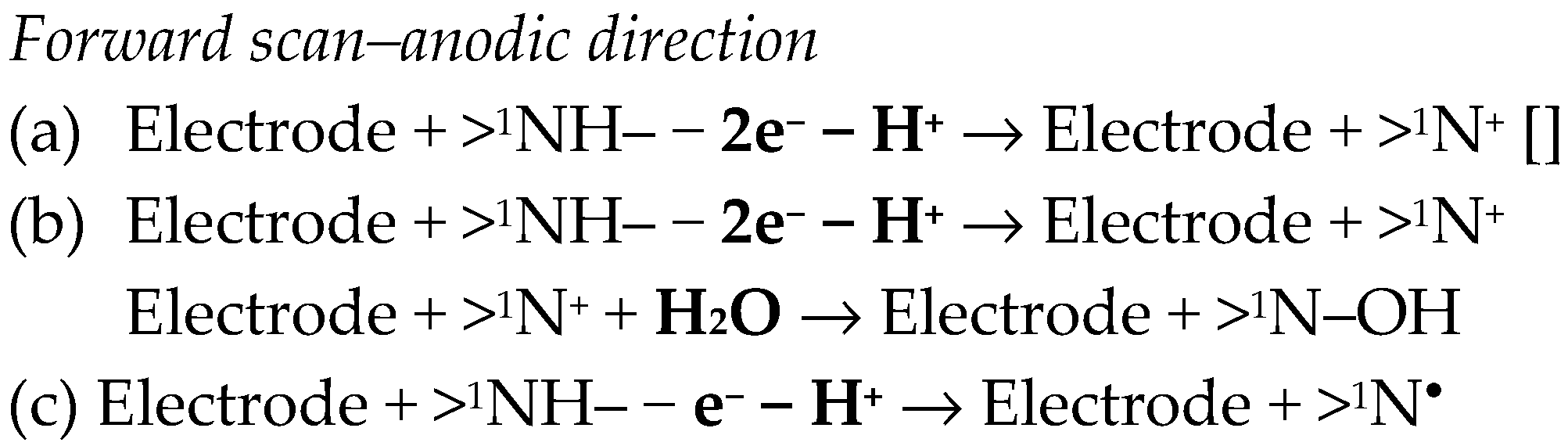Abstract
Benzodiazepines are psychoactive drugs with wide clinical applications. Unfortunately, due to their sedative effects, benzodiazepines are frequently used as date rape drugs or in drug-facilitated crimes. Considering the electroactive nature of benzodiazepines and the unique advantages of electrochemical techniques, this review presents a critical discussion of the state of the art of benzodiazepine electroanalysis. Aspects related to sample preparation as well as electrodes (from mercury electrodes to bare or modified solid electrodes and to disposable sensors) and techniques (mainly voltammetry) used for the quantification of benzodiazepines in different matrices (pharmaceuticals, body fluids, alcoholic and soft drinks) were discussed. Considering the actual achievements in the field, some general suggestions for possible further research were given.
1. Introduction
BZDs are psychoactive agents that act as CNS depressants. Their rapid entry into the brain, enabled by their high lipid solubility, allows them to enhance the activity of GABA, the brain’s primary inhibitory neurotransmitter. BZDs quickly won favor in clinical practice because they reduce anxiety within minutes and at the same time act as anticonvulsants, hypnotics, skeletal–muscle relaxants, and general sedatives. In both generalized anxiety disorder and panic disorder, BZDs produce outcomes that match or surpass other drug classes while causing fewer side-effects [1] and outperform tricyclic antidepressants in preventing panic attacks [2].
BZDs are widely used for acute symptom relief across a range of conditions: insomnia, anxiety disorders, seizures, spasticity, and muscle tension. Primary indications are: anxiolysis (ALP, CDZO, DZP, LZP, OZP with an elimination half-time ranging between 6 and 100 h) and hypnosis (FNZP, NZP, TMP, with an elimination half-time ranging between 2 and 100 h) [3].
Because BZDs amplify GABA A-receptor signaling, it has been proposed that they might partially modify the expression of AD. Brain tissue analyses and spectroscopy studies consistently show that GABA levels fall in AD. However, while BZDs theoretically compensate for the GABA shortfall in AD, their long-term impact on memory and neuropathology has not been settled: evidence points simultaneously to potential benefits, neutral effects, and harm, depending on the compound, dose, duration, and clinical context [4].
Pharmacologically, BZDs have different actions, where, in addition to anxiolytic effects, various analogs have demonstrated anticancer, anticonvulsant (CLZP), anesthetic (MDZ), antipsychotic (OLP, clozapine), muscle-relaxing, antitubercular, and antimicrobial properties.
However, BZDs are frequently implicated in polydrug abuse, especially among users of opioids (oxycodone and heroin). Studies report that over half of those abusing oxycodone also misuse BZDs (most commonly DZP). This combination increases the risk of overdose due to compounded respiratory and CNS depression [5]. BZDs’ use has expanded among individuals, especially among those with substance dependence, and has become increasingly common in drug-facilitated assaults and road-traffic accidents. Enhancing analytical methods for detecting these drugs in biological samples is crucial in toxicology, as it enables the detection of exposure or intoxication [6].
A 34% surge in anxiolytic prescriptions has been seen concurrently with COVID-19-related stress and with the wider mental-health awareness movement. In polysubstance abuse, BZDs are commonly combined with opioids or alcohol to enhance euphoria or ease comedowns, making them the second-most common prescription drug class implicated in fatal overdoses, after opioids [7]. Survey data shows stark age gradients: about half (~51%) of individuals aged 18–25 admit to non-medical BZD use, whereas misuse falls to ~4% in those individuals aged ≥ 65 [8].
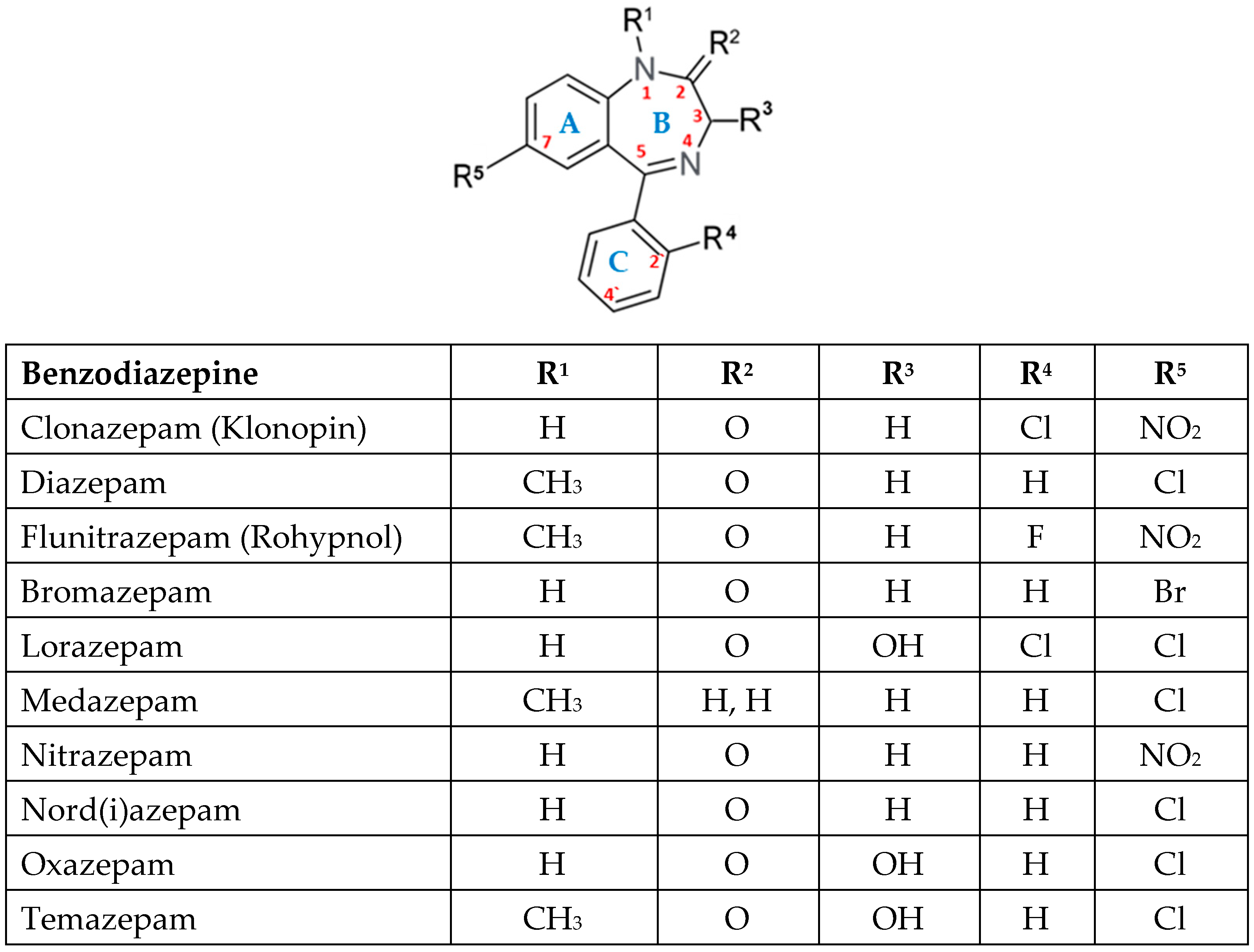
Every benzodiazepine has a 1,4-benzodiazepine core, with sidechain substitutions generating several subfamilies (2-keto, 3-hydroxy, 7-nitro, triazolo- and imidazo- derivatives) (Figure 1). These drugs bind to the GABA A receptor, a ligand-gated chloride channel, which is allosterically modulated. The neurotransmitter GABA allows Cl− to enter the cell, hyperpolarizing neurons and decreasing excitability. BZDs boost this effect allosterically, yielding an even larger chloride current [1].
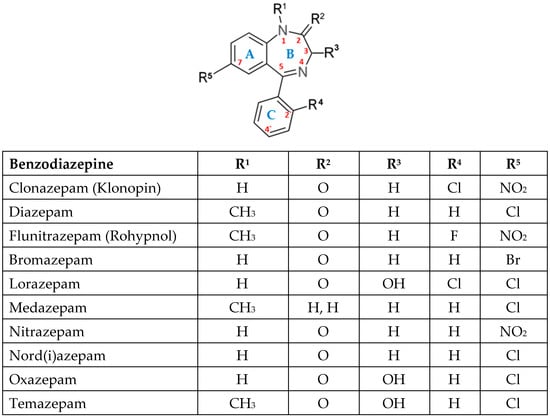
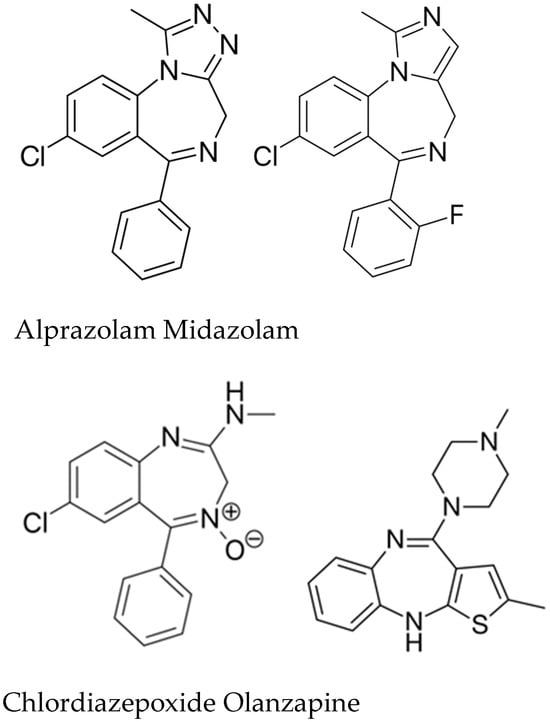
Figure 1.
Chemical structures of the most well-known BZD.
The relationship between chemical structure and biological activity is illustrated in Table 1 [9].

Table 1.
Chemical structure-biological activity relationship [9].
BZDs can be classified based on their chemical structure in 2-keto BZDs (for example: CDZO, DZP), 3-hydroxi BZDs (for example: LZP, OZP, TMP), 7-nitro BZDs (for example: CLZP, FNZP, NZP), triazolo- BZDs (for example: ALP), imidazo- BZDs (for example: MDZ, loprazolam) [1].
According to their half-life, they can be divided into three groups: short (MDZ, OZP), intermediate (ALP, BZP, LZP, TMP), and long (CDZO, CLZP, DZP) [1], with half-lives ranging from minutes or hours (short) to days (long). Additionally, certain BDZs form active metabolites, such as DZP and desmethyldiazepam, whereas others, like oxazepam, do not [1].
In recent years, “designer” analogs have appeared and are sold online with minimal information provided as to their toxicology. One example is flubromazolam (a triazolo-benzodiazepine: 8-bromo-6-(2-fluorophenyl)-1-methyl-4H-triazolo[4,3-a][1,4]benzodiazepine) prized by recreational users for deep hypnosis, prolonged anterograde amnesia, and a steep tolerance curve. Deschloroetizolam (a thienodiazepine) is another example of a “designer” analog, which augments GABA A signaling, like its classical relatives. A third example, clonazolam, the triazolo derivative of clonazepam, is suggested to have twice the potency as alprazolam. Meanwhile, meclonazepam is structurally similar to clonazepam but presents additional anti-schistosomal activity and sedation, keeping it off the pharmaceutical market [7].
Oral preparations are generally well-absorbed, while the intravenous route is rapid but may irritate local tissues. Intramuscular administration is preferred for DZP. High lipid solubility confers large volumes of distribution. Absorption rates vary widely, with DZP, for example, reaching peak plasma levels rapidly. Lipid solubility governs blood–brain-barrier penetration and onset of action [1].
BZD therapy can occasionally trigger paradoxical behavioral effects. Patients may become irritable, uninhibited, unusually outspoken or inappropriate, and may even display anger or aggression. Regardless of class, the dose-limiting adverse effects remain confusion, daytime somnolence, anterograde amnesia, and ataxia [10].
Although therapeutic BZDs carry low intrinsic toxicity, they are not negligible. Typical acute overdose produces somnolence, slurred speech, ataxia, and altered consciousness, yet pupils remain reactive and vital signs are usually stable [7]. When outright abuse is documented, it almost invariably occurs in individuals already using other depressant substances, most commonly being alcohol, opioids, or additional sedative-hypnotics, rather than abusing only BZDs. Nonetheless, evidence does not place them among the main “gateway” or standalone drugs of abuse [11].
Considering all the above-mentioned aspects, it is clear that BZDs’ determination is essential for pharmaceutical, clinical, and forensic applications, such as purity checks, counterfeit detection, biological fluid drug level monitoring for adequate medical treatments, or to detect their presence in incidents like drug-facilitated sexual assaults or traffic accidents. There are reviews that briefly summarize the methodologies reported in the literature regarding sample preparation methods [12] and BZDs (bio)analysis in biological matrices with emphases on toxicological or clinical purposes [13,14,15,16,17]. Due to BZDs’ increasing use as date-rape drugs, especially by spiking different types of beverages, their detection and quantification in matrices other than biological ones is also important. In this respect, chromatographic techniques have usually been employed [18,19,20,21], with some fluorometric methods [22]. More recently, a portable, microfluidic device based on a colorimetric reaction was developed for the rapid naked-eye detection of DZP and MDZ in drinks [23]. Electrochemical methods were also reported for BZDs analysis in beverages [24], presenting the advantage that they allow determinations in colored or turbid solutions.
Another aspect of the widespread use of BZDs is permeation into the environment, mainly through wastewater, due to their slow degradation. BZD concentrations from 0.01 μg/L to several μg/L have been found in different types of natural waters [25], a factor that can affect aquatic life, as BZDs can be harmful to living species. This pharmaceutical class can be considered CECs. It was reported that among BZDs, lorazepam had the highest concentration (89.9 μg/L) in aquatic environments [26]. While chromatography is the technique of choice for the analysis of complex mixtures of structurally related species, for example, using the UHPLC-MS/MS method for the simultaneous determination of 14 BZDs residues in aquaculture water and sediments [27], voltammetry has also been applied for the quantification of various CECs, with BZDs also among them. Compared to LC-MS or LC-MS/MS, voltammetry has lower material consumption and reagents, and reduced analysis time, mostly without the need for sample pretreatment, using cheaper instruments and less qualified personnel, but is less sensitive and has lower selectivity [28].
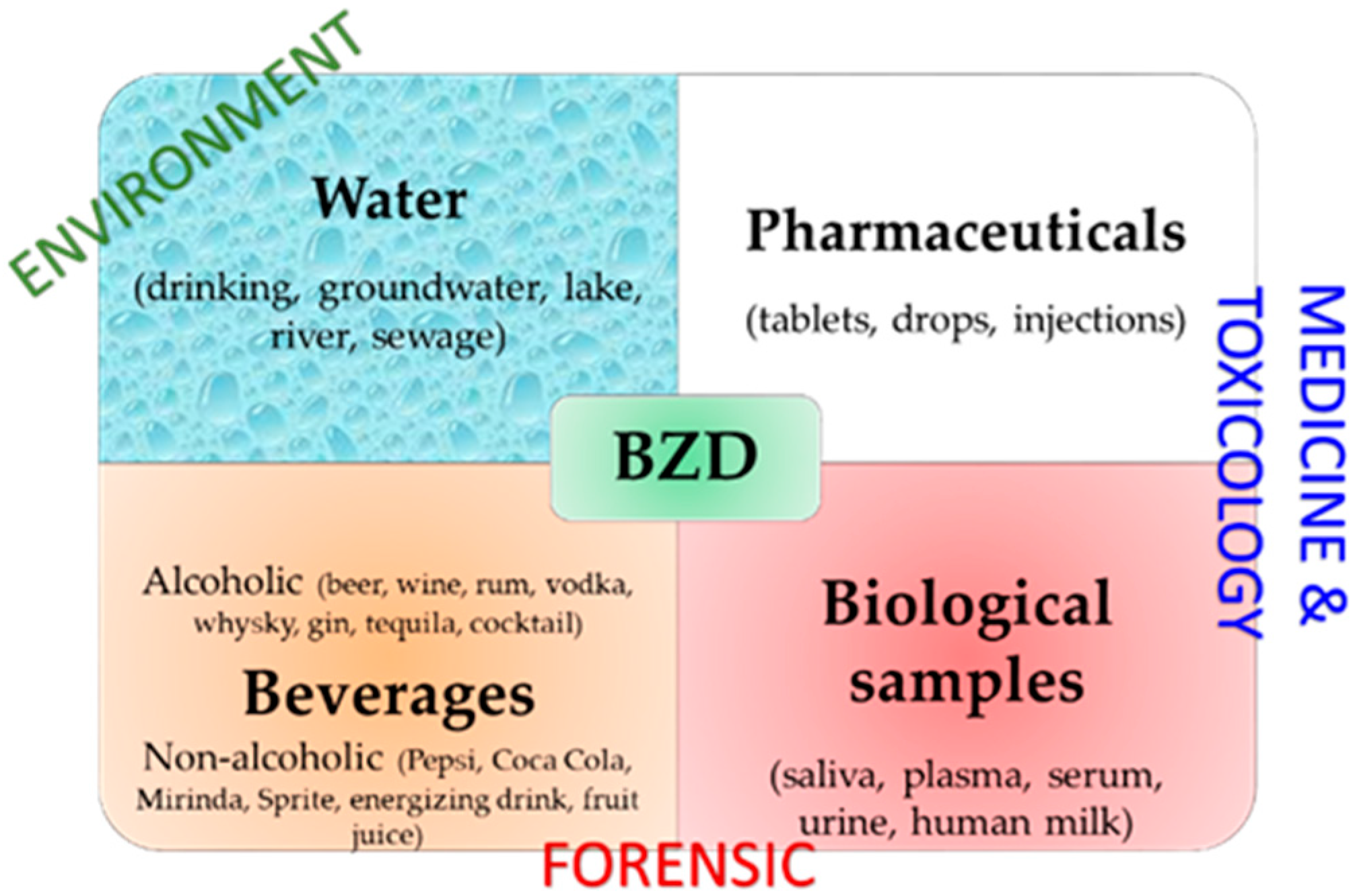
However, there is an increasing need to develop portable, cost-effective, rapid, sensitive, and selective BZD detection devices for various samples (Figure 2) [28]. Electrochemical sensors are often the right choice in this respect because they have rapid response times, are usually quite easy to prepare, have various preparation methods, and can be miniaturized. Additionally, they can have various designs [29] and can be coupled with different techniques to obtain low detection limits and linear ranges wide enough to be applied for the accurate analysis of multiple types of samples.
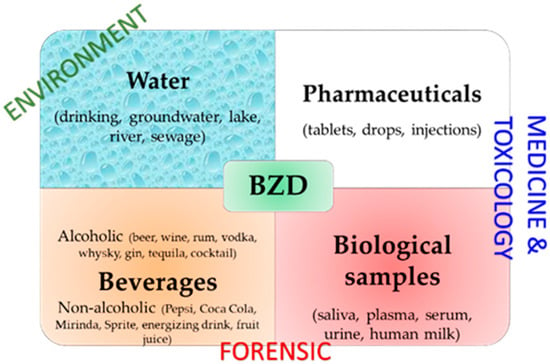
Figure 2.
Main matrices and application fields of BZD detection.
It has already been established that electrochemical techniques can be important tools for the successful assessment of drugs, for both their quantification and the investigation of their redox properties, which can help in understanding some of their biological actions. This paper aimed to review and discuss the electrochemical sensors and methods developed for the analysis of BZDs and reported in the literature over the last 10 years. A similar review was published in 2019, discussing the evolution of BZD electroanalysis from the early 1960s [30]. However, there has been recent significant progress, especially regarding modified and disposable electrodes. More recent reviews dealing with biosensing of illicit drugs [31,32], emphasizing point-of-care testing [33] or beverage screening [24] include BZDs electroanalysis, among other analysis techniques. The originality of this review paper lies in its structure, with the discussion conducted for each analyte separately, to facilitate an almost index-like approach. Since in an analytical process the sample preparation step is as important, if not more important, than the actual analysis stage, this review focuses on the sample preparation techniques employed based on sample type, and on presenting the analysis methods.
2. Benzodiazepines Analysis
Most BDZs, except for MDZ, have poor solubility at physiological pH, but can be solubilized in acidic environments. However, without the use of organic co-solvents or surfactants, most BDZs were shown to form unstable protonated forms [34]; thus, depending on the sample type and target calibration, pH can play an important role in stock and sample preparation protocols.
There are three typical analytical starting positions governing the analysis of 1,4-benzodiazepines: physiological fluids (blood or serum, urine, saliva, or other), pharmaceutical formulations (tablets, drops, or injections), or beverages (alcoholic or soft drinks). These, in turn, align with the purposes of pharmacokinetics, quality control, or forensic investigations. A fourth, less commonly explored analytical path is that of analyzing surface water and sewage; this is mainly performed for environmental purposes or community drug-use monitoring. Often, BZDs concentrations in natural waters (especially during rainy periods) are below the LoDs of even stripping voltammetric methods, so that pre-concentration techniques should be applied before the actual analysis [25].
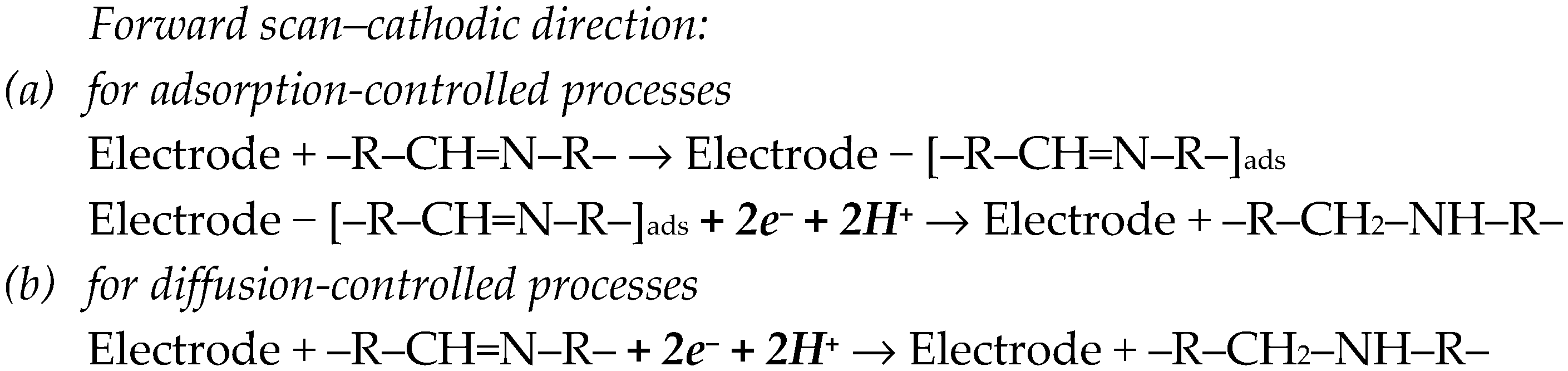
BZDs are electroactive compounds due to the existence in their molecular structure of the 4,5-azomethine group, which undergoes a reduction process, generating a cathodic signal (Scheme 1).

Scheme 1.
Electro-reduction mechanism of BZDs’ 4,5-azomethine group [25,28].
Depending on the presence of additional functional moieties, supplementary cathodic and/or anodic peaks can be observed in the voltammograms of the corresponding analytes. Voltammetric (CV and SWV) investigations of DZP, CLZP, and ALP at graphite SPE in standard and deoxygenated solutions coupled with LC-HRMS analysis of their degradation products allowed the association of characteristic voltammetric peaks of each analyte with the corresponding redox active group. Thus, based on the different electrochemical behavior, BZDs were classified into three groups, and the approach was then applied to classify illicit pills with unknown BZD content [35].
Voltammetric techniques are important tools not only for quantitative analysis, but also for the investigation of interactions between drugs and other bioactive molecules. CV and SWV studies indicated that DZP and CDZO formed an electro-inactive complex with bovine serum albumin and allowed the assessment of the molar ratio (m) and the binding constants (βs) of the formed biocomplexes, which were 1.23 and 2.00 × 106 L/mol for the DZP-complex and 1.04 and 7.76 × 104 L/mol for the CDZO-complex [36].
BZD electroanalysis has been performed with conventional unmodified or modified electrodes (GCE, SPE, CPE), as well as with newly developed designs, like PADs or wearable sensors, most of them being used in voltammetric detection. Amperometry, EIS, and potentiometry were also applied.
2.1. Alprazolam (ALP)
For biological samples, ALP has been studied in neat, spiked urine [37]. Given the high likelihood that illicit ALP tablets contain other controlled substances [38], tablets have been prepared in small amounts of alcohol, filtered and diluted with (pH 10.00), and analyzed against other potential BZDs (DZP, CLZP, and the like), opioids (fentanyl, U-4770), cannabinoids (5F-ADB), stimulants (cocaine, amphetamine, 3,4-MDMA), anesthetics (ketamine, lidocaine) with the main intent of discriminating between Xanax (commercial name for ALP) and fake Xanax tablets. For beverages [38,39,40,41], samples have been either spiked and then 25 to 100-fold diluted with the working buffer [39], diluted with water (1:1, v/v) and then spiked [40], or diluted tenfold with buffer, then spiked [41]. Studying its potential as a surface water contaminant, samples were filtered and directly analyzed [28].
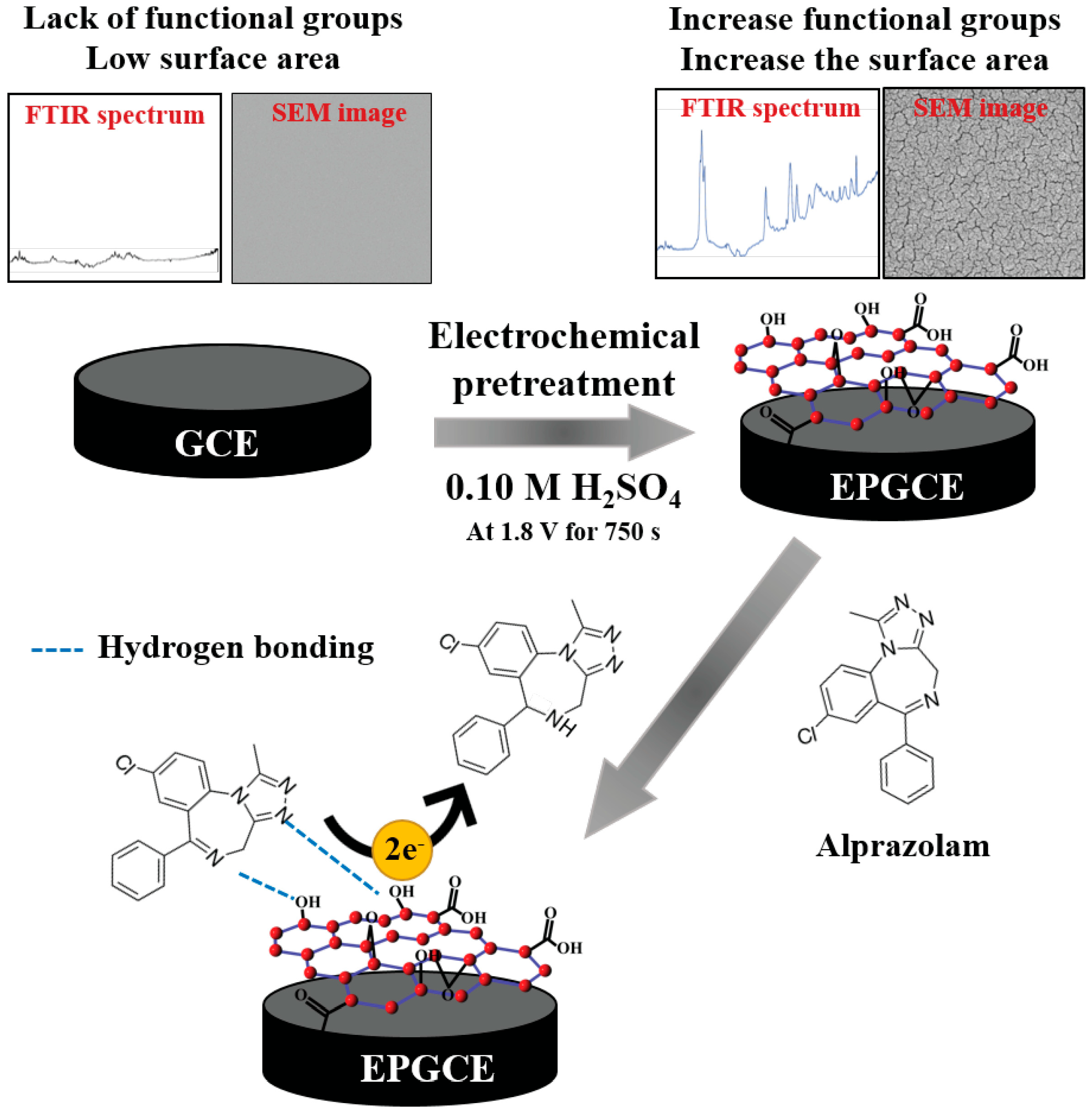
Cyclic voltammetric investigation of ALP at HMDE indicated a cathodic peak at about −0.95 V (PBS pH 7.00) attributed to the irreversible, adsorption-controlled reduction of the azomethine group involving 2e− and 2H+ according to the reaction presented in Scheme 1(a) [28]. Similar behavior was reported on BDDE [42] and CPE [43], but in this case, the ALP reduction process was diffusion-controlled (Scheme 1(b)). Among the solid electrodes, the most widely used is the GCE. At bare GCE, ALP did not show any voltammetric signal, but after the electrochemical pretreatment of the electrode (Figure 3), a well-defined cathodic peak was observed (Figure 4). This difference between ALP’s electrochemical behavior at the two investigated working electrodes was attributed to a better adsorption of the analyte on the EPGCE due to its increased electroactive surface area and the presence of oxygen-containing groups that could interact with the analyte through hydrogen bonds (Figure 4). EPGCE was applied to ALP determination from six non-alcoholic and alcoholic spiked drinks without interference from substances such as ascorbic acid, citric acid, sucrose, and acetaminophen, which are commonly contained in beverages used in sexual assault cases. Applying the standard addition method, the recoveries obtained were between 82.0 ± 0.2 and 109.0 ± 0.3% [39].
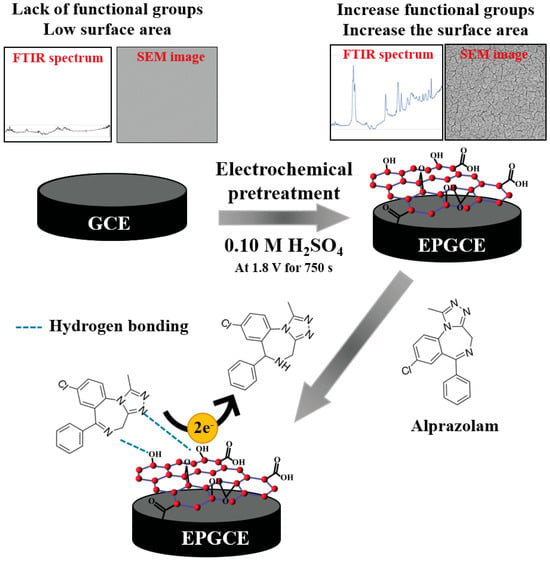
Figure 3.
Schematic representation of the EPGCE preparation and its application to ALP detection [39].
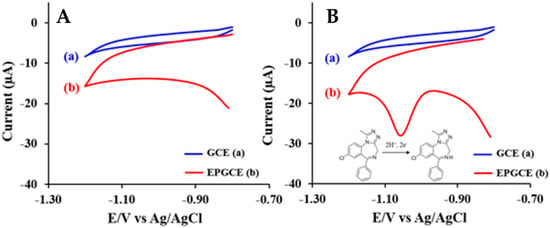
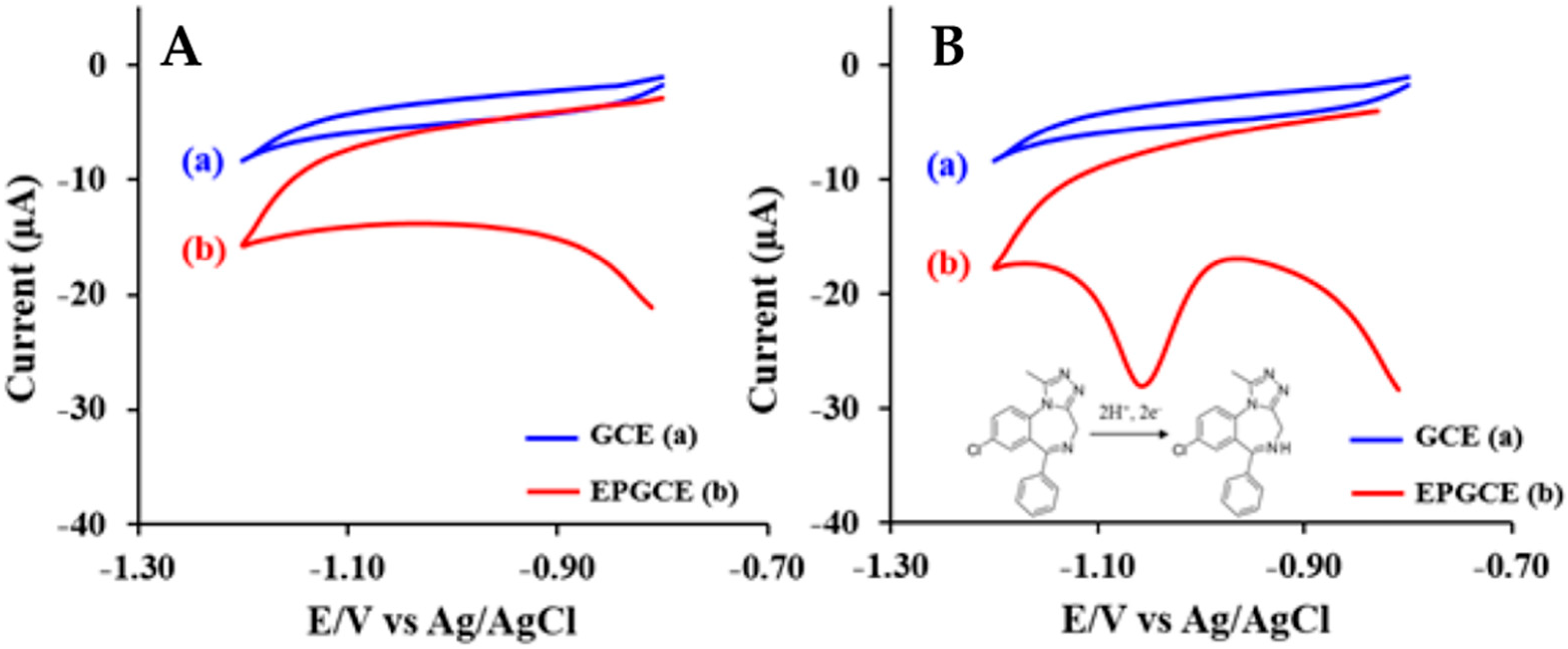
Figure 4.
Cyclic voltammograms of the (a) GCE and the (b) at a scan rate of 100 mV/s in B-R buffer pH 9.00 without ALP (A) and with 4 mg/L ALP (B) [39].
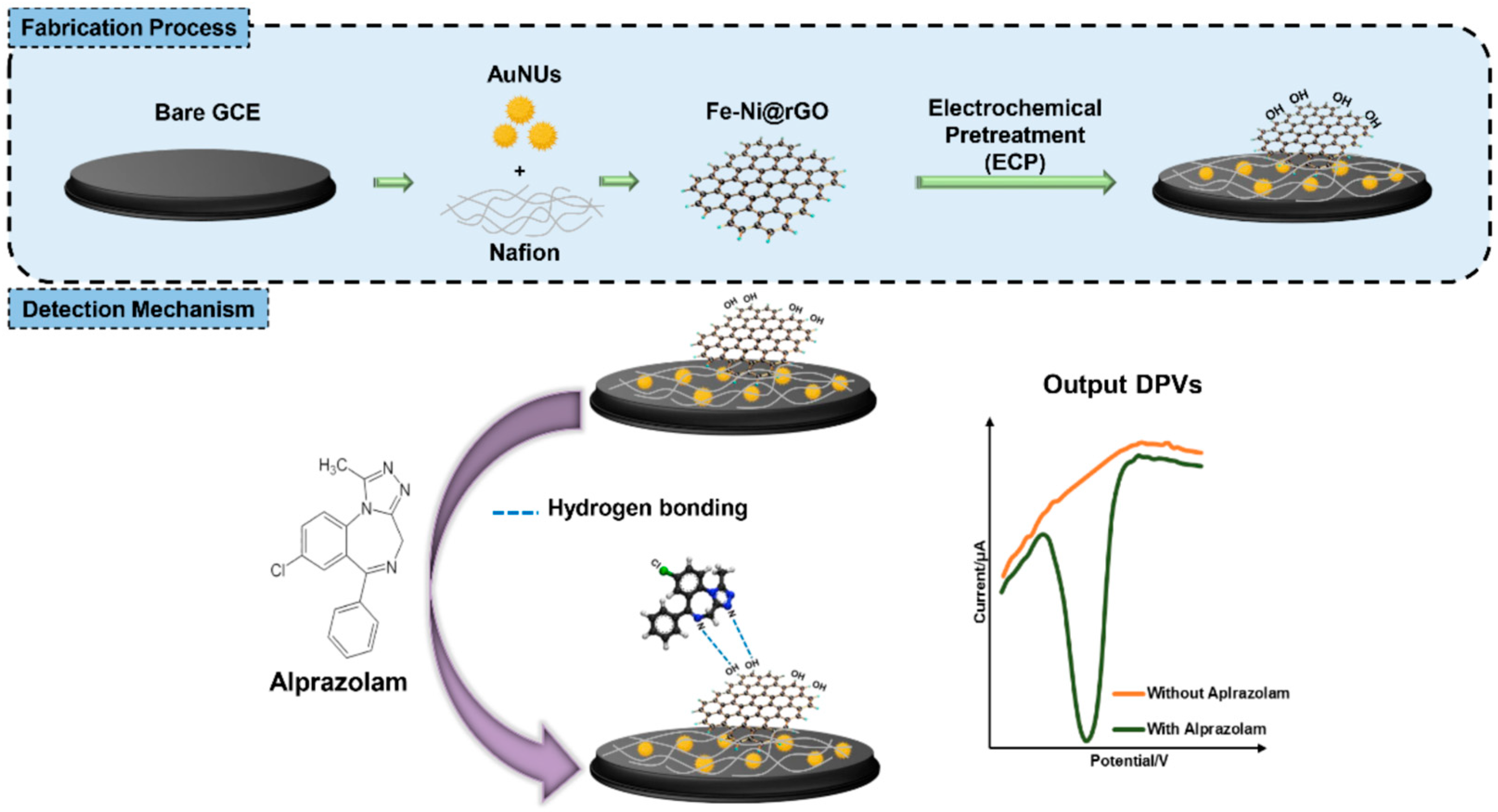
Electrode surface modification with different types of (nano)materials or combinations of them usually results in more sensitive and selective determinations, due to improved conductivity, larger electroactive surface area, and electrocatalytic properties of the modifier(s). For example, a gold nanoparticle decorated reduced graphene oxide nanocomposite, obtained in a single-step laser-assisted procedure, was drop-casted on a GCE, generating a sensor with enhanced electrochemical performances towards ALP detection. In this case, the electrode surface modification resulted in a decrease in the charge transfer resistance and an increase in the electroactive surface from 3884 kΩ and 0.080 cm2, for GCE, to 20.6 kΩ and 0.126 cm2, for the LI-AuNP-rGO/GCE. The situation of adding two 1 mg ALP tablets to a 300 mL beverage was simulated. After a ten-fold dilution of the spiked drink, the analyte concentration was within the linear range of the voltammetric method developed using this sensor, and ALP could be reliably detected without interference from other substances found in spiked drinks used in these types of scenarios [41]. The steps followed for the fabrication of AuNUs-Fe-Ni@GO/GCE developed for ALP determination are illustrated in Figure 5. The nanomaterials increased the surface area and the conductivity of the electrode, while electrochemical pretreatment enhanced the number of oxygen-containing groups, which favored ALP adsorption at the electroactive surface. This biosensor presented high selectivity in the presence of compounds normally found in blood (ascorbic acid, citric acid, and glucose) and a good long-term stability, preserving 92.2% of the original signal value after two weeks of storage in a refrigerator [44].
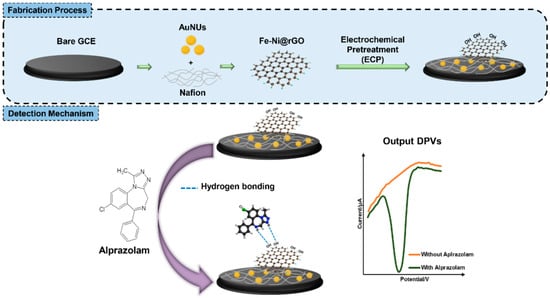
Figure 5.
Schematic representation of the AuNUs-Fe-Ni@GO/GCE preparation and ALP electrochemical detection [44].
It is worth mentioning that the above-described sensors enabled ALP analysis in the presence of other BZDs, like CLZP, DZP [28,39,40], LZP [41], DZP, CDZO, and OZP [44].
An interesting protocol for the detection of counterfeit Xanax tablets was by coupling electrochemical measurements with data analysis [38]. Using an optimized pH of 10, the voltammetric behavior of ALP and other BZDs, as well as that of common adulterants, like 5F-ADB, fentanyl, acetaminophen, ketamine, amphetamine, lidocaine, caffeine, 3,4-MDMA, cocaine, U-47700, were investigated and the corresponding oxidation and reduction peaks were attributed to different functional moieties belonging to the investigated species (e.g., reduction of thiophene ring, of fluoride atom, oxidation of secondary and tertiary amines). Based on the correlation between peak potential and functional groups, the authors developed a flow-chart system for the on-site analysis of falsified Xanax tablets.
PADs represent a good alternative for drug analysis, due to advantages, such as low cost and ease of preparation. Improved performance characteristics for ALP detection were obtained using an electrochemical microfluidic PAD having a methylene blue-doped Ag@Pd nanohybrid modified working electrode, due to the synergistic effects of the nanohybrids, which generated an increased electroactive surface area, and the redox mediator, which allowed an increased rate of electron transfer [37]. Another electrochemical microfluidic PAD, employing three working electrodes modified with AgNPs, each with an aptamer specific for ketamine, methamphetamine, or ALP, enabled the on-site, rapid (30 s), simultaneous quantification of these illicit drugs. However, the authors mention that due to the large dimensions of the platform-transducer system, it has the disadvantage of not being portable, its miniaturization being necessary [40].
The performance characteristics and the analytical applications of all these methods developed for ALP determination are summarized in Table 2. It can be observed that the LODs obtained for BDDE, CPE, and CNFs/SPE are not as low as those obtained with Hg and modified electrodes, but they are satisfactory for the analysis of pharmaceutical samples [38,42]. Although stripping voltammetry at HMDE obtained very low detection limits, this is not sufficient for BZDs detection in natural waters, their concentration in this situation being lower. Moreover, humic acid interfered with the determination [28].

Table 2.
Performance characteristics of electrochemical methods reported in the literature for ALP quantification.
2.2. Bromazepam (BZP)
BZP was determined from tap water and wastewater effluents from a pharmaceutical industrial plant, after pH adjustment to 4.00 and filtration, if necessary [45].
CV studies of BZP at BDDE emphasized that, only in the direct scan, one peak due to the 2e− and 1H+ diffusion-controlled, irreversible reduction of the azomethine group, most probably with the generation of a radical anion [42]. At CPE, the BZP reduction process proceeded according to Scheme 1(a) [43].
Two membrane ISEs containing either BZP-PTA or BZP-TPB as ion-pair complexes were reported for rapid (10 s–20 s) BZP potentiometric determination. The electrodes presented Nernstian responses of 54 ± 0.321 mV/decade (BZP-PTA) and 57 ± 0.223 mV/decade (BZP-TPB), a linear concentration range of 1.00 × 10−6 mol/L–1.00 × 10−3 mol/L, and a LoD of 7.94 × 10−7 mol/L BZP [45].
2.3. Clonazepam (CLZP)
Another widely analyzed BZD, CLZP, has been systematically studied along the three main paths. For biological samples, serum has either been simply diluted with PBS pH 7.00 [46,47], then spiked and analyzed, or processed via ethyl acetate LLE, which was then dried and reconstituted with electrolyte solution [48]. Urine was diluted with PBS [46], then spiked and analyzed, or centrifuged, filtered, then diluted with PBS [47], and analyzed via standard addition. Human blood serum [49] has been prepared by methanol precipitation, centrifugation, and filtration, with the supernatant being used for further analyses. Saliva [50] has been analyzed as-is, and human plasma [51] has been prepared by spiking, then processing via organic solvent deproteinization with acetonitrile, centrifugation, and analysis.
The standard protocol of weighing a number of tablets to determine their average mass, homogenizing all to a powder and then sampling a certain amount has been used [47,49] with dissolving either in a 2:8 methanol/water (v/v) mixture, followed by dilution with PBS pH 7.00 [47], or dissolving with just methanol, filtration, and dilution with PBS pH 7.20 [49].
Alcoholic [48] and non-alcoholic [48,52] drinks have been prepared by spiking, then diluting to a certain phosphate concentration [48] or analyzed as-is [52].
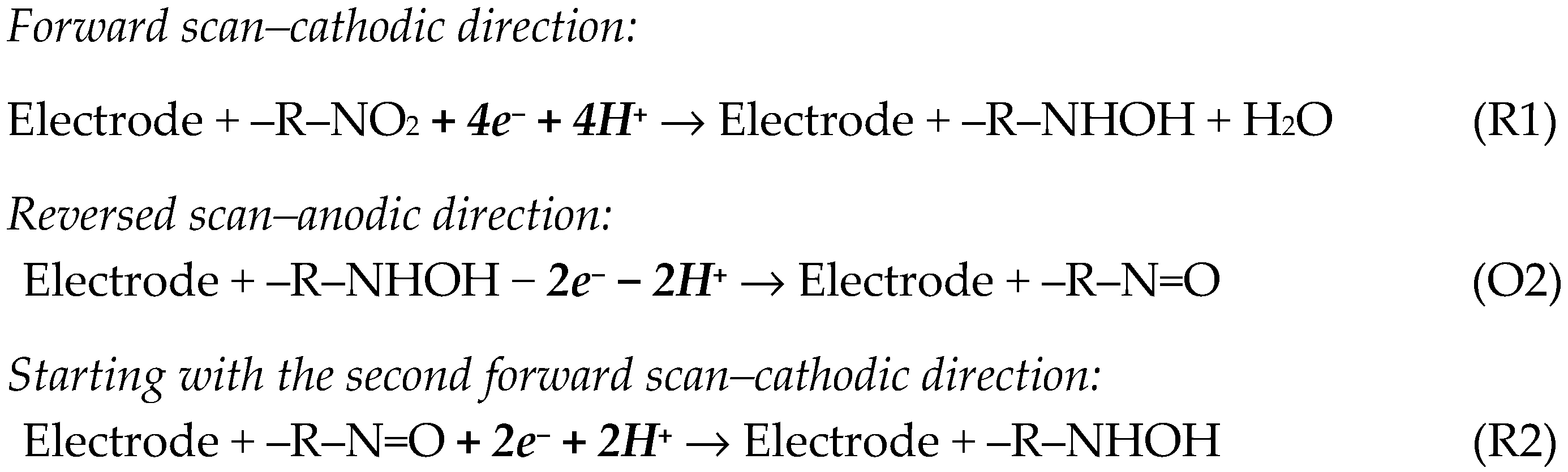
Due to the existence of a nitro group in its molecular structure, CLZP presented in the first forward cathodic scan only one reduction peak (R1) at about –0.6 V to –0.8 V, which was attributed to the irreversible, 4e− and 4H+ reduction of the –NO2 moiety to hydroxylamine. In the reversed anodic scan, an oxidation signal appeared (O2), which formed a redox peak pair with the new reduction peak (R2) recorded during the second scan in the negative direction. Peaks (O2) and (R2) (situated at less negative potentials) were associated with the oxidation of the –NHOH group to –NO and the reverse reduction of –NO to –NHOH, respectively, in processes involving 2e− and 2H+ (Scheme 2) [47,49,53].
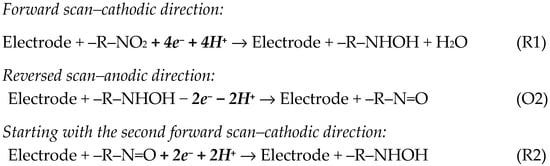
Scheme 2.
Electrode reactions involved in the electrochemistry of the –NO2 group [47,49,53].
Some investigations explore more negative potentials, recording the cathodic signal (R3) generated by reduction of the 4,5-azomethine group (Scheme 1) [48,50]. Moreover, one paper also indicated a cathodic signal, at about 0.3 V, generated by the reduction of the >C=O group (Scheme 3). However, for quantitative purposes, the -NO2 reduction signal was exploited the most [50].
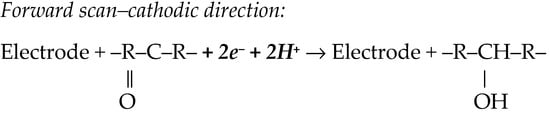
Scheme 3.
Electrode reaction involved in the electro-reduction of the >C=O moiety [50].
With mercury electrodes in a supporting electrolyte of pH 7.00, CLZP presented two irreversible, adsorption-controlled cathodic peaks associated with the reduction of the nitro group (Ep~−0.40 V) and of the azomethine group (Ep~−1.05V), respectively [25]. Using Cotrell’s relation and chronoamperometric measurements data, CLZP diffusion coefficient in PBS pH 7.00 was calculated to be 1.12 × 10−6 cm2/s [51] or 1.14 × 10−6 cm2/s [52].
Owing to their good electrical conductivity, catalytic properties, and large surface area, as-is metal or carbon-based (nano)materials or in composites were widely used to change the electrochemical surface properties of various sensors [49]. For example, the GCE modification with silver fibers and ionic liquid (1-butyl-3-methylimidazolium tetrafluoroborate) nanocomposite resulted in a 3.5-fold amplification of CLZP reduction peak and its shift by about 100 mV towards less negative potentials, in comparison to the bare GCE. The improved electrochemical properties of the modified electrode were attributed both to the Ag nanofibers, which had electrocatalytic properties and increased the electroactive surface area, and to a better electron transfer due to the ionic liquids, which enhanced the conductivity of the sensor [46]. On the other hand, the CLZP reduction peak was 3.8 times higher at the C3N4/CNH-MSN/GCE, compared to the bare electrode, due to doubling of the surface area, the high N content of the nanocomposite, which ensures a low charge-transfer resistance, and the intrinsic catalytic activity of copper. The electrode showed a stable response even after being stored for 2 weeks in ambient conditions, without being used. The results obtained for CLZP voltammetric quantification in real samples were in good agreement with those obtained by HPLC [47].
Other interesting inorganic materials for sensor construction are LDHs and MXenes, which have common features, like high surface area, electrocatalytic activity, and non-toxicity. A FeCu-LDH@MXene nanocomposite obtained by a hydrothermal synthesis involving a co-precipitation step was applied to modify a GCE. Chronocoulometric measurements emphasized that the surface area of the resulting electrode (0.267 cm2) was almost ten-fold larger than that of the bare electrode (0.029 cm2). The enhanced electroactive surface area and the synergistic electrocatalytic effects of FeCu-LDH and MXene resulted in amplified CLZP reduction signals and, consequently, a sensitive detection. Moreover, the electrode presented a good stability for at least one month [51].
pMela/MWCNT-COOH/GCE was obtained by casting onto the GCE surface and drying of a MWCNT-COOH suspension, and subsequent melamine electropolymerization by cyclic voltammetry. At the modified electrode, the CLZP main reduction peak was shifted by 140 mV in the positive direction, and its intensity increased 14 times compared to the unmodified electrode. These effects were assigned to the enhanced adsorption of the analyte on the polymer film, the catalytic activity of the MWCNT-COOH, and the higher effective surface area. Adsorptive stripping analysis of CLZP at this electrode involved the adsorption and subsequent reduction of the analyte, followed by the oxidation of the reduction product and recording of the voltammogram during the anodic stripping step. The electrode surface was cleaned between measurements by successively cycling the applied potential in the supporting electrolyte. The response of the electrode stored at room conditions decreased by only 8% after 15 days, suggesting good stability [53].
An alternative to the classical solid electrodes is represented by disposable electrodes, among them the best-known being SPEs with various types of working electrodes, which were also applied to CLZP quantification either as such [48] or modified [50,52]. The mammalian glycoprotein lubricin was used to modify the rGO surfaces of SPEs, due to its ability to act as a size-selective molecular sieve, separating the large (bio)molecules from the smaller ones (analyte) and preventing thus the electrode surface fouling. Moreover, lubricin also enabled the CLZP detection in unprocessed saliva in the presence of other similar compounds, like DZP and MDZ, suggesting that this device could be applied in POC testing [50].
The performance characteristics and the analytical applications of all these methods developed for CLZP determination are summarized in Table 3.

Table 3.
Performance characteristics of electrochemical methods reported in the literature for CLZP quantification.
2.4. Diazepam (DZP)
By far, one of the most investigated BZDs is DZP, a 1,4-benzodiazepine with an azomethine group with a pKa of 3.4 [54]. Blood, serum, or urine samples were collected from volunteers after diazepam oral administration [54,55], or from non-user volunteers [56,57,58,59,60] with sample spiking post-collection. In either case, prior to analysis, the biological matrices have been deproteinized by use of organic solvent [54], diluted with the buffer solution [55,56,57], or used without pretreatment post-spiking [56,58,59,60].
Pharmaceutical formulations are usually tablets but may also be found as injection solutions or drops. For tablets, the most common sample preparation [54,55,56,57,61,62,63] is grinding one or more tablets, after determining a tablet’s average mass, weighing a certain amount of that combined powder, usually the average weight of one tablet, and dissolving it to a certain concentration, then potentially filtering and volumetrically achieving the desired target concentration depending on the tablet’s active substance content. Various solvents have been used for the dissolution of diazepam, or for final volume diluents, such as methanol:water (3:2, v/v) [54] or (1:4, v/v) [55], water [56], buffer [57], the target beverage [61], alcohol [62,63], buffer (phosphate, pH 7.00 [54,56] or pH 4.00 [57]; acetate, pH 4.80 [55]), or acidic aqueous solutions (0.01 mol/L HCl [62]). For liquid and drop pharmaceutical formulations [63], the choice solvent was alcohol, followed by a diluent consisting of alcohol:electrolyte.
A special application encompassing testing of solid residues was the use of agarose hydrogels with 0.10 mol/L PBS [64], with direct sampling of the specimens.
Beverage analysis entailed the use of both alcoholic and soft drinks, or a mixture of those, to mimic bar settings where illicit drink spiking may occur. For analysis, samples were either diluted with buffer and water, sonicated, then spiked [61], spiked with a crushed diazepam tablet [63], followed by dilution with alcohol:electrolyte for analysis using the agarose hydrogels [64] dipped into the spiked and non-spiked drinks, or by spiking the drinks and analyzing without other treatment steps [58].
A thin three-electrode flow-cell was applied to the CV investigation of DZP reduction at GCE and its subsequent quantification by FI_DPA [61]. DZP irreversible reduction at various tested electrodes was diffusion-controlled [56,58] and pH-dependent, involving an equal number of electrons and protons [54,55,65]. An adsorption-controlled reduction of DZP was reported at SPCE [63]. Most papers stated a 2e− and 2H+ reduction of the 4,5-azomethine group from the benzodiazepine ring (Figure 1, Scheme 1) [54,63,65,66]. However, there are also some exceptions. At the BiPPGE, the cathodic process involved one electron, and the transfer coefficient, α, was found to be 0.34. The diffusion coefficient, estimated from chronoamperometric data, was 6.82 × 10−5 cm2/s [55]. In another study [54], a much lower diffusion coefficient of 3.06 × 10−6 cm2/s was obtained from CV data. It was reported that in acidic medium, DZP reduction at MWCNT/GC/CPE DZP proceeds via 1e− and 1H+ transfer. The proposed mechanism involved two chemical steps during which the benzodiazepine ring is cleaved, followed by the irreversible reduction, which finally leads to a pinacol derivative as reaction product [58].
Some literature reports indicated that DZP’s highest reduction signal was obtained at pH below its pKa, which is about 3.0, where the analyte is protonated at the N4 atom of the azomethine group. However, most analyses were performed at pH near the physiological value [54,56,59,64,65].
Mercury electrodes were the first sensors used for BZDs analysis, exploiting their azomethine group reduction peak. DPAdSV at the HMDE method for DZP quantification presented limited selectivity because other BZDs could interfere with the determination, CLZP being an example. However, quantifying CLZP based on its -NO2 reduction signal (situated at less negative potentials) allowed DZP determinations by subtracting the CLZP quantity from the common peak result [25].
GCE surface modification with various (nano)composites (e.g., by drop-casting C60–CNT/IL [56], graphene nano-sheets, and subsequent potentiostatic electrodeposition of Ag nanodendrites [54], or by drop-casting the graphene oxide and lysine on the potentiostatic pretreated GCE [65]) resulted in a lower peak potential and an amplified reduction signal of DZP due to the synergistic effects of the components [54,56]. The obtained modified electrodes presented good stabilities for two weeks [54] or more [56], but unfortunately, other BZDS could interfere in DZP determination [54]. Due to DZP’s electro-reduction being pH-dependent, and the tested beverages having various pH values, it was observed that in alcoholic drinks, with pH varying from 5 to 8.5, the analytical signal was shifted. Therefore, preliminary beverage pH measurements were necessary [65].
SPEs bare [63] or modified electrochemically ex situ with an Sb film [62] or by drop-casting SiO2 encapsulated QDs on the potentiodynamic pretreated electrode and subsequent covering with an electrogenerated MIP [60] were also reported for DZP analysis. When using SPE [63] for the analysis of beverages containing Coca Cola, the sensitivity, linear concentration range, and LoD previously established in the supporting electrolyte were altered. Therefore, for DZP determination in these kinds of samples, a matrix-matched calibration curve was necessary. Even though SPEs are disposable electrodes, it was reported that on such an electrode, Sb could be deposited three times, with an intermediate potentiostatic cleaning step before each deposition. Moreover, an Sb/SPE could be used for up to 20 measurements [62]. The MIP@SiO2-MPA-AuZnCeSeS-QDs/SPCE was applied to DZP quantification either by EIS or by SWV, using the redox probe K3[Fe(CN)6]/K4[Fe(CN)6]. The EIS analysis was based on the linear increase of the charge transfer resistance with DZP concentration, while the SWV determination relied on the linear decrease in the anodic signal of the redox probe with increasing analyte concentrations. EIS measurements were carried out using both a portable potentiostat connected to a laptop and a hand-held potentiostat connected to a smartphone. The authors stated that the portable potentiostat was about seven times more rapid but four times more expensive than the hand-held smartphone-based one. However, the use of SWV allowed obtaining a larger linearity range and a lower LoD [60].
CPEs modified with MIP grafted on functionalized MWCNTs [66] or with glassy carbon and MWCNTs [58] were also reported. The electrode containing glassy carbon presented a high stability of 15 months, while the presence of the MIP offered better performance characteristics but the analysis was more time-consuming, involving more steps, such as the MIP-modified electrode being incubated after the template extraction for 12 min in B-R buffer solution pH 4.00 containing DZP, followed by electrode washing before the actual voltammetric measurement [66].
A disposable wearable glove-based multi-sensor array was developed for the on-site detection of DZP, the explosive picric acid, and the pollutant 4-nitrophenol in liquid and powder samples. The three-electrode configuration was stencil-printed on the fingertips of a nitrile glove. The working electrodes were subsequently covered with MWCNTs/PEDOT:PSS. The thumb finger of the glove was covered with an agarose containing an ionic gel diluted in buffer, which acted as a semi-solid electrolyte. The electrical circuit was closed by contacting the thumb finger and the sensing fingertip, containing the analyte. The electrode system was connected to a portable potentiostat coupled with a smartphone. These wearable sensors cost USD 1.00, and their electrochemical stability lasted at least ten days [64].
POC testing needs cost-effective, miniaturized devices that allow the rapid, sensitive, and selective analysis of small sample volumes (20 μL). Microfluidic electrochemical PADs meet these requirements by featuring two integrated, paper-patterned electrodes that have a high surface-to-volume ratio, thus enabling low LoDs. Such a microdevice obtained by Si@GNRs drop-casted on the working electrode of the PAD was developed for DZP detection in urine spiked with the drug from tablets and injections with a recovery of 92.0% [59].
A disposable solid-state potentiometric sensor for DZP detection in biological fluids was also developed [57], consisting of a microfabricated Cu electrode on a printed circuit board. A conductive polymer, namely poly (3-octylthiophene), was drop-casted on the Cu surface to improve the stability of the potential drift. Further, the electrode surface area was covered with a PVC-based ion-sensitive membrane containing calix[4]arene as the ionophore. The sensor presented Nernstian response (55.0 ± 0.4 mV/decade), short response time (11 ± 2 s), and long-term stability (3 months). The potentiometric selectivity constants (log KpotDZP,i) of −2.26 and −3.89 for the possible interfering compounds OZP and 2-(N-methylamino)-5-chlorobenzophenone, which are a metabolite and degradation product, respectively, emphasized that DZP can be detected in the presence of these species. Other performance characteristics of this sensor are listed in Table 4. The sensor is affordable, sensitive, specific, user-friendly, and rapid; it necessitates only portable devices for on-site analysis and is easily deliverable to the end-user. All these parameters make it adequate for point-of-care diagnosis.

Table 4.
Performance characteristics of electrochemical methods reported in the literature for DZP quantification.
The performance characteristics and the analytical applications of all these sensors developed for DZP determination are summarized in Table 4.
2.5. Flunitrazepam (FNZP)
In biological samples, FNZP has been either studied from plasma [67,68] or serum [69]. Plasma samples were either centrifuged, diluted with B-R buffer (1:10 v/v, pH 3.0) and analyzed via standard addition [67], or analyzed as-is [68], while serum was processed via centrifugal filtration, dilution with PBS (1:5 v/v, pH 6.50), and spiked.
Alcoholic [70] and soft drinks [70,71] were spiked, without further treatment or dilution, or in the case of soft drinks, a 1:1 B-R buffer dilution [68] was employed. In a special application of a wearable electrode detector [72], FNZP was analyzed alongside scopolamine and ketamine spiked in alcoholic drinks.
Like the other BZDs, FNZP presented a cathodic peak at more negative potentials due to the azomethine group reduction (Scheme 1), and the characteristic signals attributed to the –NO2 group reduction (Scheme 2), as it was previously presented for CLZP. The processes were pH-dependent, and diffusion-adsorption controlled [67,69] or only diffusion-limited [68]. The charge transfer coefficient (α) and kcat for the oxidation-reduction signals corresponding to the redox pair –NH–OH/–N=O at the TiO2@CuO-N-rGO/p(L-Cys)/GCE were estimated to be 0.61 and 2.92 s−1, indicating a high electron transfer rate, while the value of the diffusion coefficient was found to be 2.26 × 10−6 cm2/s [67]. Chronoamperometric measurements allowed the estimation of FNZP diffusion coefficient in B-R buffer pH 7.00 and kcat at AGO-CuNPs/SPCE as 2.62 × 10−6 cm2/s and 0.88 s−1, respectively [71].
Voltammetric quantification of FNZP at nanomolar levels was achieved using GCEs modified with a conductive polymer (poly-L-cysteine or poly-β-cyclodextrin) and reduced graphene oxide doped with TiO2@CuO-N or with boron, respectively. Compared to GCE, the effective surface area of the modified electrodes was increased by about 7 and 27 times, while the charge transfer resistance decreased by 12 and 1.4-fold for TiO2@CuO-N-rGO/p(L-Cys)/GCE and Eβ-CD/B-rGO/GCE, respectively. In both situations [67,69], at AGO-CuNPs/SPCE [71] and at AuNPs/MnFe2O4NPs/CPE [68], the peak corresponding to the oxidation of the –NHOH group was used for FNZP quantitative determination. The stability of the electrochemical response of these sensors towards FNZP ranged from 8 days for the Eβ-CD/B-rGO/GCE up to one month [67,68] or longer [71] for the others.
A SPE with an iron-sparked graphite working electrode modified with GOx and GluHD was reported for FNZP analysis in drop-volume samples. The Fe-sparking of the graphite electrode tripled the electrode response while the immobilized enzyme performed an in situ deoxygenation of the solution, necessary for the investigation of FNZP reduction processes. The GluHD was added to provide the glucose necessary for the enzymatic reaction [70].
The performance characteristics and the analytical applications of all these sensors developed for FNZP determination are summarized in Table 5.

Table 5.
The performance characteristics of voltammetric methods developed for FNZP electrochemical quantification.
A new screening tool, which may be applied in forensic science, consisting of a cheap ($0.19 cost) in-house 3D printed electrochemical finger (e-finger), was produced for the rapid, direct multi-analyte testing of FNZP, ketamine, scopolamine, and paracetamol in undiluted alcoholic drinks. The sample was deoxygenated with an effervescent vitamin C tablet. The device, consisting of three carbon black-based plastic electrodes, was connected to a mini-potentiostat coupled to a smartphone controlled via an Android application. FNZP was detected based on its reduction signal, while the other analytes presented oxidation peaks at well-separated potentials. The FNZP was determined in the concentration range 2 mg/L–16 mg/L, and the LoDs in different beverages were 0.07 mg/L (whisky), 0.08 mg/L (vodka), 0.09 mg/L (gin), and 0.011 mg/L (beer) FNZP [72].
Chen et al.’s [73] review on analytical methods applied for FNZP detection in biological samples, which includes a small chapter dedicated to electroanalysis, is worth mentioning here.
2.6. Lorazepam (LZP)
LZP was analyzed from pharmaceutical injection liquids and drinking water, where both were diluted by PBS pH 7.00 and analyzed by the standard addition method using SWV at NiO/SWCNTs/CPE. LSV measurements indicated that LZP oxidation was a diffusion-controlled process, and the diffusion coefficient determined by chronoamperometry using Cotrell’s equation was 9.05 × 10−6 cm2/s. LZP oxidation peak (PBS pH 7.00, Ep~0.90 V) varied linearly with the analyte concentration in the range 1.00 × 10−7 mol/L–2.80 × 10−4 mol/L. The obtained LoD was 5.00 × 10−8 mol/L LZP [74].
A MWCNTs/NADES/CPE was applied for the simultaneous determination of LZP and noscapine in pharmaceutical samples. LZP diffusion coefficient calculated in this work was 7.83 × 10−7 cm2/s. DPV analysis of LZP at MWCNTs/NADES/CPE presented a linear range of 1.00 × 10−6 mol/L–2.22 × 10−3 mol/L and a LoD equal to 6.90 × 10−7 mol/L LZP [75].
2.7. Midazolam (MDZ)
In biological samples, MDZ has been analyzed in plasma after organic deproteinization and dilution with acetate buffer (pH 5.60), followed by spiking [76], or in urine by spiking, centrifuging, and B-R buffer dilution [77]. Pharmaceutical liquid dosage samples were studied post-dilution with either acetate [76] or B-R buffer [77]. Finally, alcoholic beverages were studied following both pre- and post-spike B-R dilution [78].
Cyclic voltammograms of MDZ recorded at different working electrodes [76,77,78] are characterized by at least an irreversible cathodic peak, typical for BZDs (Scheme 1). When using a MIP-CPE in the cathodic potential window, due to the structural similarity between MDZ and ALP, DZP and OZP, it was observed that these BZDs were also bound to the electrode surface, but to a much lesser degree, so using this sensor allowed for the selective detection of MDZ [77].
By applying the direct potential scan in the cathodic direction at BDDE in B-R buffer pH 2.00, MDZ presented the BZDs’ common electrochemical behavior (Scheme 1). However, in these conditions, if the forward scan was oriented towards positive potentials, an anodic peak was observed, attributed to an irreversible, diffusion-controlled electrode process. Based on voltammetric data (CV and SWV) and density functional theory (DFT) calculations, the following electro-oxidation mechanism was proposed (Scheme 4) [78].
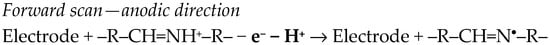
Scheme 4.
Electro-oxidation mechanism of MDZ [78].
The use of MDZ oxidation offers the advantage of higher selectivity, thus enabling its determination in the presence of other BZDs, like CLZP, DZP, and FNZP [78].
A more recent paper dealing with MDZ electrochemistry at SiNPs modified CPE covered by chronoamperometry with AgNPs emphasized that this analyte presented four reduction signals. The first two peaks were assigned to the irreversible reduction of the 4,5–C=N– with a hydroxylamine derivative as intermediate, and the last two to the >C=C< and –N=C< from the imidazole ring. This sensor was suitable for reliable MDZ detection in human plasma of healthy volunteers, but in the case of patients treated with anesthetic drugs, like ketamine and structurally related compounds, there was an interference of approximately 24% [76].
The performance characteristics and the analytical applications of all these sensors developed for MDZ determination are summarized in Table 6.

Table 6.
The performance characteristics of voltammetric methods developed for MDZ electrochemical quantification.
2.8. Nitrazepam (NZP)
In biological samples, NZP has been studied in spiked human serum [79] diluted 1:100 with PBS (pH 7.40), or in urine samples [80], following N,N-dipropylamine LLME, and evaporating onto the electrode, then read in buffer solution. Tablets [79,81] were finely ground, dissolved in either ethanol [79] or methanol [81], and analyzed following buffer dilution. In the case of both alcoholic and non-alcoholic beverages [81], crushed tablets were spiked into the target liquid and analyzed as-is.
Because NZP contains in its molecular structure the same electroactive groups as CLZP, its voltammetric behavior is similar (Scheme 1 and Scheme 2). Thus, in the first scan, NZP cyclic voltammograms emphasized, in the cathodic forward direction, two peaks (R1 at −0.60 to −0.75 V and R3 at −1.20 to −1.30 V due to the reduction of the –NO2 and 4,5-azomethine group, respectively) and, in the reversed scan, an anodic signal (O2 at −0.10 V to −0.20 V). In the subsequent scan, a new cathodic peak appears at about −0.20 to −0.3 V (R2), which forms a peak pair with (O2), which was assigned to the nitroso/hydroxylamine redox couple [79,80,81]. Peak R1 was mostly used for NZP quantification [80,81].
NZP detection was improved by using a GCE modified with GO-HNT by drop-casting, due to the synergetic effects of both components, namely, HNT increased the electroactive surface area (from 0.06 to 0.263 cm2) and GO provides electron-rich functionalities, increasing the electrocatalytic properties of the electrode [81]. Another sensor reported for NZP analysis was obtained by drop-casting at the GCE surface of an aqueous solution of rGO and drying, followed by potentiodynamic electrodeposition of AgNPs. GCE modification with AgNPs, rGO, and AuNPs-rGO increased NZP peak current about 2, 11, and 15 times, respectively [79].
NZP was voltammetrically determined after the homogeneous liquid–liquid microextraction employing N,N-dipropylamine as switchable solvent. The NZP containing extractant was dropped onto the surface of a GCE, which was previously pretreated by CV. After the evaporation of the solvent, the electrode was immersed in PBS pH 7.00, and differential pulse voltammograms were recorded [80].
The performance characteristics and the analytical applications of all these sensors developed for NZP determination are summarized in Table 7.

Table 7.
The performance characteristics of voltammetric methods developed for NZP electrochemical quantification.
2.9. Olanzapine (OLP)
OLP has been primarily studied in urine [82,83,84], serum [83,84,85], and from pharmaceutical tablets [83,84,85]. Urine samples have been analyzed by the standard addition method as-is [82,83], or either urine or serum [84] samples were spiked, followed by organic solvent deproteinization, centrifugation, and dilution with PBS pH 7.00. Serum was also prepared by organic solvent deproteinization pre- and post-spiking [83], and dilution with B-R buffer, or by a 1:10 pre-spike dilution with acetate buffer solution (pH 5.50). Pharmaceutical tablets were ground, and a certain weight was either dissolved in methanol followed by water dilution [83] or directly dissolved into buffer [84,85], prior to analysis.
Some studies emphasized an anodic signal for OLP at modified GCEs [82,84,86], which was assigned to the oxidation of the secondary amino group (>1NH) from the diazepine ring B (Figure 1). Somewhat different mechanisms were proposed for this process (Scheme 5).
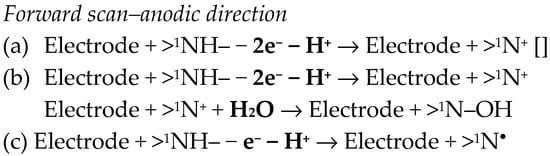
Scheme 5.
Various mechanisms have been proposed for the electro-oxidation of OLP [82,84,86].
In a study regarding OLP’s possible oxidative removal from contaminated aqueous samples, the CV recordings at BDDE revealed two anodic signals and a cathodic one. The anodic peak situated at a less positive potential (0.82 V, pH 2.00) was considered to form a pair with the reduction signal from about 0.20 V, associated with a quasi-reversible redox couple involved in surface-confined electrode processes. The more anodic signal (1.61 V) was due to an irreversible, diffusion-controlled oxidation process. Using chronoamperometric data and Cotrell’s equation, a diffusion coefficient of 6.50 × 10−4 cm2/s was estimated for OLP [87].
At AuNPs/p(L-Ala)/pre-PGE, OLP presented two anodic and one cathodic signals due to irreversible processes. It was considered that the adsorption-controlled oxidations took place at the benzodiazepine ring and involved 1e− and 1H+ (similar to Scheme 5(c)) and 1e− and no H+, respectively, while 2e− and 2H+ participated in the diffusion-controlled reduction at the imine group (similar to Scheme 1(b)). The authors have also proposed a mechanism for OLP electrochemical behavior [83].
A detailed voltammetric study showed that at GCE, OLP participated in four electrochemical processes. The first anodic signal (process I) appeared at about 0.20 V and was a reversible process, because a counter cathodic peak (process II) was observed. Two additional, ill-defined oxidation signals were observed at about 0.85 V (process III) and 1.10 V (process IV). The studies revealed that process I was followed by a chemical step so that it follows an EC mechanism. Thus, processes I/II are proton-coupled electron transfer processes involving the nitrogen oxidation with the formation of a radical which can either dimerize or participate in a reaction involving H2O addition. Process III involved 1e− and 1H+ and led to the formation of an ammonium radical in the piperazine ring. In fact, processes III and IV formed together a two-step oxidation involving 2e− and 1H+ at the piperazine group, generating as a final product an imine. In that paper, the authors proposed a mechanism for OLP electrooxidation [88].
QD-based nanocomposites were used to modify the GCE surface by drop casting to improve its electroanalytical performance towards OLP analysis. Enhanced current responses for OLP oxidation was due to the increase in the specific electroactive area (by about 1.5 and 3 times for S-CQDs/Fe2O3/GCE and NC@N,S@GQDs/GCE, respectively [82,86]) in comparison to bare GCE, the decrease in the charge transfer resistance (from 435.6 Ω for GCE to 64.5 Ω for NC@N,S@GQDs/GCE [86]) and to the synergistic effects of the modifier’s components. For example, better adsorption of OLP molecules was ensured by the H-bonds formed between the diazepine ring and the functional groups of the S-CQDs, while the Fe2O3 NPs were responsible for the better electrocatalytic oxidation of the analyte [82]. Similar results were obtained for PGEs, whose electroactive surface area increased by 1.4 and 3 times, respectively, after modification with AuNPs/p(L-Ala) [83] and N,P-CNOs/GNP [84]. At chronoamperometric pretreated PGE, OLP presented two oxidation peaks, whose intensity increased 2.5 times and 6.72 times, respectively, after AuNPs/p(L-Ala) deposition at the pretreated PGE surface [83]. Regarding the selectivity of the presented electrodes, valine, cysteine and some common sugars, like glucose, fructose and sucrose, did not present any signal at AuNPs/p(L-Ala)/pre-PGE [83] and S-CQDs/Fe2O3/GCE, while small anodic responses were observed for ascorbic acid and uric acid, but they did not influence OLP determination at the modified GCE [82]. The previously mentioned possible interferents, as well as other compounds, like metformin, paroxetine [84], or clozapine, asenapine, aripripazole, even when present in 100-fold excess [86], did not influence the OLP anodic signal recorded at N,P-CNOs/GNP/PGE and NC@N,S@GQDs/GCE, respectively.
A gold microelectrode modified with platinum black by chronopotentiometry was developed for rapid and sensitive OLP analysis in microliter volumes of undiluted serum samples. When compared to the unmodified electrode, this sensor presented a 6.7 times higher sensitivity and 5.1 times lower LoD [89]. As is obvious from the literature and from the data presented in this review, most electrochemical investigations were conducted applying different voltammetric methods. However, a hydrodynamic amperometric method, at a constant potential of 0.13 V and using a gold modified electrode, was described for the sensitive OLP determination [90].
Potentiometric analysis of OLP was also reported using either a carbon paste electrode modified with the ion-exchanger OLP-TP [85] or an ion-selective electrode with a PVC membrane containing the ion pair OLP-TPB [91]. Both electrodes presented Nernstian responses (59.2 mV/decade and 60 mV/decade) and a short response time of 4 and <10 s, for the OLP-TP/CPE [85] and the OLP-TPB/DOP/PVC, respectively [91]. OLP-TP/CPE was used in the potentiometric titration of OLP with phosphotungstic acid as well as in the study of its complexation with β-cyclodextrin. For the OLP-β-cyclodextrin complex, a molar ratio of 1:1 was found, and the inclusion constant was found to be 973.8 [85].
The performance characteristics and the analytical applications of all these sensors developed for OLP determination are summarized in Table 8.

Table 8.
The performance characteristics of voltammetric methods developed for OLP electrochemical quantification.
2.10. Oxazepam (OZP)
Biological samples (serum or urine) were spiked and analyzed as-is, while OZP tablets were analyzed by the standard addition method, but without an indication of sample preparation [92].
OZP presented only an irreversible reduction peak (Scheme 1), which was exploited for its quantitative determination using modified GCEs. The OZP cathodic signal (PBS pH 7.00, Ep~−0.60 V) was increased 4.1 times and shifted by 0.22 V in the positive direction when the GCE surface was covered with (Ag-Pt)NPs/GNs nanocomposite. DPV enabled OZP quantification in the concentration range 5.00 × 10−8 mol/L–1.50 × 10−4 mol/L with a LoD 4.20 × 10−8 of mol/L. The electrode presented good selectivity from common ions and molecular species (sugars, urea, acid uric, acetaminophen, folic acid), and its response was stable over four weeks. The applicability of the developed method was tested by OZP analysis in pharmaceuticals, serum, and urine samples [92]. A B-rGO/p(ASP)/GCE was developed for OZP determination by DPV (Ep~−1.10 V) in B-R buffer pH 7.00 after a preconcentration time of 60 s. The method presented a LoD of 3.00 × 10−10 mol/L OZP and two linear ranges, 1.00 × 10−9 mol/L–1.00 × 10−6 mol/L and 1.00 × 10−6 mol/L–8.00 × 10−4 mol/L, with different slopes, which were explained by a monolayer adsorption at low concentrations, followed by a multi-layer adsorption at higher analyte concentrations. Using this electrode, OZP was determined from plasma without interference of CLZP, LZP, fentanyl, zolpidem, and common biologically important species [93].
2.11. Tetrazepam (TZP)
TZP was analyzed by using human serum in 0.10 mol/L PBS electrolyte. DPV measurements were performed at a CPE modified with nitrogen-doped carbon nanoparticles. TZP reduction signal (PBS pH 7.40, Ep −0.83 V) was used for its quantitative determination in the concentration range 0–2.25 × 10−4 mol/L. The method presented a LoD of 1.73 × 10−8 mol/L TZP and was applied to the analyte determination in blood serum [94].
2.12. Multidrug Studies
Certain publications intended the development of similar yet standardized methods or electrodes to analyze multiple benzodiazepines, and as such, these are presented below, grouped by the target matrix.
2.12.1. Biological Samples
DZP, NDZP, BZP, and ALP were individually studied from pure weight or tablet samples with solvation in methanol. DZP was specifically determined by spiking a certain amount from a pharmaceutical tablet into synthetic urine mixed with 0.10 mol/L NaOH [95].
Urine samples were centrifuged and filtered, then diluted with ABS pH 6.00 prior to spiking. Analysis of DZP and OZP at a MIP-MMWCNTs-COOH/PGE, presented two well-separated DPV reduction peaks, thus allowing their concomitant quantification (Table 9). The central composite design method was used to establish the optimum conditions for the preparation of the modified PGE which involved the following steps: (i) the potentiodynamic activation of a 0.7 mm HB type PGE in 0.10 mol/L H2SO4, (ii) modification of the activated PGE with magnetic carboxylic MWCNTs by dip-coating; (iii) covering the whole system with a thin layer of MIP prepared using methacrylic acid as functional monomer, ethylene glycol dimethacrylate and 2,2-azobisisobutyronitrile as crosslinker and initiator, respectively, and (iv) removing of DZP and OZP from the polymeric matrix by washing with a methanol:acetic acid (9:1) mixture. Similar compounds, like ALP, CDZO, LZP, and TMP, did not interfere in DZP and OZP voltammetric determination for this electrode. The high selectivity conferred by the MIP was also demonstrated by the high imprinting factors of 2.9 and 2.6 for DZP and OZP, respectively [96].
Whole blood and urine spiked with NZP and 7-aminon-NZP were processed via microwave-assisted extraction using a pH 9.00 borax buffer and ethyl acetate, followed by two series of centrifugation, evaporation of the extractant, and a final small-volume aqueous reconstitution of the sample residue. Subsequently, NZP and 7-amino-NZP were analyzed by ITIES using ITV. In this technique, the analytical signals are represented by ionic currents generated by the ion transfer between two immiscible solutions due to an applied Galvani potential difference. The electrochemical behavior of the analytes is controlled by their partition characteristics between the two phases. Thus, this technique has the advantage that it can be applied to non-redox species. Voltammetric information obtained from measurements performed at eLLI at macroscopic and microscopic ITIES was exploited to investigate the interfacial behavior of the two analytes and for their quantitative analysis (Table 9). The diffusion coefficients of the two compounds in aqueous (DNZP,aq, D7a-NZP,aq) and organic (DNZP,o, D7a-NZP,o) media were established to be 1.41 × 10−5 cm2/s, 1.12 × 10−5 cm2/s, 1.31 × 10−5 cm2/s, and 1.11 × 10−5 cm2/s, respectively [97].
OLP, acetaminophen, and tramadol were targeted for simultaneous analysis by spiking into human plasma or urine samples previously diluted ten-fold with PBS pH 7.00. DPV at a CuO-rGNR/IL/CPE enabled the simultaneous determination of OLP, acetaminophen, and tramadol based on their well-resolved cathodic signals situated at 0.20 V, 0.44 V, and 0.90 V, respectively. The tested mixtures contained the analytes in the concentration ranges 2.00 × 10−5 mol/L–2.00 × 10−4 mol/L OLP, 2.00 × 10−4 mol/L–4.00 × 10−4 mol/L acetaminophen, and 5.00 × 10−5 mol/L–9.50 × 10−4 mol/L tramadol [98].
CLZP, ALP, DZP, OZP, and CDZO were targeted in spiked human serum samples analyzed as-is [99]. Human plasma was spiked with tenfold volumes of CLZP, DZP, ALP, CDZO, or OZP prepared in 0.10 mol/L NaOH [100]. The electrodes employed in these studies for the individual determination of BZDs were GCE electrochemically modified by layer-by-layer deposition of poly-dopamine, electrodeposition of chitosan, and subsequently chronoamperometric synthesis of AuNPs on the polymeric layers [99], as well as a similar poly-dopamine-coated electrode onto which poly-folic acid was additionally electropolymerized [100] (Table 9).
In an HPLC-ED [101] application, quetiapine, OLP, clozapine, fluphenazine, promazine, promethazine, levomepromazine, chlorpromazine, thioridazine, and perphenazine were spiked into control serum and human plasma samples, followed by precipitation with acetonitrile and centrifugation. Suspect patient samples were also diluted with water (1:1) prior to analysis. Thus, using this method, phenothiazines and BZDs belonging to the first generation of antipsychotic drugs were quantified using their oxidation signals recorded at a PGE. The method presented for all tested drugs a linear range up to 5.00 × 10−7 mol/L and LoDs in the nmol/L range.
A gold electrode covered electrochemically with poly-chitosan and subsequently by layer-by-layer deposition of a conductive ink based on AgNPs-N doped graphene QDs was developed for the quantification of several BZDs in plasma samples without any pretreatment step [102] (Table 9).
A LSG multiplex sensing platform was developed for simultaneously detecting amphetamine, cocaine, and BZDs (via metabolite detection), by using saliva extracted by centrifugation from oral pad swabs, testing both spiked and neat healthy saliva and spiked and neat patient saliva. This sensing platform with one reference, one counter, and three working electrodes, connected to a custom-made potentiostat and a smartphone running a custom application, represented a POC testing device applied to detect the mentioned analytes in saliva provided by healthy volunteers and methamphetamine patients (Table 9). The system presented high selectivity since each working electrode was modified with the corresponding antibody; namely, BZD antibody was immobilized on the first, amphetamine antibody on the second and cocaine antibody on the third working electrode [103].
2.12.2. Pharmaceutical Formulations
As in most sample preparations, tablets are generally weighed and homogenized, followed by dissolving a certain amount to make a stock solution. For DZP and OZP, the tablet portion was dissolved in water, sonicated, filtered, then diluted with buffer, and analyzed by the standard addition method [96]. NZP in tablet form was crushed and dissolved in 0.01 mol/L HCl/NaCl, sonicated, filtered, and further diluted with the same solution to bring the concentration within linearity [97]. BZP and ALP tablet [42,43] portions were dissolved in methanol, filtered, and diluted with B-R buffer (1:9) prior to analysis. BDDE [42] and CPE [43] were applied to the cyclic voltammetric analysis of ALP and BZD and their individual quantification by DPV (Table 9). Before using, the BDDE was pretreated electrochemically through an anodic (2.00 V, 60 s) and a cathodic (−2.00 V, 30 s) step, to clean the electrode surface and to generate an H-terminated electrode surface, respectively [42].
Finally, for DZP, CLZP, and ALP [35], a 4–5 mg portion of crushed tablets was dissolved in B-R buffer with ethanol and applied directly onto the electrode for analysis.
2.12.3. Beverages
Alcoholic beverages were studied by spiking DZP, CLZP, or ALP, diluting tenfold with the B-R buffer solution (pH 2.00 or 7.00), and then analyzed in standard conditions. Based on the correlation of their voltammetric peaks with the electroactive groups existing in their molecule, BZDs were classified into three classes, which were detected after spiking beer, gin, cognac, white wine, and red wine. A dual test, at pH 2.00 and pH 7.00, was necessary for red wine to be able to discriminate between the three BZDs classes [35].
Similarly, DZP and MDZ were analyzed with an LSG-PEI disposable sensor after spiking into B-R buffer pH 4.00, tenfold diluted alcoholic and non-alcoholic samples (Table 9). Before the actual voltammetric recordings, the LSG-PEI was pretreated electrostatically at −2.0 V for 120 s, to partially reduce the oxygen-containing groups from the graphene surface and to improve its conductivity. 3,4-MDMA and substances commonly found in beverages, like glucose, ethanol, ascorbic, and citric acids, did not interfere with the BDZ determination. However, the method could not discriminate between structurally related BDZs, which present reduction signals in the same potential region [104].
ITV at an eLLI-based sensor was also applied to evaluate physicochemical parameters of four “designer” BZDs, among them being the diffusion coefficients of the cationic forms of the analytes, which were 1.6 × 10−5 cm2/s, 9.7 × 10−5 cm2/s, 7.3 × 10−5 cm2/s, and 8.4 × 10−5 cm2/s for FPZM, FBZM, CLNZ, and DCZP. This technique allowed their quantification (Table 9) and the discrimination between the two very similar species CLNZ and FPZM [105].
A voltammetric ET consisting of six working electrodes (graphite epoxy composite, Pt, and graphite modified with one of the following NPs: Cu, CuO, Pt, WO3), an auxiliary, and a reference electrode was designed for the simultaneous determination of DZP, FNZP, and LZP. To obtain reproducible results every time, the 4th cycled voltammogram was considered, and before each measurement, the electrode’s surface was cleaned by applying a potential of 1.20 V for 60 s in a pH 10.00 solution. Principal Component Analysis was applied to select the sensors. For quantitative analysis (Table 9), the Discrete Wavelet Transform was used to reduce data complexity and to build an Artificial Neuronal Network model [106].

Table 9.
The performance characteristics of electrodes developed for multidrug analysis, including BZDs.
Table 9.
The performance characteristics of electrodes developed for multidrug analysis, including BZDs.
| Analyte (BZD) | Electrode | Technique/Conditions/Ep (V) | Linear Range (mol/L) | LoD (mol/L) | Sample | Ref. |
|---|---|---|---|---|---|---|
ALP BZP DZP NDZP | m-AgSAE | DPV/ PBS pH 11/~−1.06 B-R buffer pH 2.00/~−0.35 0.10 mol/L NaOH/~−1.22 B-R buffer pH 10.20/~−1.10 | 6.00 × 10−7–1.00 × 10−4 6.00 × 10−7–1.00 × 10−4 4.00 × 10−7–1.00 × 10−4 6.00 × 10−7–1.00 × 10−4 | 3.54 × 10−7 3.73 × 10−7 9.00 × 10−8 1.28 × 10−7 | Synthetic urine | [95] |
| DZP OZP | MIP-MMWCNTs-COOH/PGE | DPV/ABS pH 6.00/ DZP: −0.89 OZP: −1.10 | 9.83 × 10−8–2.00 × 10−4 4.88 × 10−8–1.50 × 10−4 | 5.97 × 10−8 2.10 × 10−8 | Spiked urine, tablets | [96] |
| NZP | eLLI/macroITIES eLLI/microITIES | ITV/0.01 mol/L NaCl, 0.01 mol/L HCl, pH 2.00 | 1.00 × 10−5–3.0 × 10−5 2.00 × 10−6–3.00 × 10−5 | 2.15 × 10−6 (+) 1.86 × 10−6 (−) 4.20 × 10−7 (+) 3.80 × 10−7 (−) | Spiked urine, blood, pharmaceuticals | [97] |
| 7-amino-NZP | eLLI/macroITIES eLLI/microITIES | ITV/0.01 mol/L NaCl, 0.01 mol/L HCl, pH 2.00 | 1.00 × 10−5–3.0 × 10−5 2.00 × 10−6–3.00 × 10−5 | 7.10 × 10−7 (+) 1.23 × 10−6 (−) 2.10 × 10−7 (+) 4.20 × 10−7 (−) | Spiked urine, blood | |
ALP CDZO CLZP DZP OZP | p(DA-CS)-AuNPs/GCE | DPV PBS pH 7.00/−0.33 PBS pH 6.00/−0.42 PBS pH 10.00/−0.58 PBS pH 8.00/−0.52 PBS pH 7.00/−0.32 | 5.00 × 10−8–4.20 × 10−7 7.50 × 10−8–4.00 × 10−7 2.80 × 10−7–6.50 × 10−7 2.00 × 10−8–1.40 × 10−7 6.30 × 10−8–1.39 × 10−6 | 5.00 × 10−8 7.50 × 10−8 2.80 × 10−7 2.00 × 10−8 6.30 × 10−8 | Human serum | [99] |
ALP CDZO CLZP DZP OZP | p(DA-FA)/GCE | DPV/0.10 mol/L NaOH ~−0.40 ~−0.25 ~−0.16 ~−0.40 ~−0.40 | 3.10 × 10−8–5.20 × 10−8 3.00 × 10−8–7.10 × 10−8 2.50 × 10−8–9.00 × 10−7 2.70 × 10−8–4.10 × 10−8 2.50 × 10−8–4.70 × 10−8 | 3.10 × 10−8 3.00 × 10−8 2.50 × 10−8 2.70 × 10−8 2.50 × 10−8 | Human plasma | [100] |
| ALP CDZO CLZP DZP OZP ALP CDZO CLZP DZP OZP ALP CDZO CLZP DZP OZP | AgNPs/N-GQD/Cs/Au | DPV/0.10 mol/L NaOH Oxidation ALP, CDZO, DZP, OZP: 0.50; CLZP: 0.60 DPV SWV | 5.60 × 10−5–1.56 × 10−4 5.20 × 10−5–2.50 × 10−4 8.40 × 10−5–6.25 × 10−4 5.40 × 10−5–1.42 × 10−4 5.40 × 10−5–4.54 × 10−4 3.80 × 10−6–2.47 × 10−4 6.19 × 10−5–9.90 × 10−4 3.80 × 10−6–4.95 × 10−4 7.70 × 10−6–4.95 × 10−4 6.30 × 10−8–1.39 × 10−6 7.30 × 10−5–1.92 × 10−4 7.04 × 10−5–2.50 × 10−4 5.40 × 10−5–1.00 × 10−3 5.60 × 10−5–4.54 × 10−4 5.60 × 10−5–2.94 × 10−4 | 5.60 × 10−5 5.20 × 10−5 8.40 × 10−5 5.40 × 10−5 5.40 × 10−5 3.80 × 10−6 6.19 × 10−5 3.80 × 10−6 7.70 × 10−6 6.30 × 10−8 7.30 × 10−6 7.04 × 10−5 5.40 × 10−5 5.60 × 10−5 5.60 × 10−5 | Electrolyte Human plasma Electrolyte | [102] |
| BZDs | Ab-LSG | DPV/5.00 mmol/L [Fe(CN)6]3−/4− in 0.10 mol/L PBS + 0.10 mol/L KCl/~−0.05 | 1 pg/mL–500 ng/mL | 9.7 ng/mL | Saliva | [103] |
| ALP BZP | BDDE | DPV/ALP: B-R buffer pH 5.00/−0.84; BZP: B-R buffer pH 11.00/−1.10 | 8.00 × 10−7–1.00 × 10−4 1.00 × 10−6–1.00 × 10−4 | 6.40 × 10−7 3.10 × 10−7 | Pharmaceuticals | [42] |
ALP BZP | CPE | DPV/B-R buffer:methanol (9:1) pH 3.00/ALP: −0.78; BZP: ~−0.50 | 8.00 × 10−7–1.00 × 10−4 8.00 × 10−7–1.00 × 10−4 | LoQ: 4.20 × 10−7 3.80 × 10−7 | Pharmaceuticals | [43] |
ALP CLZP DZP | Graphite SPE | SWV/B-R buffer pH 7.00 Standard solution ALP: ~−0.6 to ~−0.7 CLZP: ~−0.55, ~−0.75, ~−0.9 DZP: ~−0.75 to − ~0.9 | 2.50 × 10−8–1.00 × 10−6 7.50 × 10−7–6.00 × 10−4 2.50 × 10−8–1.00 × 10−6 | 1.40 × 10−8 7.50 × 10−7 3.60 × 10−8 | Pharmaceuticals | [35] |
ALP CLZP DZP | Deoxygenated solution ALP: ~−0.75, ~−0.97 CLZP: ~−0.55, ~−0.95 DZP: ~−0.85 | 1.50 × 10−5–2.00 × 10−4 1.00 × 10−6–2.00 × 10−4 5.00 × 10−6–3.00 × 10−4 | 1.10 × 10−5 7.50 × 10−7 3.11 × 10−6 | Beverages | ||
| DZP MDZ | LSG-PEI | SWV/B-R buffer pH 4.00/DZP:~−1.15; MDZ:~−1.05 | 2.50 × 10−6–2.50 × 10−5 2.50 × 10−5–1.00 × 10−4 | 6.60 × 10−7 6.10 × 10−7 | Beverages | [104] |
| CLNZ DCZP FBZM FPZM | eLLI | ITV/B-R buffer pH 2.00 | 2.00 × 10−6–5.00 × 10−5 2.00 × 10−6–5.00 × 10−5 2.00 × 10−6–5.00 × 10−5 2.00 × 10−6–5.00 × 10−5 | 1.40 × 10−7 1.60 × 10−7 2.40 × 10−7 1.90 × 10−7 | Beverages | [105] |
| DZP FNZP LZP | ET | CV/B-R buffer pH 10.00/PCA, DWT | 3.50 × 10−5–1.10 × 10−4 3.20 × 10−5–9.60 × 10−4 3.10 × 10−5–9.30 × 10−5 | 6.00 × 10−6 5.60 × 10−6 4.60 × 10−6 | Electrolyte | [106] |
(+)—positive signals defined as the cation transfer from the aqueous to the organic phase; (−)—negative signals defined as the cation transfer from the organic to the aqueous phase.
3. Conclusions
One can conclude that there is an increasing need for the development of cost-effective, sensitive platforms, like e-fingers [72], for the non-invasive simultaneous detection and monitoring of drugs. In this respect, electrodes modified with various (nano)materials (CNTs, QDs, NPs, polymeric films, etc., or a combination of them) have been reported for BZDs quantification in different matrices. However, it should be emphasized that most electrodes presented were conventional solid electrodes, bare or modified, used in batch electrochemical systems that require at least 5 mL of analyzed solutions, thus consuming more reagents than drop-based analytical systems, such as SPE and wearable sensors. Therefore, finding proper upscaling-compatible sensor fabrication methods may be a challenge for further research directions. On the other hand, in terms of time consumption, many of the presented procedures for electrode modification involved multiple steps, and therefore, future research should be directed towards finding rapid, single-step modification methods. One can consider that the first steps in these directions (miniaturization and reducing preparation time and costs) were made by the development of PADs.
It is essential that these devices can be miniaturized in order to analyze small sample volumes and to be used with portable devices so that the BZDs can be detected on-site, at the place of potential use, as well as in POC diagnosis. The present literature emphasized that there are increasing concerns in the development of various disposable (e.g., PADs) and even wearable sensors. To develop more eco-friendly analytical methods, it is important that the sample pretreatment step is minimized to avoid reagent consumption and to shorten the analysis time, and electrochemical analysis usually fulfills these requirements.
There is also a trend towards using various advanced mathematical models (chemometric tools) for data processing to enable selective multicomponent determination even with electrodes that are not very selective. ET also falls into this category.
Finally, it can be noted that the number of electrochemical methods reported in the last decade for the determination of individual BZDs is not very large, while for some components of this class, as well as for the “designer” BZDs, there is very little or even no data in this regard.
The current information contained in this review may be a useful starting point for further research in the field which may be directed towards (i) electroanalysis of more BZDs including “designer” BZDs; (ii) improving selectivity for structurally related molecules detection (e.g., by proper combination of molecularly imprinted polymers with nanomaterials with electrocatalytic activity or by using chemometric approaches); (iii) miniaturization; (iv) the further design and development of disposable sensors for POC testing and for the on-the-spot illicit drug detection; and (v) the use of more eco-friendly preparation methods and sensors.
Author Contributions
Conceptualization, I.G.D. and M.-A.C.; formal analysis, I.G.D., M.-A.C. and A.S.T.; writing—original draft preparation, I.G.D., M.-A.C. and A.S.T.; writing—review and editing, M.-C.C. and E.-E.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Being a review, no new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 3,4-MDMA | 3,4-methylenedioxymethamphetamine |
| Ab-LSG | Antibody–Laser-Scribed Graphene |
| AD | Alzheimer’s Disease |
| AgFs-IL | Ag Fibers and Ionic Liquid nanocomposite |
| AgNDs/GNs | Ag Nanodendrimers supported by Graphene Nanosheets |
| Apt-AgNPs-EPMAD | Aptamer-Ag Nanoparticles–Electrochemical Paper-based Multiplex Analytical Device |
| AgNPs/N-GQD/Cs/Au | Ag Nanoparticles-Nitrogen doped Graphene Quantum Dots deposited onto Chitosan modified gold electrode |
| AGO-CuNPs/SPCE | Amine-functionalized Graphene Oxide sheets reinforced through Cu Nanoparticles modified Screen Printed Carbon Electrode |
| (Ag-Pt)NPs/GNs | Ag-Pt core–shell Nanoparticles supported on Graphene Nanosheets |
| AuNPs/p(L-Ala)/pre-PGE | Au Nanoparticles poly(L-alanine) pre-anodized Pencil Graphite Electrode |
| AuNPs-rGO | Au Nanoparticles reduced Graphene Oxide nanocomposite |
| AuNPs/SiNPs-CPE | Au Nanoparticles-Silica Nanoparticles modified Carbon Paste Electrode |
| ALP | Alprazolam |
| BDDE | Boron-Doped Diamond Electrode |
| BIA-SWAdSV | Batch Injection Analysis coupled with Square Wave Adsorptive Stripping Voltammetry |
| BiPPGE | Bismuth-modified Pretreated Pencil Graphite Electrode |
| BMBPBP/CdS-QDs/MWCNTs/Au | 1, 4 Bis(N Methyl) Benzene bis(N Phenyl, N Benzoylphosphoramidate)/Cadmium Sulfide Quantum Dots/Multi-Walled Carbon Nanotubes modified gold electrode |
| B-R | Britton-Robinson |
| B-rGO/p(ASP) | Boron doped reduced Graphene Oxide–poly(Aspartic acid) |
| BZP | Bromazepam |
| C60–CNT/IL | Fullerene-functionalized Carbon Nanotubes and Ionic Liquid (1-butyl-3-methylimidazolium tetrafluoroborate) |
| C3N4/CNH-MSN | C3N4 decorated Copper Nitrate Hydroxide-containing Mesoporous Silica Nanoparticles |
| CECs | Contaminants of Emerging Concern |
| CDZO | Chlordiazepoxide |
| CLNZ | Clonazolam |
| CLZP | Clonazepam |
| CNFs/SPE | Carbon Nanofiber-Modified Screen-Printed Electrodes |
| CNS | Central Nervous System |
| CoOOH-rGO/SPCE | Cobalt Oxyhydroxide nanoflakes and reduced Graphene Oxide modified Screen Printed Carbon Electrode |
| CPE | Carbon Paste Electrode |
| Cu/POT/ISM | Copper/Poly(3-Octylthiophene)/Ion Selective Membrane |
| CuO-rGNR/IL/CPE | CuO/reduced Graphene Nanoribbons nanocomposites and Ionic Liquid (1-ethyl 3-methyl imidazolinium chloride) modified Carbon Paste Electrode |
| CV | Cyclic Voltammetry |
| DCZP | Diclazepam |
| DPAd(C)SV | Differential Pulse Adsorptive (Cathodic) Stripping Voltammetry |
| DPV | Differential Pulse Voltammetry |
| DZP | Diazepam |
| DWT | Discrete Wavelet Transform |
| Eβ-CD/B-rGO | Electropolymerized β-Cyclodextrin and Boron-doped reduced Graphene Oxide |
| EIS | Electrochemical Impedance Spectroscopy |
| eLLI | electrified Liquid–Liquid Interface |
| EPGCE | Electrochemically Pretreated Glassy Carbon Electrode |
| ET | Electronic Tongue |
| Fe3O4/R-SH/Pd | 2-mercaptoethanol on triazine functionalized iron oxide magnetic nanoparticles Pd nanocomposite |
| FeCu-LDH@MXene | FeCu-Layered Double Hydroxides Mxene nanocomposite |
| FI-DPA | Flow Injection–Differential Pulse Amperometry |
| FNZP | Flunitrazepam |
| FBZM | Flubromazolam |
| FPZM | Flualprazolam |
| GABA | Gamma-Aminobutyric Acid |
| GCE | Glassy Carbon Electrode |
| GO-HNT | Graphene Oxide functionalized Halloysite Nanotubes |
| GO/Lys | Graphene Oxide-Lysine composite |
| GOx/GluHD/FeSPC | Glucose Oxidase and Glucose Hydrogel Droplets Modified Iron-Sparked Graphite Electrode |
| HA/RDE | Hydrodynamic Amperometry at Rotating Disk Electrode |
| HPLC-ED | High Performance Liquid Chromatography with Electrochemical Detection |
| ISE | Ion Selective Electrode |
| ITIES | Interface between Two Electrolyte Solutions |
| ITV | Ion Transfer Voltammetry |
| kcat | catalytic rate constant |
| LC-HRMS | Liquid Chromatography-High Resolution Mass Spectrometry |
| LDHs | Layered Double Hydroxides |
| LI-AuNP-rGO | Laser-induced Au Nanoparticles reduced Graphene Oxide nanocomposite |
| LLE | Liquid–Liquid Extraction |
| LoD | Limit of Detection |
| LoQ | Limit of Quantification |
| LSG-PEI | Laser-scribed graphene device fabricated on Polyetherimide substrate |
| LSV | Linear Sweep Voltammetry |
| LUB-rGOSPE | Lubricin-modified reduced Graphene Oxide Screen-Printed Electrode |
| LZP | Lorazepam |
| m-AgSAE | Meniscus Modified Silver Solid Amalgam Electrode |
| MB-Ag@Pd/EμPAD | urchin-like Methylene Blue doped silver core shell palladium nano-hybrids Electrochemical microfluidic Paper-based Analytical Device |
| MDZ | Midazolam |
| MIP | Molecularly Imprinted Polymer |
| MIP-CPE | Molecularly Imprinted Polymer-based Carbon Paste Electrode |
| MIP@SiO2-MPA-AuZnCeSeS-QDs/SPCE | SiO2-encapsulated-3-Mercaptopropionic Acid-capped AuZnCeSeS Quantum Dots overcoated with a Molecularly Imprinted Polymer on Screen-Printed Carbon Electrodes |
| MIP-MMWCNTs-COOH/PGE | Magnetic carboxylated Multi-Walled Carbon Nanotubes modified Pencil Graphite Electrode covered with a Molecularly Imprinted Polymer |
| MIP-MWCNTs-COOH/CPE | Molecularly Imprinted Polymer grafted on carboxylated Multi-Walled Carbon Nanotubes modified Carbon Paste Electrode |
| MWCNTs | Multi-Walled Carbon Nanotubes |
| MWCNTs/GC/CPE | Multi-Walled Carbon Nanotubes/Glassy Carbon/Carbon Paste Electrode |
| MWCNTs/PEDOT:PSS | Multi-Walled Carbon Nanotubes/Poly(3,4-Ethylenedioxythiophene):Poly(Styrenesulfonate) |
| NaTKBor | Buffer containing sodium acetate, ammonium acetate, KCl, KSCN, and borax |
| NC@N,S@GQDs | Nanocellulose and Nitrogen, Sulfur co-doped Graphene Quantum Dots |
| NiO/SWCNTs/CPE | NiO/Single-Walled Carbon Nanotubes modified Carbon Paste Electrode |
| N,P-CNOs/GNP/PGE | N, P-doped Carbon Nanoonions and Graphene Nanoplates modified Pencil Graphite Electrode |
| NDZP | Nordiazepam |
| NZP | Nitrazepam |
| OLP | Olanzapine |
| OLP-TPB/DOP/PVC | Olanzapine-Tetraphenyl Boron/Dioctylphthalate/Polyvinylchloride |
| OZP | Oxazepam |
| PAD | Paper-Based Analytical Device |
| PBS | Phosphate-Buffer Solution |
| PCA | Principal Component Analysis |
| p(DA-CS)-AuNPs | Poly (Dopamine-Chitosan)–gold Nanoparticles |
| p(DA-FA) | Poly (Dopamine-Folic Acid) |
| pMela/MWCNT-COOH | Poly Melamine and carboxylated Multi-Walled Carbon Nanotubes |
| PTA | Phosphotungstic Acid |
| POC | Point-of-Care |
| PtB/Au | Platinum Black modified gold microelectrode |
| QDs | Quantum Dots |
| S-CQDs/Fe2O3 | Sulfur-Doped Carbon Quantum Dots chemically incorporated with iron oxide nanoparticles |
| Si@GNRs/EμPAD | Silica-Coated Gold Nanorods drop-casted on an electrochemical microfluidic-paper-based device |
| SbFSPE | Antimony Film Screen-Printed Electrode |
| SPE | Screen-Printed Electrode |
| SS-HLLME | Switchable Solvent-Based Homogeneous Liquid–Liquid Microextraction |
| SWV | Square Wave Voltammetry |
| SWAdASV | Square Wave Adsorptive Anodic Stripping Voltammetry |
| SWAdSV | Square Wave Adsorptive Stripping Voltammetry |
| TiO2@CuO-N-rGO/p(L-Cys) | TiO2@CuO hybrid nanoparticles-N-doped reduced Graphene Oxide-poly(L-Cysteine) |
| TMP | Temazepam |
| TPB | Tetraphenyl Borate |
| TZP | Tetrazepam |
References
- Balon, R.; Starcevic, V. Role of Benzodiazepines in Anxiety Disorders. In Anxiety Disorder: Rethinking and Understanding Recent Discoveries, 1st ed.; Kim, Y.K., Ed.; Springer Nature: Singapore, 2020; Volume 1191, pp. 367–387. [Google Scholar] [CrossRef]
- Offidani, E.; Guidi, J.; Tomba, E.; Fava, G.A. Efficacy and Tolerability of Benzodiazepines versus Antidepressants in Anxiety Disorders: A Systematic Review and Meta-Analysis. Psychother. Psychosom. 2013, 82, 355–362. [Google Scholar] [CrossRef]
- Lader, M. Benzodiazepines revisited—Will we ever learn? Addiction 2011, 106, 2086–2109. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alsayegh, A.A.; Abusudah, W.F.; Almohmadi, N.H.; Eldahshan, O.A.; Ahmed, E.A.; Batiha, G.E.S. Insights on Benzodiazepines’ Potential in Alzheimer’s Disease. Life Sci. 2023, 320, 121532. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Nix, C.A.; Hollier, J.; Sagrera, C.E.; Delacroix, B.M.; Abubakar, T.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. Benzodiazepines: Uses, Dangers, and Clinical Considerations. Neurol. Int. 2021, 13, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Freire, I.; Brunetti, P.; Cabarcos-Fernández, P.; Fernández-Liste, A.; Tabernero-Duque, M.; Bermejo-Barrera, A. Determination of Benzodiazepines in Pericardial Fluid by Gas Chromatography-Mass Spectrometry. J. Pharm. Biomed. Anal. 2018, 159, 45–52. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Nix, C.A.; Odisho, A.S.; Babin, C.P.; Derouen, A.G.; Lutfallah, S.C.; Cornett, E.M.; Murnane, K.S.; Kaye, A.M.; Kaye, A.D. Novel Designer Benzodiazepines: Comprehensive Review of Evolving Clinical and Adverse Effects. Neurol. Int. 2022, 14, 648–663. [Google Scholar] [CrossRef]
- Hirschtritt, M.E.; Olfson, M.; Kroenke, K. Balancing the Risks and Benefits of Benzodiazepines. JAMA 2021, 325, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Honeychurch, K.C.; Hart, J.P. Electrochemical Detection of Benzodiazepines, Following Liquid Chromatography, for Applications in Pharmaceutical, Biomedical and Forensic Investigations. Insciences J. 2014, 4, 1–18. [Google Scholar] [CrossRef]
- Arora, N.; Dhiman, P.; Kumar, S.; Singh, G.; Monga, V. Recent Advances in Synthesis and Medicinal Chemistry of Benzodiazepines. Bioorg. Chem. 2020, 97, 103668. [Google Scholar] [CrossRef]
- Silberman, E.; Balon, R.; Starcevic, V.; Shader, R.; Cosci, F.; Fava, G.A.; Nardi, A.E.; Salzman, C.; Sonino, N. Benzodiazepines: It’s Time to Return to the Evidence. Br. J. Psychiatry 2021, 218, 125–127. [Google Scholar] [CrossRef]
- Jain, B.; Jain, R.; Nowak, P.M.; Ali, N.; Ansari, M.N.; Kabir, A.; Chandravanshi, L.P.; Sharma, S. Comparison of Various Sample Preparation Methods for Benzodiazepines in Terms of the Principles of White Analytical Chemistry. TrAC Trends Anal. Chem. 2024, 171, 117524. [Google Scholar] [CrossRef]
- Persona, K.; Madej, K.; Knihnicki, P.; Piekoszewski, W. Analytical Methodologies for the Determination of Benzodiazepines in Biological Samples. J. Pharm. Biomed. Anal. 2015, 113, 239–264. [Google Scholar] [CrossRef]
- Qriouet, Z.; Qmichou, Z.; Bouchroutrouch, N.; Mahi, H.; Cherrah, Y.; Sefrioui, H. Analytical Methods Used for the Detection and Quantification of Benzodiazepines. J. Anal. Meth. Chem. 2019, 2019, 2035492. [Google Scholar] [CrossRef]
- Yafout, M.; Mouss, R.A.; Bouchafra, H.; Zarayby, L.; El-Otmani, I.S. Overview of the Bioanalytical Methods Used for the Determination of Benzodiazepines in Biological Samples and Their Suitability for Emergency Toxicological Analysis. J. Pharmacol. Toxicol. Meth. 2023, 123, 107294. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Zhang, Y.; Bian, Y.; Liu, Y.-J.; Ren, A.; Zhou, Y.; Shi, D.; Feng, X.-S. Benzodiazepines in Complex Biological Matrices: Recent Updates on Pretreatment and Detection Methods. J. Pharm. Anal. 2023, 13, 442–462. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, I.S.; Vázquez-Garrido, I.; Islas, G.; Flores-Aguilar, J.F. New Trends in the Methodologies of Determination of Benzodiazepine Residues in Biological Samples. Separations 2025, 12, 95. [Google Scholar] [CrossRef]
- Piergiovanni, M.; Cappiello, A.; Famiglini, G.; Termopoli, V.; Palma, P. Determination of Benzodiazepines in Beverages Using Green Extraction Methods and Capillary HPLC-UV Detection. J. Pharm. Biomed. Anal. 2018, 154, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Montesano, C.; Babino, P.; Carboni, S.; Napoletano, S.; De Sangro, G.; Di Rosa, F.; Gregori, A.; Curini, R.; Sergi, M. Finding Evidence at a Crime Scene: Sensitive Determination of Benzodiazepine Residues in Drink and Food Paraphernalia by HPLC-HRMS/MS. Forensic Chem. 2021, 23, 100327. [Google Scholar] [CrossRef]
- Gupta, N.; Thakur, R.S.; Patel, D.K. Detection, Quantification and Degradation Kinetic for Five Benzodiazepines Using VAUS-ME-SFO/LC-MS/MS Method for Water, Alcoholic and Non-Alcoholic Beverages. Talanta 2023, 260, 124572. [Google Scholar] [CrossRef]
- Miri, M.S.; Badakhshan, D.; Akhgari, M.; Ghadipasha, M. Chemical Components Analysis of Handmade Alcoholic Beverages with Special Focus on Psychoactive Drugs as Adulterants. MethodsX 2025, 14, 103230. [Google Scholar] [CrossRef]
- Yen, Y.-T.; Lin, Y.-S.; Chen, T.-H.; Chyueh, S.-C.; Chang, H.-T. A Carbon-Dot Sensing Probe for Screening of Date Rape Drugs: Nitro-containing Benzodiazepines. Sens. Actuators B Chem. 2020, 305, 127441. [Google Scholar] [CrossRef]
- Poves-Ruiz, I.; Azuaje-Hualde, E.; Corchado-Gonzalez, I.; Basabe-Desmonts, L.; Benito-Lopez, F. Autonomous Microfluidic Device for the Naked-Eye Detection of Benzodiazepines in Adulterated Beverages. Anal. Chim. Acta 2025, 1368, 344309. [Google Scholar] [CrossRef]
- Balamurugan, T.S.T.; Kwaczyński, K.; Rizwan, M.; Poltorak, L. Current Trends in Rapid Electroanalytical Screening of Date Rape Drugs in Beverages. TrAC Trends Anal. Chem. 2024, 175, 117712. [Google Scholar] [CrossRef]
- Nunes, C.N.; dos Anjos, V.E.; Quinaia, S.P. Determination of Diazepam and Clonazepam in Natural Water—A Voltammetric Study. Electroanalysis 2018, 30, 109–118. [Google Scholar] [CrossRef]
- Cunha, D.L.; de Araujo, F.G.; Marques, M. Psychoactive Drugs: Occurrence in Aquatic Environment, Analytical Methods, and Ecotoxicity—A Review. Environ. Sci. Pollut. Res. 2017, 24, 24076–24091. [Google Scholar] [CrossRef]
- Guo, H.; Chen, J.; Jiang, G.; Mei, Y.; Gong, Z.; Liu, M.; Li, J.; Gan, J. Determination of 14 Benzodiazepine Multiresidues in Aquaculture Environment by Ultra-High-Performance Liquid Chromatography—Tandem Mass Spectrometry. Molecules 2025, 30, 775. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.N.; Pauluk, L.E.; dos Anjos, V.E.; Lopes, M.C.; Quinaia, S.P. New Approach to the Determination of Contaminants of Emerging Concern in Natural Water: Study of Alprazolam Employing Adsorptive Cathodic Stripping Voltammetry. Anal. Bioanal. Chem. 2015, 407, 6171–6179. [Google Scholar] [CrossRef]
- Barhoum, A.; Naseef, A.; Ahmed, Y.M.; Zahran, M.K.; Alhashemi, Y.; Mohamed, M.S.; Rizk, M.S.; Abdel-Haleem, F.M. Modern Designs of Electrochemical Sensor for Accurate Drug Analysis in Pharmaceutical and Biological Samples: Principles, Nanofabrication, and key challenges. Mater. Chem. Phys. 2025, 337, 130588. [Google Scholar] [CrossRef]
- Honeychurch, K.C. Review of Electroanalytical-Based Approaches for the Determination of Benzodiazepines. Biosensors 2019, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Chand, R.; Kumar, S.; Mittal, N.; Srinivasan, S.; Rajabzadeh, A.R. Recent Biosensing Advances in the Rapid Detection of Illicit Drugs. TrAC Trends Anal. Chem. 2020, 131, 116006. [Google Scholar] [CrossRef]
- Anzar, N.; Suleman, S.; Parvez, S.; Narang, J. A Review on Illicit Drugs and Biosensing Advances for its Rapid Detection. Process Biochem. 2022, 113, 113–124. [Google Scholar] [CrossRef]
- Dagar, M.; Yadav, S.; Sai, V.V.R.; Satija, J.; Bhatia, H. Emerging Trends in Point-of-Care Sensors for Illicit Drugs Analysis. Talanta 2022, 238, 123048. [Google Scholar] [CrossRef]
- Loftsson, T. 1,4-Benzodiazepines: Chemical Stability and Cyclodextrin Solubilization. J. Drug Deliv. Sci. Tec. 2021, 66, 102936. [Google Scholar] [CrossRef]
- Schram, J.; Parrilla, M.; Sleegers, N.; Slosse, A.; Van Durme, F.; van Nuijs, A.L.N.; De Wael, K. Electrochemical Classification of Benzodiazepines: A Comprehensive Approach Combining Insights from Voltammetry and Liquid Chromatography—Mass Spectrometry. Talanta 2024, 279, 126623. [Google Scholar] [CrossRef]
- Naggar, A.H.; El Kaoutit, M.; Naranjo-Rodriguez, I.; El-Sayed, A.Y.; de Cisneros, J.L.H.H. Voltammetric and Spectroscopic Investigation of the Interaction Between 1,4-Benzodiazepines and Bovine Serum Albumin. J. Solut. Chem. 2016, 45, 1659–1678. [Google Scholar] [CrossRef]
- Narang, J.; Malhotra, N.; Singhal, C.; Mathur, A.; Anoop Krishna, P.N.; Pundir, C.S. Detection of Alprazolam with a Lab on Paper Economical Device Integrated with Urchin Like Ag@ Pd Shell Nano-Hybrids. Mater. Sci. Eng. C 2017, 80, 728–735. [Google Scholar] [CrossRef]
- Mazurków, J.M.; Montiel, N.F.; Van Echelpoel, R.; Kusior, A.; De Wael, K. The Potential of Electrochemical Sensors to Unveil Counterfeits: Xanax as a Case Study. Electrochim. Acta 2024, 494, 144458. [Google Scholar] [CrossRef]
- Boonmee, W.; Samoson, K.; Yodrak, J.; Thiagchanya, A.; Phonchai, A.; Limbut, W. Adsorptive Cathodic Stripping Voltammetry for Quantification of Alprazolam. Molecules 2021, 26, 2958. [Google Scholar] [CrossRef]
- Suleman, S.; Anzar, N.; Patil, S.; Ansari, S.; Jahan, F.; Narang, J. Development of an Electrochemical Paper Based Multiplex Analytical Device for the Detection of “Illicit Drugs” Employing Silver Nanoparticles. Mater. Chem. Phys. 2025, 338, 130649. [Google Scholar] [CrossRef]
- Phua, C.H.; Saisahas, K.; Soleh, A.; Promsuwan, K.; Saichanapan, J.; Limbut, W. Electrode Modified with Reduced Graphene Oxide Decorated with Gold Nanoparticles co-induced by Laser for Electrochemical Alprazolam Sensor. Microchem. J. 2023, 195, 109380. [Google Scholar] [CrossRef]
- Samiec, P.; Švorc, Ľ.; Stanković, D.M.; Vojs, M.; Marton, M.; Navrátilová, Z. Mercury-Free and Modification-Free Electroanalytical Approach Towards Bromazepam and Alprazolam Sensing: A Facile and Efficient Assay for their Quantification in Pharmaceuticals Using Boron-Doped Diamond Electrodes. Sens. Actuators B Chem. 2017, 245, 963–971. [Google Scholar] [CrossRef]
- Samiec, P.; Navrátilová, Z. Electrochemical Behaviour of Bromazepam and Alprazolam and their Determination in the Pharmaceutical Tablets Lexaurin and Xanax on Carbon Paste Electrode. Monatsh. Chem. 2017, 148, 449–455. [Google Scholar] [CrossRef]
- Sadrabadi, E.A.; Khosravi, F.; Benvidi, A.; Shiralizadeh Dezfuli, A.; Khashayar, P.; Khashayar, P.; Azimzadeh, M. Alprazolam Detection Using an Electrochemical Nanobiosensor Based on AuNUs/Fe-Ni@rGO Nanocomposite. Biosensors 2022, 12, 945. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, S.A.; Arab, H.H. Potentiometric Sensors for the Selective Determination of Benzodiazepine Drug Residues in Real Wastewater Effluents. Chemosensors 2022, 10, 74. [Google Scholar] [CrossRef]
- Khoshroo, A.; Hosseinzadeh, L.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Ahmadi, F. Silver Nanofibers/Ionic Liquid Nanocomposite Based Electrochemical Sensor for Detection of Clonazepam via Electrochemically Amplified Detection. Microchem. J. 2019, 145, 1185–1190. [Google Scholar] [CrossRef]
- Abedi, F.; Rajabi, H.R.; Roushani, M.; Rafiee, Z.; Rahmati, E. Mesoporous silica nanoparticles decorated with C3N4 framework as a novel electrocatalyst for the design of a selective clonazepam sensor. J. Mater. Res. Technol. 2024, 29, 5731–5740. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Brooks, J.; Hart, J.P. Development of a Voltammetric Assay Using Screen-Printed Electrodes for Clonazepam and its Application to Beverage and Serum Samples. Talanta 2016, 147, 510–515. [Google Scholar] [CrossRef]
- Lotfi, S.; Veisi, H. Electrochemical Determination of Clonazepam Drug Based on Glassy Carbon Electrode Modified with Fe3O4/R-SH/Pd Nanocomposite. Mater. Sci. Eng. C 2019, 103, 109754. [Google Scholar] [CrossRef]
- Russo, J.M.; Quigley, A.F.; Kapsa, R.M.I.; Moulton, S.E.; Guijt, R.; Silva, S.M.; Greene, G.W. A Simple Electrochemical Swab Assay for the Rapid Quantification of Clonazepam in Unprocessed Saliva Enabled by Lubricin Antifouling Coatings. ChemElectroChem 2020, 7, 2851–2858. [Google Scholar] [CrossRef]
- Shahparast, S.; Asadpour-Zeynali, K. Development of a Novel and Highly Sensitive Electrochemical Sensor Based on FeCu-LDH@MXene Nanocomposite for the Selective Determination of Clonazepam. Microchem. J. 2025, 211, 113095. [Google Scholar] [CrossRef]
- Garima; Sachdev, A.; Matai, I. An Electrochemical Sensor Based on Cobalt Oxyhydroxide Nanoflakes/Reduced Graphene Oxide Nanocomposite for Detection of Illicit Drug-Clonazepam. J. Electroanal. Chem. 2022, 919, 116537. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Khaloo, S.S.; Mirzaie, R.A. Klonopin Assay Using Modified Electrode with Multiwalled Carbon Nanotubes and poly Melamine Nanocomposite. Mater. Sci. Eng. C 2019, 99, 121–128. [Google Scholar] [CrossRef]
- Majidi, M.R.; Ghaderi, S.; Asadpour-Zeynali, K.; Dastangoo, H. Synthesis of Dendritic Silver Nanostructures Supported by Graphene Nanosheets and its Application for Highly Sensitive Detection of Diazepam. Mater. Sci. Eng. C 2015, 57, 257–264. [Google Scholar] [CrossRef]
- Dehghanzade, M.; Alipour, E. Voltammetric Determination of Diazepam Using a Bismuth Modified Pencil Graphite Electrode. Anal. Methods 2016, 8, 1995–2004. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Khoshroo, A.; Mazloum-Ardakani, M. Electrochemical Determination of Diazepam in Real Samples Based on Fullerene-Functionalized Carbon Nanotubes/Ionic Liquid Nanocomposite. Sens. Actuators B Chem. 2017, 240, 125–131. [Google Scholar] [CrossRef]
- Hassan, S.A.; ElDin, N.B.; Zaazaa, H.E.; Moustafa, A.A.; Mahmoud, A.M. Point-of-Care Diagnostics for Drugs of Abuse in Biological Fluids: Application of a Microfabricated Disposable Copper Potentiometric Sensor. Microchim. Acta 2020, 187, 491. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Borges, I.; Goularte, R.B.; Vanoni, C.R.; Lima, A.R.S.; Scheide, M.R.; Mezalira, D.Z.; Vitali, L.; Stulzer, H.K.; Jost, C.L. Exploiting the synergistic effects of combined carbon-based materials for rape drug diazepam electrochemical sensing: Application to simulated casework matrices. Electroanalysis 2024, 36, e202300231. [Google Scholar] [CrossRef]
- Narang, J.; Singhal, C.; Mathur, A.; Khanuja, M.; Varshney, A.; Garg, K.; Dahiya, T.; Pundir, C.S. Lab on paper chip integrated with Si@GNRs for electroanalysis of diazepam. Anal. Chim. Acta 2017, 980, 50–57. [Google Scholar] [CrossRef]
- Adegoke, O.; Oyinlola, K.; Adeniyi, K.O.; Achadu, O.J.; Yang, Z.; Daeid, N.N. An Organic-Inorganic Polyacrylamide-Based Surface Imprinted Quantum Dots for the Impedimetric and Voltammetric Detection of Diazepam in Saliva with Smartphone Readout. Talanta 2025, 285, 127400. [Google Scholar] [CrossRef]
- Antunovic, V.; Tesanovic, S.; Peruskovic, D.; Stevanovic, N.; Baosic, R.; Mandic, S.; Lolic, A. Development of a Flow Injection System for Differential Pulse Amperometry and Its Application for Diazepam Determination. J. Anal. Meth. Chem. 2018, 2018, 6121489. [Google Scholar] [CrossRef] [PubMed]
- Antunovic, V.; Baosic, R.; Lolic, A. Voltammetric Determination of Diazepam on Antimony Film Screen-Printed Electrode in Pharmaceutical Formulations. Curr. Pharm. Anal. 2021, 17, 945–950. [Google Scholar] [CrossRef]
- Haššo, M.; Kekeľáková, A.; Hanko, M.; Švorc, Ľ. Powerful Analytical Platform for Diazepam Determination in Pharmaceuticals and Alcoholic Drinks Based on Batch Injection Analysis Coupled with Adsorptive Stripping Voltammetry. J. Electrochem. Soc. 2024, 171, 047517. [Google Scholar] [CrossRef]
- Corsato, P.C.R.; de Lima, L.F.; Paschoarelli, M.V.; de Araujo, W.R. Electrochemical sensing at the fingertips: Wearable glove-based sensors for detection of 4-nitrophenol, picric acid and diazepam. Chemosphere 2024, 363, 142771. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Sharma, S.; Kaur, J.; Mehta, S.K. Graphene Oxide/Lysine Composite—A Potent Electron Mediator for Detection of Diazepam. Anal. Methods 2018, 10, 5038–5046. [Google Scholar] [CrossRef]
- Hosseini, M.R.M.; Motaharioan, A. Electroanalytical Determination of Diazepam in Tablet and Human Serum Samples Using a Multiwalled Carbon Nanotube Embedded Molecularly Imprinted Polymer-Modified Carbon Paste Electrode. RSC Adv. 2015, 5, 81650–81659. [Google Scholar] [CrossRef]
- Sohouli, E.; Ghalkhani, M.; Rostami, M.; Rahimi-Nasrabadi, M.; Ahmadi, F. A Noble Electrochemical Sensor Based on TiO2@CuO-N-rGO and Poly (L-Cysteine) Nanocomposite Applicable for Trace Analysis of Flunitrazepam. Mater. Sci. Eng. C 2020, 117, 111300. [Google Scholar] [CrossRef]
- Asiabar, B.M.; Karimi, M.A.; Tavallali, H.; Rahimi-Nasrabadi, M. Application of MnFe2O4 and AuNPs modified CPE as a sensitive flunitrazepam electrochemical sensor. Microchem. J. 2021, 161, 105745. [Google Scholar] [CrossRef]
- Ghanbari, M.H.; Norouzi, Z.; Ghanbari, M.M. Using a Nanocomposite Nonsist of Boron-Noped Reduced Graphene Oxide and Electropolymerized β-Cyclodextrin for Flunitrazepam Electrochemical Sensor. Microchem. J. 2020, 156, 104994. [Google Scholar] [CrossRef]
- Tseliou, F.; Pappas, P.; Spyrou, K.; Hrbac, J.; Prodromidis, M.I. Lab-on-a-Screen-Printed Electrochemical Cell for Drop-Volume Voltammetric Screening of Flunitrazepam in Untreated, Undiluted Alcoholic and Soft Drinks. Biosens. Bioelectron. 2019, 132, 136–142. [Google Scholar] [CrossRef]
- Mohammadnia, M.S.; Naghian, E.; Ghalkhani, M.; Nosratzehi, F.; Adib, K.; Zahedi, M.M.; Nasrabadi, M.R.; Ahmadi, F. Fabrication of a New Electrochemical Sensor Based on Screen-Printed Carbon Electrode/Amine-Functionalized Graphene Oxide-Cu Nanoparticles for Rohypnol Direct Determination in Drink Sample. J. Electroanal. Chem. 2021, 880, 114764. [Google Scholar] [CrossRef]
- Poulladofonou, G.; Freris, C.; Economou, A.; Kokkinos, C. Wearable electronic finger for date rape drugs screening: From “do-it-yourself” fabrication to self-testing. Anal. Chem. 2022, 94, 4087–4094. [Google Scholar] [CrossRef]
- Chen, S.; Huang, W.; Qiu, Y.; Zhang, X.; Kong, J. Detection of FlunitrazepamIn Biological Fluids and Beverages: A Review. ChemistrySelect 2023, 8, e202301659. [Google Scholar] [CrossRef]
- Chenarani, S.; Ebrahimi, M.; Arabali, V.; Beyramadi, S.A. Determination of Lorazepam Using the Electrocatalytic Effect of NiO/SWCNTs Modified Carbon Paste Electrode as a Powerful Sensor. Top. Catal. 2022, 65, 733–738. [Google Scholar] [CrossRef]
- Vafadar, M.; Zarei, E.; Asghari, A. Electrochemical Measurement of Noscapine and Lorazepam Using a Carbon Paste Electrode Modified with Multi-walled Carbon Nanotubes and Natural Deep Eutectic Solvent. Iran. J. Pharm. Res. 2021, 20, 490–505. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.S.; Mahmoud, A.M.; Elghobashy, M.R.; Zaazaa, H.E.; Sedik, G.A. Eco-Friendly Electrochemical Sensor for Determination of Conscious Sedating Drug “Midazolam’’ Based on Au-NPs@Silica Modified Carbon Paste Electrode. Talanta 2024, 267, 125238. [Google Scholar] [CrossRef]
- Panahi, Y.; Motaharian, A.; Hosseini, M.R.M.; Mehrpour, O. High Sensitive and Selective Nano-Molecularly Imprinted Polymer Based Electrochemical Sensor for Midazolam Drug Detection in Pharmaceutical Formulation and Human Urine Samples. Sens. Actuat. B Chem. 2018, 273, 1579–1586. [Google Scholar] [CrossRef]
- Rocha, R.G.; Silva, P.W.; Sousa, R.M.F.; Junior, M.C.; Santana, M.H.P.; Munoz, R.A.A.; Richter, E.M. Investigation of Midazolam Electro-Oxidation on Boron Doped Diamond Electrode by Voltammetric Techniques and Density Functional Theory Calculations: Application in Beverage Samples. Talanta 2020, 207, 120319. [Google Scholar] [CrossRef] [PubMed]
- Fritea, L.; Bănică, F.; Costea, T.O.; Moldovan, L.; Iovan, C.; Cavalu, S. A gold nanoparticles—Graphene based electrochemical sensor for sensitive determination of nitrazepam. J. Electroanal. Chem. 2018, 830–831, 63–71. [Google Scholar] [CrossRef]
- Shahraki, S.; Ahmar, H.; Nejati-Yazdinejad, M. Electrochemical Determination of Nitrazepam by Switchable Solvent Based Liquid-Liquid Microextraction Combined with Differential Pulse Voltammetry. Microchem. J. 2018, 142, 229–235. [Google Scholar] [CrossRef]
- Kaur, G.; Garima; Prakash, V.; Gupta, S.; Chaudhary, M.K.; Mehta, S.K.; Sharma, S. Graphene Oxide Functionalized Halloysite Nanotubes for Voltammetric Determination of Psychoactive Drug from Alcoholic and Non-Alcoholic Drinks. FlatChem 2025, 49, 100794. [Google Scholar] [CrossRef]
- Muthusankar, G.; Sangili, A.; Chen, S.-M.; Karkuzhali, R.; Sethupathi, M.; Gopu, G.; Karthick, S.; Devi, R.K.; Sengottuvelan, N. In Situ Assembly of Sulfur-Doped Carbon Quantum Dots Surrounded Iron(III) Oxide Nanocomposite; A Novel Electrocatalyst for Highly Sensitive Detection of Antipsychotic Drug Olanzapine. J. Mol. Liq. 2018, 268, 471–480. [Google Scholar] [CrossRef]
- Tüzün, Ü.N.; Yıldız, C.; Eskiköy Bayraktepe, D.; Polatt, K.; Yazan, Z. Electrochemical Fabrication of poly(L-Alanine)-Gold Nanoparticle Nanocomposite-Modified Electrode: Application for Determination and Mechanism of Antipsychotic Drug Olanzapine. Monatsh. Chem. 2023, 154, 95–104. [Google Scholar] [CrossRef]
- Khalilzadeh, A.; Soleymanpour, A.; Zarei, K.N. P-Doped Carbon Nano-Onions and Graphene Nanoplate Modified Pencil Graphite Electrode to Determine Trace Amounts of Olanzapine in Biological Environments. Microchem. J. 2024, 205, 111334. [Google Scholar] [CrossRef]
- Rouhani, M.; Soleymanpour, A. A New Selective Carbon Paste Electrode for Potentiometric Analysis of Olanzapine. Measurement 2019, 140, 472–478. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Mahnashi, M.H.; Alkahtani, S.A.; El-Wekil, M.M. Nitrogen and Sulfur Co-Doped Graphene Quantum Dots/Nanocellulose Nanohybrid for Electrochemical Sensing of Anti-Schizophrenic Drug Olanzapine in Pharmaceuticals and Human Biological Fluids. Int. J. Biol. Macromol. 2020, 165, 2030–2037. [Google Scholar] [CrossRef]
- Cardozo, J.C.; da Silva, D.R.; Martínez-Huitle, C.A.; Quiroz, M.A.; dos Santos, E.V. The Versatile Behavior of Diamond Electrodes—Electrochemical Examination of the Anti-Psychotic Drug Olanzapine (OL) Oxidation as a Model Organic Aqueous Solution. Electrochim. Acta 2022, 411, 140063. [Google Scholar] [CrossRef]
- Bacil, R.P.; Garcia, P.H.M.; de Araujo, W.R.; Serrano, S.H.P. Mechanism and Kinetics of Olanzapine and Quetiapine Oxidations at Glassy Carbon Electrode. Electrochim. Acta 2021, 368, 137683. [Google Scholar] [CrossRef]
- Shukla, R.P.; Belmaker, R.H.; Bersudsky, Y.; Ben Yoa, H. A Platinum Black-Modified Microelectrode for in situ Olanzapine Detection in Microliter Volumes of Undiluted Serum. J. Neural Transm. 2020, 127, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Behzad, L.; Gholivand, M.B.; Shamsipur, M.; Gholivand, K.; Barati, A.; Gholami, A. Highly Sensitive Voltammetric Sensor Based on Immobilization of Bisphosphoramidate-Derivative and Quantum Dots onto Multi-Walled Carbon Nanotubes Modified Gold Electrode for the Electrocatalytic Determination of Olanzapine. Mater. Sci. Eng. C 2016, 60, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, R.P. Use of sodium tetraphenyl boron for fabrication of potentiometric membrane sensor for the assay of olanzapine in pharmaceuticals and human urine. App. Chem. Eng. 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Khoshroo, A.; Hosseinzadeh, L.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Ehrlich, H. Development of electrochemical sensor for sensitive determination of oxazepam based on silver-platinum core–shell nanoparticles supported on graphene. J. Electroanal. Chem. 2018, 823, 61–66. [Google Scholar] [CrossRef]
- Behravan, M.; Aghaie, H.; Giahi, M.; Maleknia, L. A glassy carbon electrode modified with boron-doped graphene oxide/polyaspartic acid for electrochemical determination of oxazepam. Iran. J. Catal. 2022, 12, 353–363. [Google Scholar] [CrossRef]
- Ma, H.; Tian, Q. Application of Nitrogen-Doped Carbon Particles Modified Electrode for Electrochemical Determination of Tetrazepam as Muscle Relaxant Drug. Int. J. Electrochem. Sci. 2023, 18, 100084. [Google Scholar] [CrossRef]
- Samiec, P.; Navrátilová, Z.; Fischer, J. Voltammetry of benzodiazepines on meniscus-modified silver solid amalgam electrode. Monatsh. Chem. 2016, 147, 127–134. [Google Scholar] [CrossRef]
- Vahidifar, M.; Es’haghi, Z. Magnetic Nanoparticle-Reinforced Dual-Template Molecularly Imprinted Polymer for the Simultaneous Determination of Oxazepam and Diazepam Using an Electrochemical Approach. J. Anal. Chem. 2022, 77, 625–639. [Google Scholar] [CrossRef]
- Stelmaszczyk, P.; Kwaczyński, K.; Rudnicki, K.; Skrzypek, S.; Wietecha-Posłuszny, R.; Poltorak, L. Nitrazepam and 7-aminonitrazepam studied at the macroscopic and microscopic electrified liquid-liquid interface. Microchim. Acta 2023, 190, 182. [Google Scholar] [CrossRef] [PubMed]
- Shahinfard, H.; Shabani-Nooshabadi, M.; Reisi-Vanani, A.; Darabi, R. Electrochemical Sensor Based on CuO/reduced Graphene Nanoribbons and Ionic Liquid for Simultaneous Determination of Tramadol, Olanzapine and Acetaminophen. Carbon Lett. 2023, 33, 1433–1444. [Google Scholar] [CrossRef]
- Ashrafi, H.; Hasanzadeh, M.; Ansarin, K.; Ozkan, S.A.; Jouyban, A. Application of Chitosan as Biocompatible Polysaccharide in Quantification of Some Benzodiazepines Affecting Sleep Disorders: A New Platform for Preparation of Bioactive Scaffolds. Int. J. Biol. Macromol. 2018, 120, 2466–2481. [Google Scholar] [CrossRef]
- Ashrafi, H.; Mobed, A.; Hasanzadeh, M.; Babaie, P.; Ansarin, K.; Jouyban, A. Monitoring of Five Benzodiazepines Using a Novel Polymeric Interface Prepared by Layer by Layer Strategy. Microchem. J. 2019, 146, 121–125. [Google Scholar] [CrossRef]
- Riman, D.; Rozsypal, J.; Halouzka, V.; Hrbac, J.; Jirovsky, D. The use of micro carbon pencil lead electrode for sensitive HPLC-ED analysis of selected antipsychotic drugs. Microchem. J. 2020, 154, 104606. [Google Scholar] [CrossRef]
- Ashrafi, H.; Hassanpour, S.; Saadati, A.; Hasanzadeh, M.; Ansarin, K.; Ozkan, S.A.; Shadjou, N.; Jouyban, A. Sensitive Detection and Determination of Benzodiazepines using Silver Nanoparticles-N-GQDs Ink Modified Electrode: A New Platform for Modern Pharmaceutical Analysis. Microchem. J. 2019, 145, 1050–1057. [Google Scholar] [CrossRef]
- Beduk, D.; Beduk, T.; de Oliveira Filho, J.I.; Lahcen, A.A.; Aldemir, E.; Celik, E.G.; Salama, K.N.; Timur, S. Smart Multiplex Point-of-Care Platform for Simultaneous Drug Monitoring. ACS Appl. Mater. Interfaces 2023, 15, 37247–37258. [Google Scholar] [CrossRef]
- Paschoarelli, M.V.; Kavai, S.M.; de Lima, L.F.; de Araujo, W.R. Laser-Scribing Fabrication of a Disposable Electrochemical Device for Forensic Detection of Crime Facilitating Drugs in Beverage Samples. Talanta 2023, 255, 124214. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, T.S.T.; Laroui, A.; Wietecha-Posłuszny, R.; Poltorak, L. Instant On-Site Screening and Discrimination of Designer Benzodiazepine Date Rape Drugs in Spiked Beverages at Electrified Liquid-Liquid Interface. Sens. Actuators B Chem. 2025, 443, 138228. [Google Scholar] [CrossRef]
- Herrera-Chacón, A.; Torabi, F.; Faridbod, F.; Ghasemi, J.B.; González-Calabuig, A.; del Valle, M. Voltammetric Electronic Tongue for the Simultaneous Determination of Three Benzodiazepines. Sensors 2019, 19, 5002. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).